Abstract
Signaling through Toll-like receptor-9 (TLR9), a mediator of innate immune responses, could have a role in the pathogenesis of systemic lupus erythematosus (SLE). Some studies have shown an association between polymorphisms in the TLR9 gene and disease manifestations. We investigated whether two single nucleotide polymorphisms (-1486 T>C and +1174 G>A) in the TLR9 gene are associated with the risk of renal involvement in SLE. DNA samples from 112 SLE patients (62 with lupus nephritis) and 100 healthy controls were obtained. TLR9 polymorphisms (-1486 T>C and +1174 G>A) were analyzed by polymerase chain reaction–restriction fragment length polymorphism. Genotype and allelic frequencies were compared between lupus patients and healthy controls. Clinical and laboratory manifestations and activity scores on renal biopsy of patients with lupus nephritis were compared between various genotypes. There was no difference in the frequency of genotype or allele distribution at either of the two loci between lupus patients and controls and in lupus patients with or without nephritis. Patients with CC/CT genotype at the -1486 position had higher serum creatinine (P = 0.03) and Austin activity scores (P = 0.015). Patients with AA/AG genotype at +1174 position showed higher serum creatinine (P = 0.04), proteinuria (P = 0.011), anti-dsDNA titers (P < 0.001) and Austin activity scores (P = 0.003) than the GG genotype. Variations at the -1486 and +1174 positions of TLR9 gene are not associated with increased risk of SLE or that of kidney involvement in North Indians. CC/CT genotypes at -1486 and AA/AG at +1174 positions are associated with more severe kidney disease at presentation.
Keywords: Genetics, lupus nephritis, systemic lupus erythematosus, toll-like receptor
Introduction
Systemic lupus erythematosus (SLE), the prototypic systemic autoimmune disease, is characterized by B-cell hyperreactivity and production of autoantibodies against antigens consisting of nucleic acid–protein complexes such as chromatin and small nuclear ribonuceoproteins (snRNPs).[1] According to one hypothesis, failure in the clearance of apoptotic lymphocytes resulting in the release of nucleosomes with abnormally CG-rich DNA is the primary trigger for this phenomenon.[2,3]
The family of toll-like receptors (TLR) is a key component of innate immunity, and identifies conserved microbial patterns. Activation of TLRs is followed by recruitment of adapter proteins; the resulting signaling cascade leads to generation of proinflammatory cytokines and IFN-g, similar to that seen in SLE.[4] A subset of TLRs, located in endosomes, recognize single- and double-stranded RNA and unmethylated CpG DNA, important for defense against viruses. Because of this property, TLRs have been implicated in the genesis of autoimmune diseases such as rheumatoid arthritis, multiple sclerosis and SLE.[5] Of the 11 identified members of the family, TLR 7 and 9 have been implicated in human and experimental SLE. TLR9-deficient mice exhibit defective suppressive activity of regulatory T cells and exacerbate disease in several murine models of SLE.[6] However, it remains to be clarified whether TLR9 is causative or protective for human SLE.[7,8]
The association between genetic variations of TLR9 with SLE has been examined in a few studies. Studies from UK, Korea and Hong Kong failed to find an association of TLR9 gene variations with SLE susceptibility,[9–11] whereas in Japanese patients, presence of a G allele at position +1174 located in intron 1 and C allele at position -1486 of TLR9 was associated with an increased risk of SLE.[12] Another study in the Chinese Han population showed an increased frequency of G allele at the +1174 position in subjects with lupus nephritis.[13] TLR9 expression is increased on B lymphocytes of patients with SLE.[14]
In the present study, we investigated whether patients with SLE with and without renal involvement exhibited any variation at the +1174 and -1486 loci of the TLR9 gene.
Materials and Methods
This study was carried out at the Postgraduate Institute of Medical Education and Research, Chandigarh, a major tertiary care hospital in north India. The study protocol was approved by the Institute Ethics Committee. A total of 112 consecutive patients who fulfilled the 1997 American College of Rheumatology (ACR) criteria of SLE[15] were enrolled prospectively. Patients who did not give consent and those with overlap syndromes were excluded.
The activity of SLE was measured by the systemic lupus erythematosus disease activity index (SLE-DAI).[16] Renal involvement was defined by demonstration of one or more of the following: elevated serum creatinine (>1.5 mg/ dL), proteinuria >500 mg/day or active sediments in the second morning sample of urine in the absence of any other cause. All cases with clinical evidence of kidney disease underwent renal biopsy and were classified according to the International Society of Nephrology-Renal Pathology Society 2003 classification.[17] Austin index was used for scoring activity on biopsy. Patients with Class III/IV disease were treated with a combination of cyclophosphamide and corticosteroids according to the NIH protocol or Eurolupus regimen.[18]
Complete remission was defined as decrease in proteinuria to <1g/day (or <0.3g/day in cases of lupus nephritis diagnosed in the past 6 months) and partial remission as decrease in proteinuria by > 50% (to <1.5 g/d) with normal serum albumin concentrations, inactive urine sediment and improved or stable renal function.[18]
A total of 100 healthy volunteers were also genotyped. Genomic DNA was extracted from peripheral blood mononuclear cells. The functional DNA polymorphisms in TLR9, the -1486 T>C in promoter (rs187084) and +1174 A>G in intron 1 (rs352139) have been previously described.[19] The -1486 T>C polymorphism (rs187084) was determined by polymerase chain reaction (PCR) amplification using specific forward (5‘-TCATTCAGCCTTCACTCAGAAA-3’) and reverse (5‘-ACCTCCCACCCCAGATCT-3’) primers followed by digestion with restriction endonuclease AflII. PCR products (299 bp) were digested at 37°C for 3 h and then fractionated on a 2% agarose gel. A restriction site appears when the T allele is present and two fragments are seen in heterozygotes (155 bp and 144 bp). The intron +1174 A>G polymorphism (rs352139) was determined by allele-specific PCR using two different sets of forward primers (for A: 5‘-GTGGAGTGGGTGGAGGTG3’; for G: 5‘-AAGTGGAGTGGGTGGAGGTA-3’) and a common reverse primer (5‘-CAAGGAAAGGCTGGTGACAT-3’), followed by fractionation of the amplification product at 2% agarose gel. An amplified PCR product (299 bp) shows the presence of specific allele. Sequencing was done in a subset of samples to confirm the results.
Data are presented as mean ± SD. The genotype frequencies were tested for Hardy-Weinberg equilibrium. Between-group differences were tested with the Student's t-test. Disease associations were analyzed by chi-square tests. Statistical analyses were performed with SPSS12.0 software (SPSS Inc., Chicago, IL, USA). A two-tailed P value of <0.05 was considered significant.
Results
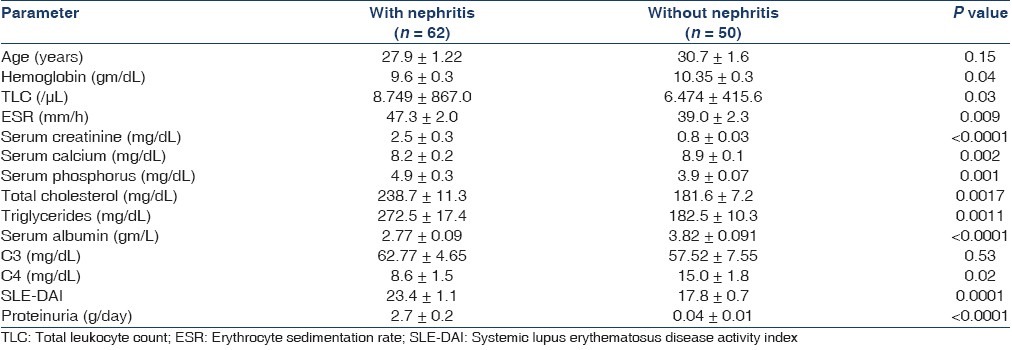
Table 1 shows the clinical characteristics of patients with SLE with and without lupus nephritis. Of the 62 patients with lupus nephritis, 58 underwent kidney biopsy: three patients showed Class II disease, five patients had class III, 36 class IV, six Class V, two class VI, two class IV and V, two Class V and III, whereas one each showed focal glomerulosclerosis and arteriosclerosis. Patients with nephritis had higher SLE-DAI scores, serum creatinine, urea, total cholesterol and triglyceride and lower total protein and albumin values compared with patients without nephritis.
Table 1.
Baseline characters of SLE patients with and without lupus nephritis
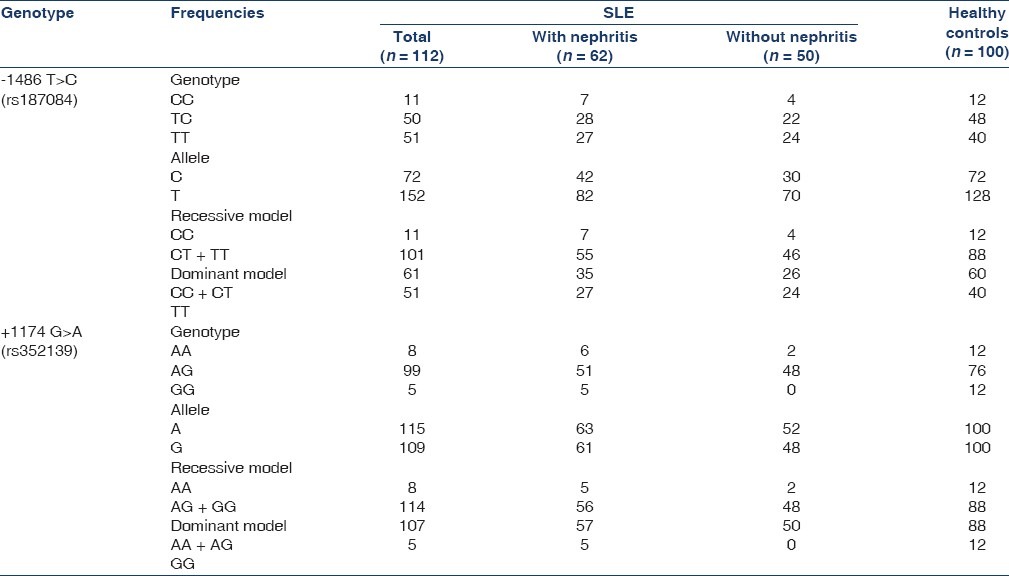
The allelic and genotype frequencies of the identified markers of the TLR9 gene are presented in Table 2. There was no difference in the frequencies of either of the alleles between the SLE patients and the healthy controls.
Table 2.
TLR9 polymorphism in SLE patients and healthy controls
Next, we divided the patients according to their genotypes and compared the clinical presentation, both in terms of disease activity and in terms of indices of renal involvement [Table 3]. Those with at least one C allele at the -1486 position exhibited higher serum creatinine at presentation; this distinction was more pronounced in the subgroup with clinical renal involvement. Individuals with the CC/CT genotype showed a higher activity score on biopsy and higher anti-dsDNA titers than those with the TT genotype. Similarly, presence of at least one A allele at the +1174 position was associated with a higher ds DNA titer, proteinuria, serum creatinine and Austin activity score.
Table 3.
Comparison of patient characteristics and treatment response in different genotypes
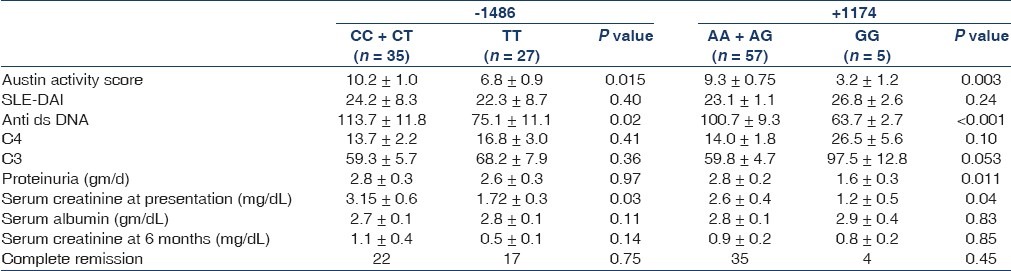
Sixty-one (98%) patients with lupus nephritis received treatment. A total of 41 patients received prednisolone and cyclophosphamide, 12 received prednisolone and mycophenolate mofetil, six received prednisolone and two received prednisolone and azathioprine. All patients received supportive therapy including angiotensin-converting enzyme inhibitors and/or angiotensin receptor blockers. Thirty-nine (64%) patients achieved complete remission and 17 (29%) achieved partial remission with the primary therapy. A total of five patients did not respond: three patients with advanced renal failure at presentation did not receive second-line therapy and remained dialysis dependent, one was treated with mycophenolate mofetil, rituximab and intravenous immunoglobulin (IVIG) sequentially and went into remission, while another received IVIG, but failed to respond. Next, we compared the impact of the genotypes on response to treatment in terms of remissions, and could not detect any difference [Table 3].
Discussion
In this report, we demonstrate that compared with healthy controls, north Indian SLE patients with or without clinical renal involvement do not exhibit alteration in genotype or allelic distribution at the -1486 and +1174 positions in the TLR9 gene. However, in those who do exhibit clinical renal involvement, variations at both loci do seem to affect the severity of presentation.
Patients with A allele at position +1174 had higher serum creatinine, proteinuria and activity score on renal biopsy than those with G allele among patients with lupus nephritis. Anti-dsDNA C4 levels were also higher in these patients. Our study also found an association between C allele at -1486 locus with the severity of lupus nephritis. These patients had higher serum creatinine and activity scores on renal biopsy compared with their counterparts, and were more likely to exhibit other markers of activity, such as higher dsDNA titers. This is the first report to document such an association.
Previous studies from the UK, Korea and Hong Kong were unable to find any association between variations at these two loci in the TLR9 gene and SLE risk.[9–11] In contrast, G allele at the +1174 TLR9 gene was associated with a decreased risk of lupus nephritis in the Chinese Han population and increased risk of SLE in Japanese patients.[12,13]
In the published studies that examined the association between TLR9 polymorphism and lupus nephritis, variations at both loci were associated with lupus nephritis, although weakly in the Chinese Han population.[13] However, they could not find any difference between genotypes in terms of activity and chronicity indices, SLEDAI, C4levels or proteinuria. In contrast, a study from the UK failed to find any association between nephritis and any of the nine studied single nucleotide polymorphisms (SNPs) in the TLR9 gene.[9] This study, however, did not examine the various parameters related to disease severity.
Our results suggest that in the Indian population, polymorphisms in these two loci on the TLR9 gene are associated with altered markers of disease severity and impact the presentation of renal disease. Other lines of evidence have hinted at the possibility of involvement of TLR9 in SLE. Compared with patients in the quiescent phase, those with active disease show increased increase in the frequency of circulating CD19+ B lymphocytes and CD14+ monocytes expressing TLR9.[20] TLR9 is also expressed on renal nonimmune cells,[21–23] and it is possible that they might bind CpG DNA and lead to active disease. Presence of G allele at the +1174 position is associated with reduced TLR9 expression at the transcriptional level and, therefore, might be protective.[13]
TLRs might offer a new therapeutic target for lupus patients. Inhibitory oligos have been shown to lead to reduced ANA production and severity of glomerular disease and mortality in (NZB×NZW) F1 mice.[24] Biologicals that can block receptor–ligand interaction are being developed as are small molecule inhibitors.[25] At least one such agent has been shown to be well tolerated in Phase I studies.
Some limitations of the study must be pointed out. The first is the small patient numbers, and it is possible that with a larger sample size, a more significant association between the SNPs and nephritis might emerge. The second is that these findings might be ethnicity specific. Our population was a homogeneous north Indian one. It is likely that TLR9 exerts only a weak effect, and the final phenotype depends on a number of other factors, which might vary between populations. Finally, although TLR9 could be a susceptibility gene for severe lupus nephritis and disease activity, the possibility of linkage disequilibrium between one of these polymorphisms with another associated polymorphism or the same or another gene cannot be ruled out.
Conclusions
Our findings suggest that genetic variation in TLR9 has an impact on the severity of lupus nephritis in the Indian population. This study needs to be extended in a larger sample size and in other population groups to establish this association more accurately.
Acknowledgments
We acknowledge an intramural grant support by the Postgraduate Institute of Medical Education and Research.
Footnotes
Source of Support: PGIMER intramural funding.
Conflict of Interest: None declared.
References
- 1.Plotz PH. The autoantibody repertoire: searching for order. Nat Rev Immunol. 2003;3:73–8. doi: 10.1038/nri976. [DOI] [PubMed] [Google Scholar]
- 2.Arbuckle MR, McClain MT, Rubertone MV, Scofield RH, Dennis GJ, James JA, et al. Development of autoantibodies before the clinical onset of systemic lupus erythematosus. N Engl J Med. 2003;349:1526–33. doi: 10.1056/NEJMoa021933. [DOI] [PubMed] [Google Scholar]
- 3.ter Borg EJ, Groen H, Horst G, Limburg PC, Wouda AA, Kallenberg CG. Clinical associations of antiribonucleoprotein antibodies in patients with systemic lupus erythematosus. Semin Arthritis Rheum. 1990;20:164–73. doi: 10.1016/0049-0172(90)90057-m. [DOI] [PubMed] [Google Scholar]
- 4.Rahman AH, Eisenberg RA. The role of toll-like receptors in systemic lupus erythematosus. Springer Semin Immunopathol. 2006;28:131–43. doi: 10.1007/s00281-006-0034-3. [DOI] [PubMed] [Google Scholar]
- 5.Clanchy FI, Sacre SM. Modulation of toll-like receptor function has therapeutic potential in autoimmune disease. Expert Opin Biol Ther. 2010;10:1703–16. doi: 10.1517/14712598.2010.534080. [DOI] [PubMed] [Google Scholar]
- 6.Wu X, Peng SL. Toll-like receptor 9 signaling protects against murine lupus. Arthritis Rheum. 2006;54:336–42. doi: 10.1002/art.21553. [DOI] [PubMed] [Google Scholar]
- 7.Schroppel B, He JC. Expression of Toll-like receptors in the kidney: their potential role beyond infection. Kidney Int. 2006;69:785–7. doi: 10.1038/sj.ki.5000190. [DOI] [PubMed] [Google Scholar]
- 8.Shigeoka AA, Holscher TD, King AJ, Hall FW, Kiosses WB, Tobias PS, et al. TLR2 is constitutively expressed within the kidney and participates in ischemic renal injury through both MyD88-dependent and -independent pathways. J Immunol. 2007;178:6252–8. doi: 10.4049/jimmunol.178.10.6252. [DOI] [PubMed] [Google Scholar]
- 9.De Jager PL, Richardson A, Vyse TJ, Rioux JD. Genetic variation in toll-like receptor 9 and susceptibility to systemic lupus erythematosus. Arthritis Rheum. 2006;54:1279–82. doi: 10.1002/art.21755. [DOI] [PubMed] [Google Scholar]
- 10.Hur JW, Shin HD, Park BL, Kim LH, Kim SY, Bae SC. Association study of Toll-like receptor 9 gene polymorphism in Korean patients with systemic lupus erythematosus. Tissue Antigens. 2005;65:266–70. doi: 10.1111/j.1399-0039.2005.00374.x. [DOI] [PubMed] [Google Scholar]
- 11.Ng MW, Lau CS, Chan TM, Wong WH, Lau YL. Polymorphisms of the toll-like receptor 9 (TLR9) gene with systemic lupus erythematosus in Chinese. Rheumatology (Oxford) 2005;44:1456–7. doi: 10.1093/rheumatology/kei120. [DOI] [PubMed] [Google Scholar]
- 12.Tao K, Fujii M, Tsukumo S, Maekawa Y, Kishihara K, Kimoto Y, et al. Genetic variations of Toll-like receptor 9 predispose to systemic lupus erythematosus in Japanese population. Ann Rheum Dis. 2007;66:905–9. doi: 10.1136/ard.2006.065961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhou XJ, Lv JC, Cheng WR, Yu L, Zhao MH, Zhang H. Association of TLR9 gene polymorphisms with lupus nephritis in a Chinese Han population. Clin Exp Rheumatol. 2010;28:397–400. [PubMed] [Google Scholar]
- 14.Migita K, Miyashita T, Maeda Y, Nakamura M, Yatsuhashi H, Kimura H, et al. Toll-like receptor expression in lupus peripheral blood mononuclear cells. J Rheumatol. 2007;34:493–500. [PubMed] [Google Scholar]
- 15.Hochberg MC. Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1997;40:1725. doi: 10.1002/art.1780400928. [DOI] [PubMed] [Google Scholar]
- 16.Bombardier C, Gladman DD, Urowitz MB, Caron D, Chang CH. Derivation of the SLEDAI. A disease activity index for lupus patients. The Committee on Prognosis Studies in SLE. Arthritis Rheum. 1992;35:630–40. doi: 10.1002/art.1780350606. [DOI] [PubMed] [Google Scholar]
- 17.Weening JJ, D’Agati VD, Schwartz MM, Seshan SV, Alpers CE, Appel GB, et al. The classification of glomerulonephritis in systemic lupus erythematosus revisited. J Am Soc Nephrol. 2004;15:241–50. doi: 10.1097/01.asn.0000108969.21691.5d. [DOI] [PubMed] [Google Scholar]
- 18.Boumpas DT, Sidiropoulos P, Bertsias G. Optimum therapeutic approaches for lupus nephritis: what therapy and for whom? Nat Clin Pract Rheumatol. 2005;1:22–30. doi: 10.1038/ncprheum0016. [DOI] [PubMed] [Google Scholar]
- 19.Shlomchik M, Mascelli M, Shan H, Radic MZ, Pisetsky D, Marshak-Rothstein A, et al. Anti-DNA antibodies from autoimmune mice arise by clonal expansion and somatic mutation. J Exp Med. 1990;171:265–92. doi: 10.1084/jem.171.1.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Papadimitraki ED, Choulaki C, Koutala E, Bertsias G, Tsatsanis C, Gergianaki I, et al. Expansion of toll-like receptor 9-expressing B cells in active systemic lupus erythematosus: implications for the induction and maintenance of the autoimmune process. Arthritis Rheum. 2006;54:3601–11. doi: 10.1002/art.22197. [DOI] [PubMed] [Google Scholar]
- 21.Allam R, Lichtnekert J, Moll AG, Taubitz A, Vielhauer V, Anders HJ. Viral RNA and DNA trigger common antiviral responses in mesangial cells. J Am Soc Nephrol. 2009;20:1986–96. doi: 10.1681/ASN.2008101067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Eleftheriadis T, Lawson BR. Toll-like receptors and kidney diseases. Inflamm Allergy Drug Targets. 2009;8:191–201. doi: 10.2174/187152809788680985. [DOI] [PubMed] [Google Scholar]
- 23.Robson MG. Toll-like receptors and renal disease. Nephron Exp Nephrol. 2009;113:e1–7. doi: 10.1159/000228077. [DOI] [PubMed] [Google Scholar]
- 24.Barrat FJ, Meeker T, Chan JH, Guiducci C, Coffman RL. Treatment of lupus-prone mice with a dual inhibitor of TLR7 and TLR9leads to reduction of autoantibody production and amelioration of disease symptoms. Eur J Immunol. 2007;37:3582–6. doi: 10.1002/eji.200737815. [DOI] [PubMed] [Google Scholar]
- 25.Hennessy EJ, Parker AE, O’Neill LA. Targeting Toll-like receptors: emerging therapeutics? Nat Rev Drug Discov. 2010;9:293–307. doi: 10.1038/nrd3203. [DOI] [PubMed] [Google Scholar]


