Abstract
Introduction:
Several series of patient studies have been published on the use of rFVIIa in traumatic haemorrhagic shock, although to date no international recommendations have been produced. France does not currently recognise traumatic haemorrhagic shock as an appropriate indication for the use of rFVIIa.
Materials and methods:
In this retrospective study, we present our experience in the use of rFVIIa in traumatic haemorrhagic shock.
Results:
Twenty-seven patients treated with rFVIIa after a traumatic injury between May 2005 and December 2008 were included. Average age was 46 years old. Eighty per cent of patients were polytransfused. Mortality rate was 33%. Adjusted mortality rate, using the Boffard study criteria, was 8.3%. We observed significant differences between the group of patients who died and the group of survivors in pH, PT, Hb, ionised calcaemia, temperature and platelet count. We observed significant differences between the successful rFVIIa group and the failed rFVIIa group in pH, Hb, platelet count and ionised calcaemia. Ten patients had an rFVIIa injection only and 17 patients had an rFVIIa injection combined with a mechanical procedure to stop the bleeding. Two patients presented with thromboembolic complications. We observed a tendency to recommend an rFVIIa injection before radical treatment is applied.
Conclusion:
It seems to us legitimate to recommend earlier use of rFVIIa in cases of traumatic haemorrhagic shock in the context of haematological damage control combined with the use of an algorithm to predict the risk involved in polytransfusion and a more aggressive transfusion strategy.
Keywords: Haemorrhagic shock, trauma, transfusion, rFVIIa
INTRODUCTION
Because of its ability to activate the coagulation cascade, the rFVIIa has not only been proposed as an adjuvant treatment in hemorrhagic shock and more particularly in trauma cases, but also in hemorrhages during placenta delivery, digestive hemorrhages, and hemorrhagic complications associated with the cardiac surgery. Many published clinical cases do indeed validate the advantages of rFVIIa as an adjuvant treatment in trauma cases or as a last resort after the failure of a radical treatment for hemorrhage and this has led to an international randomized placebo-controlled double-blind clinical trial into traumatic hemorrhagic shock.[1] The patients who were treated received rFVIIa after being transfused with eight erythrocyte concentrates. Unfortunately, this study did not reveal any difference in mortality between the group of treated patients and the control group. Nevertheless, the co-investigating pharmaceutical laboratory put a new study in place where this time the rFVIIa was injected after the transfusion of the fourth erythrocyte concentrate, as they considered rightly that the rFVIIa was less effective after the often-delayed appearance of the trauma triad of death (hypothermia, acidosis, and coagulopathy). This latest study has recently been abandoned before it reached its conclusion for two reasons: (1) the inclusion rate was relatively low and so the study timetable could not be adhered to, (2) the intermediate analysis showed a mortality rate that was too low in the two groups, which could not produce a statistically significant difference in favor of rFVIIa by the end of the study.
A group of European experts[2] has tried to define the role of rFVIIa as an adjuvant treatment for hemorrhagic shock after first applying a mechanical surgical treatment or by interventional radiology. The preferential indications selected were trauma, post-partum hemorrhages, and bleeding complications in cardiac surgery.
In the intensive care unit of the emergency service in Bordeaux, we have used rFVIIa in trauma patients since May 2005. In this paper, we share our experience (the Bordeaux experience) in using rFVIIa in trauma cases.
The main objective of this study is to describe the care given to trauma patients who have been treated with rFVIIa. The secondary objectives are to determine the role of rFVIIa in our strategy for treating traumatic hemorrhagic shock in Bordeaux and also to highlight predictive criteria for rFVIIa ineffectiveness. To do this, we compared our strategy for using rFVIIa (cf infra) with Boffard's study protocols and European recommendations, in terms of mortality and effectiveness. We also wanted to determine the impact of thromboembolic complications, in order to compare it with the data in the literature.
MATERIALS AND METHODS
This is a descriptive and retrospective study carried out in our unit from May 2005 until December 2008. Inclusion criteria were defined as all patients hospitalized in the intensive care unit of the Bordeaux casualty department during this period who had been treated with rFVIIa following a trauma.
The variables recorded were: patient's civil status, circumstances of the trauma, time lapse before being hospitalized in our unit, treatment administered before arrival in our unit, description of the lesions observed, IGSII and ISS scores, respiratory state, patient's hemodynamic and neurology on arrival, temperature, pH, and the biological report combining the haemogram and coagulation before the injection of rFVIIa, blood replacement, and transfusion before the injection of rFVIIa, time lapse between the trauma and the injection of rFVIIa, radical treatment of the bleeding process either by surgery or interventional radiology before, during or after injection of rFVIIa. Patients were stratified retrospectively according to the benefit of prescribing rFVIIa, as shown in Table 1. Thus, we noted the moment when the rFVIIa was injected and subsequently allocated the patients according to different strategies. We assessed the effectiveness of the rFVIIa treatment: (1) bleeding stopped after rFVIIa alone or in combination with a radical procedure (success), (2) hemorrhaging was not stopped or stopped after a second mechanical procedure (failure).
Table 1.
Benefit of prescribing rFVIIa
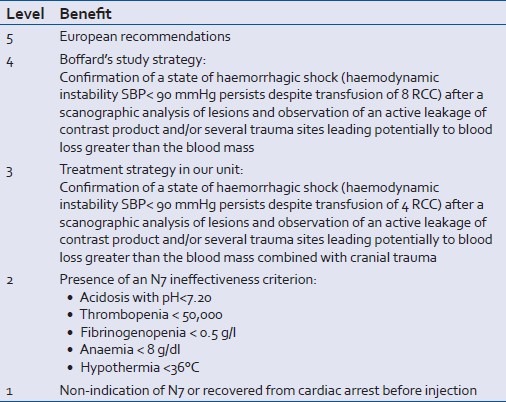
The variables recorded after the injection of rFVIIa were: polytransfusion, defined as a transfusion of more than or equal to ten erythrocyte concentrates, patient survival at D28, duration of mechanical ventilation, length of stay in the intensive care unit, occurrence of complications like ARDS, MOFS, the occurrence of thromboembolic complications until D28.
We calculated an adjusted mortality rate using the exclusion criteria from Boffard's study, i.e., patients with cranial trauma, aged over 65, pH <7, delay before injecting rFVIIa >12 h after the trauma.
Results from the patient files were computerized and analyzed using the StatView 5.0 statistical software (SAS Institute Inc., Cary, USA).
Results from the continuous variables are expressed as mean value±standard deviation.
The continuous values underwent an analysis of variance (ANOVA), combined with Bartlett's test. If the variances differed significantly, a nonparametric Mann–Whitney test was performed on the results.
A value of P<0.05 was considered significant.
RESULTS
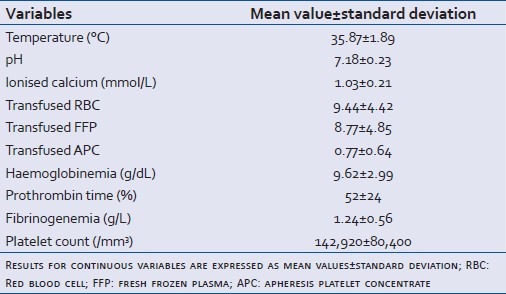
Twenty-seven patients were included in this study between May 2005 and November 2008. Five patients were also included in the French national register directed by Pr Payen (Grenoble, France).Their demographic and pathological characteristics are described in Table 2. Average age was 46 years±18 (standard deviation). The sex-ratio was 4.4. In 58% of cases, patients were admitted directly into our unit. Twenty-four patients presented with a closed trauma. All the patients were intubated, ventilated and sedated. They all received vasopressor treatment with norepinephrine. Details of the patients’ coagulation results and prescribed blood transfusion before rFVIIa injection are given in Table 3. 80% of patients (22) were polytransfused (more than 10 RBC) with an average of 15±6 erythrocyte concentrates and 14±6 fresh frozen plasma. Of five nonpolytransfused patients, three underwent autotransfusion after draining the haemothorax.
Table 2.
Patients’ demographic and pathological characteristics
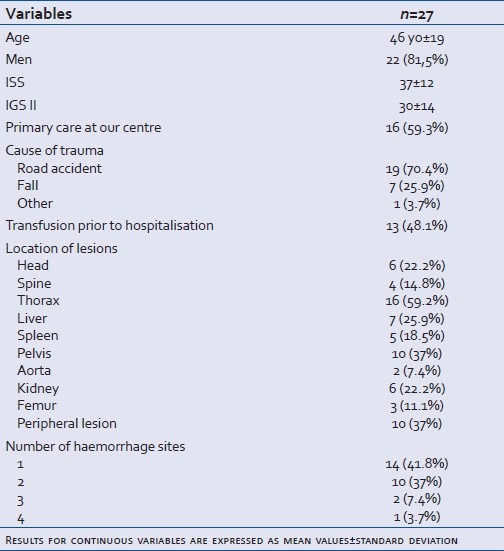
Table 3.
Clinical-biological values before rFVIIa injection
Nine patients in this study died, giving a mortality rate of 33%. The adjusted mortality rate according to Boffard's study was 8.3%. Six patients out of the nine who died received the compassionate use rFVIIa treatment, defined either by the presence of lethal visceral lesions (three patients with brain death, one patient with a disinsertion of the viscera and the submesocolic vessels, and one patient with a suspected complete rupture of the aortic isthmus associated with pelvic fracture), or by the presence of three ineffectiveness criteria including, in particular, a pH under 7.
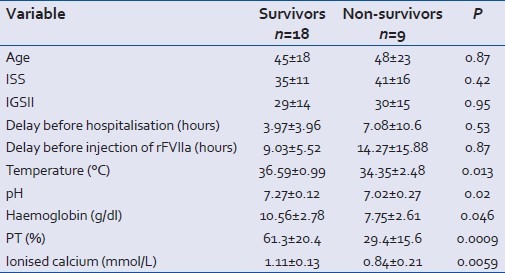
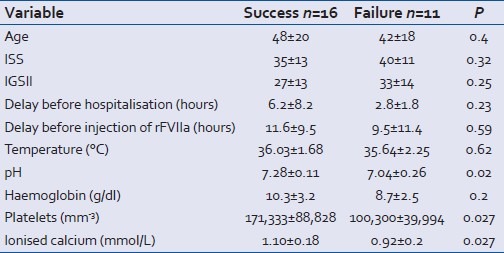
The deceased patients were no different from the survivors in terms of age, ISS and IGS2 scores, delay before hospitalization and before injection of rFVIIa. The significant differences between the two groups concerned pH, temperature, hemoglobin rate, PT and ionized calcaemia, all of which were significantly lower in the deceased group [Table 4].
Table 4.
Comparison of groups of survivors and nonsurvivors before rFVIIa injection
The distribution of deceased and surviving patients according to level of benefit from prescribing rFVIIa is shown in Figure 1. In terms of the Boffard 's study strategy and our unit protocol there were no deaths, and there was one death according to the European recommendations strategy.
Figure 1.
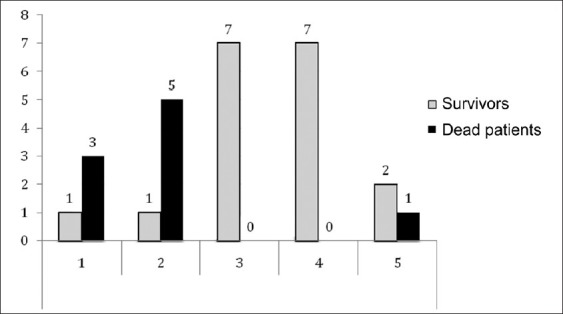
Patients distribution according to level of benefit of prescribing the rFVIIa Level 1: no indication or recovered cardiac arrest; level 2: ≥2 predictive ineffectiveness criteria; level 3: unit protocol; level 4: Boffard's study strategy; level 5: European recommendations. Grey: survivors, black: dead patients
Comparison of the rFVIIa success and failure groups shows that the failure group had significantly lower pH, platelet count and ionized calcium levels than the success group [Table 5].
Table 5.
Comparison of success versus failure of rFVIIa
Two-thirds of the patients (20/27) received rFVIIa with no associated radical procedure or before such a procedure was carried out [Table 6]. We observed a lower death rate of 24% (5/20) versus 57% (4/7) when the rFVIIa was injected without or before a mechanical procedure, but this difference was not significant. Moreover, the patients with three ineffectiveness criteria were present only in the group where rFVIIa was injected after the radical procedure.
Table 6.
Patient distribution according to when injected with rFVIIa
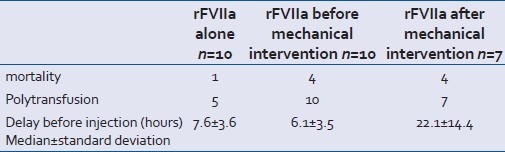
Ten patients in our series were treated with rFVIIa with no associated radical procedure. One patient died without the radical procedure, this was a patient suffering from cranial trauma and with uncontrollable bleeding from the face and who had been treated with rFVIIa on a compassionate-use basis. The possibility of a radical procedure was considered systematically. Four patients out of 10 showed an active contrast product leak in an abdomino-pelvic scan. In two cases, the radiologist chose to delay carrying out embolization. Arteriography was carried out on two patients. In one case, the leak stopped after injection of rFVIIa and in the other, the presence of an arterial stent was a counter-indication for the radiologist for embolization. Five patients were polytransfused, but of the five patients who were not polytransfused, three were able to be autotransfused with blood from a haemothorax that was not counted in the blood transfusion. In all, eight patients who underwent no mechanical procedure could be considered as having been polytransfused.
In 63% of patients (17), a mechanical procedure was used to stop the hemorrhage. Ten patients had arteriography embolization and seven underwent the exploratory surgery.
We observed few complications that could be said to be attributable to the injection of rFVIIa. Only two patients developed thromboembolic complications in the group of surviving patients, i.e., a complication rate of 11%.
The average time on mechanical ventilation for the group of survivors was 20±11 days. Half the patients presented with acute respiratory distress syndrome (ARDS) on D1. The average length of stay in intensive care was 28±17 days.
DISCUSSION
The mortality rate in our study was 33%. It is difficult to compare these results with other series as every study has different exclusion criteria. However, Sauaia et al,[3] reports a mortality rate of 39% which is similar to our own results. In the princeps study by Boffard et al,[1] the authors observe a mortality rate between 24% and 30% depending on the group. Our adjusted mortality rate is 8.3%. Our series is different from that in princeps study in two essential respects: first the delay before injection (after the eighth unit of RCC in princeps study and variable in ours) and second transfusion strategy. For Boffard et al, the biological objectives were to maintain hematocrit between 20% and 30% (i.e., hemoglobin level of about 8 g/dl and 10 g/dl), PT at 60% and platelet count above 50 000/ml. Our biological objectives[4] encouraged a more aggressive transfusion strategy, with the hemoglobin level maintained at 10 g/dl, PT at 70% and platelet count above 100 000/ml. Recent studies into hemorrhagic shock recommend a more aggressive transfusion procedure, in particular the latest review by Spinella[5] of the RBC/plasma ratio. Patients in our study who died had an average hemoglobin level of 7.75 g/dl. This result should, in our opinion, encourage us to raise the threshold for erythrocyte transfusion. Lastly, the study by Dutton et al,[6] of the ineffectiveness criteria of rFVIIa showed that to obtain an optimal coagulation efficiency a platelet count of over 140 000/mm3 was required. This new transfusion strategy is therefore based on clinical data but also on new coagulation theories, as described in our own review of blood transfusion in traumatic hemorrhagic shock.[4] Coagulation is a solid phenomenon that requires the presence of the membranes of cells involved in coagulation (erythrocytes, platelets, leucocytes, and endothelial cells) and coagulation factors. This implies an earlier compensation for the coagulation cells in the hemorrhage processes. Moreover, our transfusion criteria have been validated by the French Army medical service.[7] Lastly, United States military doctors[8] have recently reminded us of the need to compensate for blood loss as efficiently as possible. They made a retrospective comparison of two groups of transfused patients, one transfused with whole blood and the other with a combination of RBC, plasma, and platelets. The group with the whole blood had a significantly better life expectancy after 30 days. Thus, even combining RBC-plasma-platelets in restrictive ratios of 1 : 1 (RBC : plasma), 1 : 6 (platelets : RBC) does not entirely compensate for blood loss or result in a “restitutio ad integrum” of the coagulation system.
The predictive factors that we find for mortality are the elements that make up the trauma triad of death, i.e., acidosis, hypothermia, coagulopathy, and also ionized hypocalcaemia. Similarly, as predictive factors for rFVIIa ineffectiveness, we find acidosis, thrombopenia, and ionized hypocalcaemia. Our study is the first to define ionized hypocalcaemia as a predictive factor for mortality and rFVIIa ineffectiveness. The results obtained from a cohort of limited seize should be considered primilary and hypothesis generating and should be confirmed in a large prospective study. As calcium is an indispensable element in any coagulation reaction, it should be considered as a full-fledged coagulation factor. Different elements of hemorrhagic shock combine to bring about hypocalcaemia. First, there is blood loss, but there is also the massive replacement by colloids and the transfusion of RBC, of which the additive solution contains citrate, a chelating agent for calcium. Thrombopenia is traditionally described as a factor of rFVIIa ineffectiveness. Our study reported an ineffectiveness threshold of around 100 000 platelets per mm3. Dutton et al,[6] took 86 000 platelets per mm3 as the ineffectiveness threshold and European recommendations on the use of rFVIIa[2] suggest maintaining a level of 50 000 platelets, considerably lower than the level required for an optimal coagulation reaction. Hypothermia was not a criterion for rFVIIa ineffectiveness, which is in agreement with Meng et al,[9] who found no influence of temperature on the level of rFVIIa activity, contrary to pH. This also leads us to consider the significance of hypothermia in this context: is it a real major aggravation factor or does it merely reflect the overwhelming (lethal) nature of the hemorrhagic shock? Three ineffectiveness criteria were observed in two patients who died from refractory hemorrhagic shock, and we observed one survivor in the group with two ineffectiveness criteria. In our opinion, there would be justification in future not to resort to rFVIIa when three ineffectiveness criteria are observed together.
In this study, we observe that different strategies were applied when using rFVIIa, but this is mainly because our own practices have evolved over the last 3 years. This is why we wanted to define our own strategy, called the “unit protocol,” where rFVIIa was injected after transfusion of the fourth RBC and often before the haemostatic procedure was carried out. When we followed this strategy, we observed no deaths, unlike the strategy in the European recommendations. Moreover, the presence of three ineffectiveness criteria appeared only in the group receiving rFVIIa after a radical procedure. By injecting rFVIIa before the arteriography, the hemorrhaging diagnosed with tomodensitometry and the injection of a contrast product was able to be stopped in four patients out of ten. Several recent studies are in favor of an earlier injection of rFVIIa. First, Boffard et al, clearly showed that an injection of rFVIIa after the transfusion of the eighth RBC was unable to reduce mortality. In a hemorrhagic shock model using a pig, Sapsford et al,[10] showed that with rFVIIa, the pigs’ survival time could be increased during hypotensive reanimation versus placebo. Lecompte et al,[11] in France, also working on a hemorrhagic shock model, but this time using a rabbit with a wound to the carotid artery, showed that rFVIIa reduced blood loss. Lastly, in man, Spinella et al,[12] showed in a retrospective study that with an injection of rFVIIa 120 min in median after the trauma, mortality was significantly reduced compared with no injection of rFVIIa in the first 24 h and at D30. These results must therefore now be confirmed in a prospective study. All these studies therefore suggest that consideration should be given to the possible benefits of injecting rFVIIa earlier in cases of traumatic hemorrhagic shock. For our part, we are convinced that optimal control of hemostasis is an indispensable preliminary step to controlling the hemorrhaging process, as this makes it easier to carry out the radical procedure, helps gain time so that this procedure can be carried out, reduces blood loss (decrease in the number of patients polytransfused in the princeps study) and finally, in some cases the bleeding stops spontaneously, as we have shown in this study. McLaughlin et al,[13] described a predictive algorithm for massive transfusion which combines hemodynamic parameters, pH<7.25, hematocrit <32% and the presence of intra-abdominal bleeding on the ultrasound scan. With such an algorithm, once an analysis of the lesions has been carried out, it would be possible to determine which patients would be likely to benefit from rFVIIa treatment, associated with a mechanical procedure to stop the bleeding. Lastly, half the patients in this study presented with multiple hemorrhages [Table 1] and yet normally the mechanical procedure concerns only one hemorrhage site. Given this context, rFVIIa could cause blood loss to decrease at the hemorrhage site not involved in the radical procedure and thus avoid overactivity in the haemostatic system.
In our study, we report a higher percentage of ARDS (55%) than in the princeps study (between 4% and 6%). However, these patients did not require any specific care relating to the ARDS and no patient died from ARDS. On the other hand, ventilator time was entirely comparable with princeps study. The high ARDS rate may be linked with our transfusion strategy. However, it must be noted that in our series of patients there were a large number with traumatism to the thorax (16/27) and we report a higher thromboembolic complication rate than in princeps study. However, we have many fewer patients and arterial thrombosis is a frequent complication in arterial bypass surgery. In addition, American military doctors[14] have used rFVIIa for vascular bypass following trauma and observed a low complication rate.
This study does have many limitations. First of all, it is retrospective. The strategies to be used were therefore determined subsequent to the procedures being carried out. A certain amount of data were not used, in particular the hemodynamic parameter analysis. However, all the patients presented with hemorrhagic shock requiring blood replacement, blood transfusion and vasopressor support with the aim, which may or may not have been achieved, of maintaining a MAP of around 60 mmHg. The dosage of pressor amines in the acute phase was first determined empirically then adapted to the patient's MAP. We therefore believe that the best picture of the patient's hemodynamic state and the gravity of the hemorrhagic shock, which in turn represents the hemorrhagic rate, is based on the blood replacement required and the quantity of blood products transfused.
CONCLUSION
In our opinion, the earlier and cautious use of rFVIIa seems promising for treating patients in a state of traumatic hemorrhagic shock in the context of hematological damage control. In addition, this strategy requires (1) taking ineffectiveness criteria into account, (2) using a predictive polytransfusion algorithm, and (3) applying a more aggressive transfusion strategy.
ACKNOWLEDGMENT
We would like to thank the Novonordisk Laboratory for their financial support in writing up this study.
Footnotes
Source of Support: Novonordisk Laboratory
Conflict of Interest: None declared.
REFERENCES
- 1.Boffard KD, Riou B, Warren B, Choong PI, Rizoli S, Rossaint R, et al. Recombinant factor VIIa as adjunctive therapy for bleeding control in severely injured trauma patients: Two parallel randomized, placebo-controlled, double-blind clinical trials. J Trauma. 2005;59:8–15. doi: 10.1097/01.ta.0000171453.37949.b7. [DOI] [PubMed] [Google Scholar]
- 2.Vincent JL, Rossaint R, Riou B, Ozier Y, Zideman D, Spahn DR. Recommendations on the use of recombinant activated factor VII as an adjunctive treatment for massive bleeding-a European perspective. Crit Care. 2006;10:R120. doi: 10.1186/cc5026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sauaia A, Moore FA, Moore EE, Moser KS, Brennan R, Read RA, et al. Epidemiology of trauma deaths: A reassessment. J Trauma. 1995;38:185–93. doi: 10.1097/00005373-199502000-00006. [DOI] [PubMed] [Google Scholar]
- 4.Morel N, Morel O, Chimot L, Lortet V, Julliac B, Lelias A, et al. Acute traumatic haemorrhagic shock and transfusion: What's new in 2009.? Ann Fr Anesth Reanim. 2009;28:222–30. doi: 10.1016/j.annfar.2008.12.023. [DOI] [PubMed] [Google Scholar]
- 5.Spinella PC, Holcomb JB. Resuscitation and transfusion principles for traumatic hemorrhagic shock. Blood Rev. 2009;23:231–40. doi: 10.1016/j.blre.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dutton RP, McCunn M, Hyder M, D’Angelo M, O’Connor J, Hess JR, et al. Factor VIIa for correction of traumatic coagulopathy. J Trauma. 2004;57:709–19. doi: 10.1097/01.ta.0000140646.66852.ab. [DOI] [PubMed] [Google Scholar]
- 7.Ausset S, Meaudre E, Kaiser E, Sailliol A, Hugard L, Jeandel P. Acute traumatic haemorrhagic shock and transfusion: The French army policy. Ann Fr Anesth Reanim. 2009;28:707–9. doi: 10.1016/j.annfar.2009.05.011. [DOI] [PubMed] [Google Scholar]
- 8.Spinella PC, Perkins JG, Grathwohl KW, Beekley AC, Holcomb JB. Warm fresh whole blood is independently associated with improved survival for patients with combat-related traumatic injuries. J Trauma. 2009;66(4 Suppl):S69–76. doi: 10.1097/TA.0b013e31819d85fb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Meng ZH, Wolberg AS, Monroe DM, 3rd, Hoffman M. The effect of temperature and pH on the activity of factor VIIa: Implications for the efficacy of high-dose factor VIIa in hypothermic and acidotic patients. J Trauma. 2003;55:886–91. doi: 10.1097/01.TA.0000066184.20808.A5. [DOI] [PubMed] [Google Scholar]
- 10.Sapsford W, Watts S, Cooper G, Kirkman E. Recombinant activated factor VII increases survival time in a model of incompressible arterial hemorrhage in the anesthetized pig. J Trauma. 2007;62:868–79. doi: 10.1097/ta.0b013e318034204b. [DOI] [PubMed] [Google Scholar]
- 11.Durand D GA, Notet V, Hacquard M, Collignon O, Corbonnois G, Plénat F, et al. Efficacy and safety of rFVIIa in a model of hemorrhagic shock in the rabbit. Ann Fr Anesth Reanim. 2009;28:S144. [Google Scholar]
- 12.Spinella PC, Perkins JG, McLaughlin DF, Niles SE, Grathwohl KW, Beekley AC, et al. The effect of recombinant activated factor VII on mortality in combat-related casualties with severe trauma and massive transfusion. J Trauma. 2008;64:286–94. doi: 10.1097/TA.0b013e318162759f. [DOI] [PubMed] [Google Scholar]
- 13.McLaughlin DF, Niles SE, Salinas J, Perkins JG, Cox ED, Wade CE, et al. A predictive model for massive transfusion in combat casualty patients. J Trauma. 2008;64(2 Suppl):S57–63. doi: 10.1097/TA.0b013e318160a566. [DOI] [PubMed] [Google Scholar]
- 14.Fox CJ, Mehta SG, Cox ED, Kragh JF, Jr, Salinas J, Holcomb JB. Effect of recombinant factor VIIa as an adjunctive therapy in damage control for wartime vascular injuries: A case control study. J Trauma. 2009;66(4 Suppl):S112–9. doi: 10.1097/TA.0b013e31819ce240. [DOI] [PubMed] [Google Scholar]


