Abstract
Blood-brain barrier (BBB) formed by brain microvascular endothelial cells (BMVEC) regulates the passage of molecules and leukocytes in and out of the brain. Activation of matrix metalloproteinases (MMPs) and alteration of basement membrane (BM) associated with BBB injury were documented in stroke patients. While chronic alcoholism is a risk factor for developing stroke, underlying mechanisms are not well understood. We hypothesized that ethanol (EtOH)-induced protein tyrosine kinase (PTK) signaling resulted a loss of BBB integrity via MMPs activation and degradation of BM component, collagen IV. Treatment of BMVEC with EtOH or acetaldehyde (AA) for 2–48 hr increased MMP-1, -2 and -9 activities or decreased the levels of tissue inhibitors of MMPs (TIMP-1, -2) in a PTK-dependent manner without affecting protein tyrosine phosphatase activity. Enhanced PTK activity after EtOH exposure correlated with increased phosphorylated proteins of selective receptor and non-receptor PTKs. Up-regulation of MMPs activities and protein contents paralleled a decrease in collagen IV content, and inhibitors of EtOH metabolism, MMP-2 and 9, or PTK reversed all these effects. Using human BMVEC assembled into BBB models, we found that EtOH/AA diminished barrier tightness, augmented permeability, and monocyte migration across the BBB via activation of PTKs and MMPs. These findings suggest that alcohol associated BBB injury could be mediated by MMPs via BM protein degradation and could serve as a co-morbidity factor for neurological disorders like stroke or neuroinflammation. Furthermore, our preliminary experiments indicated that human astrocytes secreted high levels of MMP-1 and 9 following exposure to EtOH, suggesting the role of BM protein degradation and BBB compromise as a result of glial activation by ethanol. These results provide better understanding of multifaceted effects of alcohol on the brain and could help develop new therapeutic interventions.
Keywords: Blood-brain barrier, brain endothelial cell, ethanol metabolism, matrix metalloproteinases, protein tyrosine kinase, protein tyrosine phosphatase
Introduction
Alcohol is the most commonly used and abused drug in the United States. Deleterious alcohol-related health effects attributed to the internal organ toxicity include irreversible brain tissue injury (Harper and Matsumoto 2005). Brain tissue of chronic alcoholics features neurodegeneration (Harper 1998) paralleling neuro-cognitive deficits in these patients (Parsons 1998; Zeigler et al. 2005). White matter abnormalities seen in alcoholics (Mann et al. 2001) could be associated with blood-brain barrier (BBB) dysfunction detected in alcohol abusers (Pratt et al. 1990; Thomsen et al. 1994) and seen in animal models of chronic alcohol administration (Phillips and Cragg 1982; Rosengren and Persson 1979). The underlying mechanism of BBB dysfunction caused by alcohol abuse remains elusive. BBB formed by brain microvascular endothelial cells (BMVEC), pericytes, and astrocytes (Rubin and Staddon 1999), and tight junctions (TJ) connecting BMVEC ensure the structural tightness of the BBB (Pardridge 1983). Intracellular signaling process regulating phosphorylation of TJ proteins controls the TJ assembly and BBB integrity (Rubin and Staddon 1999). Using primary human BMVEC, we previously demonstrated that ethanol (EtOH) increases activity and content of EtOH-metabolizing enzymes leading to the production of acetaldehyde (AA) and reactive oxygen species (ROS) in brain endothelium (Haorah et al. 2005b). AA and ROS then activate myosin light chain kinase (Haorah et al. 2005a) via stimulation of inositol 1,4,5-triphosphate (IP3R)-gated intracellular Ca2+ release signaling pathway (Haorah J 2007a) resulting in phosphorylation of cytoskeletal/TJ proteins and BBB dysfunction.
Our recent findings suggested that oxidative stress activates matrix metalloproteinases (MMPs) via protein tyrosine kinase (PTK) signaling and resulted in degradation of basement membrane (BM) protein and BBB disruption (Haorah J 2007b). Similar findings have been reported in mice and rats after ischemic stroke (Kim et al. 2003; Machado et al. 2006). Chronic alcohol administration has been shown to activate MMP-2 and -9 in in vitro and in vivo models (Aye et al. 2004; Lois et al. 1999); however, mechanisms leading to such effects remain undefined. We hypothesize that alcohol abuse disrupts BBB due to activation of MMPs via PTK signaling pathway leading to degradation of BM protein and tyrosine phosphorylation TJ proteins. To test this idea, we treated human BMVEC with EtOH and studied the activity of MMP-1, -2, -9, expression of tissue inhibitors of MMPs (TIMP-1, -2), BM degradation, activity of PTK and protein tyrosine phosphatase (PTP), expression of receptor and non-receptor PTKs, phosphorylation of TJ proteins, and alterations of BBB function. Our results indicated that EtOH metabolism activated MMP-1, -2, -9 in a PTK dependent manner, and PTK/MMPs inhibitors prevented functional changes of BBB (decreased structural integrity, enhanced permeability, and leukocyte migration across the BBB).
Materials and Methods
Cell isolation and culture
Primary human BMVEC were isolated from the temporal cortex of brain tissue obtained during surgical removal of epileptogenic foci in adult patients and were supplied by Dr. M. Witte (University of Arizona). BMVEC purity evaluation and culture condition were performed as described previously (Haorah J 2007a). Optimal concentrations determined by dose-dependent response were 50 mM EtOH (concentrations tested were 10–200 mM), 100 μM AA (10–200 μM), 1 mM 4-methylpyrole (4-MP, inhibitor of EtOH metabolizing enzymes; 0.25 – 4 mM), 50 μM uric acid (UA, anti-oxidant; 10–200 μM), 100 μM genistein (GS, PTK inhibitor; 10–200 μM), 20μg/ml endostatin (ES, MMP-2 and -9 inhibitor; 1–40 μg/ml), and 100 μM phenylarsine oxide (PAO, PTP inhibitor; 10–200 μM).
MMPs activity and TIMPs expression
Following the manufacturer’s instructions (Amersham, Piscataway, NJ), MMP-1, -2 or -9 activity was detected by biotrak activity assay system; whereas, the level of total protein contents of MMP-1, -2, -9 or TIMP-1, -2 was assayed by biotrak (human) ELISA system using different concentrations of respective MMP or TIMP as standard curves. MMP-1, -2, -9 or TIMP-1, -2 protein contents determined by this system is much more sensitive and faster than Zymography or Western blot analyses. Briefly, secreted proteins were collected from cell culture conditioned media at 2 – 96 hr after treatment with EtOH or AA. Secreted proteins were immuno-conjugated with pre-coated anti-MMP-1, -2, -9, or anti-TIMP-1, -2 antibody in microplate wells. MMPs activities and level of MMPs or TIMPs protein contents were calculated from standard curve run in parallel with samples detected at 450 nm in a SpectraMax M5 microplate reader (Molecular Devices, Sunnyvale, CA). The results were expressed as nmoles or μmoles/mg protein.
Extracellular matrix/BM degradation assay
We utilized the R&D (Minneapolis, MN) thin layer BM (4–6 μm) coating system. Briefly, glass cover slips pre-coated with type I collagen (0.1 mg/ml) were layered with a mixture (1:1) of fluorescein-conjugated and unconjugated type IV collagen (0.2 mg/ml). BMVEC (10,000 cells/well) cultured on top of fluorescein-conjugated collagen IV were treated with test compounds for 72 hrs, followed by washes with phosphate buffered saline (PBS), fixation, and confocal microscopy analyses. In parallel, quantitative analysis of collagen IV degradation was performed with BMVEC cultured on the top of collagen layers in 96-well black wall clear bottom plates after activation of MMPs by EtOH or AA. Cells were washed after 72 hr treatment with EtOH or AA, and fluorescence was detected at 488 nm excitation and 515 nm emission using a microplate reader spectrophotometer (Molecular Devices). Non-degraded collagen IV from each condition was calculated using the standard curve of fluorescein-conjugated collagen IV run in parallel with samples.
PTK/PTP activity assay and PTK Array
PTK and PTP activities were assessed using the ELISA-based kits (Calbiochem, San Diego, CA). Briefly, cellular lysates protein (from 48 hr treatment) was immuno-conjugated with pre-coated anti-PTK/anti-PTP antibody in microplate wells. PTK/PTP activity was calculated from standard curve run in parallel with respective samples, detected at 450 nm in a microplate reader-spectrophotometer. PTK/PTP activity results were expressed as pmoles or mmoles/mg protein. Activation of specific PTK after EtOH treatment was assessed by human phospho-receptor tyrosine kinase (RTKs) array (R&D Systems, Inc.). This proteome profiler array kit identifies 42 different activated RTKs at a time. Briefly, cell lysates protein (100 μg) derived from control or EtOH treatment was conjugated to 42 different anti-RTKs antibodies that were spotted in duplicate on nitrocellulose membranes. The conjugates were captured by horseradish peroxidase and were detected by chemiluminescence.
TEER measurement
Transendothelial electrical resistance (TEER) measurement by sensitive electric cell-substrate impedance-sensing (ECIS) model 1600R (Applied BioPhysics, Troy, NY) assessed the tightness of BMVEC monolayers. The ECIS model 1600R system monitors the dynamic behavior of cell monolayers in culture with a live recording of TEER, mimicking closely physiological conditions. In the ECIS system, BMVEC were grown directly on gold film electrode surface (0.25 cm2) in culture wells (0.8 cm2) with cell culture medium serving as the electrolyte. A constant current source of 4 kHz was maintained between the TEER measuring electrode (0.25 cm2) and the counter electrode (0.8 cm2). TEER readings of fully confluent BMVEC monolayers were recorded after application of each test compound for 2 hr or 48 hr. TEER values were calculated as: Resistance of a unit area = Resistance (Ohms) × effective membrane area (cm2), where, effective membrane area is 0.25 cm2 (for ECIS system). Normalizing the resistance for 1.0 cm2 defines the “Resistance” as: “Ohms × 1.0 cm2 divided by 0.25 cm2 (i.e. Ohms.cm2). Thus, resistance is inversely proportional to the effective membrane surface area. Results were expressed as percent of controls.
Dextran permeability assay
We determined the rate of permeability of different molecular weights of dextrans (low to high molecular weights, 4, 10 and 40 kDa) prior to studying the effects of test compounds in permeability assay. Here, we used the low molecular weight (4 kDa) FITC-labeled dextran (Molecular Probes), because our control optimization data showed that only 0.05 – 0.22% of the initial dextran concentration was permeated after 1 – 4 hrs permeability assay. After treatment for 48 hrs with test compounds, we removed 100 μL (out of 200 μL) of media from all upper chambers of 24-transwell tissue culture inserts (pore diameter 0.4 μm) and replaced with 100 μL of media containing 20 μM FITC-labeled dextran (10 μM final concentration) with or without test compounds. Samples were collected from lower chambers at 2 hrs after addition of dextran with or without test compounds for analysis of permeability rate across the BBB. Together, with various concentrations of FITC-labeled dextran standard, fluorescence intensity of each sample was read at 488 nm excitation and 525 nm emission. Permeability flux rate in nmoles/hr was calculated from the standard curve. Results were expressed as apparent permeability coefficient (P), defined as cm/hr permeability. “P” is derived from the ratio of flux rate (nmoles/hr) to that of initial concentration (in nmoles) and surface area of permeant constant at 0.32 cm2.
Monocyte migration and Western blot
Migration of monocytes across the BBB was performed as previously described (Haorah J 2007b), and our methods for immunoconjugation, immunoprecipitation, and Western blot were also described (Haorah et al. 2005b). Antibodies to occludin, claudin-5, ZO-1, and phosphotyrosine were purchased from Zymed (Zymed, San Francisco, CA), and CD68 antibody was purchased from Dako (macrophage marker, at 1:100 dilution, Dako, Carpenteria, CA).
Statistical analysis
Results were expressed as mean values (± SEM), and a value of p < 0.05 was considered significant. Statistical significance was assessed by two-way ANOVA analyses with Newman-Keuls post-test for multiple comparisons. The specific or the non-selective inhibitors that we used did not significantly affect the activity, expression or the BBB functional integrity of the respective basal controls (data not shown).
Results
Activity of MMP-1, -2 or -9
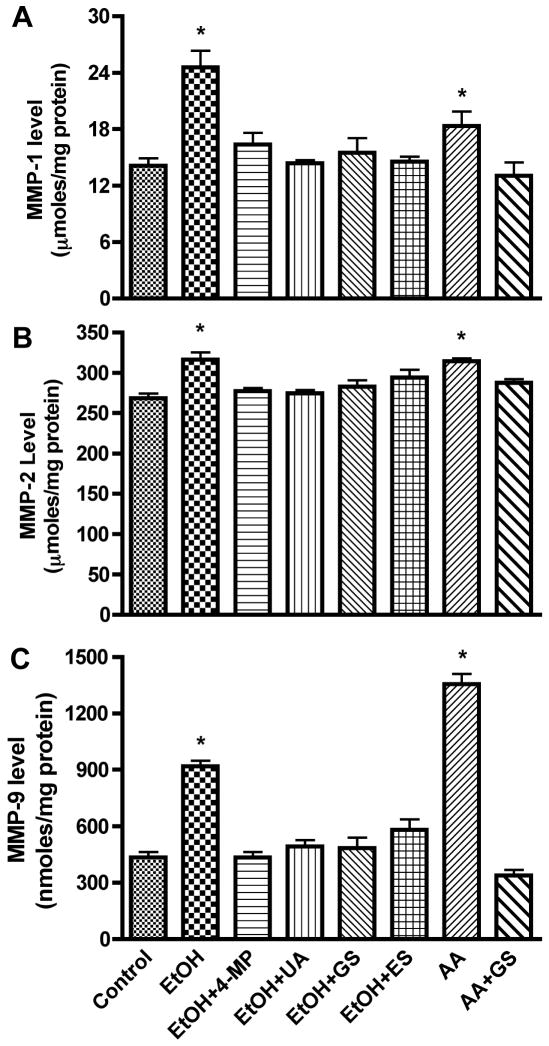
In the present study, we explored the idea that in alcohol abuse the BBB is disrupted via activation of MMPs due to alcohol metabolism in human brain endothelial cells. We analyzed the activity of endogenous free MMPs and total MMPs (free active and bound MMPs in its pro-form) in human BMVEC culture media after treatment with EtOH/AA for 2 – 96 hrs. Total MMPs were activated by p-aminophenylmercuric acetate (APMA) following the manufacturer’s instructions (Amersham). We found that MMP-1 or -9 was mainly represented by free active form while MMP-2 existed mostly in the pro-form and very little in inducible form. BMVEC treatment with EtOH/AA respectively enhanced the activity of MMP-1 by 119/141%, MMP-2 by 132/124%, and MMP-9 by 215/1380% (p<0.001) compared with controls (Figure 1 A–C). Treatment of BMVEC with EtOH (for 2, 4, 8, 24, 48, 72, and 96 hr) resulted in maximum MMP-1, -2, or -9 activity at 24 to 48 hr, while AA showed highest MMPs activity at 2 hr (tested at 2, 4, 8, 24, and 48 hr). A potent stimulator of MMPs, IL-1β (used as a positive control) augmented the MMPs activities (MMP-1 by 725%, MMP-2 by 65%, and MMP-9 by 315%, p<0.001). Increase in MMPs activities after EtOH or AA treatment were inhibited by 4-MP (inhibitor of EtOH metabolizing enzymes), ES (inhibitor of MMP-2 or -9), GS (PTK inhibitor), or UA (antioxidant), suggesting that MMPs activation was mediated by EtOH metabolism and ROS production via PTK stimulation.
Figure 1.
Secreted proteins from BMVEC cultured media after treatment with EtOH 48 hr or with AA for 2 hr were assayed for activity of (A) MMP-1, (B) MMP-2, and (C) MMP-9. Results presented are inducible endogenous active form for MMP-1 and -9, total activity for MMP-2. MMPs activities were calculated from standard curve, and results were expressed as nmoles or μmoles/mg protein (± SEM; n = 4). * indicates p-values <0.01 compared with control. 4-MP (inhibitor of ADH/CYP2E1), UA (antioxidant), GS (PTK inhibitor), ES (inhibitor of MMP-2/-9).
Expression of MMPs/TIMPs protein
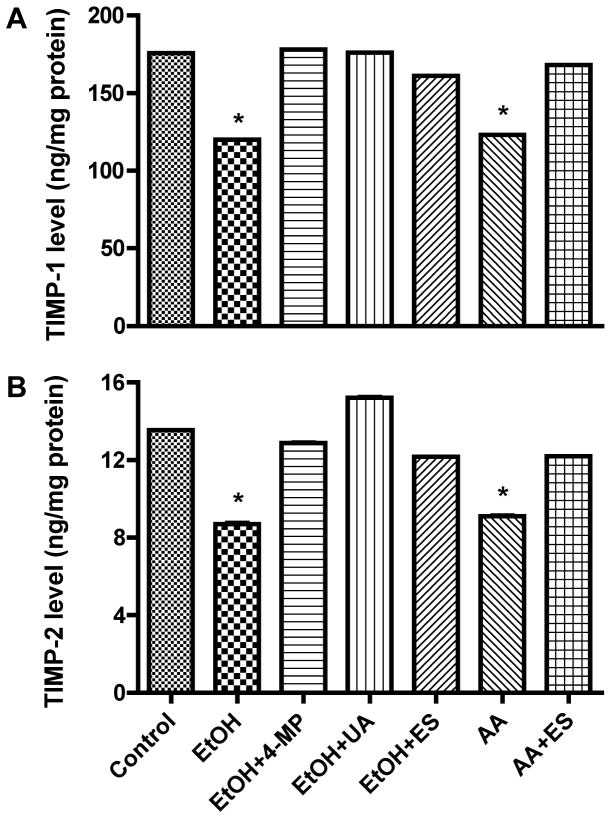
EtOH/AA-mediated increase in MMPs activity correlated with the significant up-regulation of protein content for MMP-1 (by 30–47%), MMP-2 (by 17–18%) and MMP-9 (by 111–211%, p<0.001) compared with controls (Figure 2 A–C). Treatment of BMVEC with 4-MP, UA, ES, or GS prevented the EtOH/AA-induced increase expression of MMPs protein, suggesting that EtOH metabolism and ROS production initiated the activation of MMPs via PTK signaling pathway. We expected that increased MMPs activity and expression after BMVEC treatment with EtOH/AA would diminish the levels of TIMP-1 and -2 proteins, natural inhibitors of MMPs. Indeed, EtOH significantly decreased TIMP-1 and -2 protein contents by 22% and 36% (p<0.01) respectively, and AA also lowered TIMP-1 and -2 levels by 21% and 36% (p<0.01) respectively, compared with controls (Figure 3 A and B). Application of 4-MP, UA, ES, or GS prevented decrease in TIMPs levels, indicating the role of EtOH metabolism, ROS production, and activation of MMPs via PTK signaling pathway.
Figure 2.
Secreted proteins from BMVEC cultured media after treatment with EtOH 48 hr or with AA for 2 hr were assayed for the content of (A) MMP-1, (B) MMP-2, and (C) MMP-9. Total MMP-1, -2 and -9 protein contents were calculated from standard curve, and results were expressed as ng/μg/ml condition media (± SEM; n = 4). * indicates p-values <0.01 compared with control. Inhibitors did not significantly affect MMPs activity or expression of respective basal controls.
Figure 3.
Secreted proteins from BMVEC cultured media after a 48 hr treatment with EtOH or 2 hr with AA were analyzed for levels of (A) TIMP-1 or (B) TIMP-2 expression using the ELISA kits. TIMPs levels were calculated from standard curve and results were expressed as ng/mg protein (± SEM; n = 4). Respective inhibitor did not change the TIMPs expression of basal controls.
BM protein degradation
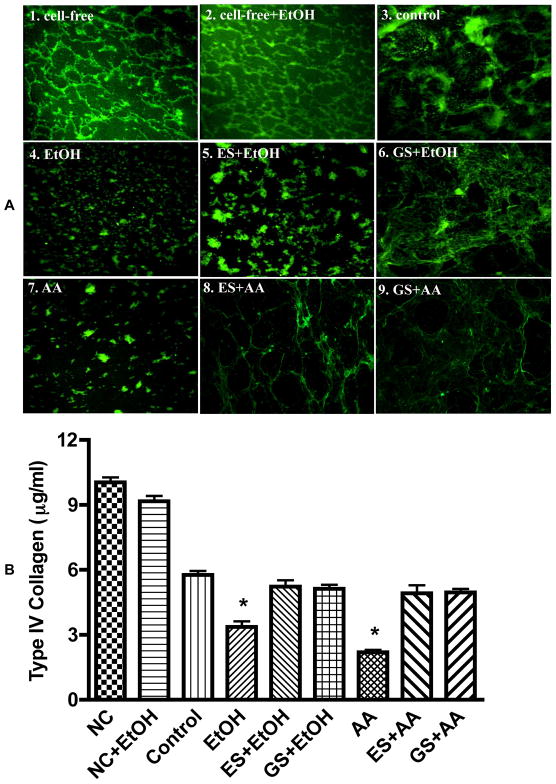
To determine whether MMPs activation could result in degradation of BM proteins, we assessed the changes in fluorescein-conjugated collagen IV after EtOH/AA exposure. EtOH/AA-induced degradation of collagen IV was prevented by GS or ES, suggesting that MMPs activation was regulated by PTK signaling in BMVEC (Figure 4 A1–9). In the absence of BMVEC monolayers (without CYP2E1/ADH), EtOH had no effect on collagen IV degradation (cell-free +EtOH), supporting the idea that EtOH metabolism was essential for MMPs activation and BM protein degradation (Figure 4 A4 and A7 compared with control, A3). These qualitative results were further confirmed by quantitative analysis. Exposure of BMVEC with EtOH or AA showed 42% or 62% reduction in collagen IV levels compared with control (Figure 4 B). GS or ES prevented the effects of EtOH or AA on type IV collagen degradation, indicating that PTK-mediated activation of MMPs led to BM protein degradation. We observed 14% of fluorescein-conjugated collagen IV initially bound to collagen I prior to BMVEC culture (14 μg/ml bound out of 100 μg/ml applied). After 72 hr of BMVEC (control) culture at 37°C, there was 42% drop in amount of collagen IV compared with cell-free, suggesting that the cells digested the bound IV collagen. Wells without BMVEC featured only 4% loss of collagen IV after 72 hr in culture media at 37°C compared with initial 14% bound collagen IV.
Figure 4.
(A) Confocal microscopy analysis of (1) fluorescein-conjugated collagen IV bound to collagen 1 (cell-free), (2) cell-free+EtOH incubated for 72 hr. BMVEC monolayers cultured on top of collagen I and IV layers after 72 hr exposure were (3) control, (4) 50 mM EtOH, (5) 20 μg/ml ES + EtOH, (6) 100 μM GS + EtOH, (7) 100 μM AA, (8) 20 μg/ml ES + AA, (9) 100 μM GS + AA. Original magnification × 600. (B) Degradation of collagen IV by MMPs activation after EtOH/AA stimulation. BMVEC cultured on top of collagen I and IV layers were treated with EtOH/AA for 72 hr followed by quantitative analysis of non-degraded collagen IV amount. Results were expressed as mean μg/ml of undigested collagen IV (± SEM; n = 4).
PTK/PTP activity and RTK array
We proposed that PTK signaling could mediate MMPs activation after BMVEC exposure to EtOH. Therefore, we next assayed the PTK activity in protein extracts from EtOH/AA treated BMVEC. We also examined PTP activity, because PTK activation would result in diminished PTP activity as PTK and PTP function in reciprocal fashion. EtOH and AA treatment increased PTK activity by 949% and 555% respectively, compared with controls without affecting PTP activity (Figure 5 A and B). The increase in PTK activity was inhibited by 4-MP or GS, suggesting that EtOH metabolism mediated the PTK activation. As expected, PAO (inhibitor of PTP) completely inhibited PTP activity (Figure 5 B). Using a proteome profiler array, we observed that EtOH activated four receptor PTKs (insulin receptor/insulin-like growth factor-1 receptor, IR/IGF1-R; ephrinB2 receptor, EphB2-R; vascular endothelial growth factor receptor, VEGFR; epidermal growth factor receptor, ErbB2-R) and two non-receptor PTKs (cellular Sarcoma, c-Src kinase; focal adhesion kinase, FAK) in BMVEC (Figure 5 C). Results of EtOH effects on PTKs are shown in descending order of magnitude, compared with controls. The specific role of the individual PTK in EtOH mediated BMVEC alterations will be studied in our future experiments.
Figure 5.
Lysate proteins (20 μg/well) derived from human BMVEC treated with EtOH for 48 hr or with AA for 2 hr were assayed for PTK/PTP activity. Activity of (A) PTK or (B) PTP was calculated from respective standard curve. Results were expressed as mmoles or pmoles/mg protein (± SEM; n = 4). *designates significant decrease (p<0.01) compared to control. PAO is an inhibitor of PTP. (C) Using 100 μg of lysate protein, EtOH-activated receptor and non-receptor PTKs detected by human proteome profiler array kit compared with untreated controls (± SEM; n = 4).
TJ protein phosphorylation
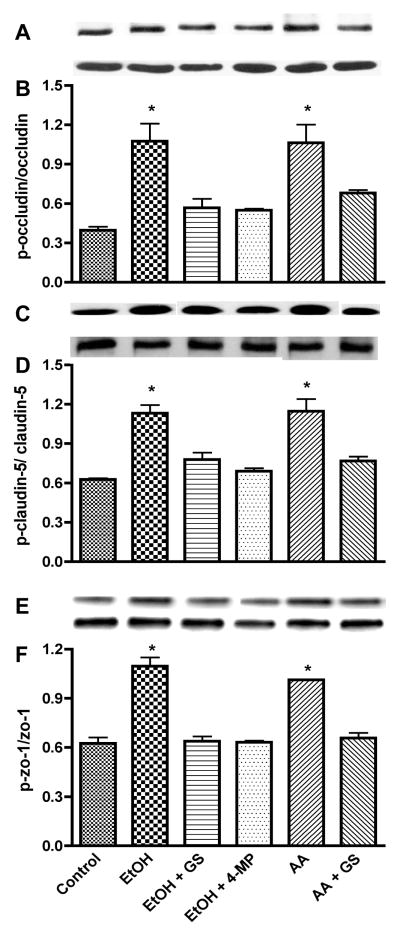
Primary function of PTK activation is the phosphorylation of cellular proteins at tyrosine residues. Therefore, we explored the idea that PTK activation would lead to an increase of phosphotyrosine levels of TJ proteins. Indeed, 48 hr EtOH treatment of BMVEC augmented the phosphotyrosine levels of occludin by 171%, claudin-5 by 80%, and ZO-1 by 75% (p<0.01) compared with respective controls (Figure 6 A–I). Similarly, AA up-regulated the tyrosine phosphorylation of TJ proteins (occludin by 168%, claudin-5 by 82%, and ZO-1 by 62%, respectively, p<0.01, Figure 6 A–I) supporting the idea that EtOH metabolites initiated the PTK activation and TJ protein phosphorylation. EtOH/AA-mediated increase in TJ protein phosphorylation was accompanied by diminution of total occludin and claudin-5 contents (18% and 28%, respectively, p<0.01) without affecting total ZO-1 level. All these changes were prevented by alcohol metabolizing enzyme inhibitor (4-MP) or by PTK inhibitor (GS), suggesting that PTK activation is due to EtOH metabolism phosphorylated TJ proteins.
Figure 6.
Lysate proteins derived from human BMVEC treated with EtOH for 48 hr or with AA for 2 hr in the presence or absence of 4-MP or GS were subjected to Western blot analysis after immunoprecipitation. Representative immunoreactive bands of (A) occludin-phosphotyrosine, (B) total occludin, (C) ratio of occludin-phosphotyrosine/total occludin, (D) claudin-5-phosphotyrosine, (E) total claudin-5, (F) ratio of claudin-5-phosphotyrosine/total claudin-5, (G) ZO-1-phosphotyrosine, (H) total ZO-1, (I) ratio of ZO-1-phosphotyrosine/total ZO-1. * indicates statistical differences (p < 0.01) compared with control. Respective inhibitor did not change the expression of phosphorylated TJ protein of the basal controls.
EtOH diminished BBB integrity
The functional significance of MMPs activation via PTK signaling resulting in BM degradation and TJ phosphorylation were further investigated by assessing TEER, permeability, and monocyte migration across the BMVEC monolayers. BMVEC monolayers treated with EtOH/AA showed a 30% decrease in TEER compared with control (Figure 7 A). Inhibitors of EtOH-metabolizing enzymes (4-MP), PTK (GS), or MMP-2 and -9 (ES) partially restored the EtOH-induced decrease in TEER, indicating that EtOH metabolism and MMPs activation caused the BBB injury via PTK signaling (Figure 7 A). Treatment of BMVEC with EtOH for 48 hr or with AA for 2 hr augmented FITC-labeled dextran permeability (by 110% or 112%, respectively, Figure 7 B) and enhanced monocyte migration across the BMVEC monolayers (by 178% or 136%, respectively, Figure 7 C), compared with respective controls. Inhibition of alcohol metabolism, PTK or MMP-2/-9 prevented increased in permeability and monocyte migration indicating that BBB injury was mediated by MMPs activation via PTK signaling. Taken together, these data suggested that EtOH associated BBB dysfunction was caused by PTK activation leading to MMPs stimulation, BM degradation, and TJ protein modifications. Figure 8 summarized these findings.
Figure 7.
Effects of 50 mM EtOH or 100 μM AA on the BBB functional assays (A) Changes in TEER, (B) 4 kDa dextran permeability, and (C) monocyte migration across the BBB. Prior to TEER, permeability or migration assay, cell monolayers were treated with EtOH for 48 hr or with AA for 2 hr in the presence or absence of specific inhibitor. Results were expressed as percent of controls or apparent permeability coefficient “P” (± SEM; n = 3). Respective inhibitor alone did not change the BBB functional integrity of the basal controls.
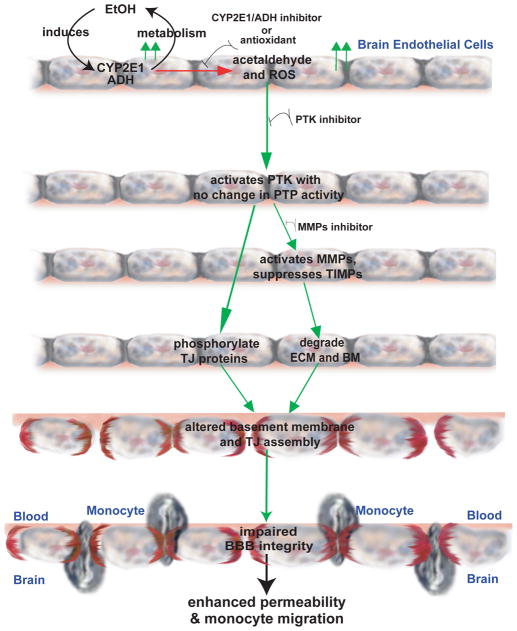
Figure 8.
Schematic representation of BBB dysfunction due to MMPs activation via PTK signaling pathway stemming from EtOH metabolism in human BMVEC as delayed effects.
Discussion
Accumulated evidence acknowledges that chronic alcohol consumption increases the risk factor for developing cerebral vascular diseases such as stroke (Hillbom and Kaste 1990; Regan 1990). The putative mechanisms of such effects still remain undefined. Our results provided the first evidence that EtOH at pathophysiological doses activated several receptor and non-receptor PTKs in primary human BMVEC leading to the diminished BBB tightness. Alcohol-induced PTK activation up-regulated activities of MMPs, led to BM degradation, and phosphorylation of TJ proteins (occludin, claudin-5 and ZO-1) at tyrosine residues, causing cumulative disruption of BBB integrity. Consistent with our previous findings, enhanced tyrosine phosphorylation of TJ proteins paralleled decrease in TEER, increased permeability to low molecular tracer, and monocyte migration across the BBB (Haorah J 2007b). These events may trigger BBB breakdown commonly observed in stroke and neurodegenerative disorders, conditions frequently associated with oxidative damage and MMPs activation (Kim et al. 2003; Rosell et al. 2006).
Importantly, 4-MP or anti-oxidant prevented the EtOH-mediated increase in MMPs activity/protein level and subsequent degradation of BBB essential BM proteins, suggesting that EtOH metabolism and ROS production initiated the MMPs activation. Treatment of BMVEC with AA also activated PTK and MMPs confirming the role of EtOH metabolism in this process. PTK signaling regulated this redox-sensitive mechanism because PTK inhibitor prevented the EtOH-mediated MMPs-2/-9 activation, BM degradation, tyrosine phosphorylation of TJ proteins, and BBB dysfunction even in the absence of 4-MP or UA. These results suggest that PTK activation occurred prior to MMPs protein expression, which was also confirmed by the fact that PTK activation started within 24 hr of EtOH treatment, whereas increase in MMPs activity or protein expression occurred after 24 hr of EtOH exposure. Activation of PTK signaling by oxidative stress has been demonstrated in human smooth muscle cells and in gerbil hippocampus (Du et al. 1999; Whisler et al. 1994; Zalewska et al. 2003). Others and we have shown that increase in PTK activity was accompanied by inactivation of PTP after ROS exposure in different cell types, including BMVEC (Chen et al. 2006; Haorah J 2007b; Tao et al. 2005). This concurrent event was due to transient oxidation of catalytic cysteine on PTP and trans-activation of PTK due to an autophosphorylation of PTK receptor in a reversible manner (Meng et al. 2002). Such alterations were associated with MMPs activation and phosphorylation of occludin and ZO-1 (Lohmann et al. 2004). Contrary to these findings, EtOH metabolism in BMVEC up-regulated PTK activity and protein level without affecting PTP activity.
The neurovascular unit composed of BMVEC, basement membrane, pericytes, microglia, astrocytes and neurons are integral components of the BBB assembly. In particular, in vitro studies of endothelial-astrocytes co-culture model support the idea that astrocytes are essential for the formation of BBB (Holash et al. 1993). Direct contact between BMVEC and astrocytes are necessary for optimal tightness of the barrier (Rubin et al. 1991), as such, astrocytes end-feet envelop about 99 % of the outer surface of the CNS capillaries (Hawkins and Davis 2005). Astrocyte-BMVEC interaction promotes the integrity of TJ assembly of brain endothelium (Abbott 2002). The fact that co-culture of astrocytes and BMVEC decreases permeability and increases TEER across BBB via increased expression of ZO-1 (Siddharthan et al. 2007), while co-culture of GFAP-deficient astrocytes and BMVEC impairs the induction of these BBB properties (Pekny et al. 1998) clearly indicate the importance role of glial cells in the maintenance of BBB function. In addition to regulation of TJ proteins expression, astrocytes also improve barrier function through enhanced expression of P-glycoprotein (Gaillard et al. 2000). Conversely, secretion of IL-1β by astrocytes mediates endothelin-1 and TNF-α induced BBB breakdown during CNS inflammation (Didier et al. 2003). Recent findings implicate the involvement of toll-like receptors (TLRs) in neuroinflammation and neurodegeneration in the CNS, in which alcohol-induced astrocytes or microglia indicate the enhanced expression of TLR2 and TLR4 via IRAK/MAPK activation pathway (Blanco and Guerri 2007). Further, our unpublished data indicate that human astrocytes secreted high levels of MMP-1 and 9 following exposure to EtOH, suggesting the role of BM protein degradation and BBB compromise as a result of glial activation by ethanol.
Using proteome profiler array for human PTKs, we observed that 50 mM EtOH selectively increased the phosphorylation/activation of receptor PTKs (IR/IGF1-R, EphB2-R, VEGFR, ErbB2-R) and non-receptor PTKs (c-Src kinase, FAK). These events were associated with MMPs activation in human BMVEC. Trans-activation of VEGFR caused MMP-2 and -9 activation in pressure-induced resistance in mouse mesenteric arteries (Lucchesi et al. 2004) was associated with MMP-2 and -9 activation. High EtOH concentration (100 – 200 mM) inhibited the tyrosine autophosphorylation of IGF-IR in neuroblastoma cells (Seiler et al. 2001); and chronic ethanol administration decreased the ligand binding properties, protein content, and the synthesis of the IGF-IR in rat hepatocytes (Haorah et al. 2003; Haorah et al. 2002). The differential effects of EtOH on IGF-IR autophosphorylation could be related to EtOH concentration, because 100 – 200 mM EtOH inhibited MMP-2 activity; whereas, lower concentrations (<100 mM) activated MMP-2 in an ErbB2-R dependent manner in breast cancer cells (Aye et al. 2004; Luo 2006).
EtOH-mediated increase in MMPs activity and protein level correlated with enhanced degradation of BM, the essential BBB component. Notably, ES prevented the increases in MMPs activity and BM degradation, indicating direct involvement of VEGFR in MMPs activation. Vascular endothelial growth factor (VEGF, a potent mediator of angiogenesis or tumorigenesis) binds to its receptor KDR/Flk-1 (VEGFR) and augments the receptor tyrosine phosphorylation leading to activation of the downstream extracellular signal-regulated kinase (ERK), p38 mitogen-activated protein kinase (MAPK), and p125FAK pathways (Ferrara 1996). The molecular mechanism is that direct binding of ES to KDR/Flk-1 prevents VEGF-KDR/Flk-1 interaction (causes VEGFR inactivation); thus, ES acts as an effective anti-angiogenetic and anti-tumorigenic agent (Kim et al. 2002). ES can form a stable complex with pro-MMPs by directly binding to the catalytic site of MMPs preventing tumor-associated angiogenesis (Kim et al. 2000; Nyberg et al. 2003). We proposed that EtOH-induced MMPs activation via PTKs signaling was also regulated by downstream p38 MAPK or p125 FAK pathway, because our results demonstrated a selective activation of both receptor (VEGFR, IR) and non-receptor (Src kinase, FAK) protein tyrosine kinase. VEGFR-mediated MMPs activation and BM disruption were demonstrated in stroke patients (Fukuda et al. 2004; Rosell et al. 2005). Ethanol consumption was shown to induce hemorrhagic and ischemic strokes in both experimental studies and epidemiological observations (Yang et al. 2001). Interestingly, PTK inhibition ameliorated deleterious effects of EtOH on arterial wall. Importantly, ES prevented MMPs activation in stroke patients (Wang et al. 2006). We conclude that MMPs activation by EtOH metabolism in the brain endothelium could be a major risk factor for stroke. We proposed that ES and specific inhibitor to selective PTK could be a beneficial therapeutic candidate for the treatment of stroke and patients with other neurological disorders.
Acknowledgments
Authors appreciate the technical assistance from David Heilman and administrative support from Ms. Debbie Baer. This work was supported in part by NIH grants MH65151, AA013846 and AA015913 (to YP).
References
- Abbott NJ. Astrocyte-endothelial interactions and blood-brain barrier permeability. J Anat. 2002;200(6):629–38. doi: 10.1046/j.1469-7580.2002.00064.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aye MM, Ma C, Lin H, Bower KA, Wiggins RC, Luo J. Ethanol-induced in vitro invasion of breast cancer cells: the contribution of MMP-2 by fibroblasts. Int J Cancer. 2004;112(5):738–46. doi: 10.1002/ijc.20497. [DOI] [PubMed] [Google Scholar]
- Blanco AM, Guerri C. Ethanol intake enhances inflammatory mediators in brain: role of glial cells and TLR4/IL-1RI receptors. Front Biosci. 2007;12:2616–30. doi: 10.2741/2259. [DOI] [PubMed] [Google Scholar]
- Chen CH, Cheng TH, Lin H, Shih NL, Chen YL, Chen YS, Cheng CF, Lian WS, Meng TC, Chiu WT, et al. Reactive oxygen species generation is involved in epidermal growth factor receptor transactivation through the transient oxidization of Src homology 2-containing tyrosine phosphatase in endothelin-1 signaling pathway in rat cardiac fibroblasts. Mol Pharmacol. 2006;69(4):1347–55. doi: 10.1124/mol.105.017558. [DOI] [PubMed] [Google Scholar]
- Didier N, Romero IA, Creminon C, Wijkhuisen A, Grassi J, Mabondzo A. Secretion of interleukin-1beta by astrocytes mediates endothelin-1 and tumour necrosis factor-alpha effects on human brain microvascular endothelial cell permeability. J Neurochem. 2003;86(1):246–54. doi: 10.1046/j.1471-4159.2003.01829.x. [DOI] [PubMed] [Google Scholar]
- Du J, Peng T, Scheidegger KJ, Delafontaine P. Angiotensin II activation of insulin-like growth factor 1 receptor transcription is mediated by a tyrosine kinase-dependent redox-sensitive mechanism. Arterioscler Thromb Vasc Biol. 1999;19(9):2119–26. doi: 10.1161/01.atv.19.9.2119. [DOI] [PubMed] [Google Scholar]
- Ferrara N. Vascular endothelial growth factor. Eur J Cancer. 1996;32A(14):2413–22. doi: 10.1016/s0959-8049(96)00387-5. [DOI] [PubMed] [Google Scholar]
- Fukuda S, Fini CA, Mabuchi T, Koziol JA, Eggleston LL, Jr, del Zoppo GJ. Focal cerebral ischemia induces active proteases that degrade microvascular matrix. Stroke. 2004;35(4):998–1004. doi: 10.1161/01.STR.0000119383.76447.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaillard PJ, van der Sandt IC, Voorwinden LH, Vu D, Nielsen JL, de Boer AG, Breimer DD. Astrocytes increase the functional expression of P-glycoprotein in an in vitro model of the blood-brain barrier. Pharm Res. 2000;17(10):1198–205. doi: 10.1023/a:1026406528530. [DOI] [PubMed] [Google Scholar]
- Haorah J, Heilman D, Knipe B, Chrastil J, Leibhart J, Ghorpade A, Miller DW, Persidsky Y. Ethanol-induced activation of myosin light chain kinase leads to dysfunction of tight junctions and blood-brain barrier compromise. Alcohol Clin Exp Res. 2005a;29(6):999–1009. doi: 10.1097/01.alc.0000166944.79914.0a. [DOI] [PubMed] [Google Scholar]
- Haorah JKB, Gorantla S, Zheng J, Persidsky Y. Alcohol-induced blood-brain barrier dysfunction is mediated via inositol 1,4,5-triphosphate receptor IP3R-gated intracellular calcium release. Journal of Neurochemistry. 2007a;100:324–336. doi: 10.1111/j.1471-4159.2006.04245.x. [DOI] [PubMed] [Google Scholar]
- Haorah J, Knipe B, Leibhart J, Ghorpade A, Persidsky Y. Alcohol-induced oxidative stress in brain endothelial cells causes blood-brain barrier dysfunction. J Leukoc Biol. 2005b;78(6):1223–32. doi: 10.1189/jlb.0605340. [DOI] [PubMed] [Google Scholar]
- Haorah J, MacDonald RG, Stoner JA, Donohue TM., Jr Ethanol consumption decreases the synthesis of the mannose 6-phosphate/insulin-like growth factor II receptor but does not decrease its messenger RNA. Biochem Pharmacol. 2003;65(4):637–48. doi: 10.1016/s0006-2952(02)01605-2. [DOI] [PubMed] [Google Scholar]
- Haorah J, McVicker DL, Byrd JC, MacDonald RG, Donohue TM., Jr Chronic ethanol administration decreases the ligand binding properties and the cellular content of the mannose 6-phosphate/insulin-like growth factor II receptor in rat hepatocytes. Biochem Pharmacol. 2002;63(7):1229–39. doi: 10.1016/s0006-2952(02)00877-8. [DOI] [PubMed] [Google Scholar]
- Haorah JRS, Schall K, Smith S, Pandya R, Persidsky Y. Oxidative stress activates protein tyrosine kinase and matrix metalloproteinases leading to blood-brain barrier dysfunction. Journal of Neurochemistry. 2007b;101:566–576. doi: 10.1111/j.1471-4159.2006.04393.x. [DOI] [PubMed] [Google Scholar]
- Harper C. The neuropathology of alcohol-specific brain damage, or does alcohol damage the brain? J Neuropathol Exp Neurol. 1998;57(2):101–10. doi: 10.1097/00005072-199802000-00001. [DOI] [PubMed] [Google Scholar]
- Harper C, Matsumoto I. Ethanol and brain damage. Curr Opin Pharmacol. 2005;5(1):73–8. doi: 10.1016/j.coph.2004.06.011. [DOI] [PubMed] [Google Scholar]
- Hawkins BT, Davis TP. The blood-brain barrier/neurovascular unit in health and disease. Pharmacol Rev. 2005;57(2):173–85. doi: 10.1124/pr.57.2.4. [DOI] [PubMed] [Google Scholar]
- Hillbom M, Kaste M. Alcohol abuse and brain infarction. Ann Med. 1990;22(5):347–52. doi: 10.3109/07853899009147918. [DOI] [PubMed] [Google Scholar]
- Holash JA, Noden DM, Stewart PA. Re-evaluating the role of astrocytes in blood-brain barrier induction. Dev Dyn. 1993;197(1):14–25. doi: 10.1002/aja.1001970103. [DOI] [PubMed] [Google Scholar]
- Kim GW, Gasche Y, Grzeschik S, Copin JC, Maier CM, Chan PH. Neurodegeneration in striatum induced by the mitochondrial toxin 3-nitropropionic acid: role of matrix metalloproteinase-9 in early blood-brain barrier disruption? J Neurosci. 2003;23(25):8733–42. doi: 10.1523/JNEUROSCI.23-25-08733.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YM, Hwang S, Kim YM, Pyun BJ, Kim TY, Lee ST, Gho YS, Kwon YG. Endostatin blocks vascular endothelial growth factor-mediated signaling via direct interaction with KDR/Flk-1. J Biol Chem. 2002;277(31):27872–9. doi: 10.1074/jbc.M202771200. [DOI] [PubMed] [Google Scholar]
- Kim YM, Jang JW, Lee OH, Yeon J, Choi EY, Kim KW, Lee ST, Kwon YG. Endostatin inhibits endothelial and tumor cellular invasion by blocking the activation and catalytic activity of matrix metalloproteinase. Cancer Res. 2000;60(19):5410–3. [PubMed] [Google Scholar]
- Lohmann C, Krischke M, Wegener J, Galla HJ. Tyrosine phosphatase inhibition induces loss of blood-brain barrier integrity by matrix metalloproteinase-dependent and -independent pathways. Brain Res. 2004;995(2):184–96. doi: 10.1016/j.brainres.2003.10.002. [DOI] [PubMed] [Google Scholar]
- Lois M, Brown LA, Moss IM, Roman J, Guidot DM. Ethanol ingestion increases activation of matrix metalloproteinases in rat lungs during acute endotoxemia. Am J Respir Crit Care Med. 1999;160(4):1354–60. doi: 10.1164/ajrccm.160.4.9811060. [DOI] [PubMed] [Google Scholar]
- Lucchesi PA, Sabri A, Belmadani S, Matrougui K. Involvement of metalloproteinases 2/9 in epidermal growth factor receptor transactivation in pressure-induced myogenic tone in mouse mesenteric resistance arteries. Circulation. 2004;110(23):3587–93. doi: 10.1161/01.CIR.0000148780.36121.47. [DOI] [PubMed] [Google Scholar]
- Luo J. Role of matrix metalloproteinase-2 in ethanol-induced invasion by breast cancer cells. J Gastroenterol Hepatol. 2006;21(Suppl 3):S65–8. doi: 10.1111/j.1440-1746.2006.04578.x. [DOI] [PubMed] [Google Scholar]
- Machado LS, Kozak A, Ergul A, Hess DC, Borlongan CV, Fagan SC. Delayed minocycline inhibits ischemia-activated matrix metalloproteinases 2 and 9 after experimental stroke. BMC Neurosci. 2006;7(1):56. doi: 10.1186/1471-2202-7-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann K, Agartz I, Harper C, Shoaf S, Rawlings RR, Momenan R, Hommer DW, Pfefferbaum A, Sullivan EV, Anton RF, et al. Neuroimaging in alcoholism: ethanol and brain damage. Alcohol Clin Exp Res. 2001;25(5 Suppl ISBRA):104S–109S. doi: 10.1097/00000374-200105051-00019. [DOI] [PubMed] [Google Scholar]
- Meng TC, Fukada T, Tonks NK. Reversible oxidation and inactivation of protein tyrosine phosphatases in vivo. Mol Cell. 2002;9(2):387–99. doi: 10.1016/s1097-2765(02)00445-8. [DOI] [PubMed] [Google Scholar]
- Nyberg P, Heikkila P, Sorsa T, Luostarinen J, Heljasvaara R, Stenman UH, Pihlajaniemi T, Salo T. Endostatin inhibits human tongue carcinoma cell invasion and intravasation and blocks the activation of matrix metalloprotease-2, -9, and -13. J Biol Chem. 2003;278(25):22404–11. doi: 10.1074/jbc.M210325200. [DOI] [PubMed] [Google Scholar]
- Pardridge WM. Brain metabolism: a perspective from the blood-brain barrier. Physiol Rev. 1983;63:1481–1535. doi: 10.1152/physrev.1983.63.4.1481. [DOI] [PubMed] [Google Scholar]
- Parsons OA. Neurocognitive deficits in alcoholics and social drinkers: a continuum? Alcohol Clin Exp Res. 1998;22(4):954–61. [PubMed] [Google Scholar]
- Pekny M, Stanness KA, Eliasson C, Betsholtz C, Janigro D. Impaired induction of blood-brain barrier properties in aortic endothelial cells by astrocytes from GFAP-deficient mice. Glia. 1998;22(4):390–400. [PubMed] [Google Scholar]
- Phillips SC, Cragg BG. Weakening of the blood-brain barrier by alcohol-related stresses in the rat. J Neurol Sci. 1982;54(2):271–8. doi: 10.1016/0022-510x(82)90187-3. [DOI] [PubMed] [Google Scholar]
- Pratt OE, Rooprai HK, Shaw GK, Thomson AD. The genesis of alcoholic brain tissue injury. Alcohol Alcohol. 1990;25(2–3):217–30. doi: 10.1093/oxfordjournals.alcalc.a044995. [DOI] [PubMed] [Google Scholar]
- Regan TJ. Alcohol and the cardiovascular system. Jama. 1990;264(3):377–81. [PubMed] [Google Scholar]
- Rosell A, Alvarez-Sabin J, Arenillas JF, Rovira A, Delgado P, Fernandez-Cadenas I, Penalba A, Molina CA, Montaner J. A matrix metalloproteinase protein array reveals a strong relation between MMP-9 and MMP-13 with diffusion-weighted image lesion increase in human stroke. Stroke. 2005;36(7):1415–20. doi: 10.1161/01.STR.0000170641.01047.cc. [DOI] [PubMed] [Google Scholar]
- Rosell A, Ortega-Aznar A, Alvarez-Sabin J, Fernandez-Cadenas I, Ribo M, Molina CA, Lo EH, Montaner J. Increased brain expression of matrix metalloproteinase-9 after ischemic and hemorrhagic human stroke. Stroke. 2006;37(6):1399–406. doi: 10.1161/01.STR.0000223001.06264.af. [DOI] [PubMed] [Google Scholar]
- Rosengren LE, Persson LI. Influence of ethanol and dexamethasone on blood-brain barrier dysfunction to albumin. Acta Neurol Scand. 1979;59(2–3):119–26. doi: 10.1111/j.1600-0404.1979.tb02918.x. [DOI] [PubMed] [Google Scholar]
- Rubin LL, Hall DE, Porter S, Barbu K, Cannon C, Horner HC, Janatpour M, Liaw CW, Manning K, Morales J, et al. A cell culture model of the blood-brain barrier. J Cell Biol. 1991;115(6):1725–35. doi: 10.1083/jcb.115.6.1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin LL, Staddon JM. The cell biology of the blood-brain barrier. Annu Rev Neurosci. 1999;22:11–28. doi: 10.1146/annurev.neuro.22.1.11. [DOI] [PubMed] [Google Scholar]
- Seiler AE, Ross BN, Rubin R. Inhibition of insulin-like growth factor-1 receptor and IRS-2 signaling by ethanol in SH-SY5Y neuroblastoma cells. J Neurochem. 2001;76(2):573–81. doi: 10.1046/j.1471-4159.2001.00025.x. [DOI] [PubMed] [Google Scholar]
- Siddharthan V, Kim YV, Liu S, Kim KS. Human astrocytes/astrocyte-conditioned medium and shear stress enhance the barrier properties of human brain microvascular endothelial cells. Brain Res. 2007;1147:39–50. doi: 10.1016/j.brainres.2007.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao Q, Spring SC, Terman BI. Comparison of the signaling mechanisms by which VEGF, H2O2, and phosphatase inhibitors activate endothelial cell ERK1/2 MAP-kinase. Microvasc Res. 2005;69(1–2):36–44. doi: 10.1016/j.mvr.2004.11.003. [DOI] [PubMed] [Google Scholar]
- Thomsen H, Kaatsch HJ, Asmus R. Magnetic resonance imaging of the brain during alcohol absorption and elimination--a study of the “rising tide phenomenon”. Blutalkohol. 1994;31(3):178–85. [PubMed] [Google Scholar]
- Wang S, Lee SR, Guo SZ, Kim WJ, Montaner J, Wang X, Lo EH. Reduction of tissue plasminogen activator-induced matrix metalloproteinase-9 by simvastatin in astrocytes. Stroke. 2006;37(7):1910–2. doi: 10.1161/01.STR.0000226923.48905.39. [DOI] [PubMed] [Google Scholar]
- Whisler RL, Newhouse YG, Beiqing L, Karanfilov BK, Goyette MA, Hackshaw KV. Regulation of protein kinase enzymatic activity in Jurkat T cells during oxidative stress uncoupled from protein tyrosine kinases: role of oxidative changes in protein kinase activation requirements and generation of second messengers. Lymphokine Cytokine Res. 1994;13(6):399–410. [PubMed] [Google Scholar]
- Yang ZW, Wang J, Zheng T, Altura BT, Altura BM. Ethanol-induced contractions in cerebral arteries: role of tyrosine and mitogen-activated protein kinases. Stroke. 2001;32(1):249–57. doi: 10.1161/01.str.32.1.249. [DOI] [PubMed] [Google Scholar]
- Zalewska T, Ziemka-Nalecz M, Sarnowska A, Domanska-Janik K. Transient forebrain ischemia modulates signal transduction from extracellular matrix in gerbil hippocampus. Brain Res. 2003;977(1):62–9. doi: 10.1016/s0006-8993(03)02742-2. [DOI] [PubMed] [Google Scholar]
- Zeigler DW, Wang CC, Yoast RA, Dickinson BD, McCaffree MA, Robinowitz CB, Sterling ML. The neurocognitive effects of alcohol on adolescents and college students. Prev Med. 2005;40(1):23–32. doi: 10.1016/j.ypmed.2004.04.044. [DOI] [PubMed] [Google Scholar]