Abstract
Hyperphosphatemia causes endothelial dysfunction as well as vascular calcification. Management of serum phosphate level by dietary phosphate restriction or phosphate binders is considered to be beneficial to prevent chronic kidney disease patients from cardiovascular disease, but it has been unclear whether keeping lower serum phosphate level can ameliorate endothelial dysfunction. In this study we investigated whether low-phosphate diet can ameliorate endothelial dysfunction in adenine-induced kidney disease rats, one of useful animal model of chronic kidney disease. Administration of 0.75% adenine-containing diet for 21 days induced renal failure with hyperphosphatemia, and impaired acetylcholine-dependent vasodilation of thoracic aortic ring in rats. Then adenine-induced kidney disease rats were treated with either control diet (1% phosphate) or low-phosphate diet (0.2% phosphate) for 16 days. Low-phosphate diet ameliorated not only hyperphosphatemia but also the impaired vasodilation of aorta. In addition, the activatory phosphorylation of endothelial nitric oxide synthase at serine 1177 and Akt at serine 473 in the aorta were inhibited by in adenine-induced kidney disease rats. The inhibited phosphorylations were improved by the low-phosphate diet treatment. Thus, dietary phosphate restriction can improve aortic endothelial dysfunction in chronic kidney disease with hyperphosphatemia by increase in the activatory phosphorylations of endothelial nitric oxide synthase and Akt.
Keywords: hyperphosphatemia, chronic kidney disease, Cardiovascular disease, endothelial nitric oxide synthase, Akt
Introduction
Hyperphosphatemia is the consequence of dysregulation of systemic mineral metabolisms in chronic kidney disease (CKD) patients, and is associated with increased cardiovascular mortality in both CKD patients and general population.(1–5) There are several reasons accounting for increased cardiovascular risk in CKD patients with hyperphosphatemia, however, the detailed mechanism has not been clarified yet. One possible mechanism is vascular calcification. Significant positive correlation between vascular calcification and cardiovascular morbidity or mortality in CKD and end stage renal failure patients have been reported.(6–9)
On the other hand, hyperphosphatemia also independently and significantly correlates with intima-media thickness that is a validated indicator of subclinical atherosclerosis, and cardiovascular events.(10) Thus, endothelium may also be considerable target in the pathogenesis of increased cardiovascular disease in CKD patients with hyperphosphatemia. Recently, we reported that hyperphosphatemia impaired endothelial function by increasing reactive oxygen species (ROS) and inhibiting endothelial nitric oxide synthase (eNOS).(11) Other researchers have also reported that higher dietary phosphate (P) intake decreased acetylcholine-dependent vasodilation in CKD rats,(12) and elevation of extracellular P level increased oxidative stress and apoptosis in endothelial cells.(13) Therefore, lowering serum P level by treatment with P binder or dietary P restriction may be beneficial for improvement of endothelial function to prevent atherosclerosis as well as vascular calcification in CKD patients.
In this study, we investigated the effect of lowering serum P levels by low-P diet on the endothelial dysfunction in adenine-induced kidney disease rats as an experimental CKD model with hyperphosphatemia.
Materials and Methods
Animals
Male, 7 week-old Sprague-Dawley (180–230 g) rats from a local breeding colony (Japan SLC, Shizuoka, Japan) were used in all experiments. Rats were divided into two groups. Age-matched healthy control rats (control rats) were given AIN93G (were purchased from Oriental Yeast, Osaka, Japan) containing 1% P (control diet), and adenine-fed rats were given AIN93G diet containing 1% P with 0.75% adenine (adenine-diet) for 21 days. After 21days, rats were subjected to blood biochemical tests, and evaluation of mineral metabolism and endothelial function as described below. The adenine-fed rats were randomly divided into two groups: adenine-LP and adenine-CP. Adenine-LP rats were fed with AIN93G diet containing 0.2% P diet for 16 days, while adenine-CP rats were fed with control diet for same days. In order to compare the phenotypes, age-matched healthy control rats were simultaneously maintained with control diet through the experimental periods (control rats). Food intake, body weight and plasma P were monitored through out the experimental period. Aortas, serum and plasma were collected after the rats were anesthetized and euthanized by decapitation. This study was approved by the University of Tokushima Animal Use Committee, and rats were maintained according to the National Institutes of Health Guidelines for Care and Use of Laboratory Animals.
Vasodilation test with thoracic aorta rings
Vasodilation test with thoracic aorta rings was performed as described previously.(11) All vessels were treated with 1–100 nM acetylcholine after preconstriction with 100 nM phenylephrine. Vasodilation responses to acetylcholine were expressed as percentage of vasodilation to a submaximal phenylephrine-induced vasoconstriction.
Biochemical analyses and ELISA assays
Plasma creatinine, blood urea nitrogen (BUN), P, and Ca were measured using each specific assay kit (Wako Pure Chem. Ind., Osaka, Japan). Serum 1,25(OH)2D was analyzed by MBC (Mitsubishi Kagaku Bio-Clinical Lab., Inc., Osaka, Japan) using radioimmuno assay. Intact PTH (iPTH) and intact FGF23 (iFGF23) were measured using each specific ELISA kit purchased from Immunotopics International (San Clemente, CA). Asymmetric dimethylarginine (ADMA) was measured by using ADMA ELISA kit from Immundiagnostik GmbH (Bensheim, Germany).
Immunoblot analysis
Approximately 2 cm of rat thoracic aorta were longitudinally dissected, and scraped the inner side with razor in 200 µl of lysis buffer (R&D Systems, Minneapolis, MN) on ice. 15 µg of the lysate were subjected to immunoblot analysis as described previously.(11)
Statistical analysis
We tested all data for normal distribution of variables of interests using Pearson’s X2 distribution test before further parametric or non-parametric statistical analysis. We determined statistical significance of the differences between the groups by ANOVA followed by post-hoc testing using Fisher’s protected least significant difference procedure for multiple comparisons. Comparison between the dose-response curves for vasodilation analysis, and univariate associations between %vasodilation and plasma P or ADMA levels were performed as previously described.(11) All statistical analyses were performed using Statview 5.0 (SAS Institute, Cary, NC), or PRISM 5 (GraphPad Software, La Jolla, CA), and considered a p value < 0.05 statistically significant.
Results
Endothelial dysfunction in adenine-induced kidney disease rats
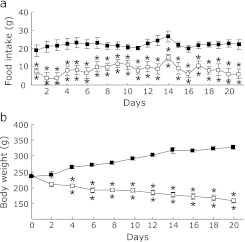
To determine whether low-P diet can ameliorate endothelial dysfunction in CKD, first we reconfirmed that endothelial dysfunction can be observed in our adenine-induced kidney disease rats. The rats were given 1% P diet containing 0.75% adenine or control diet for 21 days. Daily food consumption in adenine-fed rats was significantly lower than that in age-matched healthy control rats (control rats) (Fig. 1a), average body weight of adenine-fed rats was also lower than that of control rats (Fig. 1b). After 3 weeks, plasma P levels in adenine-fed rats were significantly higher than those in control rats (Table 1). Creatinine and BUN were also significantly increased in adenine-fed rats compared with control rats (Table 1). On the other hand, plasma Ca levels were significantly deceased in adenine-fed rats compared with control rats (Table 1). Due to low plasma Ca and high plasma P levels, plasma iPTH in adenine-fed rats levels were 20-fold higher or more than those in control (Table 1). Serum 1,25(OH)2D levels in adenine-fed rats were significantly lower than those in control (Table 1). Interestingly, plasma iFGF23 levels in adenine-fed rats tended to higher than that in control rats without statistically significant difference (Table 1).
Fig. 1.
Food intake and body weight changes during the ingestion of 0.75% adenine containing diet. SD-rats were given either control diet (closed square) or 0.75% adenine containing diet (open square) for 21 days. Food intake (a) and body weight (b) were measured. Data are expressed as means ± SEM (n = 4). **p<0.01 vs Control.
Table 1.
Blood biochemical data of control and adenine-induced kidney disease rats
| Control | Adenine | |
|---|---|---|
| P (mg/dl) | 5.55 ± 0.41 | 20.0 ± 3.14** |
| Ca (mg/dl) | 6.96 ± 0.40 | 1.69 ± 0.34* |
| Creatinine (mg/dl) | 0.69 ± 0.42 | 1.87 ± 0.70** |
| BUN (mg/dl) | 23.3 ± 0.99 | 184 ± 39.8** |
| iPTH (pg/ml) | 91.9 ± 38.6 | 2134 ± 202** |
| 1,25(OH)2D (pg/ml) | 497 ± 38.6 | 20.8 ± 9.75** |
| iFGF23 (pg/ml) | 620 ± 175 | 1242 ± 321 |
Abbreviations: P, phosphate; Ca, calcium; BUN, blood urea nitrogen; iPTH, intact parathyroid hormone; 1,25(OH)2D, 1,25-dihydroxyvitamin D; iFGF23, intact fibroblast growth factor 23. Control: age-matched healthy control rats fed with control diet. Adenine: adenine-induced kidney disease rats. Data are mean ± SEM (n = 4). *p<0.05, **p<0.01 vs Control.
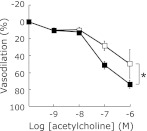
Under such a uremic condition, we evaluated acetylcholine-dependent vasodilation using thoracic aortic rings with isometric transducer. As we expected, acetylcholine-dependent vasodilation in adenine-fed rats was significantly inhibited compared with that in control rats (Fig. 2).
Fig. 2.
Inhibited acetylcholine dependent aortic vasodilation in adenine-induced kidney disease rats. Dose-response curves of vasodilation induced by acetylcholine in rat thoracic aortic rings from either control (closed square) or adenine-induced kidney disease rats (open square). Data are expressed as mean ± SEM (n = 4). There was significant difference in intercepts between the curves (p<0.05).
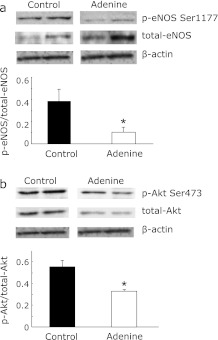
Phosphorylation of eNOS is a key regulator for its activity and regulates NO production in endothelial cells and vascular tone.(14) Akt can phosphorylate eNOS-Ser1177 which leads to increase eNOS activity. Akt can also be activated by phosphorylation at serine 473 responded to extracellular stimuli.(14) Therefore, we examined the phosphorylation of both Akt and eNOS of thoracic aorta of adenine-fed and control rats. The phosphorylated eNOS at serine 1177 in adenine-fed rats was significantly lower than that of control rats (Fig. 3a). We also found that the phosphorylation of Akt was significantly inhibited in adenine-fed rats compared with control rats (Fig. 3b). These results suggest that endothelial dysfunction in adenine-induced kidney disease rats was due to, at least in part, inhibition of eNOS activity through the inactivation of Akt in aorta.
Fig. 3.
Effect of the ingestion of adenine containing diet on the phosphorylation of Akt and endothelial nitric oxide synthase (eNOS) in rat thoracic aorta. Endothelium-enriched protein lysate was obtained from thoracic aorta of either control rats (Control) or adenine-induced kidney disease rats (Adenine). (a) Representative immunoblot of phospho-eNOS at serine 1177 (p-eNOS Ser1177), total eNOS (total-eNOS), and β-actin for internal control from two individual rats per each group are shown. Phosphorylated eNOS ratios from quantitative analysis are also shown in the lower panel. (b) Representative immunoblot of phospho-Akt at serine 473 (p-Akt Ser473), total Akt (total-Akt), and β-actin from two individual rats per each group are shown. Phopshorylated Akt ratios from quantitative analysis are also shown. Data are expressed mean ± SEM (n = 4). *p<0.05 vs Control.
Effects of low P diet on endothelial function in adenine-induced kidney disease rats
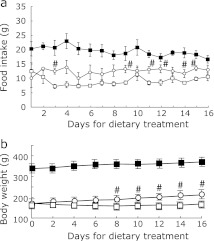
To determine whether low-P diet can ameliorate the endothelial dysfunction, adenine-induced kidney disease rats were divided into two groups, and one group was treated with low-P diet containing 0.2% P (adenine-LP) and the other group was treated with control diet containing 1% P for 16 days (adenine-CP). Food intake in adenine-LP rats was significantly larger than that in adenine-CP rats (Fig. 4a). Body weight was significantly increased in adenine-LP rats compared with adenine-CP rats (Fig. 4b). In addition, total adenine intake before the beginning of dietary intervention was not significantly different between adenine-LP (1.32 ± 0.2 g) and adenine-CP (1.38 ± 0.2 g).
Fig. 4.
Food intake and body weight changes during the ingestion of low-P diet or control diet in the adenine-induced kidney disease rats. Adenine-induced kidney disease rats were given either control diet (open square) or 0.2% low-P diet (open circle) for 16 days. Age-matched healthy control rats (control rats) were fed with control diet during all experimental periods (closed square). Food intake (a) and body weight (b) were measured. Data are expressed as mean ± SEM (n = 6–8). #p<0.05 vs adenine-induced kidney disease rats treated with control diet.
After the treatment with low-P diet, plasma P level was significantly decreased compared with control diet (Table 2), but plasma creatinine and BUN were still significantly higher in both adenine-LP and adenine-CP rats compared to age-matched healthy control rats (control rats) (Table 2). Plasma Ca level was significantly lower in adenine-CP rats than either adenine-LP or control rats. Plasma Ca level of adenine-LP rats was significantly higher than that of control rats (Table 2). Plasma iPTH of adenine-CP rats was 20-fold higher than that of either adenine-LP or control rats (Table 2). Serum 1,25(OH)2D of both adenine-LP and adenine-CP rats was 4-fold lower than that of control rats and there was no significant difference between adenine-CP and adenine-LP rats (Table 2). In addition, plasma iFGF23 in adenine-LP rats was significantly lower than adenine-CP rats (Table 2). These data indicate that, treatment of low-P diet for 16 days can correct abnormal Ca and P metabolism including PTH and FGF23 levels in adenine-induced kidney disease rats. However, despite of improvement of hyperphosphatemia, low-P diet could not improve the kidney dysfunction and uremic condition in our model.
Table 2.
Blood biochemical data of control and adenine-induced kidney disease rats treated with low-P diet (0.2% P, Adenine-LP) or control diet (1.0% P, Adenine-CP) for 16 days
| Control | Adenine-LP | Adenine-CP | |
|---|---|---|---|
| P (mg/dl) | 4.45 ± 0.33 | 3.24 ± 0.46 | 10.6 ± 1.8** |
| Ca (mg/dl) | 6.41 ± 0.40 | 10.1 ± 1.11*,† | 2.66 ± 0.49** |
| Creatinine (mg/dl) | 0.45 ± 0.04 | 2.21 ± 0.48*,# | 1.23 ± 0.07** |
| BUN (mg/dl) | 19.4 ± 1.7 | 211 ± 38**,# | 54.5 ± 4.6* |
| iPTH (pg/ml) | 83.4 ± 36 | 3.47 ± 2.1# | 1955 ± 288** |
| 1,25(OH)2D (pg/ml) | 400 ± 39 | 130 ± 72* | 202 ± 82* |
| iFGF23 (pg/ml) | 386 ± 90 | 141 ± 108# | 630 ± 125 |
| ADMA (µg/ml) | 0.30 ± 0.03 | 0.52 ± 0.05* | 0.34 ± 0.06 |
Abbreviations: P, phosphate; Ca, calcium; BUN, blood urea nitrogen; iPTH, intact parathyroid hormone; 1,25(OH)2D, 1,25-dihydroxyvitamin D; iFGF23, intact fibroblast growth factor 23. ADMA, asymmetric dimethylarginine. Control: age-matched healthy control rats fed with control diet. Data are mean ± SEM (n = 6–8). *p<0.05, **p<0.01 vs Control; #p<0.05, †p<0.01 vs Adenine-CP.
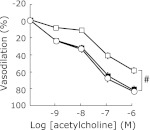
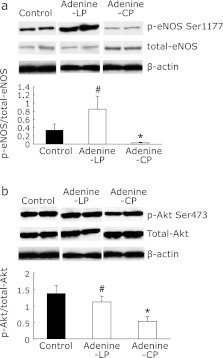
We examined the effect of treatment of low-P diet on the impaired acetylcholine-dependent vasodilation in adenine-induced kidney disease rats. As shown in Fig. 5, acetylcholine-dependent vasodilation of thoracic aortic rings of adenine-LP rats was significantly ameliorated compared with that in adenine-CP rats. Then, we investigated whether low-P diet can affect the activatory phosphorylation status of eNOS and Akt. Phosphorylation of eNOS at serine1177 in adenine-LP rats was also significantly increased (Fig. 6a). Phosphorylation of Akt at serine 473 in adenine-LP rats was significantly increased compared with adenine-CP rats (Fig. 6b).
Fig. 5.
Effect of low-P diet on the acetylcholine-dependent aortic vasodilation in adenine-induced kidney disease rats. Dose-response curves of vasodilation induced by acetylcholine in rat thoracic aortic rings from adenine-induced kidney disease rats treated with control diet (open square) or low-P diet (0.2% P containing diet, open circle). Similar dose-dependent curve from age-matched healthy control rats (control rats) was also obtained (closed square). Data are expressed as mean ± SEM (n = 6–8). #p<0.05 vs both adenine-induced kidney disease rats fed with low-P diet and control rats.
Fig. 6.
Effects of low-Pdiet on the phosphorylation of Akt and endothelial nitric oxide synthase (eNOS) in thoracic aorta of adenine-induced kidney disease rats. Endothelium-enriched protein lysate was obtained from thoracic aorta of adenine-induced kidney disease rats fed with either control (Adenine-CP) or low-P diet (Adenine-LP), or age-matched healthy control rats (Control). (a) Representative immunoblot of phospho-eNOS at serine 1177 (p-eNOS Ser1177), total eNOS (total-eNOS), and β-actin for internal control from two individual rats per each group are shown. Phosphorylated eNOS ratios from quantitative analysis are also shown in the lower panel. (b) Representative immunoblot of phospho-Akt at serine 473 (p-Akt Ser473), total Akt (total-Akt), and β-actin from two individual rats per each group are shown. Phopshorylated Akt ratios from quantitative analysis are also shown. Data are expressed mean ± SEM (n = 6–8). *p<0.05 vs Control. #p<0.05 vs Adenine-CP.
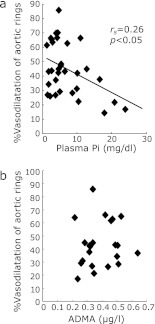
ADMA is another factor causes endothelial dysfunction under uremic condition. We measured plasma ADMA levels after the treatment of low-P diet for 16 days. As shown in Table 2, plasma ADMA levels in adenine-LP rats were not changed significantly compared with adenine-CP rats. In addition, to determine whether plasma P or ADMA levels can correlate with acetylcholine-dependent vasodilation rate of thoracic aorta, we examined the correlation between vasodilation rate and either plasma P or ADMA levels with univariate linear regression analysis. Fig. 7 demonstrates that the vasodilation rate positively and significantly correlated with plasma P levels, but not ADMA levels in our adenine-induced kidney disease rats.
Fig. 7.
Univariate association between %vasodilation and plasma P or plasma ADMA. Univariate association between %vasodilation and plasma P levels (a) or plasma ADMA levels (b). Spearman’s correlation coefficient (rs) and its p value for rs = 0 are represented if the significant association was observed.
Discussion
In this study, we demonstrate that treatment of low-P diet ameliorated acetylcholine-dependent aortic vasodilation impaired in adenine-induced kidney disease rats via improvement of phosphorylation status of Akt and eNOS.
Accumulation of endogenous inhibitor for NO production such as ADMA,(15,16) oxidative stress,(15) indoxyl sulfate,(17) FGF23,(18) fetuin-A,(19) visfatin,(20) etc. has been claimed as possible mediators for endothelial dysfunction in CKD patients. ADMA is a well-characterized endogenous inhibitor for eNOS, and plays a pivotal role in the progression of both cardiovascular disease and renal disease.(21) Therefore, we examined the effect of low-P diet on plasma ADMA levels in adenine-induced kidney disease rats. In our model, low-P diet improved both serum P levels and aortic vasodilation, but did not improve plasma ADMA levels. ADMA levels can be associated with remaining kidney function as represented by GFR.(22) However, low-P diet did not also improve plasma creatinine and BUN in this study. Previous report also demonstrated that low-P diet for 2 weeks did not improve kidney function but can ameliorate serum P levels in CKD model rats.(23) On the other hand, treatment of phosphate binder for 12 weeks can ameliorate not only serum P and calcium but also serum creatinine in chronic kidney disease rats.(24) The most important difference between the studies would be the treatment period. About 2 weeks treatment with low-P diet or phosphate binder may not enough to improve kidney function, but can improve mineral metabolisms in chronic kidney disease rats. Therefore, our results suggest that the improvement of impaired vasodilation by low-P diet was not dependent on the amelioration of renal function in the adenine-induced kidney disease rats. However, we were not able to evaluate serum indoxyl sulfate, and other inhibitors in this study. Contribution of other endogenous inhibitory factors should be investigated in the endothelial dysfunction and the improvement by low-P diet in future study.
Why can lowering plasma P level regulate endothelial function? A possible mechanism would be that changing plasma P levels can modulate phosphorylation status of eNOS and Akt in endothelium. Phosphorylation of eNOS at threonine 495 and serine 1177 are reciprocally regulated in response to both stimulatory and inhibitory stimulus for eNOS activity.(14) Our previous study demonstrated that elevation of extracellular P levels increased reactive oxygen species and decrease NO production via increase in phosphorylation of eNOS at threonine 495 which inactivated eNOS activity by conventional protein kinase C.(11) In addition, the present study demonstrates that hyperphosphatemia inhibited phosphorylation of eNOS at serine 1177, which needs to activate eNOS and can be phopshorylated by Akt.(25) On the other hand, treatment of hyperphosphatemia with low-P diet ameliorated the inhibitory status of phosphorylations in eNOS. In this study, we could not, however, obtain reciprocal regulation of the phosphorylation of threonine 495 due to antibody we used. Interestingly, the amount of phosphorylated eNOS at Ser1177 in adenine-LP rats was significantly higher than age-matched healthy control rats. Although we don’t know the exact mechanism underlying this phenomenon, our data revealed that adenine-LP rats showed lower plasma P, iPTH, iFGF23, and higher plasma Ca level than age-matched healthy control rats. These data suggest that adenine-LP rats would cause over-adaptation to the treatment of low-P diet. The over-adaptation may be involved in the hyper-phosphorylation of eNOS at Ser1177 in the adenine-LP group. Further investigation should be needed to clarify the mechanism. Taken together, we conclude at least that serum P levels can modulate the phosphorylation status of eNOS; higher serum P leads to inactive mode, oppositely lower serum P leads to active mode.
Ca2+-calmodulin pathway is another important pathway to regulate eNOS activity.(14) According to our knowledge, there is no previous report that high-P or low-P loading can regulate calmodulin. But our previous study demonstrated that high-P loading did not affect intracellular Ca2+ increase in response to vasodilator in bovine aortic endothelial cells.(11) Thus, changes in plasma P level at least would not affect the Ca2+-calmodulin pathway in the endothelilal cells.
Vascular calcification may affect the endothelial function or vasodilation directly or indirectly. Dietary P restriction or P binders can ameliorate not only hyperphosphatemia in adenine-induced or 5/6 nephrectomized CKD rats, but also vascular calcification that is a signature phenotype in hyperphosphatemia.(26–28) Although our rat model showed similar phenotype including marked hyperphosphatemia as previously described,(23,26) vascular calcification was not observed in our model (data not shown). In addition, improvement of phosphorylation of eNOS, which is specifically expressed in endothelium, by low-P diet treatment strongly indicates that low-P diet treatment, at least in part, ameliorate endothelial dysfunction rather than medial calcification in our model.
FGF23 may also be important for vascular function. Patient-oriented researches have demonstrated that serum FGF23 levels positively associated with a marked increase in cardiovascular disease in CKD patients.(29) In this study, low-P diet treatment markedly decreased plasma FGF23 levels. Therefore, we cannot exclude the role of FGF23 in the endothelial function, however, it is not clarified whether FGF23 directly or indirectly can regulate eNOS activity or other endothelial function until now. In addition, aortic dilation and constriction are also regulated by the sympathetic system in vivo. Changing dietary P intake may also affect the sympathetic system. Further study is needed to clarify the relationship between dietary P or FGF23 and the sympathetic system on the regulation of endothelial function.
In conclusion, dietary P restriction can ameliorate endothelial dysfunction by at least in part, improving the activation of Akt-eNOS signal pathway in adenine-induced kidney disease rats with hyperphosphatemia. Our results strengthen previous findings that hyperphosphatemia causes endothelial dysfunction. Prevention of both endothelial dysfunction and vascular calcification would be strait-forward targets to decrease the risk for cardiovascular morbidity and mortality in CKD patients. Recently, early initiation of P lowering therapy in non-dialysis CKD patients has also been proposed to prevent cardiovascular disease.(30) However, most of clinical studies have focused on reducing vascular calcification as a primary surrogate outcome. Our findings suggest that endothelial function may be an alternative and useful surrogate outcome, and may be evaluated as cardiovascular effect of P lowering therapy in mild-moderate CKD patients as early as vascular calcification.
Conflict of Interest
All the authors declared no competing interests.
Acknowledgments
This work was supported by Grants-in-Aid for Scientific Research on Priority Areas (YT, KM), for Scientific Research (B) (YT, ET), for Young Scientists (HY), for Exploratory Research (YT, HY), and Knowledge Cluster Initiative (ET) from the Ministry of Education, Culture, Sports, Science, and Technology in Japan, and was also supported by the Kidney Foundation (JKFB09-15).
Abbreviations
- ADMA
asymmetric dimethylarginine
- ANOVA
analysis of variance
- BUN
blood urea nitrogen
- CKD
chronic kidney disease
- eNOS
endothelial nitric oxide synthase
- GFR
glomerular filtration rate
- iFGF23
intact fibroblast growth factor 23
- iPTH
intact parathyroid hormone
- P
phosphate
- ROS
reactive oxygen species
References
- 1.Foley RN, Collins AJ, Herzog CA, Ishani A, Kalra PA. Serum phosphorus levels associate with coronary atherosclerosis in young adults. J Am Soc Nephrol. 2009;20:397–404. doi: 10.1681/ASN.2008020141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kesternbaum B. Phosphate metabolism in the setting of chronic kidney disease: significance and recommendations for treatment. Seminar Dial. 2007;20:286–294. doi: 10.1111/j.1525-139X.2007.00303.x. [DOI] [PubMed] [Google Scholar]
- 3.Tonelli M, Sacks F, Pfeffer M, Gao Z, Curhan G, Cholesterol and Recurrent Events Trial Investigators Relation between serum phosphate level and cardiovascular event rate in people with coronary disease. Circulation. 2005;112:2627–2633. doi: 10.1161/CIRCULATIONAHA.105.553198. [DOI] [PubMed] [Google Scholar]
- 4.Dhingra R, Sullivan LM, Fox CS, et al. Relations of serum phosphorus and calcium levels to the incidence of cardiovascular disease in the community. Arch Intern Med. 2007;167:879–885. doi: 10.1001/archinte.167.9.879. [DOI] [PubMed] [Google Scholar]
- 5.Menon V, Greene T, Pereira AA, et al. Relationship of phosphorus and calcium-phosphorus product with mortality in CKD. Am J Kidney Dis. 2005;46:455–463. doi: 10.1053/j.ajkd.2005.05.025. [DOI] [PubMed] [Google Scholar]
- 6.Blacher J, Guerin AP, Pannier B, Marchais SJ, London GM. Arterial calcifications, arterial stiffness, and cardiovascular risk in end-stage renal disease. Hypertension. 2001;38:938–942. doi: 10.1161/hy1001.096358. [DOI] [PubMed] [Google Scholar]
- 7.Block GA, Raggi P, Bellasi A, Kooienga L, Spiegel DM. Mortality effect of coronary calcification and phosphate binder choice in incident hemodialysis patients. Kidney Int. 2007;71:438–441. doi: 10.1038/sj.ki.5002059. [DOI] [PubMed] [Google Scholar]
- 8.Kestenbaum BR, Adeney KL, de Boer IH, Ix JH, Shlipak MG, Siscovick DS. Incidence and progression of coronary calcification in chronic kidney disease: the multi-ethnic study of atherosclerosis. Kidney Int. 2009;76:991–998. doi: 10.1038/ki.2009.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Watanabe R, Lemos MM, Manfredi SR, Draibe SA, Canziani ME. Impact of cardiovascular calcification in nondialyzed patients after 24 months of follow-up. Clin J Am Soc Nephrol. 2010;5:189–194. doi: 10.2215/CJN.06240909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Onufrak SJ, Bellasi A, Shaw LJ, et al. Phosphorus levels are associated with subclinical atherosclerosis in the general population. Atherosclerosis. 2008;199:424–431. doi: 10.1016/j.atherosclerosis.2007.11.004. [DOI] [PubMed] [Google Scholar]
- 11.Shuto E, Taketani Y, Tanaka R, et al. Dietary phosphorus acutely impairs endothelial function. J Am Soc Nephrol. 2009;20:1504–1512. doi: 10.1681/ASN.2008101106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kööbi P, Vehmas TI, Jolma P, et al. High-calcium vs high-phosphate intake and small artery tone in advanced experimental renal insufficiency. Nephrol Dial Transplant. 2006;21:2754–2761. doi: 10.1093/ndt/gfl270. [DOI] [PubMed] [Google Scholar]
- 13.Di Marco GS, Hausberg M, Hillbrand U, et al. Increased inorganic phosphate induces human endothelial cell apoptosis in vitro. Am J Physiol Renal Physiol. 2008;294:F1381–F1387. doi: 10.1152/ajprenal.00003.2008. [DOI] [PubMed] [Google Scholar]
- 14.Mount PF, Kemp BE, Power DA. Regulation of endothelial and myocardial NO synthesis by multi-site eNOS phosphorylation. J Mol Cell Cardiol. 2007;42:271–279. doi: 10.1016/j.yjmcc.2006.05.023. [DOI] [PubMed] [Google Scholar]
- 15.Yilmaz MI, Saglam M, Caglar K, et al. The determinants of endothelial dysfunction in CKD: oxidative stress and asymmetric dimethylarginine. Am J Kidney Dis. 2006;47:42–50. doi: 10.1053/j.ajkd.2005.09.029. [DOI] [PubMed] [Google Scholar]
- 16.Yilmaz MI, Sonmez A, Saglam M, et al. ADMA levels correlates with proteinuria, secondary amyloidosis, and endothelial dysfunction. J Am Soc Nephrol. 2008;19:388–395. doi: 10.1681/ASN.2007040461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yu M, Kim YJ, Kang DH. Indoxyl sulfate-induced endothelial dysfunction in patients with chronic kidney disease via an induction of oxidative stress. Clin J Am Soc Nephrol. 2011;6:30–39. doi: 10.2215/CJN.05340610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yimaz MI, Sonmez A, Saglam M, et al. FGF-23 and vascular dysfunction in patients with stage 3 and 4 chronic kidney disease. Kidney Int. 2010;78:679–685. doi: 10.1038/ki.2010.194. [DOI] [PubMed] [Google Scholar]
- 19.Caglar K, Yilmaz MI, Saglam M, et al. Serum fetuin-a concentration and endothelial dysfunction in chronic kidney disease. Nephron Clin Pract. 2008;108:c233–c240. doi: 10.1159/000120209. [DOI] [PubMed] [Google Scholar]
- 20.Yilmaz MI, Saglam M, Carrero JJ, et al. Serum visfatin concentration and endothelial dysfunction in chronic kidney disease. Nephrol Dial Transplant. 2008;23:959–965. doi: 10.1093/ndt/gfm727. [DOI] [PubMed] [Google Scholar]
- 21.Schwedhelm E, Böger RH. The role of asymmetric and symmetric dimethylarginines in renal disease. Nat Rev Nephrol. 2011;7:275–285. doi: 10.1038/nrneph.2011.31. [DOI] [PubMed] [Google Scholar]
- 22.Fliser D, Kronenberg F, Kielstein JT, et al. Asymmetric dimethylarginine and progression of chronic kidney disease: the mild to moderate kidney disease study. J Am Soc Nephrol. 2005;16:2456–2461. doi: 10.1681/ASN.2005020179. [DOI] [PubMed] [Google Scholar]
- 23.Terai K, Nara H, Takakura K, et al. Vascular calcification and secondary hyperparathyroidism of severe chronic kidney disease and its relation to serum phosphate and calcium levels. Br J Pharmacol. 2009;156:1267–1278. doi: 10.1111/j.1476-5381.2008.00108.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cozzolino M, Dusso AS, Liapis H, et al. The effects of sevelamer hydrochloride and calcium carbonate on kidney calcification in uremic rats. J Am Soc Nephrol. 2002;13:2299–2308. doi: 10.1097/01.asn.0000025782.24383.0d. [DOI] [PubMed] [Google Scholar]
- 25.Fleming I. Molecular mechanims underlying the activation of eNOS. Pflugers Arch. 2010;459:793–806. doi: 10.1007/s00424-009-0767-7. [DOI] [PubMed] [Google Scholar]
- 26.Katsumata K, Kusano K, Hirata M, et al. Sevelamer hydrochloride prevents ectopic calcification and renal osteodystrophy in chronic renal failure rats. Kidney Int. 2003;64:441–450. doi: 10.1046/j.1523-1755.2003.00126.x. [DOI] [PubMed] [Google Scholar]
- 27.Neven E, Dams G, Postnov A, et al. Adequate phosphate binding with lanthanum carbonate attenuates arterial calcification in chronic renal failure rats. Nephrol Dial Transplant. 2009;24:1790–1799. doi: 10.1093/ndt/gfn737. [DOI] [PubMed] [Google Scholar]
- 28.Shuvy M, Abedat S, Beeri R, et al. Uraemic hyperparathyroidism causes a reversible inflammatory process of aortic valve calcification in rats. Cardiovasc Res. 2008;79:492–499. doi: 10.1093/cvr/cvn088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zisman AL, Wolf M. Recent advances in the rapidly evolving field of fibroblast growth factor 23 in chronic kidney disease. Curr Opin Nephrol Hypertens. 2010;19:335–342. doi: 10.1097/mnh.0b013e328338f536. [DOI] [PubMed] [Google Scholar]
- 30.Sigrist MK, Chiarelli G, Lim L, Levin A. Early initiation of phosphate lowering dietary therapy in non-dialysis chronic kidney disease: a critical review. J Ren Care. 2009;35 (Suppl 1):71–78. doi: 10.1111/j.1755-6686.2009.00064.x. [DOI] [PubMed] [Google Scholar]