Abstract
Cleistanthus collinus, a toxic shrub, is used for deliberate self-harm in rural South India. MEDLINE (PUBMED) and Google were searched for published papers using the search/ MeSH terms “Cleistanthus collinus,” “Euphorbiaceae,” “Diphyllin,” “Cleistanthin A,” Cleistanthin B” and “Oduvanthalai.” Non-indexed journals and abstracts were searched by tracing citations in published papers. The toxic principles in the leaf include arylnaphthalene lignan lactones — Diphyllin and its glycoside derivatives Cleistanthin A and B. Toxin effect in animal models demonstrate neuromuscular blockade with muscle weakness, distal renal tubular acidosis (dRTA) and type 2 respiratory failure with conflicting evidence of cardiac involvement. Studies suggest a likely inhibition of thiol/thiol enzymes by the lignan-lactones, depletion of glutathione and ATPases in tissues. V-type H+ ATPase inhibition in the renal tubule has been demonstrated. Mortality occurs in up to 40% of C. collinus poisonings. Human toxicity results in renal tubular dysfunction, commonly dRTA, with resultant hypokalemia and normal anion gap metabolic acidosis. Aggressive management of these metabolic derangements is crucial. Acute respiratory distress syndrome (ARDS) is seen in severe cases. Cardiac rhythm abnormalities have been demonstrated in a number of clinical studies, though the role of temporary cardiac pacemakers in reducing mortality is uncertain. Consumption of decoctions of C. collinus leaves, hypokalemia, renal failure, severe metabolic acidosis, ARDS and cardiac arrhythmias occur in severe poisonings and predict mortality. Further study is essential to delineate mechanisms of organ injury and interventions, including antidotes, which will reduce mortality.
Keywords: Cleistanthin A and B, Cleistanthus collinus poisoning, Oduvanthalai
INTRODUCTION
Self-inflicted fatality accounts for 1.5% of all deaths, making it the 10th highest cause of death globally. With an approximate annual mortality of 14.5 suicides per 100,000 people, the estimated global burden of suicide deaths is 1 million annually.[1] Self-inflicted injuries and, specifically, self-poisoning are major public health problems in the developing world, with 63% of global deaths from self-harm occurring in the Asia Pacific region.[2] That being said, the overall incidence of poisoning is probably underestimated due to inadequate reporting.[3,4] Easy access to highly toxic poisons, with limited access to medical facilities and expertise contribute to high mortality rates (up to 10-20%) associated with deliberate self-harm in these regions.[3] Deliberate self-harm in developing countries occurs predominantly among individuals aged 15-40 years, is usually of low intentionality with minimal psychiatric morbidity, and occurs essentially in rural populations.[2,3]
Suicide rates in South India are high, as evidenced by an overall suicide rate of 71.4 per 100,000 people in a community-based study.[5–7] Though plant poisoning is relatively uncommon globally, it is a common method of self-poisoning in the Indian subcontinent.[3,5,8–11] The most common types of plant poisons consumed in South India are Cleistanthus collinus (C. collinus) and Thevetia peruviana (yellow oleander).[5] C. collinus poisoning appears to be almost exclusive to the southern Indian states of Tamil Nadu and Pondicherry. In excess of 1000 cases of C. collinus poisoning were reported from various parts of Tamil Nadu between 1926 and 1985.[12] Subsequently, there have been a number of published clinical reports and studies from the region, which will be referred to in this review.[13–25] Inexplicably, despite the plant being distributed in various regions of India, no reports about poisoning are available from other parts of India, though there is mention of cases from pre-independence Bengal.[17]
C. collinus poisoning appears to be a problem of the rural population, favored by young women as a method of deliberate self-harm. A study on acute poisoning in villagers reported that 87.8% of women consumed plant poisons, 44.5% of whom consumed C. collinus.[10] This female preponderance with C. collinus poisoning is evident across most clinical studies. Easy availability and free access to the plant are probably why women prefer this method of deliberate self-harm.
Methodology
MEDLINE (PUBMED), the Cochrane Database, Clinicaltrials.gov, and Google/Google Scholar were searched for published papers using the search/MeSH terms “Cleistanthus collinus,” “Euphorbiaceae,” “Diphyllin,” “Cleistanthin A,” Cleistanthin B” and “Oduvanthalai.” Non-indexed journals and abstracts were searched by tracing citations in published papers. In addition, authors and other clinicians involved in the published trials were contacted to clarify certain aspects (personal communications of unpublished data).
The plant: Cleistanthus collinus
The Euphorbiaceae (spurge family) are a family of flowering plants with 300 genera and around 7,500 species. Most are herbs, but some, especially in the tropics, are also shrubs or trees. The genus Cleistanthus, belonging to the family Euphorbiaceae, comprises 140 species native to the region between Africa and the Pacific islands. C. collinus is a toxic deciduous shrub [Figure 1] that grows in hilly deciduous forests of Central and South India, Malaysia and Africa.[21,26,27] C. collinus in India is popularly known in: Hindi as Garari; Tamil, as Oduvanthalai; Telugu, as Vadise; Malayalam, as Nilapala; and Bengali, as Karlajuri.[17,21]
Figure 1.

Cleistanthus collinus
All parts of the plant are potentially toxic.[17] The leaves are commonly used for poisoning humans (suicide or homicide) and animals (cattle and fish poison) and as an abortifacient, especially in rural South India.[21] Extracts of leaves, roots and fruits have been used in acute gastrointestinal disorders. The method of ingestion of the plant for deliberate self-harm includes swallowing the crushed plant parts;, chewing the leaves; consuming a paste/juice of the leaves or a decoction prepared by boiling the leaves in water.[17]
Toxic constituents
Analyses of extracts from the plant, including the leaves, reveal a complex group of compounds.[28–40] The toxic active principles in the leaves are arylnaphthalene lignan lactones—Diphyllin and its glycoside derivatives Cleistanthin A and Cleistanthin B; and Collinusin [Figure 2]. Diphyllin, Cleistanthin A and B were collectively known as “Oduvin” in the past.[28] In addition, the lignans Cleistanthin C, Cleistanthin D and Cleistanone, are present.[40] The toxicity of the plant has been attributed primarily to Cleistanthin A and B.[27]
Figure 2.
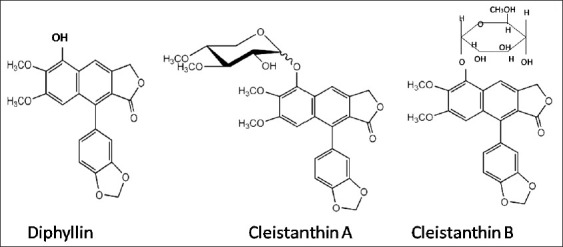
Chemical structure of Diphyllin, Cleistanthin A and B[26]
These toxic principles, the arylnaphthalene lignans, are detected in acetone extracts but, are not seen or are present in minimal amounts in aqueous extracts.[27] On spectroscopic analysis of aqueous extracts of fresh C. collinus leaves, the other major phytoconstituents detected are 3-O-methyl-d-glucose, benzenetriol (pyrogallic acid), 1.6- anhydro-â-D-glucopyranose (levoglucosan), heptacosane, 2-hydroxy-7-methoxy-4,5-diphenyl-5 hindeno [1.2-d] pyrimidine and eicosane.[27]
Animal models have revealed intriguing effects of the toxic principles at the cellular level. Cleistanthins can cause neutrophilic granulocytosis in rats, mice, cats and monkeys.[41] It can also prevent granulocytopenia induced by cyclophosphamide.[42] Alcoholic crude extracts from the entire plant have shown anti-proliferative properties against human epidermal carcinoma of the nasopharynx in tissue culture.[43] The cytotoxic effects of these glycosides have been elegantly demonstrated on tissue cell culture lines.[44] Cleistanthin A arrests cells growth by inhibiting DNA synthesis and cell division, by causing DNA strand breaks and hence DNA damage. It also induces cell apoptosis.[45–47] In addition, Cleistanthin A inhibits membrane metalloprotein-9, which reduces proliferating cell viability significantly.[48] Cleistanthin B is clastogenic and induces micronuclei formation and chromosomal aberrations. It inhibits cellular proliferation by causing a G1 phase arrest and induces cellular apoptosis.[49,50] These experimental findings suggest a possible role for these compounds as anti-neoplastic agents.
Animal models—target organ injury and proposed mechanisms of action
Studies on a murine phrenic nerve–diaphragm preparation revealed that extracts of the leaf inhibited muscle contraction by reducing excitability of nerve and muscle membranes, as well as blocking neuromuscular transmission.[51] It did not, however, affect excitation-contraction coupling or muscle fiber contractility. This effect on the neuromuscular junction with acetylcholine receptor site blockade was further delineated in sciatic nerve–tibialis anterior muscle preparations in rats, with partial response to Neostigmine.[52–54] Intra-peritoneal injection of leaf extract in rats resulted in a neuromuscular disorder resembling myasthenia gravis, with a sequential decremental response demonstrated on nerve-evoked compound muscle action potentials.[55] The model demonstrated that the neuromuscular blockade was sustained and possibly irreversible.
A detailed study of renal and cardio-respiratory functions in rat models revealed relatively more severe metabolic acidosis, lower serum potassium and alkaline urine compared to controls, suggesting a type 1 renal tubular acidosis. Additionally, a type 2 respiratory failure was observed in the test rats. The terminal event in these test rats was respiratory arrest with bradycardia, and subsequent cardiac arrest.[56] The type 2 respiratory failure could be explained by the presence of neuromuscular blockade. This study did not demonstrate the occurrence of ECG abnormalities.[56] Another study which examined the effect of the boiled extract on the rat heart had different observations. The study showed that low doses of extract caused transient tachycardia with increased myocardial contractility, while high doses caused arrhythmia and cardiac arrest. In addition, persistent renal excretion of sodium despite hyponatremia and renal failure was observed in these rats.[57]
In vitro exposure to C. collinus extract results in significant inhibition of vacuolar H+ATPase (vesicular proton pump) activity in the renal brush border membrane. Inhibition of the V-type H+ATPase in the renal tubule could explain the presence of distal renal tubular acidosis demonstrated in animal models and human subjects.[58] A separate study has demonstrated that specifically Diphyllin inhibits the V-type H+ATPase.[59] There is no evidence of inhibition of the sodium-potassium pump.[56]
Animal studies on rats and rabbits have shown an inhibition of thiol-dependent enzymes such as lactate dehydrogenase and cholinesterase (potentially explaining neuromuscular blockade effects).[60–61] It appears that C. collinus depletes glutathione and ATPases in various tissues, including the liver, kidney, brain, skeletal muscle and heart, in animal models. The loss of ATPase activity may be a result of oxidation of-SH (thiol) groups. It appears that inhibition of the thiol/thiol enzymes by lignan lactones may be an integral mechanism in the toxicity profile of C. collinus. Glutathione depletion with ATPase inhibition and resultant cellular injury results in focal hepatic necrosis, glomerular degeneration and cerebral gliosis evident in animal models.[61] Though thiol-dependent enzyme depletion has been established in animal models, it has not been clearly demonstrated in human subjects.[17,49,62]
Clinical profile of C. collinus toxicity in humans
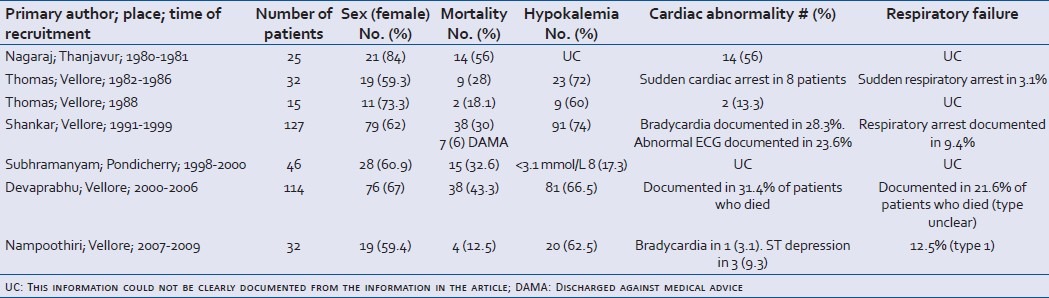
Clinical studies have described in detail the clinical profile of patients presenting with C. collinus poisoning [Table 1]. Patients may be asymptomatic or present with common gastrointestinal symptoms of vomiting, nausea and abdominal pain; and, occasionally, diarrhea, constipation, dysphagia, salivation, abdominal distension and decreased bowel sounds.[13,14,16–24] Cardio-respiratory presentations include chest pain, dyspnea, tachypnea or bradypnea, tachycardia or bradycardia, hypotension and cyanosis.[24] Documented clinical neurological abnormalities are mydriasis with visual disturbances, muscle cramps and weakness, altered sensorium, giddiness, headache, altered speech, tremors and ptosis.[14,16–24] Fever within the first 96 hours of presentation, and dehydration have also been documented.[14,24] In addition to hypokalemia with a normal anion gap metabolic acidosis, documented laboratory abnormalities include leukocytosis, elevated hepatic transaminases, elevated creatine phosphokinase, hyponatremia, hyperbilirubinemia and coagulopathy.[17,18]
Table 1.
Clinical observational studies conducted between 1980 and 2010 (>10 patients)
Renal dysfunction and electrolyte derangement
Distal renal tubular acidosis with hyperchloremic normal anion gap metabolic acidosis; alkaline urine; and hypokalemia with kaliuresis appears to be a consistent feature in patients with C. collinus poisoning.[17,25] These findings are also consistent with conclusions drawn using animal models.[56] A detailed study of renal tubular function revealed that the distal tubular cell was the most susceptible although proximal tubular injury, and in more severe forms, global tubular dysfunction with diminished glomerular filtration rate (GFR) may occur. Metabolic acidosis with defective urinary acidification was almost a universal phenomenon, often persisting even at discharge. There appeared to be a progression of the severity of renal injury — metabolic acidosis in patients with mild tubular injury; to more severe forms exhibiting metabolic acidosis and hypokalemia; to the most severe forms showing tubular dysfunction and decreased GFR—resulting in metabolic acidosis, hypokalemia, renal failure and in the majority of these patients, death.[25] Renal failure, oliguric in most instances, has been documented across clinical studies, though its etiology is probably multifactorial: direct toxin effect secondary to hypotension and, possibly, secondary to hypokalemic rhabdomyolysis.
Hypokalemia is evident in a majority of symptomatic patients, usually at presentation or within the first 48 hours of ingestion. Kaliuresis appears to be the main mechanism underlying this abnormality, though vomiting and dehydration may have a role. Hyponatremia has also been documented. Hypomagnesemia may have an additional role in refractory hypokalemia, though this requires to be confirmed by further study.
Cardio-respiratory dysfunction
The toxic principles, being glycosides, would be expected to cause cardiac effects. Clinical reports have shown cardiac rhythm abnormalities.[13–21,24] A retrospective study showed electrocardiogram (ECG) abnormalities such as sinus tachycardia, sinus bradycardia, flat “P” waves, prolonged QT and QTc intervals, ST segment depression and inverted “T” waves, in 95% of patients with C. collinus poisoning.[13] A prospective study documented premature ventricular complexes as the most common arrhythmia on continuous cardiac monitoring.[15] Malignant cardiac rhythms such as ventricular fibrillation and asystole have also been documented.[18] ST-T changes appeared to occur more frequently in patients that died.[17] Elevated cardiac enzymes have been documented in patients with poisoning, suggesting a possibility of direct cardiac myotoxicity.[17] The degree to which the dominant metabolic derangements affect cardiac function is still unclear.
Mortality occurs despite patients having prophylactic temporary cardiac pacemakers insertion (TPI).[25] In view of this fact and taking into account that recent animal studies have not shown cardiac rhythm abnormalities,[56] further studies on cardiac dysfunction in humans and the role of TPI are urgently needed.
Shock is associated with mortality. It has been hypothesized that shock occurs due to inappropriate peripheral vasodilation.[20] Whether there is a cardiogenic component in shock is unclear in literature.
Type 1 respiratory failure and adult respiratory distress syndrome (ARDS) have been documented and have been associated with mortality. A possible defect in oxygen transfer at the alveolar-capillary level has been suggested.[17] Type 2 respiratory failure has not been clearly documented in observational human studies unlike animal models, though respiratory arrests have been mentioned.
Neuromuscular weakness
Neuromuscular weakness in humans has rarely been documented as opposed to animal studies, which have shown a consistent effect of C. collinus toxins on the neuromuscular junction.[18,23] A clinical case presenting with a myasthenia gravis–like picture with response to Neostigmine has been reported.[23] Muscular weakness in patients could also be attributed to hypokalemia and possibly to hypokalemia-associated rhabdomyolysis.[63]
Mortality in C. collinus poisoning
Mortality occurs in up to 40% of patients with C. collinus poisoning. Death usually occurs within 3-7 days (majority of deaths occurring on the third day) of consumption.[17,20] Consumption of a boiled decoction of leaves (rather than other methods of ingestion) has consistently been shown to result in higher mortality rates across clinical studies. A larger number of leaves (>60) consumed was also associated with a higher rate of mortality.[17] Hypokalemia has been found to be more severe in patients who died.[17,24] Associations with mortality include older age, underlying chronic disease, altered sensorium, tachycardia (>120/min) or bradycardia (<60/min), fever, hypotension, abnormal vision, tachypnea, dyspnea, persistent abdominal pain, giddiness after 24 hours, respiratory arrest, severe acidosis, renal failure and ARDS.[17,24,25]
Management
Assessment and initial management
Airway, breathing and circulation should be assessed and appropriately stabilized. The patient's level of consciousness and ability to protect the airway, hemodynamic stability and level of hydration need to be gauged. Mechanical ventilation may be required in the setting of respiratory failure. An urgent 12-lead ECG with rhythm strip should be obtained to identify any rhythm disturbance, and the patient should be initiated on continuous cardiac monitoring. Cardiac monitoring may need to be sustained for up to 5 days in symptomatic patients. Initial lab investigations should include serum electrolytes (potassium, sodium, bicarbonate and magnesium), creatinine, arterial blood gas and a chest radiograph. In case of vomiting, an antiemetic could be used.
Decontamination
In patients who have taken decoctions, it is unlikely that gastric lavage beyond 1-2 hours post-ingestion is of benefit. A risk assessment will need to be made since patients who consume crushed leaves usually do not manifest significant toxicity. The benefit of lavage needs to be considered with caution as itsutility in this particular poisoning is unclear.
A small non-randomized study on the use of multiple doses of activated charcoal on 26 patients with C. collinus poisoning presenting within 24 hours demonstrated a mortality benefit.[22] Activated charcoal was administered every 6 hours for 4 days. All of the 12 patients in the “multi-dose charcoal” group survived and 8 (57.14%) of the 14 in the “no charcoal” group survived. The small size of the study population requires that the results be taken with caution. Large randomized controlled trials conducted for yellow oleander and the use of activated charcoal have been inconclusive in demonstrating mortality benefit,[9,64] and further study is required to determine whether activated charcoal will be of benefit in patients with C. collinus poisoning. A single dose of activated charcoal may be considered in patients presenting to the emergency department with C.collinus poisoning.
Definitive management
The mainstay of C. collinus poisoning management is monitoring and correction of electrolyte imbalances, namely, hypokalemia and metabolic acidosis [Figure 3].[25,65] In a study, the strongest protective risk factor was higher plasma potassium levels (a risk reduction of 58% in mortality per 1 mmol/L increase in plasma potassium level).[24] Aggressive preemptive correction of potassium and, acidosis is crucial. Aggressive management of shock with crystalloids and inotropes (both epinephrine and dobutamine have been used) is indicated. Renal function needs to be monitored carefully with strict intake-output monitoring, central venous access and serum creatinine levels.
Figure 3.
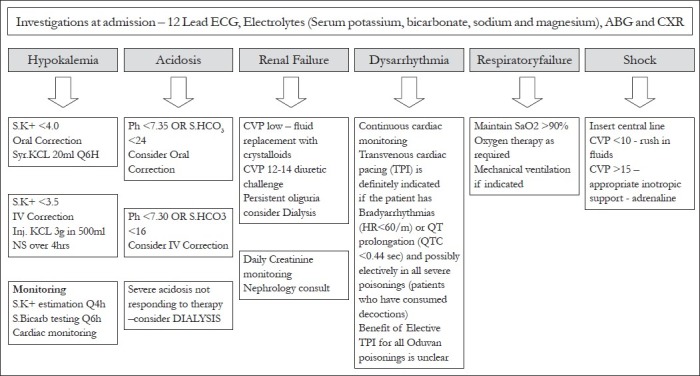
Proposed protocol of management based on current evidence
The precise role of cardiac pacing in the setting of C. collinus poisoning is a matter of conjecture as the evidence for preventing deaths through its use is inadequate.[15,25] It is likely, however, that it would be indicated in the setting of cardiac rhythm abnormalities such as bradycardia and QTc prolongation, and may need to be placed electively in patients with severe poisoning. It is unlikely that elective pacemaker insertions for all patients with C. collinus poisoning will be of benefit, and further studies on its indications and its duration of use are needed before definite recommendations can be made.
Antidotes
N-acetylcysteine, L-cysteine, melatonin and thiol-containing compounds have all been suggested as possible antidotes for management of C. collinus toxicity.[12,60,66,67] In one case report, N-acetylcysteine has been reported to be used, but the benefit of this intervention is unclear.[20] Neostigmine was used, with probable clinical benefit, for a patient who presented with a myasthenic crisis–like syndrome.[23]
Diagnostics
Enzyme-linked immunosorbent assays (ELISAs) for Cleistanthin A and B have been developed. The kits can detect levels of Cleistanthin A and B as low as 3 ng/mL and 2 ng/mL, respectively. The simplicity of the ELISA makes it a feasible test for use in clinical and forensic toxicology for confirmation of diagnosis.[68–71] Other techniques that have been used for detection of toxin in blood and urine include high performance liquid chromatography (HPLC), spectrophotometry and thin-layer chromatography.[32,37–39,72,73]
In one case series, digoxin assays were found to be positive in 16 out of 27 C. collinus poisoning cases, with higher levels of digoxin detected in fatal cases. The authors felt that the test was neither likely to be of diagnostic value nor was it likely to strengthen a case for the use of anti-Dig Fab fragment in the management as there was phytotoxin cross-reactivity.[17]
CONCLUSION
C. collinus poisoning is a problem in rural South India. The renal tubule appears to be an important target of injury. Distal renal tubular acidosis results in hypokalemia and metabolic acidosis, correction of which is integral to the management of this poisoning. The determination of the exact mechanism and extent of cardio-respiratory involvement requires further study, though evidence suggests that careful monitoring and management is important. Further research is needed to fully understand the mechanisms of toxicity in C. collinus poisoning, as well as potential antidotes, to reduce mortality.
ACKNOWLEDGMENTS
I would like to express my gratitude to Professor Anand Zachariah; and the members of Toxicology Special Interest Group, CMC, Vellore, who have done significant work in the area of Cleistanthus collinus poisoning and who helped develop the protocol of management cited in this article. I would also like to thank the staff of Department of Physiology, CMC, Vellore, who have done significant work with animal models and Cleistanthus collinus poisoning and consequently have provided crucial insights to the mechanisms of toxicity. I thank the Department of Medicine, Units 1, 2, 3; and the Department of Medical ICU, CMC, Vellore, where many of the patients of Cleistanthus collinus poisoning are treated and where data has been generated.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Hawton K, Heeringen K. Suicide. Lancet. 2009;373:1372–81. doi: 10.1016/S0140-6736(09)60372-X. [DOI] [PubMed] [Google Scholar]
- 2.Eddleston M, Phillips MR. Self poisoning with pesticides. BMJ. 2004;328:42–4. doi: 10.1136/bmj.328.7430.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Eddleston M. Patterns and problems of deliberate self-poisoning in the developing world. QJM. 2000;93:715–31. doi: 10.1093/qjmed/93.11.715. [DOI] [PubMed] [Google Scholar]
- 4.Jeyaratnam J. Acute pesticide poisoning: A major global health problem. World Health Stat Q. 1990;43:139–44. [PubMed] [Google Scholar]
- 5.Bose A, Sandal Sejbaek C, Suganthy P, Raghava V, Alex R, Muliyil J, et al. Self-harm and self-poisoning in southern India: Choice of poisoning agents and treatment. Trop Med Int Health. 2009;14:761–5. doi: 10.1111/j.1365-3156.2009.02293.x. [DOI] [PubMed] [Google Scholar]
- 6.Joseph A, Abraham S, Muliyil JP, George K, Prasad J, Minz S, et al. Evaluation of suicide rates in rural India using verbal autopsies, 1994-1999. BMJ. 2003;326:1121–2. doi: 10.1136/bmj.326.7399.1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aaron R, Joseph A, Abraham A, Muliyil J, George K, Prasad J, et al. Suicides in young people in rural southern India. Lancet. 2004;363:1117–8. doi: 10.1016/S0140-6736(04)15896-0. [DOI] [PubMed] [Google Scholar]
- 8.Eddleston M, Ariaratnam CA, Meyer WP, Perera G, Kularatne AM, Attapattu S, et al. Epidemic of self-poisoning with seeds of the yellow oleander tree (Thevetia Peruviana) in northern Sri Lanka. Trop Med Int Health. 1999;4:266–73. doi: 10.1046/j.1365-3156.1999.00397.x. [DOI] [PubMed] [Google Scholar]
- 9.Eddleston M, Juszczak E, Buckley NA, Senarathna L, Mohamed F, Dissanayake W, et al. Multiple-dose activated charcoal in acute self-poisoning: A randomised controlled trial. Lancet. 2008;371:579–87. doi: 10.1016/S0140-6736(08)60270-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Aleem MA, Paramasivam M. Spectrum of acute poisoning in villagers. J Assoc Physicians India. 1993;43:859. [Google Scholar]
- 11.Batra AK, Keoliya AN, Jadhav GU. Poisoning: An unnatural cause of morbidity and mortality in rural India. J Assoc Physicians India. 2003;51:955–9. [PubMed] [Google Scholar]
- 12.Annapoorani KS, Damodaran C, Chandrasekharan P. A promising antidote to Cleistanthus collinus poisoning. J Sci Soc Ind. 1986;2:3–6. [Google Scholar]
- 13.Thomas K, Dayal AK, Gijsbers A, Seshadri MS. Oduvanthalai leaf poisoning. J Assoc Physicians India. 1987;35:769–71. [PubMed] [Google Scholar]
- 14.Nagaraj S. Cardiac toxicity of Oduvanthalai (Cleistanthus collinus) common leaves poisoning in Tamil Nadu (Report of 25 cases) Antiseptic. 1987;84:33–5. [Google Scholar]
- 15.Thomas K, Dayal AK, Narasimhan, Ganesh A, Seshadri MS, Cherian AM, et al. Metabolic and cardiac effects of Cleistanthus collinus poisoning. J Assoc Physicians India. 1991;39:312–4. [PubMed] [Google Scholar]
- 16.Aleem HM. Oduvan leaf poisoning. J Assoc Physicians India. 1991;39:973–4. [PubMed] [Google Scholar]
- 17.Subrahmanyam DK, Mooney T, Raveendran R, Zachariah B. A clinical and laboratory profile of Cleistanthus collinus poisoning. J Assoc Physicians India. 2003;51:1052–4. [PubMed] [Google Scholar]
- 18.Eswarappa S, Chakraborty AR, Palatty BU, Vasnaik M. Cleistanthus collinus poisoning: Case reports and review of literature. J Toxicol Clin Toxicol. 2003;41:369–72. doi: 10.1081/clt-120022005. [DOI] [PubMed] [Google Scholar]
- 19.Sarathchandra G, Prabhasankar P, Sekharan PC, Annapoorani S, Murthy PB. Toxicology of Cleistanthus collinus, an indigenous plant: Acute toxicity study. Ind J Toxicology. 1996;3:9–17. [Google Scholar]
- 20.Benjamin SP, Fernando ME, Jayanth JJ, Preetha B. Cleistanthus collinus poisoning. J Assoc Physicians India. 2006;54:742–4. [PubMed] [Google Scholar]
- 21.Devaprabhu S, Manikumar S, David SS. Toxico-epidemiology and prognostic profile of patients with Cleistanthus collinus poisoning. Indian J Trauma Anaesth Crit Care. 2007;8:642–6. [Google Scholar]
- 22.Raja G, Kumaran SS, Chandrasekaran VP. Outcome of Cleistanthus collinus poisoning with and without charcoal. Acad Emerg Med. 2007;14:e111. [Google Scholar]
- 23.Damodaram P, Manohar IC, Kumar DP, Mohan A, Vengamma B, Rao MH. Myasthenic crisis-like syndrome due to Cleistanthus collinus poisoning. Indian J Med Sci. 2008;62:62–4. [PubMed] [Google Scholar]
- 24.Shankar V, Jose VM, Bangdiwala SI, Thomas K. Epidemiology of Cleistanthus collinus (oduvan) poisoning: Clinical features and risk factors for mortality. Int J Inj Contr Saf Promot. 2009;16:223–30. doi: 10.1080/17457300903307094. [DOI] [PubMed] [Google Scholar]
- 25.Nampoothiri K, Chrispal A, Begum A, Jasmine S, Gopinath KG, Zachariah A. A clinical study of renal tubular dysfunction in Cleistanthus collinus (Oduvanthalai) poisoning. Clin Toxicol (Phila) 2010;48:193–7. doi: 10.3109/15563651003641786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pinho PM, Kijjoa A. Chemical constituents of the plants of the genus Cleistanthus and their biological activity. Phytochem Rev. 2007;6:175–82. [Google Scholar]
- 27.Parasuraman S, Raveendran R, Madhavrao C. GC-MS analysis of leaf extracts of Cleistanthus collinus Roxb.(Euphorbiaceae) Int J Ph Sci. 2009;1:284–6. [Google Scholar]
- 28.Rajagopal Naidu S, Venkat Rao P, Subrahmanyam CA. The microscopy and chemistry of oduvin. J Proc Inst Chem India. 1944;16:59–63. [Google Scholar]
- 29.Maiti PC, Das AK. Chemical examination of the fruits of Cleistanthus collinus. Curr Sci. 1965;34:179–81. [Google Scholar]
- 30.Govindachari TR, Sathe SS, Viswanathan N, Pai BR, Srinivasan M. Chemical constituents of Cleistanthus collinus (Roxb.) Tetrahedron. 1969;25:2815–21. [Google Scholar]
- 31.Lakshmi TG, Srimanarayana G, Subba Rao NV. A new glucoside from Cleistanthus collinus. Curr Sci. 1970;39:395–6. [Google Scholar]
- 32.Subramanian R, Krishnamurthy G. Thin-layer chromatographic detection of the lignan lactones of Cleistanthus collinus (Roxb.) J Chromatogr. 1975;107:230–3. doi: 10.1016/s0021-9673(00)82772-7. [DOI] [PubMed] [Google Scholar]
- 33.Joshi SS, Srivastava RK. Chemical examination of Cleistanthus collinus seeds. J Oil Technol Assoc India. 1977;9:156–7. [Google Scholar]
- 34.Anjaneyulu AS, Ramaiah PA, Rao R. Crystalline constituents of Euphorbiaceae; Part XVI A new Diphyllin glycoside from Cleistanthus collinus. Indian J Chem. 1977;15B:10–1. [Google Scholar]
- 35.Anjaneyulu AS, Ramaiah PA, Row LR, Venkateswarlu R, Pelter A, Ward RS. New lignans from the heartwood of Cleistanthus collinus. Tetrahedron. 1981;37:3641–52. [Google Scholar]
- 36.Satyanarayana P, Subrahmanyam P, Koteswara RP. Chemical constituents of Cleistanthus collinus roots. Indian J Pharm Sci. 1984;46:95–6. [Google Scholar]
- 37.Annapoorani KS, Periakali P, Illangovan S, Damodaran C, Sekaran P. Spectroflurometric determination of the toxic constituents of Cleistanthus collinus. J Anal Toxicol. 1984;8:182–6. doi: 10.1093/jat/8.4.182. [DOI] [PubMed] [Google Scholar]
- 38.Annapoorani KS, Damodaran C, Sekharan PC. Spectrofluorodensitometric determination of diphyllin, a cystostatic lignan isolated from Cleistanthus collinus. Pharmazie. 1984;39:716–7. [PubMed] [Google Scholar]
- 39.Annapoorani KS, Damodaran C, Chandra Sekharan P. Solid-state fluorodensitometric quantitation of arylnaphthalene lignan lactones of Cleistanthus collinus. J Chromatogr. 1984;303:296–305. doi: 10.1016/s0021-9673(01)96081-9. [DOI] [PubMed] [Google Scholar]
- 40.Ramesh C, Ravindranath N, Ram TS, Das B. Arylnaphthalide lignans from Cleistanthus collinus. Chem Pharm Bull (Tokyo) 2003;51:1299–300. doi: 10.1248/cpb.51.1299. [DOI] [PubMed] [Google Scholar]
- 41.Rao RA, Nair TB. Investigations on induction of neutrophilic granulocytosis and toxicity of Cleistanthin-CIBA GO.4350-a new glycoside from Cleistanthus collinus (Roxb) Pharmacology. 1970;4:347–58. doi: 10.1159/000136164. [DOI] [PubMed] [Google Scholar]
- 42.Rao RA, Nair TB. Effect of cleistanthin, a new glycoside, on experimentally induced Leukopenia in rats and mice. Drug Res. 1971;21:828. [PubMed] [Google Scholar]
- 43.Bhakuni DS, Dhar ML, Dhar MM, Dhawan BN, Mehrota BN. Screening of Indian plants for biological activity part II. Indian J Exp Biol. 1969;7:250. [PubMed] [Google Scholar]
- 44.Rajkumar S, Bhatia AL, Shanmugam G. Molecular mechanisms underlying the inhibition of cell proliferation by cleistanthins. Asian J Exp Sci. 2001;15:9–16. [Google Scholar]
- 45.Pradheepkumar CP, Paneerselvam N, Shanmugam G. Cleistanthin A causes DNA strand breaks and induces apoptosis in cultured cells. Mutat Res. 2000;464:185–93. doi: 10.1016/s1383-5718(99)00179-5. [DOI] [PubMed] [Google Scholar]
- 46.Pradheepkumar CP, Shanmugam G. Anticancer potential of cleistanthin A isolated from the tropical plant Cleistanthus collinus. Oncol Res. 1999;11:225–32. [PubMed] [Google Scholar]
- 47.Meenakshi J, Shanmugam G. Cleistanthin A, a diphyllin glycoside from Cleistanthus collinus, is cytotoxic to PHA-stimulated (proliferating) human lymphocytes. Drug Dev Res. 2000;51:187–90. [Google Scholar]
- 48.Meenakshi J, Shanmugam G. Inhibition of matrix metalloproteinase-9 (MMP-9) activity by cleistanthin A, a diphyllin glycoside from Cleistanthus collinus. Drug Dev Res. 2000;50:193–4. [Google Scholar]
- 49.Pradheepkumar CP, Paneerselvam N, Rajesh S, Shanmugam G. Cytotoxic and genotoxic effects of cleistanthin B in normal and tumour cells. Mutagenesis. 1996;11:553–7. doi: 10.1093/mutage/11.6.553. [DOI] [PubMed] [Google Scholar]
- 50.Kumar CP, Pande G, Shanmugam G. Cleistanthin B causes G1 arrest and induces apoptosis in mammalian cells. Apoptosis. 1998;3:413–9. doi: 10.1023/a:1009658518998. [DOI] [PubMed] [Google Scholar]
- 51.Nandakumar NV, Pagala MK, Venkatachari SA, Namba T, Grob D. Effect of Cleistanthus collinus leaf extract on neuromuscular function of the isolated mouse phrenic nerve-diaphragm. Toxicon. 1989;27:1219–28. doi: 10.1016/0041-0101(89)90030-5. [DOI] [PubMed] [Google Scholar]
- 52.Vijayalakshmi KM, Nanadakumar NV, Pagala MK. Confirmatory in vivo electrodiagnostic and electromyographic studies for neuromuscular junctional blocking action of Cleistanthus collinus leaf extract in rat. Phytother Res. 1996;10:215–9. [Google Scholar]
- 53.Vijayalakshmi KM, Nanadakumar NV. Confirmatory in vivo electrodiagnostic and electromyographic studies for a new neuromuscular junctional blocking agent from Indian medicinal plant Cleistanthus collinus. Electroencephalogr Clin Neurophysiol. 1995;97:218. [Google Scholar]
- 54.Vijayalakshmi KM, Nanadakumar NV. Electrocardiac and electromyographic studies on the effects of Cleistanthus collinus leaf extract in rat. J Med Aromat Plant Sci. 1998;20:1009–12. [Google Scholar]
- 55.Nanadakumar NV, Vijayalakshmi KM. Experimental myasthenia gravis-like neuromuscular impairment with Cleisthanthus collinus leaf extract administration in rat. Phytother Res. 1996;10:121–6. [Google Scholar]
- 56.Maneksh D, Sidharthan A, Kettimuthu K, Kanthakumar P, Lourthuraj AA, Ramachandran A, et al. Cleistanthus collinus induces type I distal renal tubular acidosis and type II respiratory failure in rats. Indian J Pharmacol. 2010;42:178–84. doi: 10.4103/0253-7613.66843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jose VM, Anand KN, Jeyaseelan L, Ernest K, Kuruvilla A. Effect of potassium channel modulators on toxicity of Cleistanthus collinus. Indian J Exp Biol. 2004;42:81–5. [PubMed] [Google Scholar]
- 58.Kettimuthu K, Ramachandran A, Lourthuraj AA, Manickam SA, Subramani S. Mechanism of toxicity of Cleistanthus collinus: Vacuolar H+ ATPases are a putative target. Clin Toxicol. 2009;47:724. doi: 10.3109/15563650.2011.590939. [DOI] [PubMed] [Google Scholar]
- 59.Sorensen MG, Henriksen K, Neutzsky-Wulff AV, Dziegiel MH, Karsdal MA. Diphyllin, a novel and natural potent V-ATPase inhibitor, abrogates acidification of the osteoclastic resorption lacunae and bone resorption. J Bone Miner Res. 2007;22:1640–8. doi: 10.1359/jbmr.070613. [DOI] [PubMed] [Google Scholar]
- 60.Sarathchandra G, Balakrishnamurthy P. Pertubations in glutathione and adenosine triphosphatase in acute oral toxicosis of Cleistanthus collinus: An indigenous toxic plant. Indian J Pharmacol. 1997;29:82–5. [Google Scholar]
- 61.Sarathchandra G, Balakrishnamoorthy P. Acute toxicity of Cleistanthus collinus, an indigenous poisonous plant in Cavia procellus. J Environ Biol. 1998;19:145–8. [Google Scholar]
- 62.Kanthasamy A, Govidasamy S, Damodaran C. Novel inhibition of LDH isoenzymes by Cleistanthus collinus toxins. Curr Sci. 1986;55:854–6. [Google Scholar]
- 63.Eswarappa S. Renal failure and neuromuscular weakness in Cleistanthus collinus Poisoning. J Assoc Physicians India. 2007;55:85–6. [PubMed] [Google Scholar]
- 64.De Silva HA, Fonseka MM, Pathmeswaran A, Alahakone DG, Ratnatilake GA, Gunatilake SB. Multiple-dose activated charcoal for treatment of yellow oleander poisoning: A single-blind, randomised, placebo-controlled trial. Lancet. 2003;361:1935–8. doi: 10.1016/s0140-6736(03)13581-7. [DOI] [PubMed] [Google Scholar]
- 65.Thirumavalavan R. Aggressive potassium correction may halt death in Cleistanthus collinus poisonining. J Toxicol Clin Toxicol. 2004;42:801. [Google Scholar]
- 66.Jayanthi M, Raveendran R, Basu D. Role of melatonin against oxidative tissue damage induced by Cleistanthus collinus in rat brain. Indian J Med Res. 2009;130:467–74. [PubMed] [Google Scholar]
- 67.Sarathchandra G, Murthy PB. Efficacy of L-cysteine in countering Cleistanthus collinus poisoning an indigenous phytotoxin. Ind Vet J. 2000;77:209–11. [Google Scholar]
- 68.Ragupathi G, Prabhasankar P, Sundaravadivel B, Annapoorani KS, Damodaran C. Of Cleistanthus collinus toxins by enzyme-linked immunosorbent assay. Toxicol Mech Methods. 1994;4:204–13. [Google Scholar]
- 69.Ragupathi G, Prabhasankar P, Sekharan PC, Annapoorani KS, Damodaran C. Enzyme-linked immunosorbent assay for the phytotoxin cleistanthin A. J Immunoassay. 1992;13:321–38. doi: 10.1080/15321819208021236. [DOI] [PubMed] [Google Scholar]
- 70.Ragupathi G, Prabhasankar P, Sekharan PC, Annapoorani KS, Damodaran C. Enzyme-linked immunosorbent assay (ELISA) for the determination of the toxic glycoside cleistanthin B. Forensic Sci Int. 1992;56:127–36. doi: 10.1016/0379-0738(92)90170-2. [DOI] [PubMed] [Google Scholar]
- 71.Ragupathi G, Prabhasankar P, Sekharan PC, Annapoorani KS, Damodaran C. Dipstick ELISA kit for the detection of Cleistanthus collinus toxins. Hindustan Antibiot Bull. 1992;34:6–12. [PubMed] [Google Scholar]
- 72.Ragupathi G, Prabhasankar P, Sekharan PC, Annapoorani KS, Damodaran C. Novel solid-state fluorodensitometric method for the determination of haptens in protein-hapten conjugates.Demonstration with a toxic glycoside of Cleistanthus collinus. J Chromatogr. 1992;574:267–71. [PubMed] [Google Scholar]
- 73.Annapoorani KS, Damodaran C, Chandrasekharan P. High pressure liquid chromatography separation of arylnaphthalene lignan lactones. J Liq Chromatogr. 1985;8:1173–94. [Google Scholar]


