Abstract
Recently, arginase is suggested to regulate nitric oxide production by competing with nitric oxide synthase for the same substrate, L-arginine, in experimental asthma. We investigated the role of arginase and its relationship to nitric oxide production after spinal cord injury. Rats were subjected to laminectomy and complete transection of their spinal cords (injury group) or laminectomy only (sham group). In the injury group, arginase I was increased in the macrophages at the transection edge, and the peak was observed 48 h after spinal cord injury. However, nitric oxide production decreased significantly in the injury group despite increased nitric oxide synthase2 mRNA expression compared with the sham group. We also demonstrated the reduction in L-arginine concentrations, which was inversely associated with changes in arginase activity. Therefore, arginase appeared to regulate nitric oxide production by consuming L-arginine. The regulation of arginase activity and L-arginine levels may improve nitroxidative stress and reduce tissue damage in spinal cord injury.
Keywords: nitric oxide, arginase, L-arginine, nitric oxide synthase, spinal cord injury
Introduction
Neuronal regeneration is extremely limited in a variety of neurological disorders of the central nervous system (CNS). Spinal cord injury generally causes tissue damage through both primary and secondary mechanisms.(1) The primary injury, such as contusion to the spinal cord, results in the direct damage of neurons, astrocytes, oligodendrocytes and endothelial cells. The secondary injury leads to biochemical and pathological changes in the spinal cord. Neurological dysfunction is influenced by the degree of secondary damage including glutamate excitotoxicity,(2) edema,(3) ischemia,(4) Ca2+ overload,(5,6) reactive oxygen species (ROS)(7–9) and nitric oxide (NO).(10–12)
NO has been shown to play important but contradictory physiological and pathological roles in CNS. Generally, NO acts as a neurotransmitter and a vasodilator at physiologically moderate levels.(13) However, a relatively large amount of NO which is mainly produced by nitric oxide synthase 1 (NOS1) and NOS2 is neurotoxic.(14,15) Moreover, the superoxide anion radical (O2•−), which is released from the infiltrated mononuclear cells and following intracellular generation by Nox or Duox easily reacts with NO by induced NOS, and then reactive nitrogen species (RNS) such as peroxynitrite is produced.(16,17) In turn, they oxidize sulfhydryl groups, DNA, lipids and proteins causing severe tissue damage.(18,19) Therefore, reducing the production of ROS and RNS is prerequisite for the prevention or therapy of spinal cord injury.
Arginase I and II are major metabolic enzymes in the liver and kidney, and participate in the urea cycle to convert L-arginine to urea and L-citrulline.(20) In spinal cord neurons of mice, arginase I and II are expressed as key enzymes for probable pathway source from L-arginine to ornithine, glutamate and gamma-aminobutyric acid.(21) High levels of arginase I expression have been detected in neurons and macrophages after hemi-transected spinal cord injury.(22) Recently, many researchers have investigated the roles of arginase I and L-arginine homeostasis in airway inflammation and hyperresponsiveness in experimental asthma models and asthmatic patients.(23–27) These studies have shown that highly expressed arginase deprives L-arginine of NOS, and results in loss of NO and relaxation of bronchial smooth muscle in experimental asthma. However, in spinal cord injury, the relationship between NO and arginase is unclear.
Recently, the transplantation of biomaterials, such as various extracellular matrices, has been reported for the treatment of spinal cord injury. We previously reported that, after damaged spinal cord tissue was completely resected, reactive astrocytes invaded the collagen filament of amputation stump and released neurotrophic factors such as brain-derived neurotrophic factor and neurotrophin 3.(28) Precise elucidation of the tissue environment for axonal growth, including the presence of nitroxidative stress, at the site of the transection injury is of great importance.
Therefore, the aim of this study was to provide insight into the tissue environment by investigating the regulation of NO generation by arginase following spinal cord transected injury in rats.
Materials and Methods
Animals and groups
Adult female Wistar rats (Charles River Japan, Inc., Yokohama, Japan) weighing 200–220 g were used. Animals were randomly assigned to two groups: the sham group (laminectomy only, n = 9) and the injury group (laminectomy + spinal cord injury, n = 9).
Surgical procedures
For all procedures, the rats were anesthetized with an intramuscular injection of a mixture of ketamine (64 mg/kg) and xylazine (4.5 mg/kg). The surgical instruments were sterilized, and the animals were kept on a heating pad during surgery. The rectal temperature was monitored and maintained at a normal temperature throughout the surgery. The laminectomy was performed at the Th9 vertebral level, and the dorsal aspect of the dura was exposed. In the injury group, the spinal cord was then completely transected with a fine microcutter and the wound was closed. In the sham group, rats underwent laminectomy without transection injury. After surgery, the animals were caged in a warm environment and given sufficient water and feed. Manual compression was performed twice daily to empty the bladder until preparation. All animal care and procedures were performed according to the NIH guidelines; this study was approved by Okayama University School of Medicine Animal Committee.
Spinal cord tissue collection
Animals were sacrificed for collecting spinal cord tissue at the indicated times after surgery. Rats were anesthetized and perfused through the left ventricle with 100 ml of normal ice-cold saline. In the injury group, a 1-cm-long spinal cord segment (0.5 cm rostral and 0.5 cm caudal from the injury site) containing the injury epicenter was removed from each rat. In the sham group, a corresponding 1-cm-long segment of the spinal cord was removed.
NOx measurement
The generation of NO in the spinal cord was expressed as NOx, nitrite (NO2−) and nitrate (NO3−). Homogenates (25% w/v) of the spinal cord tissue were prepared using a Teflon homogenizer in 20 mM Tris-HCl buffer (pH 7.5) containing 150 mM NaCl and 1 mM EDTA at 4°C. The homogenates were then centrifuged at 12,000 × g for 20 min at 4°C. Nitrate reductase (0.66 U/ml) from Aspergillus niger (Sigma-Aldrich, St. Louis, MO) and NADPH (6.6 µM) were added to the supernatant to convert NO3− to NO2−. After incubation at room temperature for 30 min, the protein was removed by adding acetonitrile followed by centrifugation. NO2− was further reduced to NO in a PurgeVessel containing the reducing agent potassium iodide in acetic acid, and NO was subsequently detected by the ozone-chemiluminescence method using an NO analyzer 280i (Sievers, Boulder, CO).(29)
Western blot analysis
The western blot analysis was performed as previously reported.(30) Homogenates were prepared in 20 mM of Tris-HCl buffer (pH 7.5) containing 150 mM NaCl and a protease inhibitor cocktail (Complete Mini Tablets from Roche, Basel, Schweiz) at 4°C with a Teflon homogenizer. The samples were dissolved in 2% SDS and 6% β-mercaptoethanol solution by boiling for 5 min followed by centrifugation at 12,000 × g for 20 min. A total of 50 µg of protein from the supernatant of each sample was separated using SDS/PAGE in a 10% polyacrylamide gel and then transferred onto PVDF membranes (Millipore, Bedford, MA). The membranes were blocked in 5% nonfat milk in TBS-T (20 mM Tris-HCl, 150 mM NaCl, 0.05% Tween 20, pH 7.7) for 1 h at room temperature. The membranes were then incubated overnight at 4°C with the following primary antibodies: polyclonal rabbit anti-arginase I and arginase II (1:500; Santa Cruz Biochemistry, Santa Cruz, CA), and monoclonal mouse anti-nitrotyrosine (1:500; Upstate, New York, NY). The membranes were washed 3 times with TBS-T for 5 min each and then incubated for 1 h at room temperature with the following secondary antibodies conjugated with horseradish peroxidase (DAKO, Tokyo, Japan): anti-rabbit for arginase I and II (1:2000 dilution), and anti-mouse for nitrotyrosine (1:1500). After washing 3 times for 10 min each with TBS-T, a chemiluminescent marker (Western Lightning Chemiluminescence Reagent Plus, Perkin Elmer Life Sciences Inc., Boston, MA) was used for detection. The results were compared as relative values using β-actin as an internal reference. Autoradiographs were analyzed densitometrically using image analysis software (Scion Image program, Scion Corp., Frederick, MD).
Arginase activity assay
Fifty microliters of 10 mM MnCl2 in 50 mM Tris-HCl (pH 7.5) was incubated with 50 µl of tissue homogenate at 55°C for 10 min. Twenty-five microliters was then transferred into Eppendorf tubes and incubated in the presence of 25 µl of 0.5 M arginine (pH 9.7) at 37°C for 1 h. The reaction was stopped by the addition of 400 µl of an acid mixture containing H2SO4, H3PO4 and H2O (H2SO4:H3PO4:H2O = 1:3:7 volumes). Isonitrosopropiopheanone at a concentration of 9% (dissolved in 100% ethanol) was added, and the mixture was heated at 100°C for 45 min. The amount of urea formed was determined spectrophotometrically at 540 nm. The activity of arginase was expressed as UV units per min per mg protein.(31)
Measurement of L-arginine concentration by high performance liquid chromatography (HPLC)
The concentration of L-arginine in the spinal cord was quantified by fluorescence HPLC system (HITACHI, Tokyo, Japan), as previously described.(32) Briefly, L-arginine was extracted from homogenates using Oasis MCX solid phase-extraction cartridges (Waters, Milford, MA). First, the column was conditioned with 2 ml of methanol/water/ammonia solution (50:45:5, vol/vol/vol) and phosphate buffer saline (PBS). The tissue sample was then passed through the SPE column, and the column was washed with 2 ml of 0.1 M HCl and methanol. The fraction containing arginine was collected with 1 ml of methanol/water/ammonia solution (50:45:5, vol/vol/vol) and dried in a vacuum centrifuge. After the drying process, the residue was reconstituted with water and mixed with an equal amount of derivatization solution (5 mg/ml ortho-phthaldialdehyde, 10% methanol, 0.5% 3-mercaptopropionic acid in 200 mM borate buffer, pH 8.5), and the reaction was allowed to occur for 30 min at room temperature. The sample was introduced into the fluorescence HPLC system using a TSKgel ODS-100V column (4.6 × 250 mm, 5 µm, Tosoh, Yamaguchi, Japan). The mobile phase consisted of 9% acetonitrile in acetate buffer (pH 6.3) at a flow rate of 1.5 ml/min, and the excitation and emission wavelengths were 340 and 455 nm, respectively. After each measurement, 100% acetonitrile was allowed to flow in to completely eliminate any wastes remaining in the column.
Immunohistochemistry
Rats were anesthetized and perfused intracardially with 100 ml of 0.01 M PBS (pH 7.4) followed by 200 ml of 4% paraformaldehyde. The spinal cord was carefully extracted, and a 1 cm segment containing the injury epicenter was post-fixed in 4% paraformaldehyde for 6 h before cryo-protection in 20% sucrose in 0.01 M PBS overnight. The spinal cord segment was embedded in tissue freezing medium and cut into 8 µm sagittal sections using a cryostat. The sections were then collected on silanized slides (Dako). For immunostaining, frozen sections were incubated with 0.3% H2O2 in methanol for 30 min and washed with 0.01 M PBS three times for 5 min each. The sections were incubated in 5% normal goat serum. The specimens were incubated overnight at 4°C with polyclonal antibodies against arginase I (1:100) (Santa Cruz Biochemistry). Then, specimens were treated with goat anti-rabbit immunoglobulin conjugated with peroxidase labeled-dextran polymer (Dako) for 1 h at room temperature. Visualization was performed with 3,3-diaminobenzidine tetrahydrochloride (Dako) as a substrate. As a negative control, rabbit non-immune immunoglobulin (Dako) was used. Finally, the sections were counterstained with hematoxylin.
Also, to study the possible cellular colocalization of arginase, a double immunofluorescence assay for arginase and ED-1 was performed. ED-1 is widely used as a marker for macrophages. Frozen section were incubated overnight at 4°C with polyclonal rabbit anti-arginase (1:50) and monoclonal mouse anti-ED-1 (1:200) (Santa Cruz Biochemistry). The secondary antibodies were Texas red-conjugated anti-rabbit (1:200) (TAKARA BIO, Shiga, Japan) and FITC-conjugated anti-mouse (1:200) (Santa Cruz Biochemistry) antibodies. Images were obtained using a confocal laser-scanning microscope.
Reverse transcription-PCR (RT-PCR) for NOS isoforms and cytokines
Total RNA from spinal cord tissue was isolated by ISOGEN with modifications based on the manufacturer’s instructions for the strict collection of RNA. Briefly, the RNA pellet was dissolved again in ISOGEN then washed twice in chloroform to remove contaminating DNA and proteins thoroughly. The RNA samples were stored at −80°C until further analysis. Reverse transcription and PCR was performed using the TaKaRa RNA PCR Kit (AMV) ver. 3.0 (TAKARA BIO) with oligo-dT primers according to the manufacturer’s instructions. The primer sets and PCR conditions in this study are detailed in Table 1. PCR was performed using a Thermal Cycler MP (TAKARA BIO) under optimal thermal conditions specific for each primer set. Electrophoresis was performed using a 2% agarose gel containing 0.5 mg/ml of ethidium bromide. The PCR bands were visualized using an UV transilluminator. The expression of each mRNA was analyzed using the Scion Image program (Scion Corp., Frederick, MD). The results were compared as the relative values using GAPDH as an internal reference.
Table 1.
List of primers and RT-PCR conditions
| Target gene | Sense | Antisense | Denaturation | Amplification | Cycle | Elongation |
|---|---|---|---|---|---|---|
| NOS1 | AGAACGGGGAGAAATTCG | CGCAGAACACATCACAG | 94°C, 3 min | 94°C, 60 s 56°C, 60 s 72°C, 60 s | 32 | 72°C, 5 min |
| NOS2 | ATGGCTTGCCCCTGGAAGTTTCTC | CCTCTGATGGTGCCATCGGGCATC | 94°C, 3 min | 94°C, 45 s 60°C, 45 s 72°C, 90 s | 31 | 72°C, 5 min |
| NOS3 | GGGCTCCCTCCTTCCGGCTGCCACC | GGATCCCTGGAAAAGGCGGTGAGG | 94°C, 3 min | 94°C, 45 s 64°C, 45 s 72°C, 90 s | 33 | 72°C, 5 min |
| IL-4 | CTGCTTTCTCATATGTACCGGG | TTTCAGTGTTGTGAGCGTGG | 94°C, 3 min | 94°C, 60 s 61°C, 45 s 72°C, 75 s | 40 | 72°C, 7 min |
| IL-13 | CATCACACAAGACCAGAAGACTTCC | GGATGGCATTGCAACTGGAG | 94°C, 3 min | 94°C, 60 s 61°C, 45 s 72°C, 75 s | 40 | 72°C, 7 min |
| TGF-β | CTTCAGCTCCACAGAGAAGAACTGC | CACGATCATGTTGGACAACTGCTCC | 94°C, 3 min | 94°C, 60 s 54°C, 60 s 72°C, 45 s | 35 | 72°C, 5 min |
| GAPDH | ACCACAGTCCATGCCATCAC | TCCACCACCCTGTTGCTGTA | 94°C, 3 min | 94°C, 45 s 60°C, 45 s 72°C, 60 s | 30 | 72°C, 5 min |
Statistical analysis
Data were expressed as the mean ± SD. Differences were analyzed using un-paired t test or ANOVA with post hoc test. p values less than 0.05 were considered significant. Statistical analyses were performed using GraphPad Prism 5.0c for Mac (GraphPad Software, Inc., San Diego, CA).
Results
Arginase activity
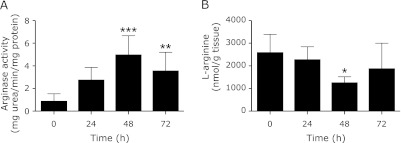
The time-course changes in arginase activity following spinal cord transection injury were investigated. The arginase activity increased gradually after the spinal cord injury. This increase was significant at 48 and 72 h after spinal cord injury compared with that at 0 h (p<0.001 at 48 h, p<0.01 at 72 h) (Fig. 1A). Therefore, our investigation was mainly conducted at 48 h after spinal cord injury.
Fig. 1.
Time-course of changes in arginase activity and L-arginine. In the injury group, arginase activity increased gradually, and was significantly higher at 48 and 72 h than at 0 h (A). L-arginine was extracted from the spinal cord homogenate using Oasis MCX SPE columns, and derivatized with OPA for the fluorescence HPLC system. Reduction in L-arginine concentration was observed at 48 h (B). Data are expressed as mean ± SD (n = 9 animals per group). *p<0.05, **p<0.01 and ***p<0.001 vs 0 h.
Concentration of L-arginine in the tissue
Contrary to arginase activity, the content of L-arginine was gradually reduced in the injury group, and the reduction reached statistical significance at 48 h (p<0.05) (Fig. 1B).
Western blot analysis for arginase
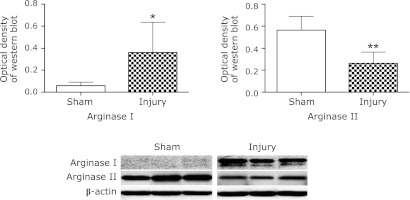
The expressions of arginase I and II protein were determined by Western blot analysis. Significant increase of arginase I was observed in the injury group at 48 h compared with low expression in the sham group (p<0.05). In contrast, arginase II was constitutively expressed in the sham group. At 48 h, expression of arginase II was down-regulated in the injury group (p<0.01) (Fig. 2).
Fig. 2.
Western blot analysis for arginase expression. Arginase expression in each group was measured by Western blot analysis at 48 h after surgery. Densitometric analysis of arginase expression is shown, and the results are normalized to the expression level of β-actin. Expression of arginase I was significantly increased in the injury group compared with the sham group. Conversely, arginase II was decreased in the injury group. Data are expressed as mean ± SD (n = 9 animals per group). *p<0.05, **p<0.01 vs sham group.
Expression of mRNA for NOS isoforms
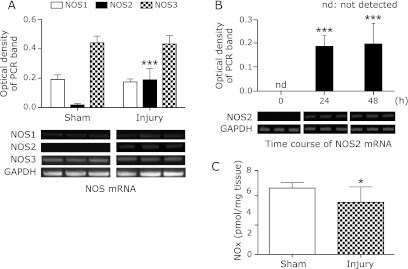
The mRNA expression of NOS isoforms at 48 h compared with that in the sham group, and the time course of changes in the expression of NOS2 mRNA in the injury group were analyzed by RT-PCR. NOS2 mRNA expression was significantly increased at 48 h compared with that in the sham group (p<0.001). Whereas, no increase in NOS1 and NOS3 mRNA expression level was observed at 48 h (Fig. 3A). Significant increase in the time course of NOS2 mRNA was observed at 24 and 48 h after spinal cord injury (p<0.001) (Fig. 3B).
Fig. 3.
Expression of mRNA for NOS isoforms and NOx concentration. The mRNA expression of NOS was measured by RT-PCR at 48 h in the injury group. Densitometric analysis is shown, and the results are normalized to the expression level of GAPDH. Significant increase of NOS2 mRNA expression was observed at 48 h. However, no increase of mRNA expression of NOS1 or NOS3 was observed compared with that in the sham group (A). Significant increase of NOS2 mRNA expression was observed from the early phase (B). NOx contents were measured in the total homogenate obtained from the sham and injury groups at 48 h. In the injury group, significant decrease was observed (C). Data are expressed as mean ± SD (n = 9 animals per group). *p<0.05, ***p<0.001 vs sham group or 0 h.
NOx measurement
At 48 h, significant decrease in NOx contents was observed in the injury group compared with that in the sham group (p<0.05) (Fig. 3C).
Western blot analysis for nitrotyrosine expression
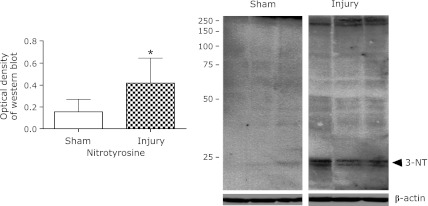
Tyrosine nitrated proteins were detected in the injury group by Western blotting. The bands of low molecular weight range were clearer than that of high molecular weight range. In contrast, in the sham group, nitrotyrosine expression was not detected. Marked increase was observed in the injury group (p<0.05) (Fig. 4).
Fig. 4.
Western blot analysis for nitrotyrosine expression. Nitrotyrosine expression was measured by Western blot analysis at 48 h. Arrowhead indicate the protein bands that intensified significantly in the injury group. Densitometric analysis of nitrotyrosine expression is shown, and the results are normalized to the expression levels of β-actin. Nitrotyrosine expression was significantly higher in the injury group than in the sham group. Data are expressed as mean ± SD (n = 9 animals per group). *p<0.05 vs sham group.
Expression of cytokine mRNA
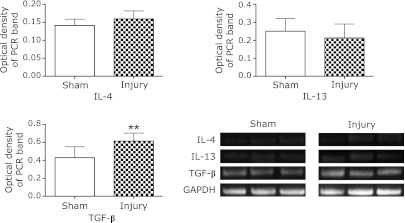
To investigate the mechanism of arginase induction, mRNA expressions of several cytokines reported to regulate arginase activity were analyzed using RT-PCR for IL-4, IL-13 and TGF-β. At 48 h, no significant increase in mRNA expression level of IL-4 or IL-13 was observed. In contrast, mRNA expression of TGF-β was markedly elevated at 48 h (p<0.01) (Fig. 5).
Fig. 5.
Expressions of cytokine mRNAs for IL-4, IL-13 and TGF-β. The mRNA expression level of TGF-β significantly increased at 48 h in the injury group compared with the sham group. In contrast, no increase in the expression level of IL-4 or IL-13 was observed. Data are expressed as mean ± SD (n = 9 animals per group). **p<0.01 vs sham group.
Localization of arginase by immunohistochemistry
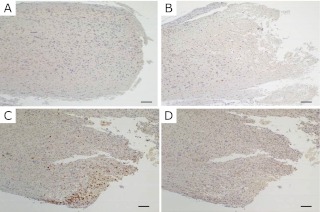
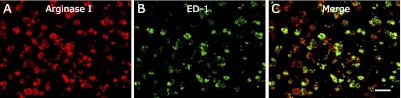
The localization of arginase was investigated by immunohistochemistry. A marked increase in the density of arginase I positive cells was observed in the vicinity of transection site at 48 h (Fig. 6C), while few cells were visible at 24 h (Fig. 6B). No infiltration of arginase-positive cells to the injury site was observed in the sham group (Fig. 6A) or in the negative control group (Fig. 6D). Double immunofluorescence staining for arginase I (Fig. 7A) and ED-1 (Fig. 7B), a marker of macrophages also revealed that most of the ED-1 positive cells were co-localized with cells expressing arginase I (Fig. 7C).
Fig. 6.
Immunohistochemistry for arginase positive cells at the injury site. No arginase positive cells were detected at 48 h in the sham group (A) or in the negative control group (D). The number of arginase positive cells in the region of transection at 48 h was significantly increased in the injury group (C), while arginase positive cells were not detectable at 24 h in the injury group (B). Bar = 100 µm.
Fig. 7.
Double immunostaining for arginase and ED-1, a marker of macrophages. In the injury group, arginase positive cells (A) were co-localized with most of the ED-1 positive macrophages (B) at the edge of transected site at 48 h (C, merge image). Bar = 50 µm.
Discussion
In this study, induction of arginase was demonstrated in transected spinal cord injury. Generally, induction of arginase occurs in two distinct isoforms, arginase I and II, which have different subcellular localization. Arginase I is predominantly expressed in cytosol of hepatic cells as a key enzyme for the urea cycle, while arginase II is expressed in mitochondria of extra-hepatic cells encoded by a different gene.(20) Especially, arginase I is highly induced in many tissues following exposure to many stimulants of Th2 cytokines such as IL-4 and IL-13, growth factors (TGF-β), endotoxin, cAMP-elevating agents, oxygen tension and ROS.(33,34) However, the inducer of arginase I in spinal cord injury has not yet been identified. In this study, based on the results of nitrotyrosine generation, ROS might be one of candidates for the inducer of arginase I. Moreover, in our study, up-regulation of TGF-β mRNA was demonstrated. Therefore, it seems likely that the TGF-β provides some contribution to the induction of arginase I, althought the precis mechanisms need to be further investigated.
In mice spinal cord, expression of arginase I and II were localized in the grey matter.(21) Although induction of arginase I was demonstrated in neurons and macrophages in hemi-transected spinal cord injury,(22) there is little evidence for interactions between arginase I and NO following spinal cord injury. In this study, we detected a remarkable increase in arginase expression in most of the macrophages located at the transection edge at 48 h. In parallel changes in arginase activity, the amount of L-arginine was significantly decreased at 48 h. Moreover, at 72 h, arginase activity had decreased and L-arginine levels had increased. On the other hand, unexpectedly, NO levels were not increased in the injury group compared with the sham group despite up-regulation of NOS2 mRNA. These results indicate that arginase activity and L-arginine levels are linked and that upregulated arginase is associated with low levels of NO for the same substrate, L-arginine. However, it has been reported that the Km for L-arginine is in the 2–20 mM range for arginase,(35) but it is in the 2–20 µM range for the NOS.(36) From this point of view, it is impossible for arginase to deprive of L-arginine from NOS because the affinity of L-arginine is much higher for NOS than for arginase. However the Vmax of arginase is more than 1,000 times that of the NOS enzymes, indicating similar rates of substrate usage for NO synthesis at low L-arginine concentrations. Sufficient quantities of arginase can limit the availability of L-arginine for NO synthesis by intact cells.(20) In fact, previous studies has been indicated that the majority of L-arginine was consumed for the production of urea rather than NO in L-arginine metabolism, and inhibition of arginase or supplementation of culture media with L-arginine promoted NO generation.(37,38) Considering this background, many researchers have focused on the induction of arginase I in experimental asthma, because increased expression of arginase would lower the concentration of L-arginine available as a substrate for NOS activity. As a result, the loss of NO inhibits dilatation of the bronchial smooth muscle.(23,24) In this study, L-arginine was possibly consumed by the up-regulation of arginase. Furthermore, the decrease of L-arginine might be progressed in spinal cord transection injury because vascular supply was significantly restricted in the injury edge. Therefore, it is probable that the elevated expression of arginase reduces L-arginine levels in the injured spinal cord and contributes to the inhibition of NO production through depriving L-arginine.
Nitroxidative stress is involved in secondary damage following spinal cord injury. Generally, there are two experimental models in spinal cord injury. One is a contusion model and the other is a transected model. In contusion spinal cord injury, there are many evidences to show the contribution of oxidative stress.(9–11) Increase in oxidative stress shown by high expression of NADPH oxidase subunits and nitrotyrosine formation is observed at 16 h after spinal cord injury.(39) Superoxide can easily react with NO, which results in the formation of peroxynitrite. In turn, peroxynitrite is readily protonated under physiological conditions, leading to the generation of peroxynitrous acid.(40) Peroxynitrous acid may react with tyrosine or undergo peroxide bond homolysis to generate hydroxyl radical and nitrogen dioxide. The reaction of the hydroxyl radical and nitrogen dioxide with tyrosine can also give nitrated tyrosine.(41) Nitrotyrosine can also be produced through a heme peroxidase-dependent mechanism. It has been shown that heme proteins with potential peroxidase activity can cause tyrosine nitration reactions using nitrite and hydrogen peroxide as a substrate.(42) In this study, augmented nitrotyrosine formation of some proteins was demonstrated in the injury group. Therefore, it is clear that nitroxidative stress was involved in transected spinal cord injury. However, increased NO production was not observed in the injury group.
The formation of peroxynitrite is supported by another theory. Usually, the coupling of NOS monomers is needed to produce NO. However, under specific conditions such as ischemia, loss of cofactor tetrahydrobiopterin (BH4) and low concentrations of L-arginine, uncoupled NOS is formed, and it can generate O2•− via the oxidase domain of NOS.(43,44) In fact, the generation of O2•− by NOS2 has been documented in vitro.(45) Similarly, in vivo, Okazaki et al.(46) reported that NOS2 derived superoxide generation was reduced by treatment with the NOS-cofactor, BH4 and inhibition of NOS2 uncoupling switched superoxide production toward NO generation in the ischemia-reperfusion injured heart isolated from diabetic rats. O2•− generated by the uncoupled oxidase domain of NOS and NO generated by coupled NOS can easily react with each other, possibly resulting in the formation of peroxynitrite.(47) Therefore, in transected spinal cord injury, under ischemia and L-arginine depletion, O2•− generation by uncoupling NOS may lead peroxynitrite formation, although there is no evidence to show that peroxynitrite was formed by uncoupling NOS in this study.
In this study, marked increase in arginase I expression and concomitant decrease in L-arginine were demonstrated at 48 h after spinal cord injury. Moreover, decrease in NOx contents and increased nitrotyrosine formation were observed. It was suggested that induced arginase might down-regulate NO production in spite of up-regulation of NOS2 because of L-arginine consumption. Therefore, regulation of arginase activity or L-arginine level may improve nitroxidative stress and attenuate tissue damage. This may provide a novel clinical approach to the treatment of spinal cord injury. However, it should be investigated more precise mechanisms of nitroxidative stress in spinal cord injury in the future.
Acknowledgment
We thank Dr. Toshikazu Gondo for advice of immunohistochemistry. This work was supported in part by Grant-in-Aid for Science Research No. 23390163 from the Ministry of Education, Culture, Sports, Science and Technology of the Japanese Government.
Abbreviations
- BH4
tetrahydrobiopterin
- cAMP
cyclic adenosine monophosphate
- DNA
deoxyribonucleic acid
- EDTA
ethylenediaminetetraacetic acid
- GAPDH
glyceraldehyde 3-phosphate dehydrogenase
- HPLC
high performance liquid chromatography
- IL
interleukin
- mRNA
messenger ribonucleic acid
- NO
nitric oxide
- NOS
nitric oxide synthase
- PBS
phosphate buffer saline
- PCR
polymerase chain reaction
- ROS
reactive oxygen species
- RNS
reactive nitrogen species
- SDS
sodium dodecyl sulfate
- TGF
transforming growth factor
Declaration of Interest
The authors have no conflicts of interest related to this manuscript.
References
- 1.Janssen L, Hansebout RR. Pathogenesis of spinal cord injury and newer treatments. A review. Spine (Phila Pa 1976) 1989;14:23–32. doi: 10.1097/00007632-198901000-00005. [DOI] [PubMed] [Google Scholar]
- 2.Demediuk P, Daly MP, Faden AI. Effect of impact trauma on neurotransmitter and nonneurotransmitter amino acids in rat spinal cord. J Neurochem. 1989;52:1529–1536. doi: 10.1111/j.1471-4159.1989.tb09204.x. [DOI] [PubMed] [Google Scholar]
- 3.Amar AP, Levy ML. Pathogenesis and pharmacological strategies for mitigating secondary damage in acute spinal cord injury. Neurosurgery. 1999;44:1027–1039. doi: 10.1097/00006123-199905000-00052. [DOI] [PubMed] [Google Scholar]
- 4.Mautes AE, Weinzierl MR, Donovan F, Noble LJ. Vascular events after spinal cord injury: contribution to secondary pathogenesis. Phys Ther. 2000;80:673–687. [PubMed] [Google Scholar]
- 5.Stokes BT, Fox P, Hollinden G. Extracellular calcium activity in the injured spinal cord. Exp Neurol. 1983;80:561–572. doi: 10.1016/0014-4886(83)90307-2. [DOI] [PubMed] [Google Scholar]
- 6.Young W. The role of calcium in spinal cord injury. Cent Nerv Syst Trauma. 1985;2:109–114. doi: 10.1089/cns.1985.2.109. [DOI] [PubMed] [Google Scholar]
- 7.Lewén A, Matz P, Chan PH. Free radical pathways in CNS injury. J Neurotrauma. 2000;17:871–890. doi: 10.1089/neu.2000.17.871. [DOI] [PubMed] [Google Scholar]
- 8.Facchinetti F, Dawson VL, Dawson TM. Free radicals as mediators of neuronal injury. Cell Mol Neurobiol. 1998;18:667–682. doi: 10.1023/A:1020685903186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu D, Liu J, Wen J. Elevation of hydrogen peroxide after spinal cord injury detected by using the Fenton reaction. Free Radic Biol Med. 1999;27:478–482. doi: 10.1016/s0891-5849(99)00073-8. [DOI] [PubMed] [Google Scholar]
- 10.Aksenova M, Butterfield DA, Zhang SX, Underwood M, Geddes JW. Increased protein oxidation and decreased creatine kinase BB expression and activity after spinal cord contusion injury. J Neurotrauma. 2002;19:491–502. doi: 10.1089/08977150252932433. [DOI] [PubMed] [Google Scholar]
- 11.Juurlink BH, Paterson PG. Review of oxidative stress in brain and spinal cord injury: suggestions for pharmacological and nutritional management strategies. J Spinal Cord Med. 1998;21:309–334. doi: 10.1080/10790268.1998.11719540. [DOI] [PubMed] [Google Scholar]
- 12.Hall ED, Braughler JM. Central nervous system trauma and stroke. II. Physiological and pharmacological evidence for involvement of oxygen radicals and lipid peroxidation. Free Radic Biol Med. 1989;6:303–313. doi: 10.1016/0891-5849(89)90057-9. [DOI] [PubMed] [Google Scholar]
- 13.Palmer RM, Ferrige AG, Moncada S. Nitric oxide release accounts for the biological activity of endothelium-derived relaxing factor. Nature. 1987;327:524–526. doi: 10.1038/327524a0. [DOI] [PubMed] [Google Scholar]
- 14.Brown GC, Bal-Price A. Inflammatory neurodegeneration mediated by nitric oxide, glutamate, and mitochondria. Mol Neurobiol. 2003;27:325–355. doi: 10.1385/MN:27:3:325. [DOI] [PubMed] [Google Scholar]
- 15.Conti A, Miscusi M, Cardali S, et al. Nitric oxide in the injured spinal cord: synthases cross-talk, oxidative stress and inflammation. Brain Res Rev. 2007;54:205–218. doi: 10.1016/j.brainresrev.2007.01.013. [DOI] [PubMed] [Google Scholar]
- 16.Beckman JS, Beckman TW, Chen J, Marshall PA, Freeman BA. Apparent hydroxyl radical production by peroxynitrite: implications for endothelial injury from nitric oxide and superoxide. Proc Natl Acad Sci USA. 1990;87:1620–1624. doi: 10.1073/pnas.87.4.1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xu J, Kim GM, Chen S, et al. iNOS and nitrotyrosine expression after spinal cord injury. J Neurotrauma. 2001;18:523–532. doi: 10.1089/089771501300227323. [DOI] [PubMed] [Google Scholar]
- 18.Radi R, Beckman JS, Bush KM, Freeman BA. Peroxynitrite oxidation of sulfhydryls. The cytotoxic potential of superoxide and nitric oxide. J Biol Chem. 1991;266:4244–4250. [PubMed] [Google Scholar]
- 19.Gatti RM, Radi R, Augusto O. Peroxynitrite-mediated oxidation of albumin to the protein-thiyl free radical. FEBS Lett. 1994;348:287–290. doi: 10.1016/0014-5793(94)00625-3. [DOI] [PubMed] [Google Scholar]
- 20.Wu G, Morris SM., Jr Arginine metabolism: nitric oxide and beyond. Biochem J. 1998;336:1–17. doi: 10.1042/bj3360001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yu H, Iyer RK, Kern RM, Rodriguez WI, Grody WW, Cederbaum SD. Expression of arginase isozymes in mouse brain. J Neurosci Res. 2001;66:406–422. doi: 10.1002/jnr.1233. [DOI] [PubMed] [Google Scholar]
- 22.Kuo HS, Tsai MJ, Huang MC, et al. The combination of peripheral nerve grafts and acidic fibroblast growth factor enhances arginase I and polyamine spermine expression in transected rat spinal cords. Biochem Biophys Res Commun. 2007;357:1–7. doi: 10.1016/j.bbrc.2007.02.167. [DOI] [PubMed] [Google Scholar]
- 23.Maarsingh H, Zuidhof AB, Bos IS, et al. Arginase inhibition protects against allergen-induced airway obstruction, hyperresponsiveness, and inflammation. Am J Respir Crit Care Med. 2008;178:565–573. doi: 10.1164/rccm.200710-1588OC. [DOI] [PubMed] [Google Scholar]
- 24.Maarsingh H, Bossenga BE, Bos IS, Volders HH, Zaagsma J, Meurs H. L-arginine deficiency causes airway hyperresponsiveness after the late asthmatic reaction. Eur Respir J. 2009;34:191–199. doi: 10.1183/09031936.00105408. [DOI] [PubMed] [Google Scholar]
- 25.Takemoto K, Ogino K, Shibamori M, et al. Transiently, paralleled upregulation of arginase and nitric oxide synthase and the effect of both enzymes on the pathology of asthma. Am J Physiol Lung Cell Mol Physiol. 2007;293:L1419–L1426. doi: 10.1152/ajplung.00418.2006. [DOI] [PubMed] [Google Scholar]
- 26.North ML, Khanna N, Marsden PA, Grasemann H, Scott JA. Functionally important role for arginase I in the airway hyperresponsiveness of asthma. Am J Physiol Lung Cell Mol Physiol. 2009;296:L911–L920. doi: 10.1152/ajplung.00025.2009. [DOI] [PubMed] [Google Scholar]
- 27.Takahashi N, Ogino K, Takemoto K, et al. Direct inhibition of arginase attenuated airway allergic reactions and inflammation in a Dermatophagoides farinae-induced NC/Nga mouse model. Am J Physiol Lung Cell Mol Physiol. 2010;299:L17–L24. doi: 10.1152/ajplung.00216.2009. [DOI] [PubMed] [Google Scholar]
- 28.Yara T, Kato Y, Kataoka H, et al. Environmental factors involved in axonal regeneration following spinal cord transection in rats. Med Mol Morphol. 2009;42:150–154. doi: 10.1007/s00795-009-0454-y. [DOI] [PubMed] [Google Scholar]
- 29.Yang BK, Vivas EX, Reiter CD, Gladwin MT. Methodologies for the sensitive and specific measurement of S-nitrosothiols, iron-nitrosyls, and nitrite in biological samples. Free Radic Res. 2003;37:1–10. doi: 10.1080/1071576021000033112. [DOI] [PubMed] [Google Scholar]
- 30.Kubo M, Kambayashi Y, Takemoto K, Okuda J, Muto M, Ogino K. Reactive nitrogen species formation in eosinophils and imbalance in nitric oxide metabolism are involved in atopic dermatitis-like skin lesions in NC/Nga mice. Free Radic Res. 2005;39:719–727. doi: 10.1080/10715760500139260. [DOI] [PubMed] [Google Scholar]
- 31.Corraliza IM, Campo ML, Soler G, Modolell M. Determination of arginase activity in macrophages: a micromethod. J Immunol Methods. 1994;174:231–235. doi: 10.1016/0022-1759(94)90027-2. [DOI] [PubMed] [Google Scholar]
- 32.Blackwell S, O’Reilly D, Talwar D. HPLC analysis of asymmetric dimethylarginine (ADMA) and related arginine metabolites in human plasma using a novel non-endogenous internal standard. Clin Chim Acta. 2009;401:14–19. doi: 10.1016/j.cca.2008.10.032. [DOI] [PubMed] [Google Scholar]
- 33.Munder M. Arginase: an emerging key player in the mammalian immune system. Br J Pharmacol. 2009;158:638–651. doi: 10.1111/j.1476-5381.2009.00291.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Maarsingh H, Zaagsma J, Meurs H. Arginine homeostasis in allergic asthma. Eur J Pahrmacol. 2008;585:375–384. doi: 10.1016/j.ejphar.2008.02.096. [DOI] [PubMed] [Google Scholar]
- 35.Grody WW, Dizikes GJ, Cederbaum SD. Human arginase isozymes. Isozymes Curr Top Biol Med Res. 1987;13:181–214. [PubMed] [Google Scholar]
- 36.Griffith OW, Stuehr DJ. Nitric oxide synthases: properties and catalytic mechanism. Annu Rev Physiol. 1995;57:707–736. doi: 10.1146/annurev.ph.57.030195.003423. [DOI] [PubMed] [Google Scholar]
- 37.Chang CI, Liao JC, Kuo L. Arginase modulates nitric oxide production in activated macrophages. Am J Physiol. 1998;274:H342–H348. doi: 10.1152/ajpheart.1998.274.1.H342. [DOI] [PubMed] [Google Scholar]
- 38.Hey C, Boucher JL, Vadon-Le Goff S, Ketterer G, Wessler I, Racké K. Inhibition of arginase in rat and rabbit alveolar macrophages by N omega-hydroxy-D,L-indospicine, effects on L-arginine utilization by nitric oxide synthase. Br J Pharmacol. 1997;121:395–400. doi: 10.1038/sj.bjp.0701143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vaziri ND, Lee YS, Lin CY, Lin VW, Sindhu RK. NAD(P)H oxidase, superoxide dismutase, catalase, glutathione peroxidase and nitric oxide synthase expression in subacute spinal cord injury. Brain Res. 2004;995:76–83. doi: 10.1016/j.brainres.2003.09.056. [DOI] [PubMed] [Google Scholar]
- 40.Kissner R, Nauser T, Bugnon P, Lye PG, Koppenol WH. Formation and properties of peroxynitrite as studied by laser flash photolysis, high-pressure stopped-flow technique, and pulse radiolysis. Chem Res Toxicol. 1997;10:1285–1292. doi: 10.1021/tx970160x. [DOI] [PubMed] [Google Scholar]
- 41.Gunaydin H, Houk KN. Mechanisms of peroxynitrite-mediated nitration of tyrosine. Chem Res Toxicol. 2009;22:894–898. doi: 10.1021/tx800463y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Eiserich JP, Hristova M, Cross CE, et al. Formation of nitric oxide-derived inflammatory oxidants by myeloperoxidase in neutrophils. Nature. 1998;391:393–397. doi: 10.1038/34923. [DOI] [PubMed] [Google Scholar]
- 43.Pou S, Pou WS, Bredt DS, Snyder SH, Rosen GM. Generation of superoxide by purified brain nitric oxide synthase. J Biol Chem. 1992;267:24173–24176. [PubMed] [Google Scholar]
- 44.Xia Y, Tsai AL, Berka V, Zweier JL. Superoxide generation from endothelial nitric-oxide synthase. A Ca2+/calmodulin-dependent and tetrahydrobiopterin regulatory process. J Biol Chem. 1998;273:25804–25808. doi: 10.1074/jbc.273.40.25804. [DOI] [PubMed] [Google Scholar]
- 45.Xia Y, Roman LJ, Masters BS, Zweier JL. Inducible nitric-oxide synthase generates superoxide from the reductase domain. J Biol Chem. 1998;273:22635–22639. doi: 10.1074/jbc.273.35.22635. [DOI] [PubMed] [Google Scholar]
- 46.Okazaki T, Otani H, Shimazu T, et al. Reversal of inducible nitric oxide synthase uncoupling unmasks tolerance to ischemia/reperfusion injury in the diabetic rat heart. J Mol Cell Cardiol. 2011;50:534–544. doi: 10.1016/j.yjmcc.2010.12.010. [DOI] [PubMed] [Google Scholar]
- 47.Xia Y, Dawson VL, Dawson TM, Snyder SH, Zweier JL. Nitric oxide synthase generates superoxide and nitric oxide in arginine-depleted cells leading to peroxynitrite-mediated cellular injury. Proc Natl Acad Sci USA. 1996;93:6770–6774. doi: 10.1073/pnas.93.13.6770. [DOI] [PMC free article] [PubMed] [Google Scholar]