Abstract
The aim of this study was to investigate the influence of rice bran oil consumption on plasma lipids and insulin resistance in patients with type 2 diabetes. Thirty-five patients with type 2 diabetes were randomly assigned to a placebo group or a rice bran oil group. The placebo group consumed 250 mL soybean oil-modified milk (18 g soybean oil) daily for 5 weeks, and the rice bran oil group consumed 250 mL rice bran oil modified milk (18 g rice bran oil) daily for 5 weeks. At week 0 and week 5, anthropometric measurements, hematology tests, and an oral-glucose-tolerance test were conducted. The results showed that the homeostasis model assessment index of insulin resistance, the area under the curve for postprandial serum insulin, and serum low-density-lipoprotein cholesterol concentrations increased significantly in the placebo group. In the rice bran oil group, fasting and 2-h postprandial blood glucose concentrations and the area under the curve for postprandial plasma glucose increased significantly; however, total serum cholesterol and low-density-lipoprotein cholesterol concentrations decreased significantly. However, the homeostasis model assessment index of insulin resistance was not significantly different. Consumption of 18 g rice bran oil modified milk daily for 5 weeks significantly decreased total serum cholesterol concentrations and tended to decrease low-density-lipoprotein cholesterol concentrations in patients with type 2 diabetes. However, no significant influence on insulin resistance was observed.
Keywords: rice bran oil, type 2 diabetes, blood lipids
Introduction
The World Health Organization reported that 50–80% of patients with type 2 diabetes die from cardiovascular diseases. In diabetes mellitus complicated by cardiovascular disease, insulin resistance and abnormal blood lipid concentrations play an important role.(1) Dietary oil intake has an important influence on blood lipid concentrations.(2) In 1980, researchers found that both the quantity and quality of dietary oil are associated with lipid metabolic disorders. Different compositions of fatty acids have different influences on lipid metabolic disorder.(3)
In Asia, rice bran oil (RBO) consumption has increased year by year.(4) Researchers have reported that oleic acid is the predominant fatty acid (38.4%) in RBO.(2,5) In a streptozotocin-induced diabetic rat model, plasma and liver triglyceride (TG) concentrations and insulin resistance decreased significantly and fecal neutral sterol concentrations and bile acid excretion increased after 4 wk of feeding with 15% RBO.(6) Total serum cholesterol concentrations decreased significantly in healthy subjects who consumed 75 mL RBO daily for 50 d.(7) Also, the use of RBO as a cooking oil can significantly decrease plasma TC and TG concentrations in hyperlipidemic patients.(8) However, the effect of RBO consumption on patients with type 2 diabetes remains unclear. Therefore, the aim of this study was to investigate the effect of RBO consumption on plasma lipids and insulin resistance in patients with type 2 diabetes.
Materials and Methods
A total of 40 patients with type 2 diabetes aged 30–80 years old were recruited from the Endocrinology and Metabolism Departments of Taipei Medical University Hospital and of Lotung Poh-Ai Hospital. The study subjects had a fasting blood glucose concentration ⩾126 mg/dL and no acute complications. Cardiovascular disease, cerebrovascular disease, kidney disease, gastrointestinal disease, liver disease (hepatitis B & C virus carrier, liver cirrhosis), thyroid disease, cancer, stroke, type 1 diabetes, and pregnancy were exclusion factors. This study was approved by the Human Experiment & Ethics Committees of Taipei Medical University Hospital and Lotung Poh-Ai Hospital. Written inform consent was obtained from each subject. However, 2 subjects can not co-operate with our experimental time, severe hemolysis occurred on 3 subjects, so 35 DM patients completed the all experiment finally.
This was a randomized, single blind, placebo, comparison study. The subjects were randomly assigned to a placebo group or an RBO group. The placebo group consumed 250 mL soybean oil–modified milk (18 g soybean oil) daily for 5 weeks, and the RBO group consumed 250 mL RBO-modified milk (18 g RBO) daily for 5 weeks. During the study period, breakfast was replaced with either the placebo or the RBO-modified milk; the reminder of the diet, lifestyle habits, and medication use were unchanged. At weeks 0 and 5, anthropometric measurements, hematology tests, and an oral-glucose-tolerance test (OGTT) were conducted. For the OGTT, fasting blood samples were collected and then the subjects ingested a 75-g glucose solution. Blood samples were then collected 30, 60, 90, 120, and 180 min after consumption of the glucose solution. During the study period, the subjects completed a diet diary 3 d/week, including 2 weekdays and 1 holiday. The modified milk (Tetra Pak; Jia Ge Food Co., Ltd.) contained 7.1 g sucrose, 11.6 g casein, and 18 g soybean oil (placebo group) or 18 g RBO (RBO group)—12.0% sugar, 19.6% protein, and 68.4% lipid. The composition of fatty acids in the modified milk is shown in Table 1.
Table 1.
Percentage of fatty acids composition in modified milk1
| Fatty acids % | Placebo | RBO |
|---|---|---|
| C14:0 | 0.1 ± 0.0 | 0.4 ± 0.0 |
| C16:0 | 13.5 ± 0.3 | 19.4 ± 0.4 |
| C18:0 | 9.3 ± 0.4 | 6.8 ± 0.6 |
| C20:0 | 0.3 ± 0.0 | 0.7 ± 0.0 |
| C22:0 | 0.3 ± 0.0 | 0.2 ± 0.0 |
| Σ SFA2 | 23.6 ± 0.7 | 27.3 ± 1.1 |
| C16:1 | 0.1 ± 0.0 | 0.2 ± 0.0 |
| C18:1 | 19.6 ± 0.1 | 39.7 ± 0.6 |
| Σ MUFA2 | 19.7 ± 0.1 | 39.9 ± 0.6 |
| C18:2 | 49.4 ± 0.5 | 31.7 ± 0.5 |
| C18:3 | 7.2 ± 0.1 | 1.0 ± 0.0 |
| Σ PUFA2 | 56.7 ± 0.6 | 32.8 ± 0.5 |
1 Values are expressed as mean ± SEM, n = 3. 2 SFA, saturated fatty acid; MUFA, monounsaturated fatty acid; PUFA, polyunsaturated fatty acid.
The diet diaries were analyzed by using EK-NUTN8 to calculate the consumption of calories and the 3 major macronutrients. The blood samples were centrifuged at 3000 rpm for 10 min at ⩽4°C. The Glucose Kit (Randox Laboratories Ltd., Co. Antrim, UK) was used to measure plasma glucose concentrations. The Human Insulin ELISA Kit (Mercodia, Uppsala, Sweden) was used to measure serum insulin concentration. Insulin resistance was reported as the homeostasis model assessment of insulin resistance (HOMA-IR) index, and the formula used was as follows: fasting insulin (µU/mL) × glucose (mmol/L)/22.5.(9) High-performance liquid chromatography (HLC-723 V, Tosoh, San Francisco, CA) was used to measure glycated hemoglobin (Hb A1c). Serum TG, TC, high-density-lipoprotein cholesterol (HDL-C), and LDL-C concentrations were analyzed with kits from Beckman (Fullerton, California, United States). Gas chromatography was performed by using a G-3000 gas chromatograph, and an FID detector and D-2500 integrator (Hitachi, Ltd., Japan) were used to analyze the composition (%) of modified milk. The chromatography column used was a Restek capillary column (Stabilwax DA 30 m, 0.53 mm ID). The total fatty acid composition was quantified by using a methylation process.(7) The C17:0 fatty acid was used as an internal standard, and the types of fatty acid were determined according to hold time and expressed as a percentage (each fatty acid/total fatty acid).
Statistical analysis
The data are presented as means ± SEMs. SAS 9.0 software (SAS Institute, Cary, NC) was used for the statistical analysis. Student’s t test was used to compare baseline data between the placebo and RBO groups and to compare percentage changes before and after the study intervention. A paired t test was used to compare data from before and after the study intervention between the placebo group and the RBO group. p<0.05 indicated a statistical difference.
Results
Two subjects did not meet the time requirement and 3 subjects had serious hemolysis; therefore, only 35 subjects completed the study. The average ages of the placebo group and the RBO group were 57.5 and 56.2 years, respectively. No significant differences in weight, BMI, or systolic and diastolic blood pressures were observed in a comparison of values before and after the study intervention (data not shown).
The results of the dietary analysis are shown in Table 2. No significant differences in calorie intakes, macronutrient intakes, or macronutrient intakes as a percentage of calories were observed in a comparison of values before and after the study intervention between the 2 groups.
Table 2.
Average daily intake of energy, protein, fat and carbohydrate in 3-day dietary records in subjects during intervention periods1
| Placebo (n = 17) |
RBO (n = 18) |
||||
|---|---|---|---|---|---|
| baseline | week 5 | baseline | week 5 | ||
| Kcal/day | 1430.2 ± 98.4 | 1448.1 ± 89.2 | 1402.8 ± 90.3 | 1509.4 ± 63.2 | |
| Protein (g) | 57.9 ± 4.7 | 54.3 ± 4.6 | 53.4 ± 5.4 | 59.0 ± 3.1 | |
| Fat (g) | 50.5 ± 4.5 | 52.8 ± 3.6 | 51.0 ± 5.3 | 58.5 ± 3.5 | |
| CHO (g)2 | 187.0 ± 20.3 | 191.3 ± 13.0 | 183.3 ± 11.8 | 187.3 ± 9.0 | |
| Protein (%E)2 | 16.3 ± 0.9 | 14.8 ± 0.5 | 14.9 ± 0.8 | 15.7 ± 0.5 | |
| Fat (%E)2 | 32.3 ± 2.7 | 32.9 ± 1.5 | 32.2 ± 2.0 | 34.6 ± 1.2 | |
| CHO (%E)2 | 51.8 ± 3.0 | 52.9 ± 1.6 | 53.2 ± 2.5 | 50.0 ± 1.5 | |
1 Values are expressed as mean ± SEM. 2 CHO, carbohydrate; %E, % of energy.
Blood biochemistry values are shown in Table 3. In the RBO group, the fasting glucose concentration increased significantly by 6.5% after the study intervention. The 2-h postprandial blood glucose concentration increased significantly by 10.6%. In the placebo group, fasting and 2-h postprandial blood sugar concentrations did not change significantly after the study intervention. No significant differences in fasting serum insulin concentrations were found in a comparison of values before and after the study intervention in the placebo and RBO groups. In the RBO group, the HOMA-IR value increased by 20.7% after the study intervention, but the change was not significantly different. In the placebo group, the HOMA-IR value increased by 36.3% after the study intervention. No significant differences in Hb A1C were observed in a comparison of values before and after the study intervention in the placebo and RBO groups. In the RBO group, the serum TC concentration was significantly lower after the intervention than before the intervention. LDL-C decreased from 111.9 ± 6.6 to 108.0 ± 6.6 mg/dL and had the tendency of decreasing, but TG and HDL-C concentrations did not change significantly. However, LDL-C was significantly higher after the study intervention than before the intervention in the placebo group. No significant differences in TC, TG, or HDL-C concentrations were observed in a comparison of values before and after the study intervention in the placebo group. A comparison of the percentage change in TC before and after the study intervention showed a significant 1.6 ± 1.6% increase in the placebo group but a 3.6 ± 1.2% significant decrease in the RBO group. A comparison of the percentage change in LDL-C before and after the study intervention showed a significant 6.4 ± 3.1% increase in the placebo group but a significant 2.7 ± 3.1% decreased in the RBO group.
Table 3.
Blood glucose, insulin, HbA1c levels and HOMA-IR index in subjects during intervention periods1,2,3
| Placebo (n = 17) |
RBO (n = 18) |
||||||
|---|---|---|---|---|---|---|---|
| baseline | week 5 | Change (%) | baseline | week 5 | Change (%) | ||
| Fasting glucose (mg/dL) | 126.3 ± 5.5 | 132.4 ± 6.7 | 3.5 ± 3.7 | 144.0 ± 10.9 | 153.2 ± 12.8* | 6.5 ± 2.8 | |
| 2 h glucose (mg/dL) | 266.1 ± 17.8 | 275.3 ± 17.8 | 4.4 ± 3.5 | 268.2 ± 23.5 | 291.8 ± 25.9* | 10.6 ± 3.6 | |
| Fasting insulin (µIU/mL) | 6.4 ± 0.9 | 7.6 ± 1.2 | 24.1 ± 13.1 | 8.0 ± 1.7 | 8.3 ± 1.6 | 10.6 ± 9.3 | |
| HOMA-IR4 | 1.8 ± 0.2 | 2.3 ± 0.3* | 36.3 ± 12.3 | 2.7 ± 0.6 | 3.2 ± 0.6 | 20.7 ± 11.9 | |
| HbA1c (%)4 | 7.3 ± 0.3 | 7.4 ± 0.3 | 1.5 ± 1.1 | 7.8 ± 0.4 | 7.8 ± 0.4 | 0.6 ± 1.0 | |
| TC5 (mg/dL) | 189.1 ± 8.8 | 192.1 ± 9.3 | 1.6 ± 1.6 | 184.3 ± 5.2 | 177.6 ± 5.4* | −3.6 ± 1.2# | |
| TG5 (mg/dL) | 151.7 ± 32.9 | 149.1 ± 24.0 | 22.4 ± 18.4 | 112.4 ± 9.2 | 130.1 ± 11.6 | 19.6 ± 9.1 | |
| HDL-C5 (mg/dL) | 43.0 ± 2.5 | 42.7 ± 2.2 | 0.3 ± 2.5 | 43.3 ± 3.0 | 43.4 ± 3.0 | 0.7 ± 1.7 | |
| LDL-C5 (mg/dL) | 108.4 ± 8.9 | 113.6 ± 8.9* | 6.4 ± 3.1 | 111.9 ± 6.6 | 108.0 ± 6.6 | −2.7 ± 3.1# | |
1 Values are expressed as mean ± SEM. 2 Values with asterisk (*) are significantly different from baseline values. 3 Values with sharp (#) are significantly different from placebo group. 4 HOMA-IR index are presented as insulin sensitivity; HbA1c, glycated hemoglobin. 5 TC, total cholesterol; TG, triglyceride; HDL-C, high density lipoprotein cholesterol; LDL-C, low density lipoprotein cholesterol.
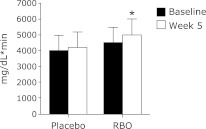
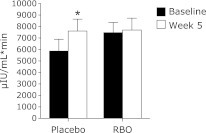
Results of the OGTT showed that the area under the curve (AUC) for postprandial plasma glucose, after the study intervention, was significantly higher than that before the intervention in the RBO group. However, no significant difference in the AUC of postprandial plasma glucose before and after the intervention was observed in the placebo group. In the placebo group, the AUC for postprandial serum insulin was higher after the study intervention than before the intervention. However, no significant difference in the AUC of postprandial serum insulin was found in a comparison of values before and after the study intervention in the RBO group.
Fig. 1.
Mean effect of intervention periods on postprandial plasma glucose areas under curves (AUC) response. Values with asterisk (*) are significantly different from baseline values. Values are expressed as mean ± SEM.
Fig. 2.
Mean effect of intervention periods on postprandial serum insulin areas under curves (AUC) response. Values with asterisk (*) are significantly different from baseline values. Values are expressed as mean ± SEM.
Discussion
The aim of this study was to determine the effects of RBO on the blood lipids profiles and insulin resistance in type 2 diabetes patients. A significantly increasing of fasting and two hour postprandial blood glucose were observed in the RBO group compared to the placebo group. In addition, the total serum cholesterol and LDL-C concentration decreased significantly by 5-week dietary intervention involving consumption of RBO.
The reason why a significantly increasing of fasting and two hour postprandial blood glucose were observed in the RBO group compared to the placebo group could be associated with the AUC for insulin. In our study, we found the AUC for insulin at 5 week did not increase significantly than that at 0 week in RBO group. We supposed there could be two reasons resulted in this situation. First, according to our results, we found that there is a higher amount of palmitic acid (PA) in RBO group compared to placebo group. In Lam’s study,(10) they demonstrated that PA would induce insulin resistance in L6 skeletal myotubes via inflammatory (nuclear factor kappa B/mTOR) and nutrient (ceramide) pathways. In Han’s study,(11) they mentioned that PA would induce insulin resistance in L6 skeletal myotubes through C-Jun N-terminal kinase (JNK) and insulin receptor substrate-1 (IRS-1) Ser307 phosphorylation. In Yang’s study,(12) they examined the antidiabetic effect of palmitoleic acid and PA in KK-Ay mice. They found the PA increased the plasma glucose concentration and decreased the insulin sensitivity by up-regulated mRNA expressions of proinflammatory adipocytokine genes (TNFα and resistin) in white adipose compared to palmitoleic acid. So we think a higher amount of PA in RBO could not improve insulin sensitivity in our type 2 DM patients and may result in a significantly increasing of fasting and two hour postprandial blood glucose. Second, we found more MUFA exists in the RBO group compared to placebo group. In Chou’s study,(13) they found that type 2 DM rats fed the RBO diet had a suppressed hyperinsulinemic response that may have resulted from the higher MUFA dietary intake. This is also may be the reason why the AUC for insulin at 5 week did not increase significantly than that at 0 week in RBO group and a significantly increasing of fasting and two hour postprandial blood glucose were observed in the RBO group compared to the placebo group in our study.
Many studies have indicated that the effective control of LDL-C in patients with type 2 diabetes is far more important than the control of HDL-C, TG, or blood glucose.(14,15) LDL, which has smaller particles and a higher particle density, is easily oxidized into oxidized-LDL (ox-LDL); ox-LDL easily enters the vascular wall and causes less release of nitric oxide (NO) and endothelial cell function impairment, attracting more monocytes to adhere to the vascular endothelium and eventually leads to atherosclerosis.(16) According to the National Cholesterol Education Program Adult Treatment Panel III, diabetes and cardiovascular disease are considered to be equally dangerous diseases, and the major target of treatment is LDL-C—the goal being a concentration ⩽100 mg/dL.
Insulin can regulate the gene expression of LDL receptors.(17) Previous studies observed that LDL clearance in diabetic patients with insulin resistance is low and that LDL particles are smaller and have a higher density.(18) Another study indicated that blood LDL-C concentrations in type 2 diabetes patients are elevated and that insulin resistance and LDL-C concentrations are positively correlated.(19) Rivellese et al.(20) indicated that a higher HOMA-IR value indicates a higher LDL concentration. The results of the present study indicate that the consumption of soybean oil–modified milk for 5 weeks significantly increased serum LDL-C concentrations, which may have been due to an increase in insulin resistance.
Many studies have shown that RBO consumption can reduce blood cholesterol concentrations. A 10-week dietary intervention with RBO significantly reduced total plasma cholesterol concentrations in hypercholesterolemic hamsters.(21) Clinically, consumption of 50 g RBO daily for 4 weeks by hypercholesterolemia male subjects can significantly decrease total serum cholesterol concentrations,(22) and consumption of 75 mL RBO daily for 50 d by healthy subjects can reduce total serum cholesterol concentrations.(7) Moreover, total plasma cholesterol concentrations were significantly lower in hyperlipidemic patients who used RBO as cooking oil for 3 month than in those who used sunflower oil as cooking oil for 3 month.(8) Madigan et al.(23) showed that total plasma cholesterol concentrations were significantly lower in patients with type 2 diabetes who consumed 30 mL olive oil and abundant oleic acid for 2 weeks than in those who consumed 30 mL sunflower oil and abundant linolenic acid. The results of the present study showed that total serum cholesterol concentrations decreased significantly in patients with diabetes after a 5-week dietary intervention with RBO-modified milk. This result is consistent with the results of previous studies and supports the recommendation of the American Diabetes Association that patients with type 2 diabetes should consume vegetable oil containing abundant amounts of oleic acid to improve abnormal blood lipid concentrations.(1)
In conclusion, the 5-week dietary intervention involving consumption of 18 g RBO daily significantly decreased total serum cholesterol concentrations and tended to decrease LDL-C concentrations. This result is consistent with the results of previous studies and supports the recommendation of the American Diabetes Association that patients with type 2 diabetes should consume vegetable oil to improve abnormal blood lipid concentrations. However, insulin resistance was not significantly influenced.
Abbreviations
- AUC
area under curve
- HbA1c
glycosylated hemoglobin A1c
- HDL
high-density-lipoprotein
- HOMA-IR
homeostasis model assessment index of insulin resistance
- LDL
low-density-lipoprotein
- NO
nitric oxide
- OGTT
oral-glucose-tolerance test
- RBO
rice bran oil
- TG
triglyceride
References
- 1.Hanley AJ, Williams K, Stern MP, Haffner SM. Homeostasis model assessment of insulin resistance in relation to the incidence of cardiovascular disease: the San Antonio Heart Study. Diabetes Care. 2002;25:1177–1184. doi: 10.2337/diacare.25.7.1177. [DOI] [PubMed] [Google Scholar]
- 2.Keys A. Serum cholesterol response to dietary cholesterol. Am J Clin Nutr. 1986;44:309. doi: 10.1093/ajcn/44.2.309. [DOI] [PubMed] [Google Scholar]
- 3.Franz MJ, Bantle JP, Beebe CA, et al. Evidence-based nutrition principles and recommendations for the treatment and prevention of diabetes and related complications. Diabetes Care. 2002;25:148–198. doi: 10.2337/diacare.25.1.148. [DOI] [PubMed] [Google Scholar]
- 4.Sugano M, Tsuji E. Rice bran oil and cholesterol metabolism. J Nutr. 1999;127:521S–524S. doi: 10.1093/jn/127.3.521S. [DOI] [PubMed] [Google Scholar]
- 5.Hunnicutt JW, Hardy RW, Williford J, McDonald JM. Saturated fatty acid-induced insulin resistance in rat adipocytes. Diabetes. 1994;43:540–545. doi: 10.2337/diab.43.4.540. [DOI] [PubMed] [Google Scholar]
- 6.Chen CW, Cheng HH. A rice bran oil diet increases LDL-receptor and HMG-CoA reductase mRNA expressions and insulin sensitivity in rats with streptozotocin/nicotinamide-induced type 2 diabetes. J Nutr. 2006;136:1472–1476. doi: 10.1093/jn/136.6.1472. [DOI] [PubMed] [Google Scholar]
- 7.Rajnarayana K, Prabhakar MC, Krishna DR. Influence of rice bran oil on serum lipid peroxides and lipids in human subjects. Indian J Physiol Pharmacol. 2001;45:442–444. [PubMed] [Google Scholar]
- 8.Kuriyan R, Gopinath N, Vaz M, Kurpad AV. Use of rice bran oil in patients with hyperlipidaemia. Natl Med J India. 2005;18:292–296. [PubMed] [Google Scholar]
- 9.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 10.Lam YY, Hatzinikolas G, Weir JM, et al. Insulin-stimulated glucose uptake and pathways regulating energy metabolism in skeletal muscle cells: the effects of subcutaneous and visceral fat, and long-chain saturated, n-3 and n-6 polyunsaturated fatty acids. Biochem Biophys Acta. 2011;1811:468–475. doi: 10.1016/j.bbalip.2011.04.011. [DOI] [PubMed] [Google Scholar]
- 11.Han MS, Lim YM, Quan W, et al. Lysophosphatidylcholine as an effector of fatty acid-induced insulin resistance. J Lipid Res. 2011;52:1234–1246. doi: 10.1194/jlr.M014787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yang ZH, Miyahara H, Hatanaka A. Chronic administration of palmitoleic acid reduces insulin resistance and hepatic lipid accumulation in KK-Ay mice with genetic type 2 diabetes. Lipids Health Dis. 2011;10:120. doi: 10.1186/1476-511X-10-120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chou TW, Ma CY, Cheng HH, Chen YY, Lai MH. A rice bran oil diet improves lipid abnormalities and suppress hyperinsulinemic responses in rats with streptozotocin/nicotinamide-induced type 2 diabetes. J Clin Biochem Nutr. 2009;45:29–32. doi: 10.3164/jcbn.08-257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goldberg RB, Mellies MJ, Sacks FM, et al. Cardiovascular events and their reduction with pravastatin in diabetic and glucose-intolerant myocardial infarction survivors with average cholesterol levels: subgroup analyses in the cholesterol and recurrent events (CARE) trial. The Care Investigators. Circulation. 1998;98:2513–2519. doi: 10.1161/01.cir.98.23.2513. [DOI] [PubMed] [Google Scholar]
- 15.Turner RC, Millns H, Neil HA, et al. Risk factors for coronary artery disease in noninsulin dependent diabetes mellitus: United Kingdom Prospective Diabetes Study (UKPDS: 23) BMJ. 1998;316:823–828. doi: 10.1136/bmj.316.7134.823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hobbs FD. Reducing cardiovascular risk in diabetes: beyond glycemic and blood pressure control. Int J Cardiol. 2006;110:137–145. doi: 10.1016/j.ijcard.2005.09.011. [DOI] [PubMed] [Google Scholar]
- 17.Streicher R, Kotzka J, Müller-Wieland D, et al. SREBP-1 mediates activation of the low density lipoprotein receptor promoter by insulin and insulin-like growth factor-I. J Biol Chem. 1996;271:7128–7133. doi: 10.1074/jbc.271.12.7128. [DOI] [PubMed] [Google Scholar]
- 18.Kissebah AH. Low density lipoprotein metabolism in non-insulin-dependent diabetes mellitus. Diabetes Metab Rev. 1987;3:619–651. doi: 10.1002/dmr.5610030302. [DOI] [PubMed] [Google Scholar]
- 19.Garvey WT, Kwon S, Zheng D, et al. Effects of insulin resistance and type 2 diabetes on lipoprotein subclass particle size and concentration determined by nuclear magnetic resonance. Diabetes. 2003;52:453–462. doi: 10.2337/diabetes.52.2.453. [DOI] [PubMed] [Google Scholar]
- 20.Rivellese AA, Patti L, Kaufman D, et al. Lipoprotein particle distribution and size, insulin resistance, and metabolic syndrome in Alaska Eskimos: the GOCADAN study. Atherosclerosis. 2008;200:350–358. doi: 10.1016/j.atherosclerosis.2007.12.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wilson TA, Nicolosi RJ, Woolfrey B, Kritchevsky D. Rice bran oil and oryzanol reduce plasma lipid and lipoprotein cholesterol concentrations and aortic cholesterol ester accumulation to a greater extent than ferulic acid in hypercholesterolemic hamsters. J Nutr Biochem. 2007;18:105–112. doi: 10.1016/j.jnutbio.2006.03.006. [DOI] [PubMed] [Google Scholar]
- 22.Berger A, Rein D, Schäfer A, et al. Similar cholesterol-lowering properties of rice bran oil, with varied gamma-oryzanol, in mildly hypercholesterolemic men. Eur J Nutr. 2005;44:163–173. doi: 10.1007/s00394-004-0508-9. [DOI] [PubMed] [Google Scholar]
- 23.Madigan C, Ryan M, Owens D, Collins P, Tomkin GH. Dietary unsaturated fatty acids in type 2 diabetes: higher levels of postprandial lipoprotein on a linoleic acid-rich sunflower oil diet compared with an oleic acid-rich olive oil diet. Diabetes Care. 2000;23:1472–1477. doi: 10.2337/diacare.23.10.1472. [DOI] [PubMed] [Google Scholar]