Abstract
Background:
Continued hemorrhage remains a major contributor of mortality in massively transfused patients and controversy regarding the optimal management exists although recently, the concept of hemostatic resuscitation, i.e., providing large amount of blood products to critically injured patients in an immediate and sustained manner as part of an early massive transfusion protocol has been introduced. The aim of the present review was to investigate the potential effect on survival of proactive administration of plasma and/or platelets (PLT) in trauma patients with massive bleeding.
Materials and Methods:
English databases were searched for reports of trauma patients receiving massive transfusion (10 or more red blood cell (RBC) within 24 hours or less from admission) that tested the effects of administration of plasma and/or PLT in relation to RBC concentrates on survival from January 2005 to November 2010. Comparison between highest vs lowest blood product ratios and 30-day mortality was performed.
Results:
Sixteen studies encompassing 3,663 patients receiving high vs low ratios were included. This meta-analysis of the pooled results revealed a substantial statistical heterogeneity (I2 = 58%) and that the highest ratio of plasma and/or PLT or to RBC was associated with a significantly decreased mortality (OR: 0.49; 95% confidence interval: 0.43-0.57; P<0.0001) when compared with lowest ratio.
Conclusion:
Meta-analysis of 16 retrospective studies concerning massively transfused trauma patients confirms a significantly lower mortality in patients treated with the highest fresh frozen plasma (FFP) and/or PLT ratio when compared with the lowest FFP and/or PLT ratio. However, optimal ranges of FFP: RBC and PLT : RBC should be established in randomized controlled trials.
Keywords: Coagulopathy, damage control resuscitation, FFP, meta-analysis, platelet concentrate, RBC, transfusion ratios, trauma
INTRODUCTION
Death due to traumatic injury is the leading cause of life years lost throughout the world and hemorrhage is responsible for 30 to 40% of total trauma mortality, accounting for almost 50% of the deaths in the first 24 hours following trauma. Coagulopathy, together with hypothermia and acidosis, forms a “lethal” triad associated with a poor prognosis.[1] Recently, an early and previously unrecognized acute coagulopathy of trauma (ACT) was described in 25% of admitted patients and occurring before the above-mentioned traditional causes of traumatic coagulopathy.[2] This ACT has injury severity and shock and hypoperfusion as the key drivers, and is characterized by activation of the protein C system and hyperfibrinolysis.[2] In the last decade, the concepts of damage control surgery have evolved, prioritizing early control of the cause of bleeding by temporary, non-definitive means. Similarly, the concept of damage controls resuscitation, i.e., providing large amount of blood products to critically injured patients in an immediate and sustained manner as part of an early massive transfusion protocol, reducing the amount of crystalloid administered, has been introduced.[3,4] The rationale behind this resuscitation concept is to transfuse red blood cells, plasma, and platelets (PLT) in the same proportion as found in circulating whole blood, thus leading toward a unit-for-unit ratio to prevent and treat coagulopathy due to massive hemorrhage. It should be emphasized, however, that such a practice at best will result in a hematocrit around 30%, coagulation factor concentration of about 65%, and a PLT count of approximately 90, and thus being far from what normally circulated in the vascular system.[4]
The aim of the present review, therefore, was to investigate the potential effect on survival of hemostatic resuscitation with proactive administration of plasma and PLT in comparison with red blood cells (RBCs) in trauma patients with massive bleeding.
MATERIALS AND METHODS
English databases, including MEDLINE, Science Direct, Cochrane Central Register of Controlled Trials, and Blackwell Science, were searched for reports of massive transfusions in a trauma setting that tested the effects of administration of plasma and/or PLT in relation to RBCs on survival from January 2005 to November 2010. The starting time point was selected as 2005, because this was the first time early and aggressive administration of fresh frozen plasma (FFP) and PLT was suggested as a mean of resuscitation for massively bleeding patients.[3,4] The keywords, used both individually and in combination, were massive transfusion, damage control resuscitation, hemostatic control resuscitation, massive transfusion protocol, FFP transfusion, PLT transfusion, RBC transfusion, FFP : RBC ratio, PLT : RBC ratio, and blood product ratios.
The inclusion criteria were (1) Massively transfused patients, defined as receiving 10 or more RBCs within 24 hours or less; (2) The presence of two or more patient groups with different transfusion strategies with regard to administration of plasma and/or PLT in relation to RBC; (3) Presenting outcome data defined as 30-day mortality or mortality rate at discharge from hospital with regard to FFP and/or PLT transfusion; and (4) A retrospective observational investigation or interventional study. Given the large heterogeneity between studies in reporting different ratios of FFP and/or PLT in relation to RBCs, we chose to compare the highest vs the lowest transfusion ratios from each study included.
Statistical meta-analyses were conducted using the RevMan 5.0.25 software package. The overall pooled risk ratio (relative risk) and 95% confidence interval (CI) were calculated with the inverse variance method.[5] We used the random-effects model in anticipation of clinical and methodological diversity between trials.[6] Statistical heterogeneity between trials was measured by the I2 statistic, which describes the proportion of heterogeneity not ascribed to random error.[7] For all calculations, two-tailed P values of less than 0.05 were considered statistically significant.
RESULTS
No randomized studies evaluating the effect of different transfusion ratios were identified. Instead, sixteen studies for evaluation encompassing 3 663 patients receiving either high or low transfusion ratios and the characteristics of these studies are presented in Table 1.[8–23] Confounding factors not possible to obtain when analyzing the data were time of transfusion, age of the blood products, and status of the patients other than being traumatized and in need for massive transfusion. There were ten studies that tested the effect on survival in relation to FFP or PLT to RBC ratio, two studies investigated the effect of FFP and PLT to RBC ratios. Four studies evaluated implementation of massive transfusion protocols with preemptive FFP and PLT administration vs historic controls [Figure 1].
Table 1.
Studies evaluating the effect of high vs low blood product ratios on 30-day mortality in massively transfused trauma patients
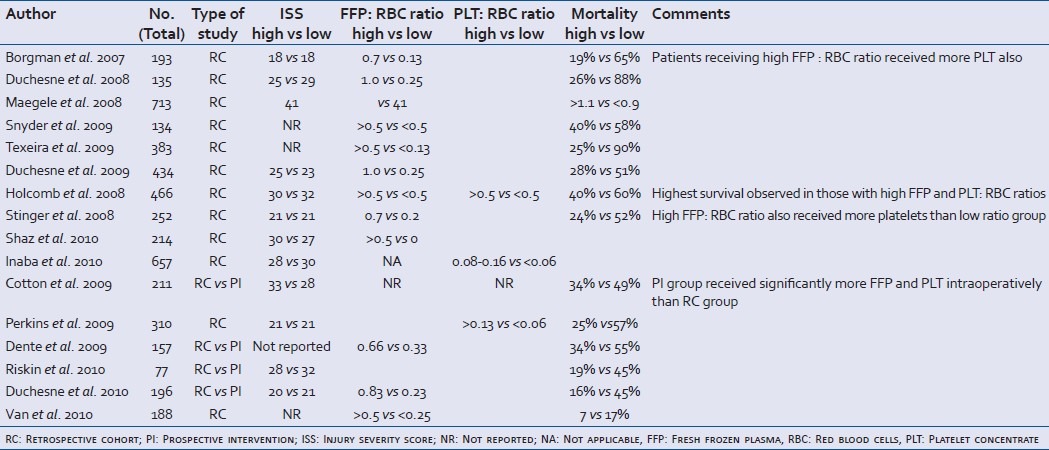
Figure 1.
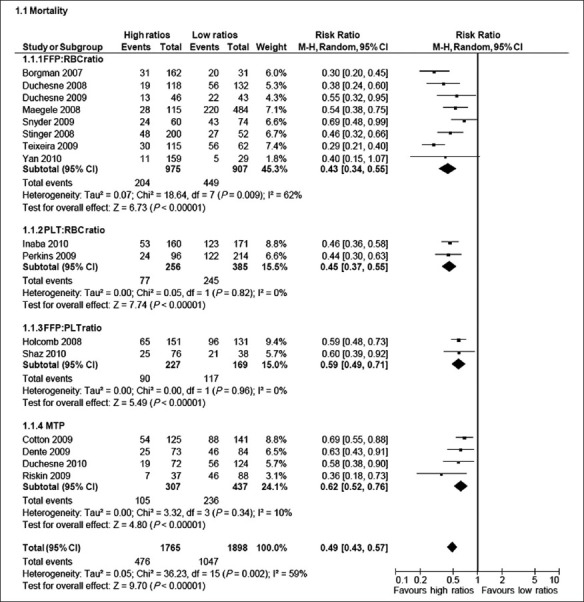
Stratified forest plot of studies included in the meta-analysis
FFP/RBC ratios ranged from the highest 1: 1 (0.5) to 1 : 9 (0.1) among all studies. Concerning FFP : RBC ratios, the eight studies included a total of 975 patients receiving a high FFP : RBC ratio and 907 patients receiving a low FFP : RBC ratio. Two studies were reports from the combat setting in Iraq encompassing 633 patients of whom 521 patients received a high FFP : RBC ratio.[8,15] One study was from Germany[10] and the remaining were reports from different Level 1 trauma centers in USA.[9,11–13,23] The pooled analysis of the studies furnishing data shows that there was a significantly lower mortality in patients receiving a high FFP: RBC ratio (Odds ratio (OR): 0.43, 95% CI: 0.34 – 0.55). There was significant heterogeneity between studies (I2 = 62%).
Two studies addressed the effect of high vs low PLT transfusion rates in 641 massively bleeding trauma patients of whom 333 received high PLT: RBC ratio.[17,19] One study was from the combat setting in Iraq, whereas the other reported from an American Level 1 trauma center. The analysis of the two studies furnishing data shows that there was a significantly lower mortality in patients receiving a high PLT : RBC ratio (OR: 0.45, 95% CI: 0.37–0.55), and this finding was not associated with heterogeneity between the studies (I2 = 0%).
One study evaluated the effect of both FFP and PLT : RBC ratios in 466 patients at 16 US Level 1 trauma centers of whom 216 patients received high ratios, and this was associated with a significantly lower mortality (OR: 0.59, 95% CI: 0. 0.48–0.73) compared with the low ratios group.[14] Shaz et al. reported that high vs low transfusion ratios were associated with improved 30-day survival: plasma : RBC, 59% vs 44%, P=0.03; PLT : RBC, 63% vs 33%, P<0.01; and cryoprecipitate : RBC, 66% vs 41%, P<0.01.[16]
Four American studies evaluated the effect of implementing a massive transfusion protocol on mortality in 858 patients of whom 513 patients received Massive transfusion protocol (MTP)[18,20–22] and this was associated with significantly lower mortality (OR: 0.63, 95% CI: 0.53 – 0.74) and this finding was not associated with significant statistical heterogeneity between the studies (I2 = 0%).
When pooling all the 16 studies, there was a significantly lower mortality in patients receiving high ratios of plasma and/or PLT or MTP (OR: 0.49, 95% CI: 0.43–0.57). The finding was associated with significant statistical heterogeneity between the studies (I2 = 59%).
DISCUSSION
The result of the present meta-analysis confirms that early aggressive administration of plasma and PLT in addition to RBCs is associated with a lower mortality in massively bleeding patients as compared with patients resuscitated with low ratios. In each of the subgroups investigated, there was a significantly lower mortality observed in those resuscitated with high FFP and/or PLT to RBC ratios when the analysis was based on dichotomous stratification of blood product ratios. Contrary to this, Snyder et al. reported that when adjusting for the timing of the FFP transfusion, no significant improvement in survival was detected between patients receiving more FFP at 24 hours post-admission and those receiving less.[11] They reported of a possible survivorship bias related to those patients who lived long enough were the ones who received a higher FFP: RBC ratio, suggesting that the difference in mortality was related to factors other than the transfusion therapy. This is in agreement with other recently published studies.[24,25] Scalea et al, retrospectively assessed the relationship between FFP transfusion and survival in trauma patients and reported that in a subset of 81 patients who received more than 10 units of RBCs within 24 hours of admission, no significant difference was found between those who received an FFP: RBC ratio of 1 : 1 compared with those who received a lower FFP : RBC ratio when controlling for age, gender ISS, closed head injury, laparotomy status, and length of Intensive care unit (ICU) stay.[24] These diverging results may be explained by the timing of the FFP administration in relation to when the patients arrive at the hospital, which is important for the outcome of massively bleeding patients. Johansson et al. introduced thawed plasma, enabling immediate administration of FFP together with RBC upon arrival at the trauma center while at the same time reducing the amount of crystalloids and colloids administered to these patients; this resulted in a reduction in mortality.[3] The same conclusion was reported by Cotton et al.[18] also having thawed plasma available and hence, avoiding a delay in plasma transfusions. Administration of large amounts of crystalloids to maintain adequate intravascular volume dilutes the level of coagulation factors, fibrinogen, and PLT, and administration of colloids further worsens the coagulopathy by interfering with fibrin polymerization, resulting in a clot with reduced strength and stability.[26] Furthermore, Cotton et al. also reported that not only was the 30-day survival higher in the trauma exsanguination protocol (TEP) group compared with controls, but the incidence of pneumonia, pulmonary failure, and abdominal compartment syndrome was lower in TEP patients. Severe sepsis or septic shock and multiorgan failure were also lower in TEP patients (9% vs 20% and 16% vs 37%, respectively).[18] Furthermore, the TEP group received more blood products intraoperatively, although the 24-hour total transfusion requirements were lower than in controls, suggesting that early and aggressive administration of plasma and PLT reduces the need for later blood transfusions due to improved hemostasis, and that this is important for survival in massively bleeding patients. Interestingly, Riskin et al. reported that implementation of a massive transfusion protocol did not change the transfusion ratios but this may be related to that the time to first FFP and PLT transfusions were above 2.5 hours and consequently severe coagulopathy must have occurred before balanced transfusion therapy was instituted.[21] This is in alignment with Dutton et al.[27] reporting that the median survival for those dying of uncontrollable hemorrhage was 2 hours and these patients are consequently not resuscitated by delayed balanced transfusion therapy as outlined by Riskin et al.[21]
The current meta-analysis investigated the highest vs the lowest transfusion ratios and consequently do not reveal an “optimal ratio” of FFP to RBCs. Furthermore, equally important, the timing of the FFP transfusions in relation to when administration of RBC was commenced were not reported in most of the studies evaluated and without this information, mere ratios provide insufficient information. This is illustrated by that the literature demonstrates conflicting results: Maegele et al. showed that an FFP : RBC ratio >1 : 1 was associated with the highest survival in German trauma patients,[10] whereas Kashuk et al. reported that patients receiving an FFP : RBC ratio of 1 : 2-1 : 3 had the highest survival and that a higher ratio was not associated with better outcome, but instead might be harmful.[28] This is in alignment with Davenport et al. who recently reported that FFP : PRBC ratios of ≥1 : 1 do not confer any additional advantage over ratios of 1 : 2 to 3 : 4, although it should be noted that in this study, the most severely bleeding patients were excluded due to blood sampling failure.[29] It should be noted, however, that there might be more than one optimal resuscitation ratio according to trauma severity, degree, and dynamics of blood loss, and previous fluid administration among other factors.
The importance of the ACT for mortality reported by Frith and colleagues is established, whereas the optimal treatment of this condition remains elusive.[30] Duchesne et al. specifically address this issue finding that in approximately 30% of those requiring massive transfusion presented with a prothrombin time (PT) >1.2 and, hence, trauma induced coagulopathy:[13] They found that administration of a high ratio of FFP was associated with a significantly lower mortality than patients treated with low ratios. This finding is especially interesting considering that activation of the protein C system is driven by thrombin generation and, therefore, an intervention supporting thrombin generation could have been expected to worsen the ACT.
Also, given that ACT is associated with endothelial damage[31] and glycocalyx degradation,[32] it is notable that freshly thawed FFP exerts a protective effect on the endothelium and glycocalyx and reduces its permeability in animal models of hemorrhagic shock models.[33,34] It could be speculated that early administration of high levels of freshly thawed FFP in patients with acute traumatic coagulopathy may contribute to improved survival by protecting the endothelium and thereby attenuating the downstream organ damage associated with capillary leakage.
The role of PLT for intact hemostasis is well established and in the present review, nine of the studies reported improved survival in the group of patients receiving the highest ratios of PLT to RBC, i.e., the most PLT. This is in agreement with Johansson et al,[35] who demonstrated that administration of PLT together with plasma and RBC immediately upon arrival in the operation theatre and throughout surgery was associated with reduced postoperative bleeding and a 50% increase in the 30-day survival in patients undergoing surgery for a ruptured abdominal aortic aneurysm. This was further corroborated by Perkins et al, who reported in a multiple regression analysis that PLT transfusion was independently associated with survival.[26]
Whole blood viscoelastical assays such as TEG and ROTEM have been shown to be superior to conventional coagulation assays in identifying clinically relevant coagulopathies as well as in reducing the need for blood transfusion in patients undergoing major surgery.[36,37] In massively transfused trauma patients, TEG has been shown to predict the need for blood transfusion[38] as well as identify ATC including hyperfibrinolysis.[39] The use of TEG/ROTEM in massively bleeding trauma patients is now recommended by current guidelines[40] and teaching books[41] and it could be speculated that systematic use of TEG/ROTEM to identify coagulopathy and guide transfusion therapy might be superior to blind transfusion based on different ratios and this warrants further evaluation.
The main limitation of this review is that it only compared the highest vs lowest transfusion ratios and consequently, no information of a potential optimal transfusion ratio in between was evaluated. Furthermore, a significant statistical heterogeneity between the studies included was found and this should be considered when interpreting the results.
CONCLUSION
This meta-analysis of retrospective studies concerning massively transfused trauma patients confirms a significantly lower mortality in patients treated with the highest FFP and/or PLT ratio when compared with the lowest FFP and/or PLT ratio. The optimal FFP : RBC and PLT : RBC ratios remain to be established.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Hardy JF, de Moerloose P, Samama M. Massive transfusion and coagulopathy: Pathophysiology and implications for clinical management. Can J Anaesth. 2004;51:293–310. doi: 10.1007/BF03018233. [DOI] [PubMed] [Google Scholar]
- 2.Brohi K, Cohen MJ, Ganter MT, Schultz MJ, Levi M, Mackersie RC, et al. Acute coagulopathy of trauma: Hypoperfusion induces systemic anticoagulation and hyperfibrinolysis. J Trauma. 2008;64:1211–7. doi: 10.1097/TA.0b013e318169cd3c. [DOI] [PubMed] [Google Scholar]
- 3.Johansson PI, Hansen MB, Sorensen H. Transfusion practice in massively bleeding patients: Time for a change? Vox Sang. 2005;89:92–6. doi: 10.1111/j.1423-0410.2005.00668.x. [DOI] [PubMed] [Google Scholar]
- 4.Hess JR, Holcomb JB, Hoyt DB. Damage control resuscitation: The need for specific blood products to treat the coagulopathy of trauma. Transfusion. 2006;46:685–6. doi: 10.1111/j.1537-2995.2006.00816.x. [DOI] [PubMed] [Google Scholar]
- 5.Deeks JJ, Higgins JP, Altman DG. Analysing data and undertaking meta-analyses. In: Higgins JP, Green S, editors. Cochrane handbook for systematic reviews of interventions. Chichester: John Wiley & Sons; 2008. pp. 243–96. [Google Scholar]
- 6.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–88. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 7.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539–58. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 8.Borgman MA, Spinella PC, Perkins JG, Grathwohl KW, Repine T, Beekley AC, et al. The ratio of blood products transfused affects mortality in patients receiving massive transfusions at a combat support hospital. J Trauma. 2007;63:805–13. doi: 10.1097/TA.0b013e3181271ba3. [DOI] [PubMed] [Google Scholar]
- 9.Duchesne JC, Hunt JP, Wahl G, Marr AB, Wang YZ, Weintraub SE, et al. Review of current blood transfusions strategies in a mature level I trauma center: Were we wrong for the last 60 Years? J Trauma. 2008;65:272–8. doi: 10.1097/TA.0b013e31817e5166. [DOI] [PubMed] [Google Scholar]
- 10.Maegele M, Lefering R, Paffrath T, Tjardes T, Simanski C, Bouillon B, et al. Red blood cell to plasma ratios transfused during massive transfusion are associated with mortality in severe multiply injury: A retrospective analysis from the trauma registry of the deuthche gesellschaft für unfallschirurie. Vox Sang. 2008;95:112–9. doi: 10.1111/j.1423-0410.2008.01074.x. [DOI] [PubMed] [Google Scholar]
- 11.Snyder CW, Weinberg JA, McGwin G, Jr, Melton SM, George RL, Reiff DA, et al. The relationship of blood product ratio to mortality: Survival benefit or survival bias? J Trauma. 2009;66:358–64. doi: 10.1097/TA.0b013e318196c3ac. [DOI] [PubMed] [Google Scholar]
- 12.Teixeira GR, Inaba K, Shulman I, Salim A, Demetriades D, Brown C, et al. Impact of plasma transfusion in massively transfused trauma patients. J Trauma. 2009;66:693–7. doi: 10.1097/TA.0b013e31817e5c77. [DOI] [PubMed] [Google Scholar]
- 13.Duchesne JC, Islam TM, Stuke L, Timmer JR, Barbeau JM, Marr AB, et al. Hemostatic resuscitation during surgery improves survival in patients with traumatic-induced coagulopathy. J Trauma. 2009;67:33–9. doi: 10.1097/TA.0b013e31819adb8e. [DOI] [PubMed] [Google Scholar]
- 14.Holcomb JB, Wade CE, Michalek J, Chisholm GB, Zarzabal LA, Schreiber MA, et al. Increased plasma and platelet to red blood cell ratios improves outcome in 466 massively transfused civilian trauma patients. Ann Surg. 2008;248:447–58. doi: 10.1097/SLA.0b013e318185a9ad. [DOI] [PubMed] [Google Scholar]
- 15.Stinger HK, Spinella PC, Perkins JG, Grathwohl KW, Salinas J, Martini WZ, et al. The ratio of fibrinogen to red cells transfused affects survival in casualties receiving massive transfusions at an army combat support hospital. J Trauma. 2008;64(2 Suppl):S79–85. doi: 10.1097/TA.0b013e318160a57b. [DOI] [PubMed] [Google Scholar]
- 16.Shaz BH, Dente CJ, Nicholas J, MacLeod JB, Young AN, Easley K, et al. Increased number of coagulation products in relationship to red blood cell products transfused improves mortality in trauma patients. Transfusion. 2010;50:493–500. doi: 10.1111/j.1537-2995.2009.02414.x. [DOI] [PubMed] [Google Scholar]
- 17.Schnüriger B, Inaba K, Abdelsayed GA, Lustenberger T, Eberle BM, Barmparas G, et al. The impact of platelets on the progression of traumatic intracranial hemorrhage. J Trauma. 2010;68:881–5. doi: 10.1097/TA.0b013e3181d3cc58. [DOI] [PubMed] [Google Scholar]
- 18.Cotton BA, Au BK, Nunez TC, Gunter OL, Robertson AM, Young PP. Predefined massive transfusion protocols are associated with a reduction in organ failure and postinjury complications. J Trauma. 2009;66:41–9. doi: 10.1097/TA.0b013e31819313bb. [DOI] [PubMed] [Google Scholar]
- 19.Perkins JG, Cap AP, Spinella PC, Blackbourne LH, Grathwohl KW, Repine TB, et al. An evaluation of the impact of apheresis platelets used in the setting of massively transfused trauma patients. J Trauma. 2009;66(4 Suppl):S77–85. doi: 10.1097/TA.0b013e31819d8936. [DOI] [PubMed] [Google Scholar]
- 20.Dente CJ, Shaz BH, Nicholas JM, Harris RS, Wyrzykowski AD, Patel S, et al. Improvements in early mortality and coagulopathy are sustained better in patients with blunt trauma after institution of a massive transfusion protocol in a civilian level I trauma center. J Trauma. 2009;66:1616–24. doi: 10.1097/TA.0b013e3181a59ad5. [DOI] [PubMed] [Google Scholar]
- 21.Riskin DJ, Tsai TC, Riskin L, Hernandez-Boussard T, Purtill M, Maggio PM, et al. Massive transfusion protocols: The role of aggressive resuscitation versus product ratio in mortality reduction. J Am Coll Surg. 2009;209:198–205. doi: 10.1016/j.jamcollsurg.2009.04.016. [DOI] [PubMed] [Google Scholar]
- 22.Duchesne JC, Kimonis K, Marr AB, Rennie KV, Wahl G, Wells JE, et al. Damage control resuscitation in combination with damage control laparotomy: A survival advantage. J Trauma. 2010;69:46–52. doi: 10.1097/TA.0b013e3181df91fa. [DOI] [PubMed] [Google Scholar]
- 23.van PY, Sambasivan CN, Wade CE, Jones JA, Holcomb JB, Schreiber MA, et al. High transfusion ratios are not associated with increased complication rates in patients with severe extremity injuries. J Trauma. 2010;69(Suppl 1):S64–8. doi: 10.1097/TA.0b013e3181e453ec. [DOI] [PubMed] [Google Scholar]
- 24.Scalea TM, Bochicchio KM, Lumpkins K, Hess JR, Dutton R, Pyle A, et al. Early aggressive use of fresh frozen plasma does not improve outcome in critically injured trauma patients. Ann Surg. 2008;248:578–84. doi: 10.1097/SLA.0b013e31818990ed. [DOI] [PubMed] [Google Scholar]
- 25.Magnotti LJ, Zarzaur BL, Fischer PE, Williams RF, Myers AL, Bradburn EH, et al. Improved survival after hemostatic resuscitation: Does the emperor have no clothes? J Trauma. 2011;70:97–102. doi: 10.1097/TA.0b013e3182051691. [DOI] [PubMed] [Google Scholar]
- 26.Perkins JG, Cap AP, Weiss BM, Reid TJ, Bolan CD. Massive transfusion and nonsurgical hemostatic agents. Crit Care Med. 2008;36(7 Suppl):S325–39. doi: 10.1097/CCM.0b013e31817e2ec5. [DOI] [PubMed] [Google Scholar]
- 27.Dutton RP, Stansbury LG, Leone S, Kramer E, Hess JR, Scalea TM. Trauma mortality in mature trauma systems: Are we doing better.? An analysis of trauma mortality patterns, 1997-2008. J Trauma. 2010;69:620–6. doi: 10.1097/TA.0b013e3181bbfe2a. [DOI] [PubMed] [Google Scholar]
- 28.Kashuk JL, Moore EE, Johnson JL, Haenel J, Wilson M, Moore JB, et al. postinjury life threatening coagulopathy: Is 1:1 fresh frozen plasma: Packed red blood cells the answer? J Trauma. 2008;65:261–71. doi: 10.1097/TA.0b013e31817de3e1. [DOI] [PubMed] [Google Scholar]
- 29.Davenport R, Curry N, Manson J, De’Ath H, Coates A, Rourke C, et al. Hemostatic effects of fresh frozen plasma may be maximal at red cell ratios of 1:2. J Trauma. 2011;70:90–6. doi: 10.1097/TA.0b013e318202e486. [DOI] [PubMed] [Google Scholar]
- 30.Frith D, Goslings JC, Gaarder C, Maegele M, Cohen MJ, Allard S, et al. Definition and drivers of acute traumatic coagulopathy: Clinical and experimental investigations. J Thromb Haemost. 2010;8:1919–25. doi: 10.1111/j.1538-7836.2010.03945.x. [DOI] [PubMed] [Google Scholar]
- 31.Ganter MT, Cohen MJ, Brohi K, Chesebro BB, Staudenmayer KL, Rahn P, et al. Angiopoietin-2, marker and mediator of endothelial activation with prognostic significance early after trauma? Ann Surg. 2008;247:320–6. doi: 10.1097/SLA.0b013e318162d616. [DOI] [PubMed] [Google Scholar]
- 32.Johansson PI, Stensballe J, Rasmussen LS, Ostrowski SR. A high admission syndecan-1 level, a marker of endothelial glycocalyx degradation, is associated with inflammation, protein C depletion, fibrinolysis and increased mortality in trauma patients. Ann Surg. 2011;254(2):194–200. doi: 10.1097/SLA.0b013e318226113d. [DOI] [PubMed] [Google Scholar]
- 33.Pati S, Matijevic N, Doursout MF, Ko T, Cao Y, Deng X, et al. Protective effects of fresh frozen plasma on vascular endothelial permeability, coagulation, and resuscitation after hemorrhagic shock are time dependent and diminish between days 0 and 5 after thaw. J Trauma. 2010;65:55–3. doi: 10.1097/TA.0b013e3181e453d4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kozar RA, Peng Z, Zhang R, Holcomb JB, Pati S, Park P, et al. Plasma restoration of endothelial glycocalyx in a rodent model of hemorrhagic shock. Anesth Analg. 2011;112(6):1289–95. doi: 10.1213/ANE.0b013e318210385c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Johansson PI, Stensballe J, Rosenberg I, Hilsøv T, Jørgensen L, Secher NH. Proactive administration of platelets and plasma for patients with a ruptured abdominal aortic aneurysm: Evaluating a change in transfusion practice. Transfusion. 2007;47:593–8. doi: 10.1111/j.1537-2995.2007.01160.x. [DOI] [PubMed] [Google Scholar]
- 36.Salooja N, Perry DJ. Thrombelastography. Blood Coagul Fibrinolysis. 2001;12:327–37. doi: 10.1097/00001721-200107000-00001. [DOI] [PubMed] [Google Scholar]
- 37.Di Benedetto P, Baciarello M, Cabetti L, Martucci M, Chiaschi A, Bertini L. Thrombelastography.Present and future perspectives in clinical practice. Minerva Anestesiol. 2003;69:501–15. [PubMed] [Google Scholar]
- 38.Plotkin AJ, Wade CE, Jenkins DH, Smith KA, Noe JC, Park MS, et al. A reduction in clot formation rate and strength assessed by thrombelastography is indicative of transfusion requirements in patients with penetrating injuries. J Trauma. 2008;64(2 Suppl):S64–8. doi: 10.1097/TA.0b013e318160772d. [DOI] [PubMed] [Google Scholar]
- 39.Carroll RC, Craft RM, Langdon RJ, Clanton CR, Snider CC, Wellons DD, et al. Early evaluation of acute traumatic coagulopathy by thrombelastography. Transl Res. 2009;154:34–9. doi: 10.1016/j.trsl.2009.04.001. [DOI] [PubMed] [Google Scholar]
- 40.Rossaint R, Bouillon B, Cerny V, Coats TJ, Duranteau J, Fernández-Mondéjar E, et al. Management of bleeding following major trauma: An updated European guideline. Crit Care. 2010;14:R52. doi: 10.1186/cc8943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hess JR, Johansson PI, Holcomb JB. In: Trauma and massive transfusion. Transfusion Therapy: Clinical principles and practice. 3rd ed. Mintz PD, editor. Bethesda: AABB Press; 2010. [Google Scholar]


