Abstract
The aim of this study was to investigate the possible effects of sulphite oxidase (SOX, E.C. 1.8.3.1) deficiency on xenobiotic metabolism. For this purpose, SOX deficiency was produced in rats by the administration of a low molybdenum diet with concurrent addition of 200 ppm tungsten to their drinking water. First, hepatic SOX activity in deficient groups was measured to confirm SOX deficiency. Then, aminopyrine N-demethylase, aniline 4-hydroxylase, aromatase, caffeine N-demethylase, cytochrome b5 reductase, erythromycin N-demethylase, ethoxyresorufin O-deethylase, glutathione S-transferase, N-nitrosodimethylamine N-demethylase and penthoxyresorufin O-deethylase activities were determined to follow changes in the activity of drug metabolizing enzymes in SOX-deficient rats. Our results clearly demonstrated that SOX deficiency significantly elevated A4H, caffeine N-demethylase, erythromycin N-demethylase and N-nitrosodimethylamine N-demethylase activities while decreasing ethoxyresorufin O-deethylase and aromatase activities. These alterations in drug metabolizing enzymes can contribute to the varying susceptibility and response of sulphite-sensitive individuals to different drugs and/or therapeutics used for treatments.
Keywords: sulphite oxidase deficiency, sulphite sensitivity, drug metabolizing enzymes, cytochrome P450s, rat
Introduction
Sulphite is generated endogenously in mammalian tissues from the normal processing of sulphur-containing amino acids. ”Sulphites” is a generic term for a group of compounds including sulphur dioxide, sodium sulphite, sodium and potassium bisulphite and sodium and potassium metabisulphite. They are antioxidants useful for their antimicrobial action and prevention of enzymatic and nonenzymatic discoloration (browning) of foods.(1) Sulphites are used in vinegar, pickles, relishes, olives and sauerkraut and in concentrates of bulk juices and purees such as tomato. They are used in the processing of many food ingredients such as gelatin, beet sugar, corn sweeteners and food starches. In addition, they may be found in a number of parenteral medications within the following categories: antiemetics, cardiovascular preparations, injectable antibiotics, antiarrhythmics, psychotropic drugs, analgesics, local anaesthetics, antishock agents, injectable corticosteroids and nebulized bronchodilator solutions.(1,2)
There are substantial data from in vitro studies indicating that sulphites have the ability to interact with several molecules of biological importance in potentially toxic reactions.(3,4) In mammals, tissues are protected from exposure to sulphite primarily by its direct oxidation to the relatively non-toxic sulphate ion. This metabolic step is catalysed by sulphite oxidase. Sulphite oxidase (SOX, E.C. 1.8.3.1) is a molybdopterin-containing enzyme that catalyses the oxidation of sulphite (SO32−) to sulphate (SO42−). This reaction is the terminal step in the oxidative degradation of sulphur-containing amino acids (cysteine and methionine) and membrane components such as the sulphatides.(5)
Cytochrome P450 (CYP) represents a superfamily of enzymes expressed predominantly in the liver but also in the respiratory tract, lung, brain and small intestine.(6) CYPs are the most important phase I drug-metabolizing enzyme system and metabolise a variety of drugs. In addition to CYPs, phase II enzymes, such as glutathione S-transferase (GST), are important in drug metabolism. GST represents a complex multigene family of cytosolic enzymes(7) that are widely distributed in the animal kingdom. GSTs play an important role in detoxification by conjugating reduced glutathione to a large number of electrophilic metabolites derived from a variety of xenobiotics, including carcinogens, toxins and drugs.
Deficiency of SOX in humans leads to progressive cerebral degeneration, major neurological abnormalities, dislocated ocular lenses, mental retardation, severe seizures, and early death, usually between 2 and 6 years of age.(8) SOX deficiency can occur for two reasons. The first is a defect in the synthesis of its molybdenum cofactor, which also affects xanthine dehydrogenase and aldehyde oxidase. The second is a specific sulphite oxidase defect due to mutations in the gene encoding SOX on chromosome 12q.(9)
Partial SOX deficiency is a possible mechanism involved in sulphite sensitivity. Adverse reactions, including anaphylactic reactions, dermatitis, urticaria, flushing, hypotension, abdominal pain and diarrhea, have been reported in sulphite-sensitive individuals. Despite numerous studies addressing adverse responses in sulphite sensitivity, the clinical importance of changes in drug metabolizing enzymes in sulphite-sensitive individuals due to SOX deficiency remains to be elucidated. For example, there are no reports concerning sulphite-related changes of drug metabolizing enzymes ingested by SOX-deficient rats. It was demonstrated that a molybdenum deficient diet would result in a sulfur handling defect at the level of transformation of sulfite to sulfate and SOX-deficiency could be induced in rats by supplying low molybdenum diet and concomitantly administrating tungsten, which had been shown to be competitive antagonist of molybdenum utilization.(10–12) In this paper, we have investigated the in vivo effects of SOX deficiency on GST and on the most common CYPs known to metabolize drugs and many other xenobiotics in the liver, lung, kidney and small intestine of rats.
Materials and Methods
Chemicals
The following chemicals were purchased from Sigma-Aldrich Chemical Company (St. Louis, MO); p-aminophenol, aniline, bovine serum albumin (BSA), butylated hydroxytoluene (BHT), CaCl2, 1-chloro-2,4-dinitrobenzene (CDNB), cholate, ethylene diamine tetra acetic acid disodium salt (EDTA), Folin phenol reagent, glycerol, glycine, D-glucose-6-phosphate monosodium salt, D-glucose-6-phosphate dehydrogenase, reduced glutathione (GSH), N-2-hydroxyethylpiperazine-N'-2, ethane sulfonic acid (HEPES), β-nicotinamide adenine dinucleotide phosphate (β-NADPH), N-nitrosodimethylamine (NDMA), 2-amino-2-(hydroxymethyl)-1,3-propanediol (TRIS), phenylmethylsulphonylfluoride (PMSF), polyoxyethylene sorbitan monolaurate (TWEEN 20), potassium dihydrogen phosphate, dipotassium hydrogen phosphate, sodium potassium tartarate. The reagents used for hepatic SOX activity assay were obtained from Merck (Darmstadt, Germany). All other chemicals and solvents were obtained from commercial sources at the highest grade of purity available.
Animals and treatment
Healthy male Wistar rats, about 3 months old, weighing 200–250 g, were obtained from University Animal House. They were housed in small cages at standard conditions (24 ± 2°C and 50 ± 5% humidity) with a 12 h light-dark cycle and were fed ad libitum with standard rat chow and tap water. All experimental procedures in animals were performed under appropriate regimes with veterinary services and licensed projects. Rats were divided into two groups: a control group (C) and a SOX-deficient group (D). SOX deficiency was produced in rats by the administration of a low molybdenum diet (AIN 76, Research Dyets Inc., Bethlehem, PA) with concurrent addition of 200 ppm tungsten to their drinking water in the form of sodium tungstate. At the end of the experimental period and following a 16 h fasting period, the rats were killed and the livers, lungs, kidneys and small intestines were removed, rinsed with cold physiological saline and stored at −80°C until analyzed.
Preparation of tissues subcellular fractions
The tissues were homogenized in 4 parts homogenization solution [1.15% KCl containing 250 mM EDTA, 100 mM PMSF, 100 mM BHT, 0.025% Triton X-100] using a tissue homogenizer with a teflon pestle at 4°C. Subcellular fractions of rat tissues were prepared by standard differential centrifugation with calcium-induced aggregation as previously described.(13) Briefly, tissue homogenates were centrifuged (12,100 g for 25 min at 4°C) to obtain postmitochondrial supernatant. Addition of CaCl2 (16 mM final concentration) to the postmitochondrial supernatant allowed complete sedimentation of the microsomes at 30,000 g for 20 min at 4°C. The pellet was then washed by resuspension in an excess volume of homogenization solution and microsomes were resedimented at 30,000 g for 20 min at 4°C. The resultant pinkish opalescent microsomal pellet was suspended in 10% (v/v) glycerol containing 2 mM EDTA. Aliquots of the microsomal fractions were stored at −80°C for subsequent enzyme assays. The amount of protein in individual fractions was measured using the method of Lowry et al.(14) with BSA as the standard.
Enzyme assays
Hepatic SOX activity was determined as an indicator of SOX status of the body according to the method of Cohen and Fridovich.(15) Microsomal cytochrome P450-dependent aniline 4-hydroxylase (A4H) activities of rat microsomes were determined by measuring the quantity of p-aminophenol formed, as described by Imai et al.(16) Aminopyrene N-demethylase (APND), caffeine N-demethylase (CN3D), erythromycin N-demethylase (ERND), N-nitrosodimethylamine N-demethylase (NDMA-ND) activities were determined by measuring the quantity of formaldehyde formed, according to the method of Nash.(17) Aromatase activities were determined using fluorescein as a standard in the presence of erythromycin.(18) 7-ethoxyresorufin O-deethylase (EROD) and penthoxyresorufin O-depenthylase (PROD) activities were assayed as describe elsewhere.(19) GST activities were determined in the cytosolic fractions by the method of Habig et al.(20) using 1-chloro-2,4-dinitrobenzene (CDNB) as a substrate. NADH-cytochrome b5 reductase activity in microsomes was determined by the method of Strittmatter and Velick,(21) in which ferricyanide acted as the electron acceptor.
Gel elecrophoresis and western blotting
SDS-PAGE and western blotting were performed as described previously.(19) Briefly, 120 µg protein samples were separated on 8.5% polyacrylamide gels using the discontinuous buffer system of Laemmli.(22) Proteins were transferred to nitrocellulose membrane by means of the iBlot dry blotting system (20 V, 12 min), using iBlot gel transfer stacks. Following transfer, the membranes were blocked using 5% non-fat dry milk in TBST (20 mM Tris-HCl, pH 7.4, 400 mM NaCl and 0.1% (v/v) Tween 20) for 60 min and incubated with rabbit polyclonal anti-rat CYP1A2, CYP2B1, CYP2C6, CYP2E1 or CYP3A1 antibodies (diluted 1:1000 in blocking solution) for 120 min at room temperature. The membranes were then washed with TBST (3 × 5 min), incubated with secondary antibody (alkaline phosphatase-conjugated anti-rabbit IgG at a 1:5000 or 1:10000 dilution) for 60 min and again washed with TBST (3 × 5 min). Visualization of the bands was carried out using the NBT/BCIP substrate system. The final images were photographed by using computer based gel imaging instrument (DNR LightBIS Pro Image Analysis System, Israel). Protein bands were quantified using Scion Image Version Beta 4.0.2 software.
Statistical analysis
Statistical analyses were performed with Minitab 13 Statistical Software (Minitab Inc., State College, PA) and all values were expressed as mean ± SD. Analysis of variance (ANOVA) and the Mann-Whitney U test were performed to compare the effects of sulphite oxidase deficiency on drug metabolizing enzymes between groups. Differences were considered to be statistically significant when p<0.05 (*).
Results and Discussion
SOX-deficient rats were phenotypically normal and indistinguishable from wild-type animals. Induction of SOX-deficiency was confirmed by measuring hepatic SOX activity in control and treated rats (Table 1). There was no significant difference between the control and treated groups in either food consumption or body weight (data not shown). Table 1 summarizes the levels of drug metabolizing enzymes in four different tissues of control and SOX-deficient animals. In general, large variations were observed with hepatic enzyme activities as compared to the other tissues.
Table 1.
The effect of sulphite oxidase deficiency on the activity of xenobiotic-metabolizing enzymes
| Enzyme§ | Liver | Lung | Kidney | Small intestine | ||||
|---|---|---|---|---|---|---|---|---|
| Control | SOX-Deficient | Control | SOX-Deficient | Control | SOX-Deficient | Control | SOX-Deficient | |
| SOX (Units/mg protein) | 2.22 ± 0.32 | 0.0035 ± 0.0001*** | nm | nm | nm | nm | nm | nm |
| b5 RED (nmol/min/mg protein) | 0.252 ± 0.01 | 0.194 ± 0.008 | nm | nm | nm | nm | nm | nm |
| NDMA-ND (nmol/min/mg protein) | 0.277 ± 0.007 | 0.456 ± 0.011** | 0.071 ± 0.006 | 0.097 ± 0.009 | 0.239 ± 0.006 | 0.211 ± 0.009 | 0.052 ± 0.001 | 0.060 ± 0.001 |
| A4H (nmol/min/mg protein) | 0.306 ± 0.028 | 0.79 ± 0.023*** | 0.051 ± 0.006 | 0.054 ± 0.004 | 0.163 ± 0.003 | 0.168 ± 0.004 | 0.058 ± 0.004 | 0.061 ± 0.003 |
| EROD (pmol/min/mg protein) | 128 ± 3.5 | 92.8 ± 1.8* | nd | nd | 13.3 ± 1.3 | 6.93 ± 2.1** | 31.6 ± 2.5 | 9.47 ± 0.9*** |
| CN3D (nmol/min/mg protein) | 0.29 ± 0.066 | 0.657 ± 0.003*** | 0.129 ± 0.008 | 0.098 ± 0.004 | 0.165 ± 0.003 | 0.081 ± 0.006** | 0.115 ± 0.008 | 0.102 ± 0.007 |
| ERND (nmol/min/mg protein) | 0.159 ± 0.02 | 0.535 ± 0.016*** | 0.052 ± 0.006 | 0.051 ± 0.006 | 0.064 ± 0.008 | 0.068 ± 0.007 | 0.131 ± 0.008 | 0.212 ± 0.005** |
| APND (nmol/min/mg protein) | 0.779 ± 0.03 | 0.948 ± 0.041* | 0.233 ± 0.021 | 0.223 ± 0.009 | 0.582 ± 0.048 | 0.540 ± 0.034 | 0.336 ± 0.022 | 0.355 ± 0.024 |
| Aromatase (pmol/min/mg protein) | 4.02 ± 0.058 | 2.93 ± 0.061* | nm | nm | nm | nm | nm | nm |
| PROD (pmol/min/mg protein) | 21.0 ± 1.66 | 13.5 ± 0.6** | 23.3 ± 1.89 | 22.2 ± 1.72 | 19.2 ± 1.54 | 20.7 ± 1.59 | 39.9 ± 2.4 | 45.3 ± 3.2 |
| GST (nmol/min/mg protein) | 140 ± 11.4 | 128 ± 22.1 | nm | nm | nm | nm | nm | nm |
§Data are presented as the mean ± SD at least four sets of triplicate determinations. *p<0.05, **p<0.01, ***p<0.001 vs Control. nd: not detectable, nm: not measured.
We first examined the effects on NADH-cytochrome b5 reductase activity because it participates in a variety of metabolic pathways, such as steroid biosynthesis and CYP-dependent reactions, and it is known to be neither induced nor suppressed. There was no significant difference in hepatic b5 reductase activity of rats in the experimental groups compared with controls. As a result, this indicates that there was no significant difference between the experimental groups regarding the pathophysiological conditions of the tissues.
Hepatic NDMA-ND activity increased about two-fold in SOX-deficient rats. However, NDMA-ND activities in the lung, kidney and small intestine were not changed significantly. Similar changes were observed with A4H activities. These two activities are known to be catalyzed by the CYP2E1 isoform. It is involved in the oxidative metabolism of mostly small range molecules such as ethanol and acetone.(23) There are many important drugs metabolized by CYP2E1, such as paracetamol (analgesic, antipyretic), dapsone (antibiotic), theophylline (stimulant) and ethanol (psychoactive). CYP2E1 is of interest because of its ability to metabolize and activate many toxicological substrates to more reactive and toxic products. In addition, levels of CYP2E1 are elevated under a variety of physiological and pathophysiological conditions.(23,24) Another notable feature of CYP2E1 is its ability to generate free radical species, such as the superoxide anion and hydroxyl radical. Therefore, care should be given while medicating sulphite-sensitive individuals with CYP2E1 substrates in order to avoid drug and related toxicities.
We found that SOX deficiency had statistically significant effects on EROD and CN3D activities in the liver, kidney and small intestines of rats (Table 1). Although EROD activities were significantly depressed to some extent in liver, kidney and intestine of SOX-deficient animals, controversial CN3D activities were found in the liver and kidney in SOX-deficient rats. EROD and CN3D activities are known to be quite specific probes for CYP1A1 and CYP1A2, respectively.(25) Both are members of the CYP1A family, which often mediates the metabolic bioactivation of carcinogens and procarcinogens, such as PAHs, polycyclic arylamines and aromatic and heterocyclic amines.(26) Thus, potential inhibition of CYP1A may be beneficial to reduce the further risk associated with exposure to such compounds.(27) On the other hand, special precautions should be taken while treating sulphite-sensitive individuals with tricyclic antidepressants, such as amitriptyline and imipramine, because CYP1A2 is one of the major enzymes responsible for the metabolism of these drugs.(28)
Hepatic and intestinal ERND activity increased about three- and two-fold, respectively, in SOX-deficient rats. There was no significant difference in the pulmonary and renal ERND activity of rats (Table 1). Erythromycin is the prototype substrate used for the measurement of CYP3A4, which is considered to have the greatest impact on human pharmacotherapy since it is the most abundant CYP in human liver and intestine. CYP3A4 is known to metabolize more than 50% of all known therapeutic drugs.(29) The molecular mechanism underlying activation of CYP3A4 is highly complex and involves many nuclear receptors. However, the net result of activation is an increase in the metabolism of those compounds and, therefore, increased clearance. An important side effect of this activation is that administered drugs metabolized by CYP3A may also have their pharmacokinetics altered. Such changes can result in reduced clinical efficacy of drugs, which results in poor patient response or the development of an adverse drug response. Activation of CYP3A4 in a SOX-deficient state is noteworthy because sulphite-sensitive individuals should not avoid using any of its substrates as drugs at least some time in their life.
Other than CYP1A- and CYP3A-dependent activities examined in this study showed alterations only in the liver. None exhibited extrahepatic variations. In addition, the hepatic modulation of ANPD, aromatase and PROD activities are significant but quite small as compared to the above-mentioned CYP activities. Changes in hepatic APND activity evoked by a SOX deficiency that we observed in this study provide convincing evidence for activation of CYP2C metabolic activity, which is involved in the metabolism of drugs such as warfarin, tolbutamide, phenytoin, and many non-steroidal anti-inflammatory drugs.(30) Contrary to our results for ANPD, aromatase activity is depressed in a SOX-deficient state. Aromatase (CYP19) is important in sexual development because it catalyzes the key step in the biosynthesis of estrogens. Depression of aromatase deserves further study because it is involved in sexual development and in certain diseases, such as estrogen-dependent cancers. Suppression of PROD activity only in liver confirm the accuracy of the results because it known to be associated with CYP2B and expressed primarily in liver.(31) Although it is involved in the metabolism of small number of drugs, it drives attention due to its role in metabolism of drugs, such as cyclophosphamide, used to treat various types of cancer and some autoimmune disorders.(32) Therefore, sulphite sensitive individuals taking cyclophosphamide may encounter adverse side effects. In contrast with the up- and down-modulation of CYP activities, activities of the hepatic conjugation enzyme, cytosolic GST, remained unchanged in a SOX-deficient state.
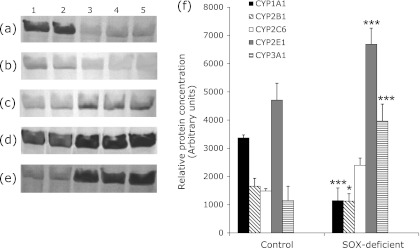
Observed activation of catalytic activities was generally consistent with the protein levels of related CYP isoforms in rat liver microsomes prepared from control and SOX-deficient rats (Fig. 1). The densitometric scanning of Western blot results revealed that hepatic CYP2E1 protein level was increased significantly around 1.42-fold in the SOX-deficient rats relative to the control. Similarly, CYP2C6 and CYP3A1 protein level was increased (1.60 and 3.45-fold). Moreover, SOX-deficiency caused induction of CYP1A1 protein level (2.97-fold). On the other hand, 1.47-fold inhibition of CYP2B1 protein level was observed in SOX-deficiency (Fig. 1).
Fig. 1.
The expression level of CYP1A1, CYP2B1, CYP2C6, CYP2E1 and CYP3A1 proteins in control rats and SOX-deficient rats. Treatments were carried out as described in Materials and Methods (a–e). Representative immunoblot analysis of liver microsomal CYP1A1, CYP2B1, CYP2C6, CYP2E1 and CYP3A1 proteins in experimental groups with rabbit anti-rat CYP1A1, CYP2B1, CYP2C6, CYP2E1 and CYP3A1 IgG. Lane 1–2, control; lane 3–5, SOX-deficiency. (f) Comparison of the CYPs protein levels among experimental groups. The bar graphs represent the mean intensity of the bands obtained from Western blot results. Experiments were repeated at least 3 times. Results are presented as the mean ± SD. *p<0.05, **p<0.01, ***p<0.001
Sulphite and sulfiting agents, such as sulfur dioxide and the salts of bisulphite and of metabisulphite, are currently used for a variety of preservative properties that include controlling microbial growth, preventing browning and spoilage, and bleaching some foods.(33) It is estimated that up to 500,000 sulphite-sensitive individuals live in the United States.(33) Although sulphites are apparently safe for consumption by most subjects, numerous studies have described individuals with sulphite sensitivity that experience adverse reactions on ingestion of sulfiting agents.(34,35) Thus, based on the observed alterations in CYP450 activities, changes in the disposition of many essential medications might be expected in partial SOX-deficient and sulphite-sensitive individuals.
In this study, we examined the in vivo effects of sulphite oxidase deficiency on the catalytic activities of major phase I drug metabolizing enzymes in rat livers. We found that sulphite oxidase deficiency had statistically significant effects on many of the cytochrome P450-dependent monooxygenase systems examined in the liver, lung, kidney and small intestine of rats. The activity of CYP1A, 2E1 and 3A appeared to be particularly vulnerable to the effect of SOX deficiency while CYP19, 2B and 2C are less affected. Knowledge of the impact and nature of these alterations associated with SOX deficiency may help to advance the individualization of medication management in this population.
Acknowledgments
This work was supported in part by a grant from the Pamukkale University Research Fund 2006FBE002. We would also like to thank the Scientific and Technological Research Council of Turkey (106Y244).
Abbreviations
- A4H
aniline 4-hydroxylase
- BHT
butylated hydroxytoluene
- BSA
bovine serum albumin
- CDNB
1-chloro-2,4-dinitrobenzene
- CN3D
caffeine N-demethylase
- CYP
cytochrome P450
- EDTA
ethylene diamine tetra acetic acid disodium salt
- ERND
erythromycin N-demethylase
- EROD
ethoxyresorufin O-deethylase
- GSH
reduced glutathione
- GST
glutathione S-transferase
- HEPES
N-2-hydroxyethylpiperazine-N'-2, ethane sulfonic acid
- NADPH
β-nicotinamide adenine dinucleotide phosphate
- NDMA
N-nitrosodimethylamine
- NDMA-ND
N-nitrosodimethylamine N-demethylase
- PMSF
phenylmethylsulphonylfluoride
- SOX
sulphite oxidase deficiency
- TRIS
2-amino-2-(hydroxymethyl)-1,3-propanediol
- TWEEN 20
polyoxyethylene sorbitan monolaurate
References
- 1.Papaioannou R, Pfeiffer CC. Sulphite sensitivity-unrecognized threat: is molybdenum deficiency the cause? J Orthom Psych. 1984;13:105–110. [Google Scholar]
- 2.Yang WH, Purchase ECR. Adverse reactions to sulphites. CMAJ. 1985;133:865–868. [PMC free article] [PubMed] [Google Scholar]
- 3.Meng Z, Qin G, Zhang B, Bai J. DNA damaging effects of sulfur dioxide derivatives in cells from various organs of mice. Mutagenesis. 2004;19:465–468. doi: 10.1093/mutage/geh058. [DOI] [PubMed] [Google Scholar]
- 4.Oztürk OH, Küçükatay V, Yönden Z, Ağar A, Bağci H, Delibaş N. Expressions of N-methyl-D-aspartate receptors NR2A and NR2B subunit proteins in normal and sulphite-oxidase deficient rat’s hippocampus: effect of exogenous sulphite ingestion. Arch Toxicol. 2006;80:671–679. doi: 10.1007/s00204-006-0125-x. [DOI] [PubMed] [Google Scholar]
- 5.Woo WH, Yang H, Wong KP, Halliwell B. Sulphite oxidase gene expression in human brain and in other human and rat tissues. Biochem Biophys Res Commun. 2003;305:619–623. doi: 10.1016/s0006-291x(03)00833-7. [DOI] [PubMed] [Google Scholar]
- 6.Hedlund E, Gustafsson JA, Warner M. Cytochrome P450 in the brain: a review. Curr Drug Metab. 2001;2:245–263. doi: 10.2174/1389200013338513. [DOI] [PubMed] [Google Scholar]
- 7.Mannervik B, Alin P, Guthenberg C, et al. Identification of three classes of cytosolic glutathione transferase common to several mammalian species: correlation between structural data and enzymatic properties. Proc Natl Acad Sci USA. 1985;82:7202–7206. doi: 10.1073/pnas.82.21.7202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Johnson JL, Wadman SK.Molybdenum cofactor deficiency and isolated sulphite oxidase deficiency Scriver CR, Beaudet AL, Sly WS, Valle D.The Metabolic and Molecular Basis of Inherited Disease New York: McGraw-Hill, 1985; 2271–2283 [Google Scholar]
- 9.Kisker C, Schindelin H, Pacheco A, et al. Molecular basis of sulphite oxidase deficiency from the structure of sulphite oxidase. Cell. 1997;91:973–983. doi: 10.1016/s0092-8674(00)80488-2. [DOI] [PubMed] [Google Scholar]
- 10.Hickey RJ, Clelland RC, Bowers EJ, Boyce DE. Health effects of atmospheric sulfur dioxide and dietary sulfites. The fallacy of typology. Arch Environ Health. 1976;31:108–112. doi: 10.1080/00039896.1976.10667201. [DOI] [PubMed] [Google Scholar]
- 11.Abumrad NN, Schneider AJ, Steel D, Rogers LS. Amino acid intolerance during prolonged total parenteral nutrition reserved by molybdate therapy. Am J Clin Nutr. 1981;34:2551–2559. doi: 10.1093/ajcn/34.11.2551. [DOI] [PubMed] [Google Scholar]
- 12.Kucukatay V, Bor-Kucukatay M, Atsak P, Ağar A. Effect of ingested sulfite on hippocampus antioxidant enzyme activities in sulfite oxidase competent and deficient rats. Int J Neurosci. 2007;117:971–983. doi: 10.1080/00207450600934085. [DOI] [PubMed] [Google Scholar]
- 13.Sen A, Kirikbakan A. Biochemical characterization and distribution of glutathione S-transferases in leaping mullet (Liza saliens) Biochemistry (Mosc) 2004;69:993–1000. doi: 10.1023/b:biry.0000043541.80075.fd. [DOI] [PubMed] [Google Scholar]
- 14.Lowry OH, Rosenburg NJ, Farr AL, Randall RJ. Protein measurements with Folin-phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 15.Cohen HJ, Fridovich I. Hepatic sulphite oxidase. Purification and properties. J Biol Chem. 1971;246:359–366. [PubMed] [Google Scholar]
- 16.Imai Y, Ito A, Sato R. Evidence for biochemically different types of vesicles in the hepatic microsomal fraction. J Biochem. 1966;60:417–428. doi: 10.1093/oxfordjournals.jbchem.a128453. [DOI] [PubMed] [Google Scholar]
- 17.Nash T. The colorimetric estimation of formaldehyde by means of the Hantzsch reaction. Biochem J. 1953;55:416–421. doi: 10.1042/bj0550416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stresser DM, Turner SD, McNamara J, et al. A high-throughput screen to identify inhibitors of aromatase (CYP19) Analytical Biochem. 2000;284:427–430. doi: 10.1006/abio.2000.4729. [DOI] [PubMed] [Google Scholar]
- 19.Sen A, Arinç E. Preparation of highly purified cytochrome P4501A1 from leaping mullet (Liza saliens) liver microsomes and its biocatalytic, molecular and immunochemical properties. Comp Biochem Physiol C Pharmacol Toxicol Endocrinol. 1998;121:249–265. doi: 10.1016/s0742-8413(98)10046-4. [DOI] [PubMed] [Google Scholar]
- 20.Habig WH, Pabst MJ, Jakoby WB. Glutathione S-transferases. The first enzymatic step in mercapturic acid formation. J Biol Chem. 1974;249:7130–7139. [PubMed] [Google Scholar]
- 21.Strittmatter P, Velick SF. The purification and properties of microsomal cytochrome reductase. J Biol Chem. 1957;228:785–799. [PubMed] [Google Scholar]
- 22.Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 23.Kessova I, Cederbaum AI. CYP2E1: biochemistry, toxicology, regulation and function in ethanol-induced liver injury. Curr Mol Med. 2003;3:509–518. doi: 10.2174/1566524033479609. [DOI] [PubMed] [Google Scholar]
- 24.Arinç E, Arslan S, Bozcaarmutlu A, Adali O. Effects of diabetes on rabbit kidney and lung CYP2E1 and CYP2B4 expression and drug metabolism and potentiation of carcinogenic activity of N-nitrosodimethylamine in kidney and lung. Food Chem Toxicol. 2007;45:107–118. doi: 10.1016/j.fct.2006.07.026. [DOI] [PubMed] [Google Scholar]
- 25.Burke MD, Mayer RT. Ethoxyresorufin: direct fluorometric assay of microsomal O-dealkylation which is preferentially induced by 3-methylcholanthrene. Drug Metab Dispos. 1974;2:583–588. [PubMed] [Google Scholar]
- 26.Gonzalez FJ, Gelboin HV. Role of human cytochromes P450 in the metabolic activation of chemical carcinogens and toxins. Drug Metab Rev. 1994;26:165–183. doi: 10.3109/03602539409029789. [DOI] [PubMed] [Google Scholar]
- 27.Ozkarsli M, Sevim H, Sen A. In vivo effects of Urtica urens (dwarf nettle) on the expression of CYP1A in control and 3-methylcholanthrene-exposed rats. Xenobiotica. 2008;38:48–61. doi: 10.1080/00498250701713968. [DOI] [PubMed] [Google Scholar]
- 28.Landi MT, Sinha R, Lang NP, Kadlubar FF. Human cytochrome P4501A2. IARC Sci Publ. 1999;148:173–195. [PubMed] [Google Scholar]
- 29.Coutts RT, Su P, Baker GB. Involvement of CYP2D6, CYP3A4, and other cytochrome P-450 isozymes in N-dealkylation reactions. J Pharmacol Toxicol Methods. 1994;31:177–186. doi: 10.1016/1056-8719(94)90001-9. [DOI] [PubMed] [Google Scholar]
- 30.Flockhart DA. Drug interactions and the cytochrome P450 system. The role of cytochrome P4502 C19. Clin Pharmacokinet. 1995;29 (Suppl 1):45–52. doi: 10.2165/00003088-199500291-00008. [DOI] [PubMed] [Google Scholar]
- 31.Mimura M, Baba T, Yamazaki H, et al. Characterization of cytochrome P450 2B6 in human liver microsomes. Drug Metab Dispos. 1993;21:1048–1056. [PubMed] [Google Scholar]
- 32.Xie HJ, Yasar U, Lundgren S, et al. Role of polymorphic human CYP2B6 in cyclophosphamide bioactivation. Pharmacogenomics J. 2003;3:53–61. doi: 10.1038/sj.tpj.6500157. [DOI] [PubMed] [Google Scholar]
- 33.Lester MR. Sulfite sensitivity: significance in human health. J Am Coll Nutr. 1995;14:229–232. doi: 10.1080/07315724.1995.10718500. [DOI] [PubMed] [Google Scholar]
- 34.Meggs WJ, Atkins FM, Wright R, Fishman M, Kaliner MA, Metcalfe DD. Failure of sulphites to produce clinical responses in patients with systemic mastocytosis or recurrent anaphylaxis: results of a single-blind study. J Allergy Clin Immunol. 1985;76:840–846. doi: 10.1016/0091-6749(85)90758-4. [DOI] [PubMed] [Google Scholar]
- 35.Bush RK, Taylor SL, Holden K, Nordlee JA, Busse WW. Prevalence of sensitivity to sulfiting agents in asthmatic patients. Am J Med. 1986;81:816–820. doi: 10.1016/0002-9343(86)90351-7. [DOI] [PubMed] [Google Scholar]