Abstract
Exposure to soy isoflavones has been associated with low mortality of prostate cancer. In this study, we examined the effects of (±)equol and two representative isoflavones, daidzein and genistein, on migration and invasion in human prostate cancer DU145 cells. First of all, the three regents did not show significant growth inhibitive effect in DU145 cells until the treatments last for 72 h. Treatment with 5 µM, 10 µM, 50 µM (±)equol, 0.5 µM, 1 µM, 5 µM daidzein and genistein for 24 h decreased cell migration and invasion significantly. (±)equol activated phosphatase and tensin homologue deleted on chromosome ten at protein level but not mRNA level, which activated antioxidants, including superoxide dismutase and nuclear factor (erythroid-derived 2)-like 2. A reduction of malondialdehyde concentration, the product of lipid per-oxidation, was observed as well. Moreover, matrix metalloproteinase-2, matrix metalloproteinase-9, and urokinase-type plasminogen activator, the crucial members in metastasis, were down-regulated. Overall, our data indicate that (±)equol, daidzein and genistein may have significant anti-invasion effect in DU145 cells (in vitro). The effects induced by (±)equol may relate to its anti-oxidant effect mediated by phosphatase and tensin homologue deleted on chromosome ten.
Keywords: (±)equol, prostate cancer, DU145 cell, invasion, antioxidant
Introduction
Prostate cancer is ranked as the second cause of cancer-related deaths in men in western countries due to its high prevalence and metastasis rate.(1,2) In contrast, the mortality of prostate cancer is relatively low in Asian men. The difference may be associated with the high consumption of soy products in Asian countries according to epidemiologic studies.(3–5) Isoflavones derived from soy have shown preventive effects on prostate cancer development in vivo and in vitro.(6,7)
Genistein and daizein are two major soy isoflavones that have been extensively studied. It’s reported that their effects mainly focused on inhibition of cell proliferation, block of cell cycle, and apoptosis induction.(7,8) A number of studies about their effects on metastasis were carried out as well, but the conclusions were not consistent. A study by Li et al.(9) showed that genistein inhibited cell invasion in six prostate cell lines (PC3-M, PC3, 1532NPTX, 1542NPTX, 1532CPTX and 1542CPTX). Genistein, daidzein, glycitein were also demonstrated to decrease the motility of breast cancer cells MDA-MB-231 markedly.(10) However, another study showed that genistein had a biphasic effect on metastasis. It suggested that genistein had an invasion promotion effect at a relatively low concentration (0.5 µM).(11)
Equol [7-hydroxy-3-(4'-hydroxyphenyl)-chroman] is an isoflavone metabolite formed by intestinal bacteria from daidzin and daidzein. Only about 30–50% individuals called ”equol producers” are able to convert daidzein to equol. The prevalence of equol producers is much lower in western countries (30%)(12) compared with that in Asian countries (58% in Japan, 60.4% in China).(13,14) Equol exists as diastereoisomer R-equol and S-equol. S-equol is the natural diastereoisomer, while (±)equol is commercially available and generally used in studies. (±)equol is reported to have stronger bioactivities than genistein and daidzein, particular the anti-oxidant activity.(15) As demonstrated by previous studies, (±)equol may have anti-proliferation effects in breast and pancreatic cancer cells.(16,17) While there are few studies on the anti-invasion effects of (±)equol.
To assess the effects of (±)equol on cell motility and invasion, we performed a series of experiments in human prostate cancer DU145 cells. In addition, we examined the mRNA and protein expression of some related genes in cancer progression and treated the cells with (±)equol to clarify the mechanism. We also compared anti-invasion effects of two representative isoflavones, daidzein and genistein.
Materials and Methods
Materials
(±)equol, daidzein and genistein were purchased from LC Laboratories (Woburn, MA). Fetal bovine serum (FBS) was purchased from Hyclone Laboratories (Logan, Utah). RPMI1640 medium was purchased from Maichen biotechnology (Beijing, China). Matrigel was purchased from BD Biosciences (Billerica, MA). Primary antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA), and secondary antibodies were purchased from Rockland (Montgomery, PA). The cDNA synthesis kit and PCR Master Mix kit were purchased from Fermentas (Vilnius, Lithuania).
Cell culture
Androgen-dependent human prostate cancer cell line DU145 was purchased from cell center of Peking Union Medical College (Beijing, China). The DU145 cells were cultured in RPMI 1640 medium supplemented with 10% fetal bovine serum, together with 1% L-glutamine, 100 units/ml penicillin and 100 µg/ml streptomycin (Maichen biotechnology, Beijing, China). Cells were incubated in a humidified atmosphere containing 5% CO2 at 37°C (SANYO MCO-175 Cell Culture CO2 Incubator, Osaka, Japan).
Determination of the reagents’ concentrations
The concentrations of the reagents for the following experiments were determined to be the same as in a previous study,(18) which were 5 µM, 10 µM, 50 µM for (±)equol and 0.5 µM, 1 µM, 5 µM for genistein and daidzein. The concentrations of the reagents used for our study were high but still physiologically achievable. Morton et al.’s study in 1997 showed that the prostate fluid concentrations of equol/daidzein reached up to 13.5 µM (7 µM in average)/2.1 µM (0.3 µM in average) in Asian men.(19) Another study performed by them in 2005 reported that the prostate fluid concentrations of genistein were about 0.5 µM (5.1 µM in maximum) in high-soy consumers in Caucasian.(20)
MTT assay
Cell viability was estimated by the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenil tetrazolium bromide (MTT) assay. The DU145 cells in the logarithmic growth phase were resuspended to 1 × 105/ml and plated in 96-well plates for 12 h to adhere in complete medium. Then the medium was substituted with 5 µM, 10 µM, 50 µM of equol or 0.1% DMSO treated complete medium. The incubation lasted for 24 h, 48 h or 72 h. Subsequently, MTT (Sigma, MO) solution was added to each well to yield a final concentration of 0.5 mg/ml. After another 4-hour-incubation, supernatant fluid was removed, and 150 µl of DMSO was added to each well to dissolve formazan crystals. Plates were shaken for 10 min, and the absorbance (A) was recorded at 492 nm (Thermo Scientific Multiskan MK3, Thermo Scientific, Milford, MA). Independent experiments were performed five times.
Wound healing assay
For cell motility determination, DU145 cells were cultured to 90% confluence in 24-well plates. After serum-starved for 12 h, the cells were carefully wounded with sterile pipette tips and washed with PBS, then incubated in serum-free medium in the presence of various concentrations of (±)equol/genistein/daidzein or 0.1% DMSO for 24 h. Photographs of cells invading the wound were taken at 0 h and 24 h after wounding. The distance between the wound edges was measured by Image-pro-plus software to examine cell migration. Independent experiments were performed six times.
Invasion assay
The spontaneous invasion of DU145 cells was measured by Transwell invasion assay using 24-well transwell invasion chambers (Corning, Lowell, MA). The upper chamber of the transwells (fitted with 8-µm-pore-size polycarbonate filters) were coated with matrigel and placed in a sterile hood to dry overnight. Then 200 µl of serum-free medium in the presence of (±)equol/genistein/daidzein or 0.1% DMSO was added into the upper chambers while 600 µl of complete medium into the lower ones. DU145 cells were seeded in the upper chambers (5000/chamber) after being serum-starved for 24 h. After 24 h incubation, cells on the upper filter surfaces were scraped, while cells on the lower surfaces were stained with 0.2% Crystal violet (Maichen biotechnology, Beijing, China) and photographed under light microscopy (Nikon TE2000-S, Nikon, Tokyo, Japan). The numbers of invaded cells were counted. Independent experiments were performed three times.
Gelatin zymography
Enzymatic activities of matrix metalloproteinase-2 and matrix metalloproteinase-9 were assayed by gelatin zymography. DU145 cells were treated with various concentrations of (±)equol or 0.1% DMSO in non-phenol red, serum-free medium for 24 h. Conditioned media was collected, centrifuged at 10000 revolutions per minute for 10 min to remove debris, and concentrated approximately 10-fold through a dialysis bag. Equivalent amounts (50 µg) of total proteins were determined by BCA kits (Maichen biotechnology, Beijing, China). The zymography was performed with MMPs zymography electrophoresis kits (Genmed, Shanghai, China) according to the manufacture’s protocols. Experiments were performed three times and a representative blot was shown.
Determination of superoxide dismutase (SOD), malondialdehyde (MDA)
DU145 cells were cultured in serum-free medium together with various concentrations of (±)equol or 0.1% DMSO for 24 h. 100 µl of the conditioned media was used to assess the activity of Total Superoxide Dismutase (T-SOD) with Total Superoxide Dismutase Detection Kit and the concentration of Malondialdehyde (MDA) with MDA Detection Kit according to the manufacturer’s instructions (Jiancheng Bioengineering Institute, NanKing, China). Independent experiments were performed five times.
Western blot analysis
Western blot analysis was performed as follows to analyze the related proteins. DU145 cells grown to 90% confluence were treated with various concentrations of (±)equol or 0.1% DMSO for 24 h, and then lysed in RIPA buffer on ice for 30 min. Equivalent amount (30 µg) of total proteins from lysates determined by BCA kits was separated by 12% SDS-polyacrylamide gels and transferred to a polyvinylidene fluoride membrane. Subsequently, the membranes were blocked in TBST (20 mmol/l Tris pH 7.5, 150 mmol/l NaCl, and 0.05% Tween 20) containing 5% nonfat dry milk for 2 h, incubated with primary antibodies (diluted 1:1000 in blocking buffer) overnight at 4°C, washed three times in TBST, and incubated with secondary antibodies (diluted 1:10000 in TBST) for 2 h at room temperature. Complexes were visualized using Odyssey imaging system (Li-Cor Biosciences Company, NE). Equivalent protein loading was confirmed with anti-actin antibody. Experiments were performed three times and a representative blot was shown.
RT-PCR analysis
In order to analyze the related gene expression, RT-PCR analysis was performed. Total RNA was extracted from various concentrations of (±)equol or DMSO treated DU145 cells using Trizol reagent (Invitrogen, Foster City, CA), and then used to synthesize first-strand cDNAs with RevertAidTM first-strand cDNA synthesis kits (Fermentas, Vilnius, Lithuania) according to the manufacture’s protocols. The RNA purity was determined by the ratio of absorbance at 260 nm and 280 nm, and its concentration was quantified by absorbance at 260 nm spectrophotometrically. PCR amplification was carried out using Dream Taqtm Green PCR Master Mix (Fermentas, Vilnius, Lithuania) under the following conditions: 30 cycles of denaturing for 1 min at 95°C, annealing at 52°C (MMP-9), 55°C (urokinase-type plasminogen activator (u-PA)), 58°C (phosphatase and tensin homologue deleted on chromosome ten (PTEN)) or 63°C (GAPDH) for 1 min, extension at 72°C for 1 min; followed by a final extension at 72°C for 5 min. Sequences of the primers were as follows: MMP-9 forward (CAACATCACCTATTGGATCC), reverse (GGGTGTAGAGTCTCTCGCT); u-PA, forward (AGA ATTCACCATCGAGA), reverse (ATCAGCTTCACAACAGT CAT); PTEN, forward (CAGAAAGACTTGAAGGCGTAT), reverse (CCTGGTGTGGGTCCTGA), GAPDH, forward (CAA GGTCATCCATGACAACTTTG), reverse (GTCCACCACCCT GTTGCTGTAG). Negative controls omitting RT were included in each PCR reaction. Experiments were performed three times and a representative blot was shown.
Statistical analysis
The results were expressed as means ± SD. Analyses of variance (ANOVA) were performed to assess the differences between treatment and control groups, and LSD post-hoc tests were used for further analyses. All tests of statistical significance were two-sided, and p<0.05 was considered significant.
Results
Effect of (±)equol, daidzein and genistein on DU145 cell viability
We first assayed toxicity of (±)equol, daidzein and genistein in DU145 cells at various concentrations for 24, 48, 72 h by MTT assay. As shown in Table 1, the three regents did not show significant growth inhibitive effect in DU145 cells until the treatments last for 72 h. Hence, treatments for 24 h were applied in the following experiments.
Table 1.
Effect of (±)equol, genistein and daidzein on the viability in DU145 cells
| Treatment | Cell viability (% of control) |
||
|---|---|---|---|
| 24 h | 48 h | 72 h | |
| (±)Equol (5.0 µM) | 94.30 ± 11.56 | 91.57 ± 4.05 | 95.93 ± 4.53 |
| (±)Equol (10.0 µM) | 97.47 ± 11.12 | 91.25 ± 8.76 | 92.35 ± 2.97 |
| (±)Equol (50.0 µM) | 92.15 ± 9.36 | 91.83 ± 7.27 | 97.75 ± 6.19 |
| Genistein (0.5 µM) | 93.04 ± 7.61 | 91.63 ± 9.55 | 86.96 ± 5.35** |
| Genistein (1.0 µM) | 87.72 ± 9.11 | 95.40 ± 8.77 | 97.65 ± 4.34 |
| Genistein (5.0 µM) | 106.96 ± 11.47 | 100.96 ± 4.96 | 93.81 ± 1.96 |
| Daidzein (0.5 µM) | 129.62 ± 7.30 | 103.83 ± 3.51 | 95.44 ± 10.39 |
| Daidzein (1.0 µM) | 97.72 ± 14.00 | 94.13 ± 3.51 | 84.31 ± 6.79 |
| Daidzein (5.0 µM) | 95.82 ± 12.55 | 89.78 ± 7.29 | 77.82 ± 6.32** |
**p<0.01.
Effects of (±)equol, daidzein and genistein on DU145 cell migration and invasion
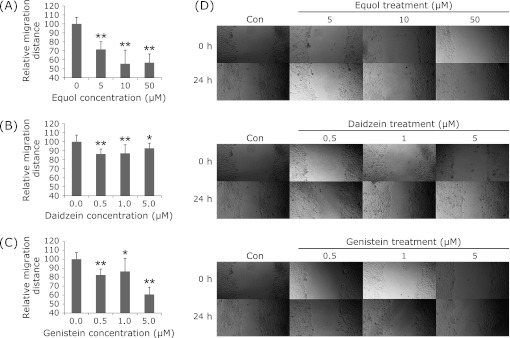
As shown in Fig. 1, (±)equol, daidzein and genistein all showed significant inhibitive effects on cell motility. The relative migration distances were decreased by 28.4%, 44.4% and 43.2% at 5 µM, 10 µM, 50 µM of (±)equol, and decreased by 13.61%, 12.81%, 7.21%, and 17.61%, 13.61%, 39.20% at 0.5 µM, 1 µM, 5 µM of daidzein and genitein, respectively.
Fig. 1.
Effect of (±)equol (A), daidzein (B) and genistein (C) on cell motility of prostate cancer DU145 cells. Relative migration distance in comparison with the 0.1% DMSO group is indicated. The relative migration distances were decreased by 28.4%, 44.4% and 43.2% at 5 µM, 10 µM, 50 µM of (±)equol, by 13.61%, 12.81%, 7.21% and 17.61%, 13.61%, 39.20% at 0.5 µM, 1 µM, 5 µM of daidzein and genistein, respectively. **p<0.01. (D) DU145 cell monolayers were wounded by a sterile micropipette tip and treated with different concentrations of (±)equol, daidzein and genistein.
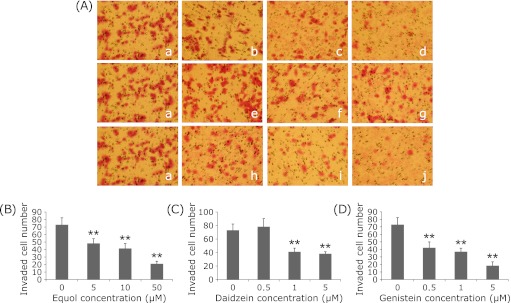
The results of the invasion assay demonstrated that (±)equol, daidzein and genistein all significantly inhibited the invasion of DU145 cells in a dose-dependent manner (Fig. 2). When treated with 5 µM, 10 µM and 50 µM of (±)equol, the numbers of invaded cells decreased to 48.25 ± 6.13, 41.50 ± 6.45 and 21.00 ± 3.37, respectively (73 ± 9.27 in the control group). Meanwhile, 0.5 µM, 1 µM and 5 µM of genistein decreased the invaded cell number to 42.2 ± 7.79, 37 ± 4.64 and 18.4 ± 5.08. 1 µM and 5 µM of daidzein decreased the invaded cell number to 41.17 ± 5.38 and 38.2 ± 3.03, respectively.
Fig. 2.
Effect of (±)equol, daidzein and genistein on cell invasion of prostate cancer DU145 cells. (A) Invaded cells were observed by microscopy (×200). Treatment groups: a: 0.1% DMSO, b: 5 µM (±)equol, c: 10 µM (±)equol, d: 50 µM (±)equol, e: 0.5 µM Daidzein, f: 1 µM Daidzein, g: 5 µM Daidzein, h: 0.5 µM Genistein, i: 1 µM Genistein, j: 5 µM Genistein. (B–D) The percentages of relative invaded cell number treated with (±)equol, daidzein, genistein in comparison with the 0.1% DMSO group is indicated in (B–D), respectively. **p<0.01.
Effects of (±)equol on MMP-2, MMP-9 activities and u-PA, MMP-9 expression
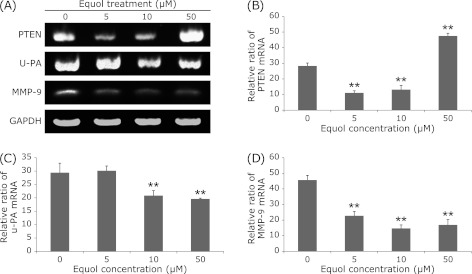
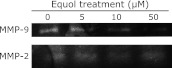
As presented in Fig. 3, the MMP-9 mRNA expression was markedly down-regulated at 5 µM, 10 µM, 50 µM of (±)equol. We also found that the mRNA expression of u-PA was down-regulated in 10 µM, 50 µM of (±)equol treatment groups (Fig. 3). Moreover, the results of gelatin zymography assay showed that 10 µM, 50 µM of (±)equol inhibited the activities of secreted MMP-2 and MMP-9 significantly (Fig. 4).
Fig. 3.
RT-PCR analysis of some gene expression in DU145 cells under the treatment of (±)equol at various concentrations (0 µM, 5 µM, 10 µM, 50 µM) incubation for 24 h. PTEN expression in the 50 µM (±)equol treatment group is significantly higher than that in control group as shown by the densitometric assessments (B). u-Pa expression in the (±)equol treatment groups of higher concentrations is significantly lower than that in control group (C). MMP-9 expression in the (±)equol treatment groups is significantly lower than that in control group (D). Columns, the densitometric values of gene expression under the treatment of different concentrations; bars, SE. **p<0.01.
Fig. 4.
Gelatin zymography analysis showed that 10 µM, 50 µM of (±)equol inhibited the activities of secreted MMP-2 and MMP-9.
Effects of (±)equol on T-SOD activity, MDA concentration and Nrf2, PTEN expression
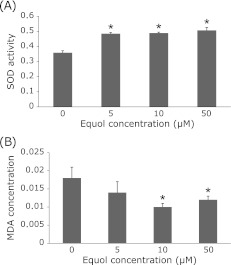
We assessed the T-SOD activity and MDA concentration (an indicator of ROS insult) to examine the antioxidant activity of (±)equol on DU145 cells. Compared with the control group, SOD activity was significantly increased by 35.1%, 36.2% and 41.2% when treated with 5 µM, 10 µM and 50 µM of (±)equol, accompanied with a reduction of MDA concentration by 22.2%, 44.4% and 33.3%, respectively (Fig. 5).
Fig. 5.
DU145 cells were exposed to (±)equol at various concentrations (0 µM, 5 µM, 10 µM, 50 µM) and incubated for 24 h. SOD activity was significantly increased by 35.1%, 36.2% and 41.2% when treated with 5 µM, 10 µM and 50 µM of (±)equol (A), accompanied with a reduction of MDA concentration by 22.2%, 44.4% and 33.3%, respectively (B). All data are reported as means ± SD (n = 5). *p<0.05.
Fig. 6.
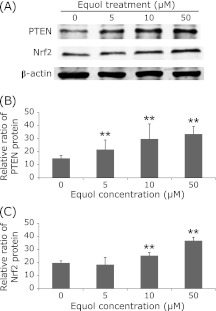
Western blot analysis of some gene expression in protein level in DU145 prostate cancer cells under the treatment of (±)equol at various concentrations (0 µM, 5 µM, 10 µM, 50 µM) incubation for 24 h. PTEN expression in the (±)equol treatment groups is significantly higher than that in control group as shown by the densitometric assessments (B). Nrf2 expression in the (±)equol treatment groups of higher concentration is significantly higher than that in control group (C). Columns, the densitometric values of gene expression under the treatment of different concentrations; bars, SE. **p<0.01.
Moreover, an up-regulated expression of antioxidant relative gene nuclear factor (erythroid-derived 2)-like 2(Nrf2) at protein level was observed when treated with (±)equol. PTEN, an important antioxidant defense system regulator, was significantly increased in a dose-dependent manner at protein level. At mRNA level, 5 µM and 10 µM of (±)equol showed weak inhibitive effects on PTEN mRNA expression while 50 µM of (±)equol showed significant promotion effects on PTEN mRNA expression (Fig. 4). The inhibitive effect at low concentrations may because that the expression of PTEN at mRNA level had passed the peak at that time point. However, studies about the mechanism are still required.
Discussion
In the present study, we compared the anti-invasion effects of soy isoflavone metabolite, (±)equol, and soy isoflavone aglycones (daidzein and genistein). The results demonstrated that all the three reagents had notable inhibitive effects on cell migration and invasion. (±)equol showed a moderate anti-invasion effect at 5 µM among the three reagents. To the authors knowledge, it is the first study to determine the anti-invasion effect of (±)equol on DU145 cells. And the results were consistent with the previous studies on anti-invasion effects of (±)equol in MDA-MB-231 breast cancer cells and PC-3 prostate cancer cells.(16,21)
We also explored the possible mechanisms of (±)equol’s effects on cell invasion. For cancer metastasis, three steps are necessary: cell-to-extracellular matrix (ECM) adhesion, proteolytic cleavage and cell migration.(22) Matrix metalloproteinases (MMPs) is a family of enzymes mainly responsible for ECM degradation (the critical step of metastasis).(23) Urokinase-type plasminogen activator (u-PA) is another important ECM-degrading enzyme.(24) Our study indicated that (±)equol inhibited the activity of secreted MMP-2, MMP-9 and mRNA expression of u-PA and MMP-9, suggesting that effects of (±)equol on invasion and migration might be mediated through down-regulation of MMP-2, MMP-9 and u-PA expression. That was consistent with other studies investigating effects of isoflavones on cancer development. It was reported that genistein inhibited glioma cell invasion by down-regulating MMP-9 and MMP-2 activity and gene expression. (9,25)
Oxidative stress and increased production of reactive oxygen species (ROS) are involved in various processes of cancer metastasis by up-regulating ECM-degrading enzymes.(26) Previous studies have identified that MMP-2, MMP-9 and u-PA were regulated by oxidative stress at expression and activity stages.(26,27) Hence, the scavenging of ROS by antioxidants can be an effective approach of inhibiting cancer progression.
Moreover, (±)equol demonstrated the greatest antioxidant activity in ferric-reducing ability of plasma (FRAP) assay and Trolox equivalent antioxidant capacity (TEAC) assay of all the soy isoflavones tested.(28) So we assessed oxidative stress levels and related gene expression in DU145 cells treated with (±)equol. Since lipid per-oxidation is one of the most important expressions of oxidative stress induced by ROS, its end product Malondialdehyde (MDA) was determined as the oxidative stress indicator in our study. The results showed that MDA concentrations were decreased in (±)equol treated cells, indicating that invasion suppression by equol may be associated with oxidative stress level and ROS decreasing.
Studies have reported that SOD family played a crucial role in ROS scavenging.(26) Moreover, Nrf2 has been suggested to regulate SOD activity via Nrf2-ARE pathway.(29) We found that exposure to (±)equol significantly increased both T-SOD activity and Nrf2 expression, which implied that T-SOD and Nrf2 played an important role here. As demonstrated by previous studies, PTEN is identified as a tumor suppressor that may activate antioxidant defense system and negatively regulate the produce of ROS.(30) As demonstrated by a previous study, PTEN-PI3K/AKT pathway may be associated with the activation of Nrf2/ARE element.(31) In our study, (±)equol showed a dose-response promotion effect on PTEN expression at protein level. That suggested that the effect of (±)equol on PTEN may be involved in regulation of the antioxidant defense system.
Taken together, our data suggested that (±)equol together with genistein and daidzein obviously inhibited prostate cancer DU145 cell migration and invasion. The anti-metastasis effect induced by (±)equol might be mediated through the inhibition of secretion of MMP-2, MMP-9 and u-PA by activating antioxidant defense system.
Acknowledgments
This work was supported by the National Natural Science Foundation (No. 30471449, No. 30671759, No. 30872114 and No. 30872115), PR China.
References
- 1.Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010;60:277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 2.Bubendorf L, Schöpfer A, Wagner U, et al. Metastatic patterns of prostate cancer: an autopsy study of 1,589 patients. Hum Pathol. 2000;31:578–583. doi: 10.1053/hp.2000.6698. [DOI] [PubMed] [Google Scholar]
- 3.Löf M, Weiderpass E. Epidemiologic evidence suggests that dietary phytoestrogen intake is associated with reduced risk of breast, endometrial, and prostate cancers. Nutrition Research. 2006;26:609–619. [Google Scholar]
- 4.Sonoda T, Nagata Y, Mori M, et al. A case-control study of diet and prostate cancer in Japan: possible protective effect of traditional Japanese diet. Cancer Sci. 2004;95:238–242. doi: 10.1111/j.1349-7006.2004.tb02209.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Armstrong B, Doll R. Environmental factors and cancer incidence and mortality in different countries, with special reference to dietary practices. Int J Cancer. 1975;15:617–631. doi: 10.1002/ijc.2910150411. [DOI] [PubMed] [Google Scholar]
- 6.Ozasa K, Nakao M, Watanabe Y, et al. Serum phytoestrogens and prostate cancer risk in a nested case-control study among Japanese men. Cancer Sci. 2004;95:65–71. doi: 10.1111/j.1349-7006.2004.tb03172.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rabiau N, Kossaï M, Braud M, et al. Genistein and daidzein act on a panel of genes implicated in cell cycle and angiogenesis by polymerase chain reaction arrays in human prostate cancer cell lines. Cancer Epidemiol. 2010;34:200–206. doi: 10.1016/j.canep.2009.12.018. [DOI] [PubMed] [Google Scholar]
- 8.Choi EJ, Kim GH. Daidzein causes cell cycle arrest at the G1 and G2/M phases in human breast cancer MCF-7 and MDA-MB-453 cells. Phytomedicine. 2008;15:683–690. doi: 10.1016/j.phymed.2008.04.006. [DOI] [PubMed] [Google Scholar]
- 9.Li Y, Kucuk O, Hussain M, Abrams J, Cher ML, Sarkar FH. Antitumor and antimetastatic activities of docetaxel are enhanced by genistein through regulation of osteoprotegerin/receptor activator of nuclear factor-kappaB (RANK)/RANK ligand/MMP-9 signaling in prostate cancer. Cancer Res. 2006;66:4816–4825. doi: 10.1158/0008-5472.CAN-05-3752. [DOI] [PubMed] [Google Scholar]
- 10.Haberland J, Bertz J, Wolf U, Ziese T, Kurth BM. German cancer statistics 2004. BMC Cancer. 2010;10:52. doi: 10.1186/1471-2407-10-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.El Touny LH, Banerjee PP. Identification of a biphasic role for genistein in the regulation of prostate cancer growth and metastasis. Cancer Res. 2009;69:3695–3703. doi: 10.1158/0008-5472.CAN-08-2958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rowland IR, Wiseman H, Sanders TA, Adlercreutz H, Bowey EA. Interindividual variation in metabolism of soy isoflavones and lignans: influence of habitual diet on equol production by the gut microflora. Nutr Cancer. 2000;36:27–32. doi: 10.1207/S15327914NC3601_5. [DOI] [PubMed] [Google Scholar]
- 13.Morton MS, Arisaka O, Miyake N, Morgan LD, Evans BA. Phytoestrogen concentrations in serum from Japanese men and women over forty years of age. J Nutr. 2002;132:3168–3171. doi: 10.1093/jn/131.10.3168. [DOI] [PubMed] [Google Scholar]
- 14.Liu B, Qin L, Liu A, et al. Prevalence of the equol-producer phenotype and its relationship with dietary isoflavone and serum lipids in healthy Chinese adults. J Epidemiol. 2010;20:377–384. doi: 10.2188/jea.JE20090185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Setchell KD, Brown NM, Lydeking-Olsen E. The clinical importance of the metabolite equol-a clue to the effectiveness of soy and its isoflavones. J Nutr. 2002;132:3577–3584. doi: 10.1093/jn/132.12.3577. [DOI] [PubMed] [Google Scholar]
- 16.Magee PJ, Raschke M, Steiner C, et al. Equol: a comparison of the effects of the racemic compound with that of the purified S-enantiomer on the growth, invasion, and DNA integrity of breast and prostate cells in vitro. Nutr Cancer. 2006;54:232–242. doi: 10.1207/s15327914nc5402_10. [DOI] [PubMed] [Google Scholar]
- 17.Choi EJ, Ahn WS, Bae SM. Equol induces apoptosis through cytochrome c-mediated caspases cascade in human breast cancer MDA-MB-453 cells. Chem Biol Interact. 2009;177:7–11. doi: 10.1016/j.cbi.2008.09.031. [DOI] [PubMed] [Google Scholar]
- 18.Liu CQ, Zhang YM, Zheng W, et al. The Effects of equol and bioactive components of soybean on growth of human prostatic cancer cells Dul45 in vitro (in Chinese) Food and Nutrition in China. 2010;6:70–72. [Google Scholar]
- 19.Morton MS, Chan PS, Cheng C, et al. Lignans and isoflavonoids in plasma and prostatic fluid in men: samples from Portugal, Hong Kong, and the United Kingdom. Prostate. 1997;32:122–128. doi: 10.1002/(sici)1097-0045(19970701)32:2<122::aid-pros7>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 20.Hedlund TE, Maroni PD, Ferucci PG, et al. Long-term dietary habits affect soy isoflavone metabolism and accumulation in prostatic fluid in caucasian men. J Nutr. 2005;135:1400–1406. doi: 10.1093/jn/135.6.1400. [DOI] [PubMed] [Google Scholar]
- 21.Magee PJ, McGlynn H, Rowland IR. Differential effects of isoflavones and lignans on invasiveness of MDA-MB-231 breast cancer cells in vitro. Cancer Lett. 2004;208:35–41. doi: 10.1016/j.canlet.2003.11.012. [DOI] [PubMed] [Google Scholar]
- 22.Woodhouse EC, Chuaqui RF, Liotta LA. General mechanisms of metastasis. Cancer. 1997;80 (Suppl):1529–1537. doi: 10.1002/(sici)1097-0142(19971015)80:8+<1529::aid-cncr2>3.3.co;2-#. [DOI] [PubMed] [Google Scholar]
- 23.Itoh Y, Nagase H. Matrix metalloproteinases in cancer. Essays Biochem. 2002;38:21–36. doi: 10.1042/bse0380021. [DOI] [PubMed] [Google Scholar]
- 24.Duffy MJ, Duggan C. The urokinase plasminogen activator system: a rich source of tumour markers for the individualised management of patients with cancer. Clin Biochem. 2004;37:541–548. doi: 10.1016/j.clinbiochem.2004.05.013. [DOI] [PubMed] [Google Scholar]
- 25.Xu L, Ding Y, Catalona WJ, et al. MEK4 function, genistein treatment, and invasion of human prostate cancer cells. J Natl Cancer Inst. 2009;101:1141–1155. doi: 10.1093/jnci/djp227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim SC, Magesh V, Jeong SJ, et al. Ethanol extract of Ocimum sanctum exerts anti-metastatic activity through inactivation of matrix metalloproteinase-9 and enhancement of anti-oxidant enzymes. Food Chem Toxicol. 2010;48:1478–1482. doi: 10.1016/j.fct.2010.03.014. [DOI] [PubMed] [Google Scholar]
- 27.Kunigal S, Lakka SS, Gondi CS, Estes N, Rao JS. RNAi-mediated downregulation of urokinase plasminogen activator receptor and matrix metalloprotease-9 in human breast cancer cells results in decreased tumor invasion, angiogenesis and growth. Int J Cancer. 2007;121:2307–2316. doi: 10.1002/ijc.22962. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 28.Mitchell JH, Gardner PT, McPhail DB, Morrice PC, Collins AR, Duthie GG. Antioxidant efficacy of phytoestrogens in chemical and biological model systems. Arch Biochem Biophys. 1998;360:142–148. doi: 10.1006/abbi.1998.0951. [DOI] [PubMed] [Google Scholar]
- 29.Warabi E, Takabe W, Minami T, et al. Shear stress stabilizes NF-E2-related factor 2 and induces antioxidant genes in endothelial cells: role of reactive oxygen/nitrogen species. Free Radic Biol Med. 2007;42:260–269. doi: 10.1016/j.freeradbiomed.2006.10.043. [DOI] [PubMed] [Google Scholar]
- 30.Huo YY, Li G, Duan RF, et al. PTEN deletion leads to deregulation of antioxidants and increased oxidative damage in mouse embryonic fibroblasts. Free Radic Biol Med. 2008;44:1578–1591. doi: 10.1016/j.freeradbiomed.2008.01.013. [DOI] [PubMed] [Google Scholar]
- 31.Li MH, Cha YN, Surh YJ. Peroxynitrite induces HO-1 expression via PI3K/Akt-dependent activation of NF-E2-related factor 2 in PC12 cells. Free Radic Biol Med. 2006;41:1079–1091. doi: 10.1016/j.freeradbiomed.2006.06.010. [DOI] [PubMed] [Google Scholar]