Abstract
The role of gamma amino butyric acid A receptors/neurons of the hypothalamic, endocrine and alimentary systems in the food intake seen in hunger was studied in 20 h food-deprived rats. Food deprivation decreased blood glucose, serum insulin and produced hyperphagia. The hyperphagia was inhibited by subcutaneous or ventromedial hypothalamic administration of gamma amino butyric acid A antagonists picrotoxin or bicuculline. Although results of blood glucose was variable, insulin level was increased by picrotoxin or bicuculline. In contrast, lateral hypothalamic administration of these agents failed to reproduce the above changes. Subcutaneous administration of picrotoxin or bicuculline increased gastric content, decreased gastric motility and small bowel transit. In contrast, ventromedial or lateral hypothalamic administration of picrotoxin or bicuculline failed to alter the gastric content but decreased the small bowel transit. The results of alimentary studies suggest that gamma amino butyric acid neurons of both ventromedial and lateral hypothalamus selectively regulate small bowel transit but not the gastric content. It may be concluded that ventromedial hypothalamus plays a dominant role in the regulation of food intake and that picrotoxin or bicuculline inhibited food intake by inhibiting gamma amino butyric acid receptors of the ventromedial hypothalamus, increasing insulin level and decreasing the gut motility.
Keywords: GABAA receptors, food deprivation, food intake, hypothalamus, satiety
Introduction
The search for food and the act of feeding are the most important part of the lives of animals. Hence it is maintained with great precision by the involvement of the nervous, endocrine and alimentary systems and metabolic activities. Further, this system is fine-tuned by the nutrients in the blood, especially glucose, neurotransmitters, peptides and hormones.(1–3) The coordination of all these systems and factors towards the regulation of food intake appears to lie in the hypothalamus and its surrounding regions of the brain.(4,5) Among the hypothalamic nuclei, ventromedial hypothalamus (VMH) and lateral hypothalamus (LH), considered the satiety and appetite center respectively, play critical role in the energy homeostasis, possess receptors for nutritional signals and receive firsthand information on the nutritional status of the organism.(2,6–8)
Several studies suggested that the organisms’ nutritional information reaches the brain through blood glucose and the sensor for glucose is present in the hypothalamus.(9–11) Flooding of the VMH area of the brain with glucose led to inhibition of food intake while its deprivation produced hyperphagia.(12) Glucoprivation was accompanied by the increased levels of gamma aminobutyric acid (GABA), an inhibitory neurotransmitter, in the VMH; this finding suggests that GABA may be a link between glucose and the VMH.(13–16) The functional relationship between glucose and GABA is supported by one of the recent publications also.(11) The link between glucose and GABA was substantiated as the injection of GABA or muscimol, a GABAA agonist or L-glutamic acid, a precursor for GABA, into the VMH facilitated feeding; in contrast, injection of these agents into the LH inhibited the food intake.(17) On the other hand, subcutaneous (SC) or VMH injection of GABAA antagonists inhibited glucoprivation-induced food intake while LH administration of these drugs failed to modulate the food intake.(18,19) Further studies supported the above findings by showing that insulin or 2-deoxy-D-glucose (2-DG) led to an increase in VMH glutamate decarboxylase (GAD), the synthetic enzyme for GABA which was accompanied by increased food intake.(20,21)
The evidence shown above studied the relation between GABA, hypothalamus and food intake following glucoprivation induced by pharmacological means. However, the contribution of GABA and hypothalamus is not known in the glucoprivation caused by fasting, a physiological means. In other words, the present study examines the molecular mechanisms underlying hunger. Hence, in this investigation, we studied the roles of GABA and hypothalamus in food deprivation employing rats and GABAA antagonists. Further, we also examined the contribution of hormonal and alimentary systems in this mechanism.
Materials and Methods
Animals
Adult male Sprague-Dawley rats weighing 200–300 g were used throughout the study. All the animals were housed individually in plastic cages with bedding. They were transferred to clean plastic cages for the duration of the food intake measurement. The temperature of the vivarium was maintained at 24–27°C with 10 h artificial light (08:00–18:00 h). Animals were trained to take the food pellets for 4 h (10:00 to 14:00 h), while they were deprived of food for the rest of the day. Water was provided ad libitum. The animal protocol for this research was approved by the Animal Care and Use Committee (ACUC) of University of Virginia, Charlottesville, VA.
Cannula implantation
Animals were implanted with a stainless steel cannula (22 ga; Plastics One Inc., Roanoke, VA) either in VMH or LH under pentobarbital sodium (40 mg/kg i.p.) anesthesia as described before.(18,19) Briefly, these unilaterally placed cannulae were fastened to the skull with dental cement such that the tip was 3.0 mm above VMH (with skull flat: 2.7 mm behind bregma, 0.5 mm lateral to sagittal suture and 8.5 mm vertical) or LH (with skull flat: 2.8 mm behind bregma, 1.6 mm lateral to sagittal suture and 8.5 mm vertical) according to the stereotaxic coordinates of Konig and Klippel.(22) A separate group of rats was implanted with the cannula in the lateral ventricle (ICV) (with skull flat: 2.0 mm posterior to bregma; 2.0 mm lateral to sagittal suture and 4.0 mm vertical) for testing the extra-hypothalamic sites of action of the administered drugs. The animals were allowed a week for recovery following surgery. The food and water intake and gross behaviors were monitored during this period.
General Procedure
Food intake measurement schedule
Pre-drug baseline was obtained in the week prior to the commencement of drug treatment with vehicle injections and employing the 4 h feeding and 20 h fasting schedule. The drug treatment was performed after the food intake during the 4 h period had been stabilized. Each animal served as its own control. The food was presented to the animals 15 min after the administration of the drug. Cumulative food intake was measured at 1, 2 and 4 h (i.e. 11:00, 12:00 and 14:00 h) after subtracting the spillage. In peripheral as well as VMH studies, different groups of animals were used for picrotoxin (Fluka AG, Switzerland) and bicuculline methiodide (Sigma, St. Louis, MO). However, in LH study, one group of LH cannulated rat was used for both picrotoxin and bicuculline. If the animals underwent more than one treatment, an interval of 3 days was allowed between each testing. Moreover, vehicle was injected a day prior to each treatment to ascertain the return of food intake scores to pre-drug baseline. Picrotoxin or bicuculline methiodide were dissolved in saline; peripheral studies were conducted after the subcutaneous (SC) injection of these drugs in a volume of 0.1 ml. VMH or LH injection was made with a 10 µl microsyringe which extended 3.0 mm beyond the tip of the cannula, whose total length was 5.5 mm. Further, these injections were made over a period of 15 s in a volume of 0.5 µl. The volume of the ICV injection was 2 µl. The doses of picrotoxin and bicuculline used were determined in a separate study. They were well below their ED 50 (3.1 and 3.4 mg/kg SC respectively) for convulsive effect. The concentration of picrotoxin or bicuculline found to have produced the maximum effect in peripheral or central study (of food intake determination) were employed in the endocrine and alimentary studies.
Endocrine study
Blood glucose and serum insulin concentration were determined in this study. These animals were not fed on the day of the experiment and only water was made available to them. Blood samples were collected from tail vein of these animals at 1/2, 1, 2 and 4 h respectively (i.e. 10:30, 11:00, 12:00 and 14:00 h). In contrast to the food intake experiments, 1/2 h value was also taken, as significant changes in both blood glucose and serum insulin were expected within an hour. The blood glucose level was determined using a Glucometer (Glucometer Elite, Bayer Corporation Diagnostics GMBH, Leverkusen, Germany). The serum was separated and stored at –20°C for the estimation of insulin. The insulin concentration was examined using immunoassay [Insulin (Rat) Ultra-Sensitive ELISA, Alpco Diagnostics, Salem, NH] following the supplier’s instructions.
Alimentary study
The animals were not fed while only water was available on the day of the experiment. Two parameters viz., gastric content (an index of gastric emptying and distention) and SBT (which indicates intestinal motility) were determined. Charcoal meal (gastric load; 10 ml/kg) consisting of 20% activated vegetable charcoal and 5% gum acacia in water was used as a marker. The vehicle or the drug injections were made 15 min (at 9:45 h) before the gastric load which was administered intragastrically. Followed by these procedures, the animals were sacrificed by cervical dislocation at 1/2, 1, 2 and 4 h (10:30, 11:00, 12:00 and 14:00 h). The 1/2 h value was also included as the gastric emptying and SBT are very fast in small animals. The abdominal cavity was dissected open and the pyloric and esophageal ends were ligated. The stomach was dissected out and the net weight was determined in a sensitive balance to the nearest 50 mg. It was opened along its greater curvature, contents removed, rinsed with water, blotted and the empty stomach was weighed again. Gastric content was determined by subtracting the weight of the empty stomach from the net weight. The percent change was calculated, by taking the load given as 100%.
In SBT study, the movement of the charcoal meal from the duodenum to the ileocaecal junction was measured at the time intervals mentioned above. From this value the percent SBT was calculated by taking the total length of the small intestine as 100% and was plotted against time.
Histology
At the conclusion of the study, the cannulated animals were killed by decapitation and the brain was processed for histological verification. Serial sections of 10 µm were cut, stained with toluidine blue and examined under light microscope. The greatest diameter of non-specific damage around the cannulation site was 1.0 mm (including the cannula). Analysis of neuropathological changes showed a small glial infiltration with an occasional cavitation along the cannula pathway.
Statistical analysis
The results were analyzed by one-way ANOVA and expressed as mean ± SEM.
Results
SC or VMH administration of GABAA antagonists inhibit deprivation-induced food intake
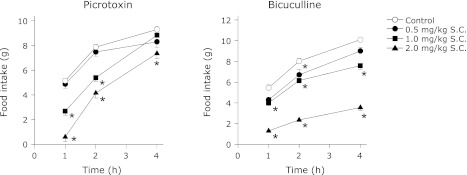
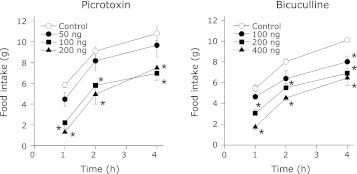
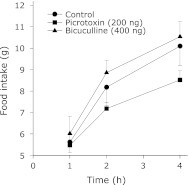
Following their habituation, animals which underwent 4 h feeding and 20 h fasting schedule showed uniform and stable food intake. In these animals, the food intake was in the range of 5 to 12 g in the 4 h test period. SC administration of either picrotoxin or bicuculline inhibited the food intake in these animals in a dose dependent fashion throughout the 4 h feeding period (Fig. 1). Similar results were obtained when either of these agents was administered in the VMH (Fig. 2). Contrarily, even the maximal effective dose (in the VMH) of picrotoxin or bicuculline failed to significantly alter the food intake when injected into the LH (Fig. 3). In addition, injection of these agents into the ICV also did not affect the food intake in the food-deprived rats (data not shown).
Fig. 1.
Cumulative food intake by 20 h food deprived rats treated with SC administration of picrotoxin or bicuculline. The rats underwent 20 h fasting and 4 h feeding as mentioned in the methods. Each point represents mean ± SEM; for picrotoxin, n = 5 to 6; for bicuculline, n = 6; *p<0.05 compared to control.
Fig. 2.
Cumulative food intake by 20 h food deprived rats treated with VMH administration of picrotoxin or bicuculline. The unilateral cannula was implanted in VMH following the stereotaxic coordinates as mentioned under cannula implantation. The drug testing was started following the recovery from surgery and the stabilization of food intake during the 4 h period. Separate groups of rats were used for picrotoxin and bicuculline. Each point represents mean ± SEM; n = 5 to 6; *p<0.05 compared to control.
Fig. 3.
Cumulative food intake by 20 h food deprived rats treated with LH administration of picrotoxin or bicuculline. Unilateral cannula in LH was implanted as mentioned in the methods. The drug testing was started following the recovery from surgery and the stabilization of food intake during the 4 h period. Both picrotoxin and bicuculline were tested in the same group of cannulated rats. However, an interval of 3 days was allowed between the testing with these agents. Each point represents mean ± SEM; n = 5 to 6.
SC, VMH or LH administration of GABAA antagonists produced varying results on blood glucose and serum insulin levels
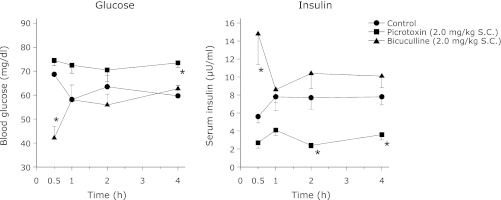
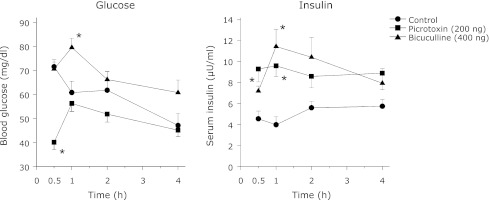
Blood glucose and serum insulin levels were estimated in the free feeding and fasted animals in order to determine the effect of food deprivation on these two parameters. Food deprivation produced a significant (p<0.05) decrease in both blood glucose and serum insulin levels when compared to free feeding rats (free feeding: glucose = 84.6 ± 3.6 mg/dl and insulin = 11.04 ± 0.86 µU/ml; food deprivation: glucose = 61.2 ± 3.6 mg/dl and insulin = 6.21 ± 0.51 µU/ml). SC administration of either picrotoxin or bicuculline showed contrasting results in both the blood glucose and serum insulin levels (Fig. 4). Picrotoxin decreased the serum insulin levels throughout the 4 h study period though it was significant at the 2nd and 4th h only. Although the decreased insulin level was accompanied with increased glucose it was significant at the 4th h only. Conversely, bicuculline led to a sharp increase in insulin which was accompanied with decreased glucose level at 1/2 h (Fig. 4).
Fig. 4.
The effect of SC administration of picrotoxin or bicuculline on the blood glucose and serum insulin levels in rats deprived of food for 20 h as described in methods. Same group of rats was used for bleeding at the 4 different time intervals. However, different groups of rats were used for picrotoxin and bicuculline. The animals were not fed on the day of the experiment whereas water was available ad libitum. Each point represents mean ± SEM; n = 5 or 6; *p<0.05 compared to control.
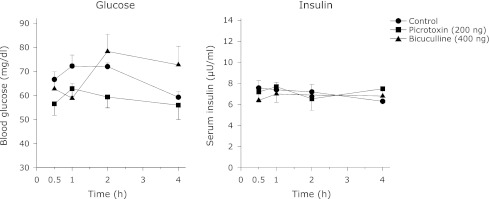
In contrast to peripheral studies, VMH injection of picrotoxin or bicuculline increased serum insulin concentration throughout the 4 h study period, though it was significant in the 1/2 and 1 h only (Fig. 5). The blood glucose concentrations were not consistent with the increased serum insulin levels seen with the VMH administration of these agents. Picrotoxin-induced increase in insulin level was accompanied with a generalized decrease in blood glucose concentration which was significant only at the 1/2 h. Contrary to picrotoxin, bicuculline produced a sharp increase in the blood glucose concentration at 1 h (Fig. 5). LH administration of these agents did not alter either serum insulin or blood glucose levels at the times tested (Fig. 6).
Fig. 5.
The effect of VMH administration of picrotoxin or bicuculline on the blood glucose and serum insulin levels in food deprived rats. Unilateral cannula was implanted in VMH as mentioned in methods. After a week period of recovery the animals underwent 20 h fasting and 4 h feeding schedule. The animals were not fed on the day of the experiment whereas water was available ad libitum. Each point represents mean ± SEM; n = 5; *p<0.05 compared to control.
Fig. 6.
The effect of LH administration of picrotoxin or bicuculline on the blood glucose and serum insulin levels in food deprived rats. The animals had unilateral cannula and underwent 20 h fasting and 4 h feeding schedule after one week period of recovery. The animals were not fed on the day of the experiment whereas water was available ad libitum. Each point represents mean ± SEM; n = 5.
Gastric content and SBT are differentially affected after SC, VMH or LH administration of GABAA antagonists
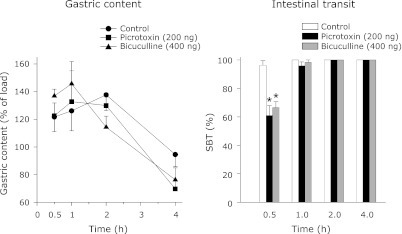
Figs. 7, 8, 9 display the effect of the GABAA antagonists on gastric content and SBT. The amount of orally administered charcoal meal together with the possible gastric secretion retained in the stomach (at various time intervals) was taken as the gastric content. As expected, the gastric content was high at the 1/2 h and at its lowest at the 4th h. Although the control gastric content varied between the SC, VMH and LH injection groups in the 1/2 and 1 h periods, it declined and consistently reached its lowest level at the 4th h in all these groups. In contrast, the control SBT was stable and similar in the peripheral, VMH and LH studies (Figs. 7, 8, 9). Further, the control SBT was below 100% in the 1/2 h period and reached 100% in the 1st h.
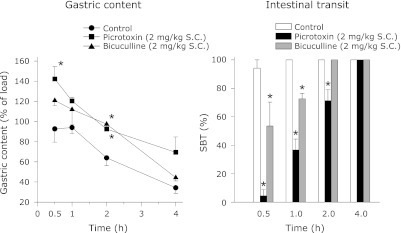
Fig. 7.
The effect of SC administration of picrotoxin or bicuculline on gastric content and SBT in rats. All these animals were trained to take the food pellets in the 4 h period as described in methods. The experiments were followed after the stabilization of food intake during this period. The animals were not fed on the day of the experiment whereas water was available ad libitum. Each point represents mean ± SEM; n = 5; *p<0.05 compared to control.
Fig. 8.
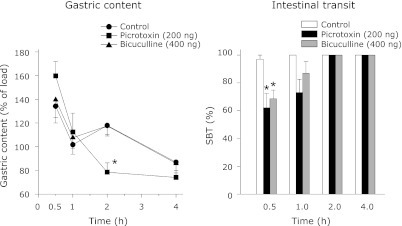
The effect of VMH administration of picrotoxin or bicuculline on gastric content and SBT in rats deprived of food. The animals had unilateral cannula and underwent training to take food in the 4 h period that was preceded by 20 h food deprivation. The experiments were followed after the stabilization of food intake during this period. The animals were not fed on the day of the experiment whereas water was available ad libitum. Each point represents mean ± SEM; n = 5; *p<0.05 compared to control.
Fig. 9.
The effect of LH administration of picrotoxin or bicuculline on gastric content and SBT. The rats had unilateral cannula and deprived of food for 20 h and fed for 4 h as described in methods. The experiments were followed after the stabilization of food intake during this period. The animals were not fed on the day of the experiment whereas water was available ad libitum. Each point represents mean ± SEM; n = 5; *p<0.05 compared to control.
SC administration of picrotoxin or bicuculline increased the gastric content and decreased the SBT (Fig. 7). The changes in the gastric content and SBT were significantly different from the control for a period of 2 h only (Fig. 7). In the VMH study, the gastric content was more than 100% in the control, picrotoxin and bicuculline groups. However, it sharply decreased in the picrotoxin group at the 2nd h (Fig. 8). Contrary to the gastric content, SBT was decreased in both the picrotoxin and bicuculline groups in the 1/2 h period (Fig. 8). In the LH study also, the gastric content was more than 100% in the control, picrotoxin and bicuculline groups. In contrast, SBT was decreased significantly in both the picrotoxin and bicuculline groups in the 1/2 h period compared to the control (Fig. 9).
Behavioral changes after SC or VMH administration of GABAA antagonists
Administration of 2 mg/kg of picrotoxin showed hypermotility and hyperexcitability in five rats. VMH injection of 400 ng of bicuculline produced frothy salivary secretion in two rats. These changes occurred 5 min after the administration of drugs and lasted for 20 min. Even though no significant change in the feeding behavior of these rats was observed, the data obtained from these rats were not included in the results.
Discussion
The presence of GABAergic neurons in various hypothalamic areas and their contribution in the regulation of food intake is well known.(23–25) An increase and a decrease of GABAergic activity was observed in the VMH and LH respectively during insulin-induced hypoglycemia and hyperphagia.(26) The above finding was supported by our earlier observation that insulin-induced hyperphagia was inhibited by SC or VMH, but not in LH administration of GABAA antagonists.(18) Similarly, hyperphagia induced by other types of hypoglycemic agents viz., 2-DG or 5-thio glucose was also inhibited by the SC or VMH, but not LH administration of GABAA antagonists.(19) Other investigators showed that 2-DG induced food intake was associated with a simultaneous increase of GABA and GAD in VMH.(14,21) Further, VMH injection of l-glutamic acid, a precursor of GABA produced an increase of food intake in satiated sheep.(17) Such elevated GABA activity in VMH would have inhibited the VMH satiety center from sending inhibitory output to LH feeding center, thereby facilitating a desire for food intake.
In the present study, both hypoglycemia and hyperphagia were induced by physiological means i.e., food deprivation. Hence, as seen with insulin or 2-DG, food deprivation may have also increased the GABAergic activity in the VMH. The increased GABAergic activity in the VMH may have inhibited VMH and the subsequent activation of LH and the food intake (or hyperphagia) in hunger. The GABAA receptor-induced inhibition of VMH was blocked by the SC or VMH GABAA antagonists and thus the food intake caused by hunger or deprivation was decreased (Figs. 1 and 2). This view is in agreement with the report that bicuculline mainly influences those postsynaptic neurons in which the GABAergic inputs prevail.(27) The failure of the GABAA antagonists to influence food intake, when administered through LH, may be due to the lack of GABA activity in that area (Fig. 3). The specificity of VMH may be further stressed as, ICV administration of picrotoxin or bicuculline failed to alter the food intake under a similar condition (data not shown).
Insulin is important in the regulation of food intake as it acts as a central satiety factor in normal animals, a process that is perturbed in obesity.(28) Although the satiety caused by the GABAA antagonists may be related to insulin, the differential levels of insulin after the SC administration of these agents (Fig. 4) is not in agreement and suggest that the inhibition of food intake and endocrine effects may be parallel. This may be agreeable since VMH administration of picrotoxin or bicuculline increased insulin for a short duration (0.5 to 1.0 h) (Fig. 5) whereas the inhibition of food intake was observed for 4 h (Fig. 2). The contribution of extra-hypothalamic factors such as the pancreas, liver and skeletal muscle and the different mechanisms of action of these agents(29) have to be considered in this context. In spite of the possible parallel action, the contribution of insulin to satiety cannot be neglected. The link between insulin and VMH may be explained from the observation that bilateral destruction of VMH produced a syndrome characterized by hyperinsulinemia, hyperphagia and obesity and these changes were reversed by vagotomy.(30,31) The role of VMH in the food intake as well as in the insulin secretion is further stressed since the injection of the GABAA antagonists in the LH neither affected the food intake nor insulin levels (Figs. 3 and 6).
It has been reported that in normal condition, GABA might inhibit the insulin release through the vagal pathway.(32,33) Thus in normal rats, the efferent vagus that reaches the pancreas is under the powerful tonic GABA inhibitory action, probably to prevent hypoglycemia.(34) The increased insulin level produced by the VMH administration of either bicuculline or picrotoxin (Fig. 5) may be the result of the blockade of the tonic inhibitory action of GABA. It may have happened at the level of the VMH since GABAergic inhibitory tone within the VMH is hypothesized to modulate glucose counter regulatory responses.(35) Direct action of GABAA antagonists at the pancreas is also possible since mRNA for GABAA receptors and secretion of GABA has been demonstrated in the pancreas.(36,37) Further, perfusion of the isolated pancreas with GABA inhibited insulin release.(38)
Although picrotoxin at VMH decreased glucose level based on the modulation of insulin, it was not so with bicuculline. The increased insulin level was accompanied with enhanced glucose concentration after VMH administration of bicuculline. It has been reported that ICV administration of bicuculline methiodide (10 nmol) increased glucose level due to an enhanced hepatic gluconeogenesis and glycogenolysis.(39,40) Direct evidence for the role of VMH in the increased glucose level is available as, VMH administration of 500 µM bicuculline led to an increase in the glucose concentration.(41)
Although GABAA antagonists produced varying effects on the gastric content, they consistently decreased gastric emptying as can be seen from the decreased intestinal transit after the SC, VMH or LH administration of these agents (Figs. 7–9). However SC GABAA antagonists were most potent in decreasing the intestinal transit compared to the VMH or LH administrations. These changes may have increased the gastric distension and led to the stimulation of the distension receptors located in the esophagus, stomach, duodenum, jejunum and small intestine.(42) It is known that the stimulation of distension receptors is one of the reasons for satiety.(43) Gastric distension induced by GABAA antagonists is substantiated as stimulation of GABA receptors of intestine led to the contraction of ileum.(44,45) Considerable amount of GABA is present in the gut as it is synthesized by the bacteria in the gut and metabolized by the hepatocytes in the liver.(46,47) In addition, GABA, GAD and high affinity GABA uptake sites are all present in the myenteric plexus (Auerbach’s plexus) of rat, guinea-pig and cat.(48–50)
GABAergic neurons of the VMH may not contribute to the gastric content or emptying since there was no significant increase in the gastric content after VMH administration of GABAA antagonists. Moreover, a decrease in the gastric content was found in the second h after VMH administration of picrotoxin (Fig. 8). As bicuculline did not show a similar change it is hard to explain this on the basis of GABAA receptor blockade. Furthermore, the gastric content was more than the load given in the control as well as in the VMH and LH groups. Since the gastric content was uniformly increased in the control as well as in the treatment groups, it may be a nonspecific effect due to the microinjection in these brain regions. Contrary to the gastric content, SBT was delayed in the 1/2 h after the VMH and LH administration of GABAA antagonists (Figs. 8 and 9). It is possible that the GABAergic neurons of both these areas are involved in the intestinal motility. It also suggests that gastric motility and SBT are regulated by mechanisms that are independent of each other.
Taken together food intake and insulin secretion seem to be controlled almost similarly by the peripheral mechanisms and VMH; in contrast, gastrointestinal activity may be modulated differently. While the GABAergic neurons of VMH and LH seem to contribute equally to the intestinal transit, it appears that VMH plays a dominant role in the regulation of food intake and insulin secretion compared to LH. Evidence of VMH’s dominance over LH comes from the observation that axons from VMH project laterally and caudally and produce inhibition of the LH feeding center, either by inhibition of the facilitatory area (feeding center), or by projecting caudally into the brainstem and inhibiting feeding reflexes directly, or both.(51) An another possibility is that VMH GABAA antagonists interfered with the transmission of impulses at the level of the brain stem, since unilateral injection of these agents modified food intake and insulin secretion in this study.
Acknowledgments
This work was supported by National Institutes of Health, General Medicine (Grant 65214) to GLK, NIH funding IP20MD001822-1 (NSV) and by the Department of Anesthesiology at the University of Virginia, Charlottesville, VA. The authors are not in any way linked to the industries or affiliations mentioned in the text of this manuscript.
Abbreviations
- ANOVA
analysis of variance
- 2-DG
2-deoxy-D-glucose
- GABAA
gamma amino butyric acid A
- GAD
glutamate decarboxylase
- ICV
intra-cerebro ventricle or lateral ventricle
- LH
lateral hypothalamus
- SBT
small bowel transit
- SC
subcutaneous
- VMH
ventromedial hypothalamus
References
- 1.Zheng H, Corkern M, Stoyanova I, Patterson LM, Tian R, Berthoud H-R. Peptides that regulate food intake: appetite-inducing accumbens manipulation activates hypothalamic orexin neurons and inhibits POMC neurons. Am J Physiol Regl Integr Comp Physiol. 2003;284:R1436–R1444. doi: 10.1152/ajpregu.00781.2002. [DOI] [PubMed] [Google Scholar]
- 2.Abizaid A, Gao Q, Horvath TL. Thoughts for food: brain mechanisms and peripheral energy balance. Neuron. 2006;51:691–702. doi: 10.1016/j.neuron.2006.08.025. [DOI] [PubMed] [Google Scholar]
- 3.Field B, Chaudhri OB, Bloom SR. Bowels control brain: gut hormones and obesity. Nat Rev Endocrinol. 2010;6:444–453. doi: 10.1038/nrendo.2010.93. [DOI] [PubMed] [Google Scholar]
- 4.King BM. The rise, fall and resurrection of the ventromedial hypothalamus in the regulation of feeding behavior and body weight. Physiol Behav. 2006;87:221–244. doi: 10.1016/j.physbeh.2005.10.007. [DOI] [PubMed] [Google Scholar]
- 5.Delaere F, Magnan C, Mithieux G. Hypothalamic integration of portal glucose signals and control of food intake and insulin sensitivity. Diabetes Metab. 2010;36:257–262. doi: 10.1016/j.diabet.2010.05.001. [DOI] [PubMed] [Google Scholar]
- 6.Horvath TL, Gao XB. Input organization and plasticity of hypocretin neurons: possible clues to obesity’s association with insomnia. Cell Metab. 2005;1:279–286. doi: 10.1016/j.cmet.2005.03.003. [DOI] [PubMed] [Google Scholar]
- 7.Zigman JM, Jones JE, Lee CE, Saper CB, Elmquist JK. Expression of ghrelin receptor mRNA in the rat and the mouse brain. J Comp Neurol. 2006;494:528–548. doi: 10.1002/cne.20823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yadav R, Suri M, Mathur R, Jain S. Effect of procainization of ventromedial nucleus of hypothalamus on the feeding behavior of rats. J Clin Biochem Nutr. 2009;44:247–252. doi: 10.3164/jcbn.08-158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen O, Zhu W, Ding Y, McCrimmon RJ, Sherwin RS. Blockade of GABAA receptors in the ventromedial hypothalamus further stimulates glucagon and sympathoadrenal but not the hypothalamo-pituitary-adrenal response to hypoglycemia. Diabetes. 2006;55:1080–1087. doi: 10.2337/diabetes.55.04.06.db05-0958. [DOI] [PubMed] [Google Scholar]
- 10.Dunn-Meynell AA, Sanders NM, Compton D, et al. Relationship among brain and blood glucose levels and spontaneous and glucoprivic feeding. J Neurosci. 2009;29:7015–7022. doi: 10.1523/JNEUROSCI.0334-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhu W, Czyzyk D, Paranjape SA, et al. Glucose prevents the fall in ventromedial hypothalamic GABA that is requirred for full activation of glucose counterregulatory responses during hypoglycemia. Am J Physiol Endocrinol Metab. 2010;298:E971–E977. doi: 10.1152/ajpendo.00749.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Geiselman P. Control of food intake: a physiologically complex, motivated behavioral system. Endocrinol Metab Clin North Am. 1996;25:815–829. doi: 10.1016/s0889-8529(05)70356-x. [DOI] [PubMed] [Google Scholar]
- 13.van Houten M, Posner BI. Cellular basis of direct insulin action in the central nervous system. Diabetologia. 1981;20:S255–S267. doi: 10.1007/BF00254491. [DOI] [PubMed] [Google Scholar]
- 14.Beverly JL, Beverly MF, Meguid MM. Alterations in extracellular GABA in the ventral hypothalamus of rats in response to acute glucoprivation. Am J Physiol. 1995;269:R1174–R1178. doi: 10.1152/ajpregu.1995.269.5.R1174. [DOI] [PubMed] [Google Scholar]
- 15.Beverly JL, de Vries MG, Beverly MF, Arseneau LM. Norepinephrine mediates glucoprivic-induced increase in GABA in the ventromedial hypothalamus of rats. Am J Physiol Regul Integr Comp Physiol. 2000;279:R990–R996. doi: 10.1152/ajpregu.2000.279.3.R990. [DOI] [PubMed] [Google Scholar]
- 16.Beverly JL, de Vries MG, Bouman SD, Arseneau LM. Noradrenergic and GABAergic systems in the medial hypothalamus are activated during hypoglycemia. Am J Physiol Regul Integr Comp Physiol. 2001;280:R563–R569. doi: 10.1152/ajpregu.2001.280.2.R563. [DOI] [PubMed] [Google Scholar]
- 17.Wandji SA, Seoane JR, Roberge AG, Bédard L, Thibault L. Effects of intrahypothalamic injections of GABA, muscimol, pentobarbital, and L-glutamic acid on feed intake of satiated sheep. Can J Physiol Pharmacol. 1989;67:5–9. doi: 10.1139/y89-002. [DOI] [PubMed] [Google Scholar]
- 18.Kamatchi GL, Bhakthavatsalam P, Chandra D, Bapna JS. Inhibition of insulin hyperphagia by gamma aminobutyric acid antagonists in rats. Life Sci. 1984;34:2297–2301. doi: 10.1016/0024-3205(84)90220-0. [DOI] [PubMed] [Google Scholar]
- 19.Lenin Kamatchi G, Veeraragavan K, Chandra D, Bapna JS. Antagonism of acute feeding response to 2-deoxyglucose and 5-thioglucose by GABA antagonists: the relative role of ventromedial and lateral hypothalamus. Pharmacol Biochem Behav. 1986;25:59–62. doi: 10.1016/0091-3057(86)90230-3. [DOI] [PubMed] [Google Scholar]
- 20.Beverly JL, Martin RJ. Influence of serum glucose on glutamate decarboxylase activity in the ventromedial nucleus of rats. Am J Physiol. 1990;258:R697–R703. doi: 10.1152/ajpregu.1990.258.3.R697. [DOI] [PubMed] [Google Scholar]
- 21.Beverly JL, Martin RJ. Effect of glucoprivation on glutamate decarboxylase activity in the ventromedial nucleus. Physiol Behav. 1991;49:295–299. doi: 10.1016/0031-9384(91)90046-q. [DOI] [PubMed] [Google Scholar]
- 22.Konig JFR, Klippel RA. The rat brain: A Stereotaxic Atlas. Baltimore: Williams and Wilkins; 1963. [Google Scholar]
- 23.Woods SC, Seeley RJ. Hap1 and GABA: thinking about food intake. Cell Metab. 2006;3:388–390. doi: 10.1016/j.cmet.2006.05.007. [DOI] [PubMed] [Google Scholar]
- 24.Tong Q, Ye CP, Jones JE, Elmquist JK, Lowell BB. Synaptic release of GABA by AgRP neurons is required for normal regulation of energy balance. Nat Neurosci. 2008;11:998–1000. doi: 10.1038/nn.2167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Turenius CI, Charles JR, Tsai DH, et al. The tuberal lateral hypothalamus is a major targe for GABAA- but not GABAB-mediated control of food intake. Brain Res. 2009;1283:65–72. doi: 10.1016/j.brainres.2009.05.064. [DOI] [PubMed] [Google Scholar]
- 26.Kimura H, Kuriyama K. Distribution of gamma aminobutyric acid (GABA) in the hypothalamus: functional correlates of GABA with activities of appetite controlling mechanism. J Neurochem. 1975;24:903–907. doi: 10.1111/j.1471-4159.1975.tb03654.x. [DOI] [PubMed] [Google Scholar]
- 27.Olgiati VR, Netti C, Guidobono F, Pecile A. The central GABAergic system and control of food intake under different experimental conditions. Psychopharmacology (Berl) 1980;68:163–167. doi: 10.1007/BF00432135. [DOI] [PubMed] [Google Scholar]
- 28.Pliquett R, Führer D, Falk S, Zysset S, von Cramon DY, Sturnvoll M. The effects of insulin on the central nervous system: focus on appetite regulation. Horm Metab Res. 2006;38:442–446. doi: 10.1055/s-2006-947840. [DOI] [PubMed] [Google Scholar]
- 29.Johnston GA. Neuropharmacology of amino acid inhibitory transmitters. Annu Rev Pharmacol Toxicol. 1978;18:269–289. doi: 10.1146/annurev.pa.18.040178.001413. [DOI] [PubMed] [Google Scholar]
- 30.Woods SC, Porte D., Jr Neural contral of the endocrine pancreas. Physiol Rev. 1974;54:596–619. doi: 10.1152/physrev.1974.54.3.596. [DOI] [PubMed] [Google Scholar]
- 31.Powley TL. The ventromedial hypothalamic syndrome, satiety, and a cephalic phase hypothesis. Psychol Rev. 1977;84:89–126. [PubMed] [Google Scholar]
- 32.Le Magnen J. Body energy balance and food intake: a neuroendocrine regulatory mechanism. Physiol Rev. 1983;63:314–386. doi: 10.1152/physrev.1983.63.1.314. [DOI] [PubMed] [Google Scholar]
- 33.Woods SC, Porte D, Jr., Bobbione E, et al. Insulin: its relationship to the central nervous system and to the control of food intake and bodyweight. Am J Clin Nutr. 1985;42:1063–1071. doi: 10.1093/ajcn/42.5.1063. [DOI] [PubMed] [Google Scholar]
- 34.Bereiter DA, Berthoud HR, Becker MJ, Jeanrenaud B. Brain stem infusion of the gamma-aminobutyric acid antagonist bicuculline increases insulin levels in the rat. Endocrinology. 1982;111:324–328. doi: 10.1210/endo-111-1-324. [DOI] [PubMed] [Google Scholar]
- 35.Chan O, Zhu W, Ding Y, McCrimmon RJ, Sherwin RS. Blockade of GABAA receptors in the ventromedial hypothalamus further stimulates glucagon and sympathoadrenal but not the hypothalamo-pituitary-adrenal response to hypoglycemia. Diabetes. 2006;55:1080–1087. doi: 10.2337/diabetes.55.04.06.db05-0958. [DOI] [PubMed] [Google Scholar]
- 36.Ahnert-Hilger G, Stadtbäumer A, Strübing C, et al. γ-Aminobutyric acid secretion from pancreatic neuroendocrine cells. Gastroenterology. 1996;110:1595–1604. doi: 10.1053/gast.1996.v110.pm8613067. [DOI] [PubMed] [Google Scholar]
- 37.Yang W, Reyes AA, Lan NC. Identification of the GABAA receptor subtype mRNA in human pancreatic tissue. FEBS Lett. 1994;346:257–262. doi: 10.1016/0014-5793(94)00485-4. [DOI] [PubMed] [Google Scholar]
- 38.Kawai K, Unger RH. Effects of γ-aminobutyric acid on insulin, glucagon, and somatostatin release from isolated perfused dog pancreas. Endocrinology. 1983;113:111–113. doi: 10.1210/endo-113-1-111. [DOI] [PubMed] [Google Scholar]
- 39.Nonogaki K, Iguchi A, Sakamoto N. Bicuculline methiodide influences the central nervous system to produce hyperglycemia in rats. J Neuroendocrinol. 1994;6:443–446. doi: 10.1111/j.1365-2826.1994.tb00605.x. [DOI] [PubMed] [Google Scholar]
- 40.Lang CH. Inhibition of central GABAA receptors enhances hepatic glucose production and peripheral glucose uptake. Brain Res Bull. 1995;37:611–616. doi: 10.1016/0361-9230(95)00052-g. [DOI] [PubMed] [Google Scholar]
- 41.Narita K, Nishihara M, Takahashi M. Concomitant regulation of running activity and metabolic change by the ventromedial nucleus of the hypothalamus. Brain Res. 1994;642:290–296. doi: 10.1016/0006-8993(94)90933-4. [DOI] [PubMed] [Google Scholar]
- 42.Koopmans HS. Satiety signals from the gastrointestinal tract. Am J Clin Nutr. 1985;42:S1044–S1049. doi: 10.1093/ajcn/42.5.1044. [DOI] [PubMed] [Google Scholar]
- 43.Phillips RJ, Powley TL. Gastric volume rather than nutrient content inhibits food intake. Am J Physiol. 1996;271:R766–R769. doi: 10.1152/ajpregu.1996.271.3.R766. [DOI] [PubMed] [Google Scholar]
- 44.Ong J, Kerr DI. GABAA- and GABAB-receptor-mediated modification of intestinal motility. Eur J Pharmacol. 1982;86:9–17. doi: 10.1016/0014-2999(82)90390-9. [DOI] [PubMed] [Google Scholar]
- 45.Erdö SL. Peripheral GABAergic mechanisms. Trends Pharmacol Sci. 1985;6:205–208. [Google Scholar]
- 46.Minuk GY, Vergalla Y, Ferenci P, Jones EA. Identification of an acceptor system for gamma-aminobutyric acid on isolated rat hepatocytes. Hepatology. 1984;4:180–185. doi: 10.1002/hep.1840040203. [DOI] [PubMed] [Google Scholar]
- 47.Schafer DF, Fowler JM, Jones EA. Colonic bacteria: a source of g-aminobutyric acid in blood. Proc Soc Exp Biol Med. 1981;167:301–303. doi: 10.3181/00379727-167-41169. [DOI] [PubMed] [Google Scholar]
- 48.Jessen KR, Mirsky R, Dennison ME, Burnstock G. GABA may be a neurotransmitter in the vertebrate peripheral nervous system. Nature. 1979;281:71–74. doi: 10.1038/281071a0. [DOI] [PubMed] [Google Scholar]
- 49.Krantis A. GABA in the mammalian enteric nervous system. In: Okada Y, Roberts E, editors. Problems in GABA Research From Brain to Bacteria. Amsterdam: Excerpta Medica; 1982. p. 128. [Google Scholar]
- 50.Krantis A, Kerr DI. Autoradiographic localization of [3H] gamma-aminobutyric acid in the myenteric plexus of the guinea pig small intestine. Neurosci Lett. 1981;23:263–268. doi: 10.1016/0304-3940(81)90008-2. [DOI] [PubMed] [Google Scholar]
- 51.De Groot J. Handbook of Physiology, Sec 6, Alimentary Canal, Control of food and water intake. Washington DC: American Physiological Society; 1967. Organisation of hypothalamic feeding mechanisms; pp. 239–247. [Google Scholar]