Abstract
Purpose.
To investigate the anti-inflammatory effect of an adenosine monophosphate (AMP) analog, aminoimidazole carboxamide ribonucleotide (AICAR), in experimental autoimmune uveoretinitis (EAU).
Methods.
C57BL/6 mice were injected daily with AICAR (200 mg/kg, intraperitoneally [IP]) from day 0, the day of interphotoreceptor retinoid-binding protein (IRBP) immunization, until day 21. The severity of uveitis was assessed clinically and histopathologically. T-cell proliferation and cytokine production of IFN-γ, IL-17, and IL-10 in response to IRBP stimulation were determined. In addition, regulatory T-cell (Treg) populations were measured. Co-stimulatory molecule expression (CD40, 80, 86, and I-Ab) on dendritic cells (DCs) in EAU and on bone marrow–derived dendritic cells (BMDCs) treated with AICAR was measured.
Results.
AICAR treatment significantly reduced clinical and histologic severity of EAU as well as ocular cytokine production. An anti-inflammatory effect associated with the inhibition of T-cell proliferation and Th1 and Th17 cytokine production was observed. Increases in the Th2 response and Treg population were not observed with AICAR treatment. AICAR did significantly inhibit BMDC maturation by reducing co-stimulatory molecule expression.
Conclusions.
AICAR attenuates EAU by preventing generation of Ag-specific Th1 and Th17 cells. Impaired DC maturation may be an underlying mechanism for this anti-inflammatory effect observed with AICAR.
Systemic treatment with AICAR attenuates EAU by preventing generation of Ag-specific Th1 and Th17 cells. As a possible mechanism underlying this anti-inflammatory effect, the authors showed impaired DC maturation by AICAR.
Introduction
Uveitis is a sight-threatening intraocular inflammatory disease.1–4 Experimental autoimmune uveitis (EAU) is an animal model of human autoimmune uveitis and has been widely used for dissecting mechanisms and developing treatment strategies.5 EAU is induced by immunization with retinal antigens such as interphotoreceptor retinoid-binding protein (IRBP),6,7 and activated and sensitized Th1 and Th17 cells are considered to play a major role in initiation and maintenance of the intraocular inflammation.8,9 For the antigen-specific T-cell proliferation, naïve CD4 T cells must interact with antigen-presenting cells (APC), which express co-stimulatory molecules (second signals) such as CD40, CD80, and CD86 in addition to major histocompatibility complex (MHC) molecules (first signals).10,11 Similar to other autoimmune animal models, co-stimulatory signals are also involved in the course of EAU, and blockade of these signals ameliorates intraocular inflammation.12–14
Adenosine monophosphate (AMP)-activated protein kinase (AMPK) is a Ser/Thr kinase that serves as a sensor of cellular energy status. It is activated by energy deficiency, increases the AMP/adenosine triphosphate (ATP) ratio, and promotes cells to convert from ATP-consuming pathways to catabolic pathways.15,16 Although AMPK has been referred to as a metabolic master switch, it is also implicated in the inflammatory response. Activation of AMPK changes macrophage function to an anti-inflammatory phenotype17 and inhibits dendritic cell (DC) maturation,18 which is essential to induce lymphocyte activation. AMP analog 5-aminoimidazole-4-carboxamide-1-β-d-ribofuranoside (AICAR) is a cell-permeable activator of AMPK. It is taken up into cells through nucleoside transporter and is phosphorylated by adenosine kinase to the monophosphorylated form (ZMP), leading to AMPK activation by mimicking AMP.19 AICAR has been shown to have anti-inflammatory effects in lipopolysaccharide (LPS)-induced in vitro inflammation20 and in vivo lung injury model.21 It is also reported to have therapeutic effects in experimental autoimmune encephalomyelitis (EAE) via AMPK activation.22,23 We previously reported the anti-inflammatory effect of AICAR in the endotoxin-induced uveitis (EIU) model through the suppression of monocyte susceptibility to LPS.24 However, its ability to suppress autoimmune-mediated intraocular inflammation has not yet been examined.
In the present study, we investigated whether AICAR can suppress ocular inflammation in EAU and then sought to understand the underlying molecular mechanisms, with particular focus on T cells and DCs.
Methods
Experimental Animals
Six- to 8-week-old female C57BL/6 wild type (WT) mice were purchased from Charles River Inc. (Wilmington, MA) and AMPKα1 knockout (KO) mice were provided as previously described.25 All experiments were conducted in accordance with the Association for Research in Vision and Ophthalmology (ARVO) Statement for the Use of Animals in Ophthalmic and Vision Research and were approved by the Animal Care and Use Committee of the Massachusetts Eye and Ear Infirmary.
Induction of EAU and AICAR Treatment
To induce EAU, WT mice were immunized subcutaneously (SC) in the neck and one footpad with 200 μg human interphotoreceptor retinoid-binding protein (hIRBP) 1-20 (GPTHLFQPSLVLDMAKVLLD) (Biomatik, Wilmington, DE) emulsified in complete Freund's adjuvant (CFA) (1:1 vol/vol) containing 2.5 mg/mL Mycobacterium tuberculosis (Difco, Detroit, MI). Concurrent with immunization, 0.1 g purified Bordetella pertussis toxin (PTX, Sigma, St. Louis, MO) was injected IP, as an additional adjuvant. Treatment was conducted as a daily IP injection of AICAR (100 or 200 mg/kg body weight; Toronto Research Chemicals, Ontario, Canada) diluted in 0.15 mL PBS from day 0 to day 21 after immunization. Injection of the same volume of PBS was used in control animals. For effector phase treatment, AICAR or PBS was administered from day 8 to day 21 after immunization.
Evaluation of EAU
Clinical scoring of EAU was performed by funduscopic examination in a masked fashion as previously described.26 On days 14 and 21 after immunization, vascular dilation, white focal vascular lesions, white linear vascular lesions, retinal hemorrhage, and retinal detachment were evaluated, and the severity of EAU was graded on scale of 0 to 4 as described by Thurau et al.27 For the histologic assessment, eyes were enucleated on day 21 and immediately frozen in optimal cutting temperature compound (Sakura Finetek, Torrance, CA). Ten-micrometer-thick sections were cut near the optic nerve head, air-dried, and fixed in 4% paraformaldehyde and stained with hematoxylin and eosin. The severity of EAU in each eye was scored on a scale of 0 to 4, as previously described,28 based on the number, type, and size of lesions.
Preparation of Lymph Node (LN) Cells, CD4 T Cells, and Bone Marrow–Derived DCs (BMDCs)
Sixteen days after immunization, draining LN cells (cervical, submandibular, and inguinal) from six to eight mice were isolated and pooled, and a single cell suspension was made through a cell strainer (BD, Franklin Lakes, NJ). CD4 T-cell–enriched fractions were prepared using CD4 Microbeads (Miltenyi Biotec GmbH, Bergisch Gladbach, Germany). The purity of the suspension was determined by staining with anti-CD4 (GK1.5; Biolegend, San Diego, CA) and flow cytometry. A threshold of 95% of the cell being CD4 positive was required for the experiments.
BMDCs were generated as previously described.29 Briefly, bone marrow was flushed from the femurs and tibias of naïve mice (6- to 8-week-old WT or AMPKα1 KO mice). The red blood cells were lysed with Red Blood Cell Lysing Buffer (Sigma). A sample of cells (2 × 106) was cultured in complete medium (RPMI 1640 medium containing 10% fetal bovine serum [FBS], 50 mM 2mercaptoethanol (ME), 10 mM HEPES [pH 7.4], 2 mM glutamine, 100 U/mL penicillin, and 100 mg/mL streptomycin) including 10 ng/mL granulocyte macrophage—colony-stimulating factor (GM-CSF; Pepro Tech, London, UK). On day 4, fresh medium was added, and then on day 7, nonadherent cells and loosely adherent cells were collected as immature DCs. Immature DCs were stimulated with 100 ng/mL LPS (Salmonella typhimurium; Sigma) for 24 hours or 1 μg/mL M. tuberculosis for 48 hours to obtain mature DCs. After LPS stimulation for 24 hours, supernatant of the BMDC culture was collected and IL-6, IL12 p70, IL-23, and TNF-α concentrations were measured (R&D Systems, Minneapolis, MN). In some experiments, the indicated volume of AICAR, 5-iodotubericidin (IODO, Sigma), and dipyridamole (DPY, Sigma) was added to the culture.
Proliferation Assay and Cytokine Analysis
LN cells were suspended at 5 × 105 per 200 μL of medium in 96-well flat-bottom plates. Triplicated cells were stimulated with or without IRBP at the indicated concentrations or with 5 μg/mL anti-CD3 monoclonal antibody (eBioscience, San Diego, CA). In some experiments, AICAR was added at the indicated concentrations. Cells were incubated for 72 hours, and proliferation during the last 12 hours was measured by using a bromodeoxyuridine (BrdU) cell proliferation assay kit (Millipore, Billerica, MA). Supernatant in the culture medium was collected at 48 hours, and cytokine production in the supernatant was measured by ELISA: Quantikine mouse IFN-γ, IL-4, IL-10, and IL-17 (R&D Systems).
Mixed Lymphocyte Reaction (MLR)
BMDCs were stimulated with 100 ng/mL LPS, in the presence or absence of 1 mM AICAR for 24 hours. Then BMDCs (4 × 104 cells) were co-cultured with CD4 T cells (4 × 105) obtained from EAU mouse (day 14) in the presence of IRBP (10 μg/mL). Three days later, supernatant was collected, and cytokine concentrations of IFN-γ and IL-17 were examined by ELISA (R&D Systems).
Measurement of mRNA Expression by Real-Time PCR
Whole retina and CD4 T cells from draining LN were used for PCR. Total RNA was harvested using the RNeasy Kit (Qiagen, Valencia, CA). Complementary DNA (cDNA) was generated with Oligo-dT primer (Invitrogen, Camarillo, CA) and Superscript II (Invitrogen) according to the manufacturer's instructions. Real-time PCR was carried out using the following TaqMan gene expression assays (Applied Biosystems, Foster City, CA): IL-1β (Mm01336189_m1), IL-6 (Mm99999064_m1), IL-17 (Mm00439618_m1), IL-21 (Mm00517640_m1), IFN-γ (Mm01168134_m1), TNF (Mm99999068_m1), actin (Mm00607939_s1), T-bet (Mm00450960_m1), and RORγt (Mm01261022_m1). Quantitative expression data were acquired and analyzed with a Step One Plus real-time PCR system (Applied Biosystems).
Flow Cytometry Analysis
For the detection of Foxp3, LN cells from five mice were harvested 21 days after immunization and stained with Mouse Regulatory T-Cell Staining Kit No. 2 (eBioscience) according to the manufacturer's instructions. Surface marker expression on pooled spleen cells from four to five EAU mice (day 12) and BMDCs was measured. Cells were incubated with the following monoclonal antibodies: anti-CD11c (N418), CD40 (3/23), CD80 (16-10A1), CD86 (GL-1), I-Ab (AF6-120.1) (Biolegend). Live cells were gated on the basis of forward and side-scatter profile and propidium iodide or DAPI exclusion. Samples of cells (1 × 106) were analyzed by BD-LSR flow cytometer (Becton Dickinson, Franklin Lakes, NJ).
Western Blot Analyses
Twenty micrograms lysate from BMDCs, liver, and spleen from WT mice and AMPKα1 KO mice was electrophoresed in a 4% to 20% gradient SDS-PAGE (Invitrogen) and electroblotted to polyvinylidene fluoride membrane (Millipore). After blocking with blocking buffer (Thermo Scientific, Rockford, IL), the membranes were incubated with a rabbit polyclonal antibody against AMPKα1, AMPKα2 (1:1000, Abcam, Cambridge, MA) or GAPDH antibody (1:1000, Cell Signaling, Danvers, MA). The membranes were washed three times (5 minutes each time) with tris-buffered saline (TBS)/Tween (TBST) and incubated for 30 minutes at room temperature with horseradish peroxidase-labeled anti-rabbit secondary antibody (1:20,000; Jackson ImmunoResearch, West Grove, PA). The membranes were washed again three times (5 minutes each time) in TBST, and the proteins were visualized by ECL plus (GE Healthcare, Piscataway, NJ).
Statistical Analysis
All results are expressed as mean ± SD. EAU scores were compared by the Mann-Whitney U test. Continuous variables from the other experiments were analyzed with the unpaired Student's t-test. P < 0.05 was considered to be statistically significant.
Results
AICAR Treatment Suppresses Uveitis in EAU
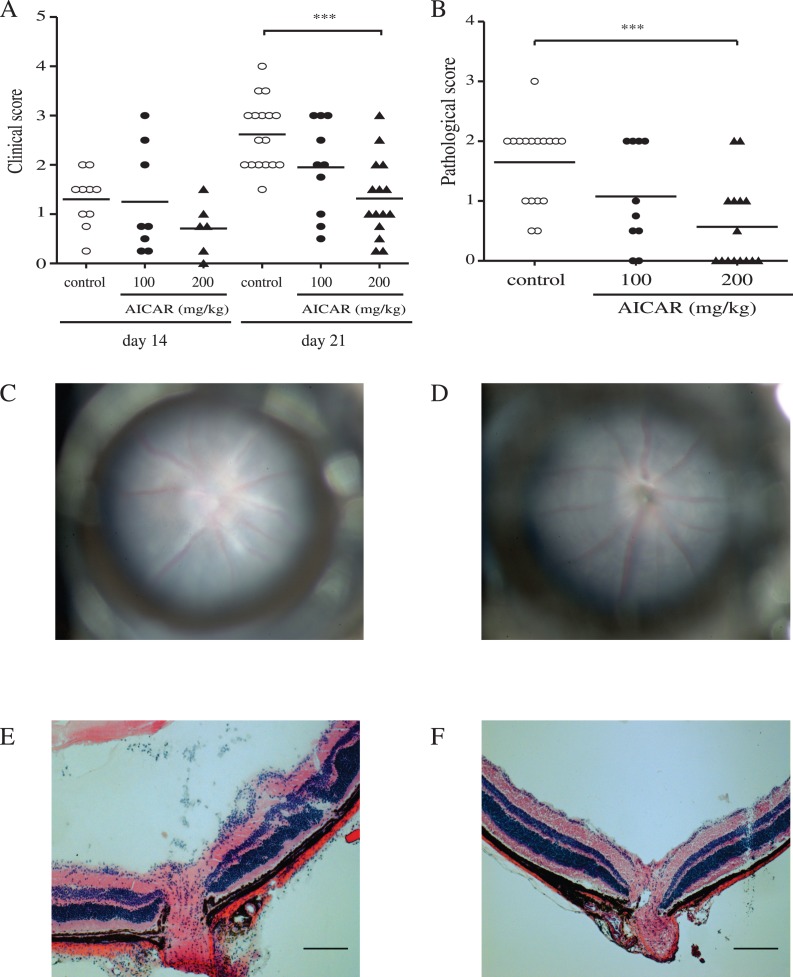
Clinical severity of EAU was assessed by fundus examination on day 14 and 21 (Figs. 1A, 1C, 1D). On day 21, PBS-treated control mice showed severe inflammation (mean clinical score, 2.6 ± 0.70; n = 17), and AICAR treatment suppressed inflammation in a dose-dependent manner. There was a statistical difference in the mean clinical scores between the control group and group treated with AICAR 200 mg/kg (mean clinical score, 1.32 ± 0.95; n = 15; P = 0.0002). Similarly, histopathologic findings on day 21 also revealed the suppressive effect of AICAR when mice were treated with 200 mg/kg (Figs. 1B, 1E, 1F; mean pathologic score, 1.65 ± 0.68 in controls vs. 0.53 ± 0.73 in AICAR-treated mice; P = 0.0008). Since 200 mg/kg AICAR showed the most robust results compared with controls, the remainder of the experiments were conducted with this dose.
Figure 1. .
Clinical and histopathologic evaluation of EAU at 21 days after immunization. (A) EAU clinical score was assessed by funduscopic examination (controls: open circles, n = 10 to 17; AICAR 100 mg/kg mice: closed circles, n = 8 to 10; AICAR 200 mg/kg mice: closed triangles, n = 6 to 15). (B) Histopathologic score was assessed with H&E sections. Mean scores are indicated by horizontal bars. Representative fundus pictures (C, D) and histopathologic (E, F) findings of vehicle-treated EAU mice (C, E) and AICAR-treated (200 mg/kg) mice (D, F). Clinical papilledema and vasculitis (C) as well as histopathologic cellular infiltration, papilledema, and retinal folds (E) were seen in vehicle-treated EAU mice. Data are shown as mean ± SD; ***P < 0.001. Results were combined from three separate experiments.
Effects of AICAR on Intraocular Inflammation
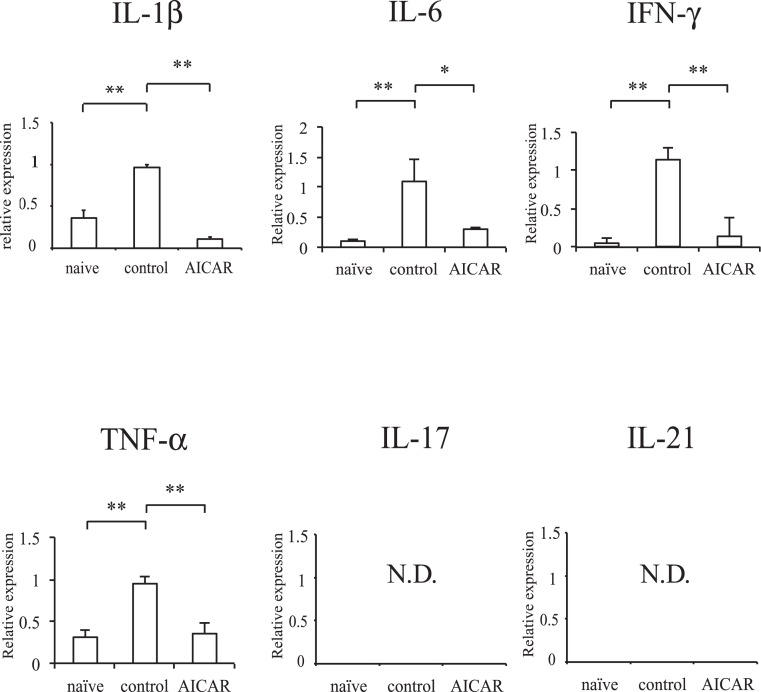
Retinal inflammation was assessed by inflammatory cytokine mRNA expression (Fig. 2). Compared with naïve mice, mRNA expression of IL-1β, IL-6, IFN-γ, and TNF-α was elevated in EAU mice. However, these cytokine expressions were significantly decreased in AICAR-treated mice. IL-17 and IL-21 mRNA levels were below the detection limit of our techniques.
Figure 2. .
Effect of AICAR on retinal inflammation. Inflammatory gene expression in the retina was compared with naïve mice by real-time PCR. The relative expression was normalized to beta-actin (naïve, n = 3; control, n = 5; AICAR, n = 5). *P < 0.05, **P < 0.01. ND, not detectable.
Effect of AICAR on Proliferation and Cytokine Production by LN Cells
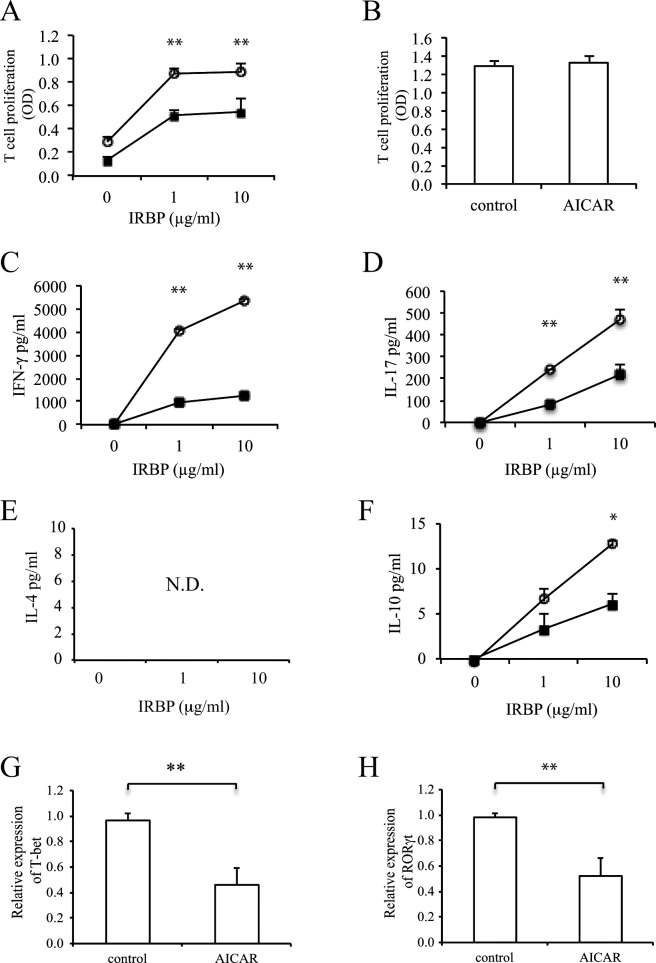
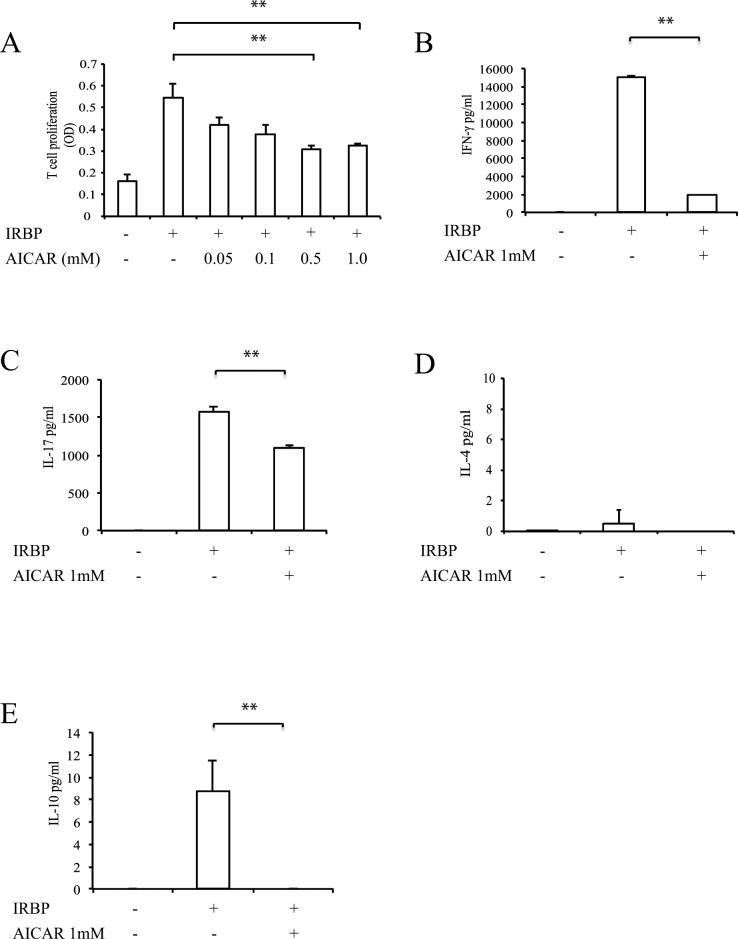
To determine the mechanism by which AICAR suppressed the severity of uveitis, IRBP-specific T-cell responses and cytokine profiles were examined in LN. While antigen-specific T-cell proliferation was observed in a dose-dependent manner in untreated mice, AICAR treatment suppressed this T-cell proliferation (Fig. 3A). There was no difference between the two groups when LN cells were stimulated with a nonspecific T-cell stimulator, anti-CD3 (Fig. 3B). Similarly, IFN-γ and IL-17 production by LN cells stimulated with IRBP was significantly decreased in AICAR-treated mice (Figs. 3C, 3D). IL-4 production was not detected, and IL-10 production was also decreased in the AICAR-treated group (Figs. 3E, 3F). CD4 T cells taken from AICAR-treated mice also revealed low expression of the transcription factors T-bet and RoRγt for Th1 and Th17 (Figs. 3G, 3H).
Figure 3. .
Effect of AICAR on the development of IRBP-reactive T cells in vivo. LN cells from controls (open circles) and AICAR-treated mice (closed squares) were stimulated with IRBP (A) and anti-CD3 (B), and the proliferative response was measured with BrdU incorporation. Cytokine production of IFN-γ (C), IL-17 (D), IL-4 (E), and IL-10 (F) was measured by ELISA. CD4 T cells were examined for the mRNA expression of T-bet (G) and RORγt (H) by real-time PCR. The relative expression was normalized to β-actin (n = 6 to 8). Data are expressed as mean ± SD and representative of two to three independent experiments. *P < 0.05, **P < 0.01.
Effect of AICAR on Fox-P3 Expression (Treg) on LN Cells
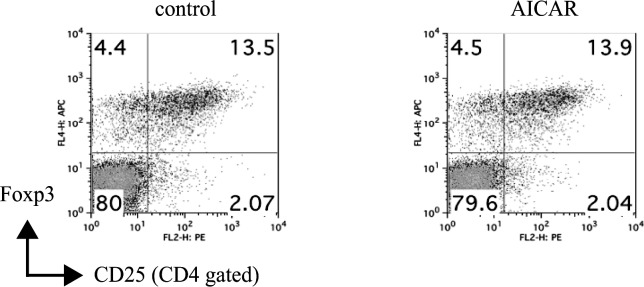
As a possible mechanism of suppressing effect of Th1 and Th17 cells, we next examined the regulatory T (Treg) cell population in the EAU mice. As shown in Figure 4, the CD4+CD25+Foxp3+ Treg population was not significantly different between controls and AICAR-treated mice.
Figure 4. .
Effect of AICAR on Treg population. Pooled LN cells from controls and AICAR-treated mice at day 21 were collected (n = 5), and the frequency of FoxP3+CD25+CD4+ T cells was analyzed by FACS. Numbers represent percentage of cells staining positive. Data are representative of two independent experiments.
Effect of AICAR on Effector Phase of EAU
To examine whether AICAR might have an effect on the effector phase of EAU, AICAR or PBS was given starting at 8 days after immunization. Both clinical and histopathologic findings revealed that AICAR treatment beginning during the effector phase significantly suppressed EAU (mean clinical score: 2.44 ± 0.56 in controls vs. 1.50 ± 0.86 in AICAR-treated mice, P = 0.031; mean pathologic score: 1.03 ± 0.75 in controls vs. 0.13 ± 0.27 in AICAR-treated mice, P = 0.004, n = 8). To assess the effect of AICAR on already developed IRBP-specific T cells, LN cells from untreated EAU mice were cultured with IRBP peptide in the presence of AICAR (Fig. 5). In vitro treatment with AICAR suppressed T-cell proliferation and IFN-γ, IL-17, and IL-10 production. IL-4 production was not significantly induced in either group.
Figure 5. .
Effect of AICAR on IRBP-reactive T cells in vitro. LN cells of untreated EAU mice (day14) were cultured with or without AICAR in the presence of IRBP. The proliferative response (A) and cytokine production of IFN-γ (B), IL-17 (C), IL-4 (D), and IL-10 (E) were measured. Data are expressed as mean ± SD and are representative of three independent experiments. **P < 0.01.
Effects of AICAR on DC Phenotype in Vivo and in Vitro
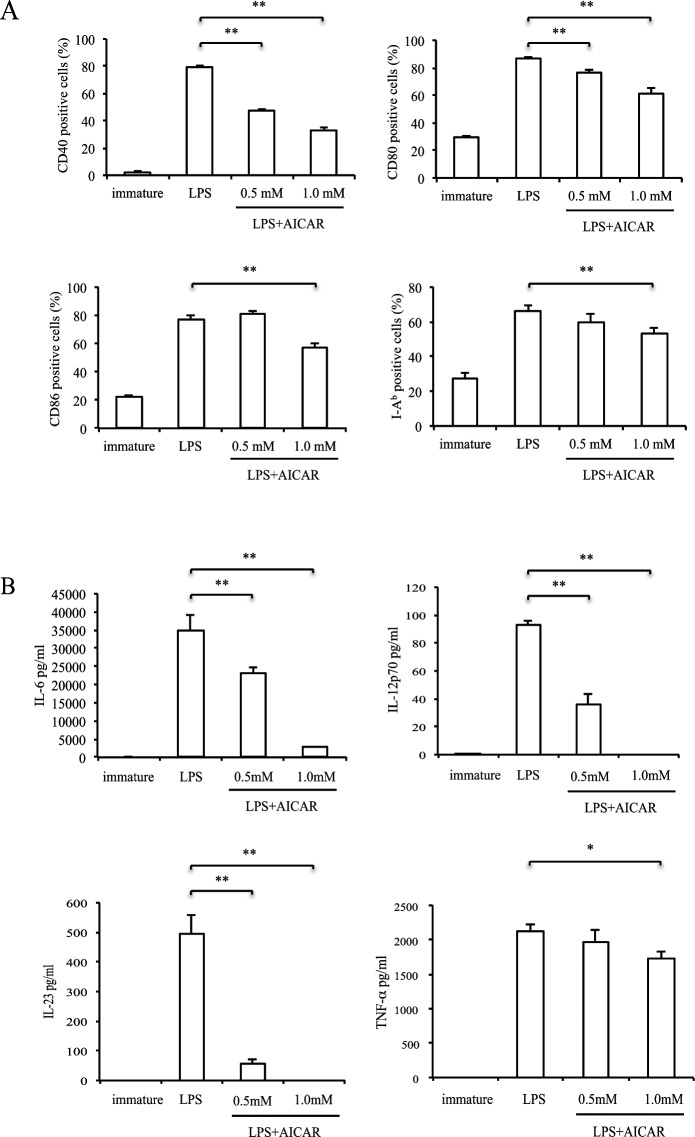
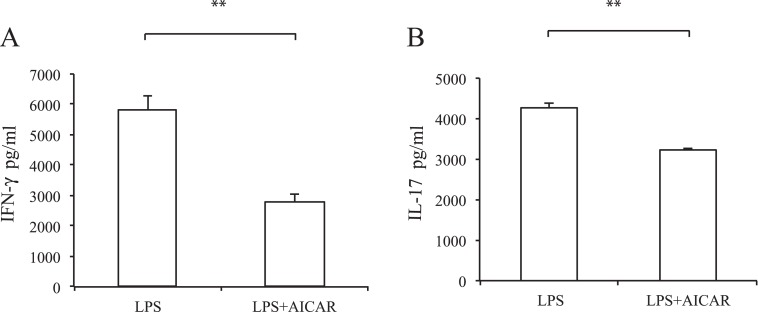
To investigate whether DC maturation was affected by AICAR, we next examined co-stimulatory molecule expression on splenic DCs in EAU. Twelve days after immunization, spleen cells were stained with an antibody against CD11c and other co-stimulatory molecules (see Supplementary Material and Supplementary Fig. S1, http://www.iovs.org/content/53/7/4158/suppl/DC1). Compared with naïve mice, the expression of CD80 and I-Ab was increased in EAU mice; however, there was no difference between controls and AICAR-treated mice. The lack of an effect may be due to DC population heterogeneity in vivo, thus to further examine the potential role of AICAR on DC maturation we performed in vitro experiments using BMDCs (Fig. 6A). After stimulation of BMDCs with LPS, the expression of CD40, CD80, CD86, and I-Ab was markedly elevated. However, AICAR significantly suppressed these elevations in a dose-dependent manner. Although its effect was mild, similar suppression was observed when BMDCs were stimulated with M. tuberculosis and treated with AICAR (see Supplementary Material and Supplementary Fig. S2, http://www.iovs.org/content/53/7/4158/suppl/DC1). IL-6, IL-12 p70, IL-23, and TNF-α production were also significantly suppressed by AICAR (Fig. 6B). Next we examined whether the suppression of DC maturation affected the IRBP-specific T-cell response. BMDCs stimulated with LPS with or without AICAR were co-cultured with CD4 T cells obtained from EAU mouse. As shown in Fig. 7, IFN-γ and IL-17 production was suppressed when CD4 T cells were co-cultured with DCs pretreated with AICAR.
Figure 6. .
Effect of AICAR on BMDC maturation. Immature BMDCs were stimulated with LPS for 24 hours in the presence or absence of AICAR (1 mM). (A) CD11c-gated CD40, CD80, CD86, and I-Ab positive cell numbers were measured by FACS. Bars represent mean ± SD from three independent experiments. (B) IL-6, IL-12 p70, IL-23, and TNF-α production were measured by ELISA. Data are expressed as mean ± SD and are representative of two independent experiments. *P < 0.05, **P < 0.01.
Figure 7. .
Effect of AICAR-treated DCs on IRBP-specific T cells. BMDCs were stimulated with LPS (100 ng/mL) with or without AICAR (1 mM) and co-cultured with CD4 T cells isolated from EAU mouse in the presence of IRBP (10 μg/mL). Cytokine production of IFN-γ (A) and IL-17 (B) was measured by ELISA. Data are expressed as mean ± SD and are representative of two independent experiments. **P < 0.01.
Effects of AICAR on DC Maturation and AMPK
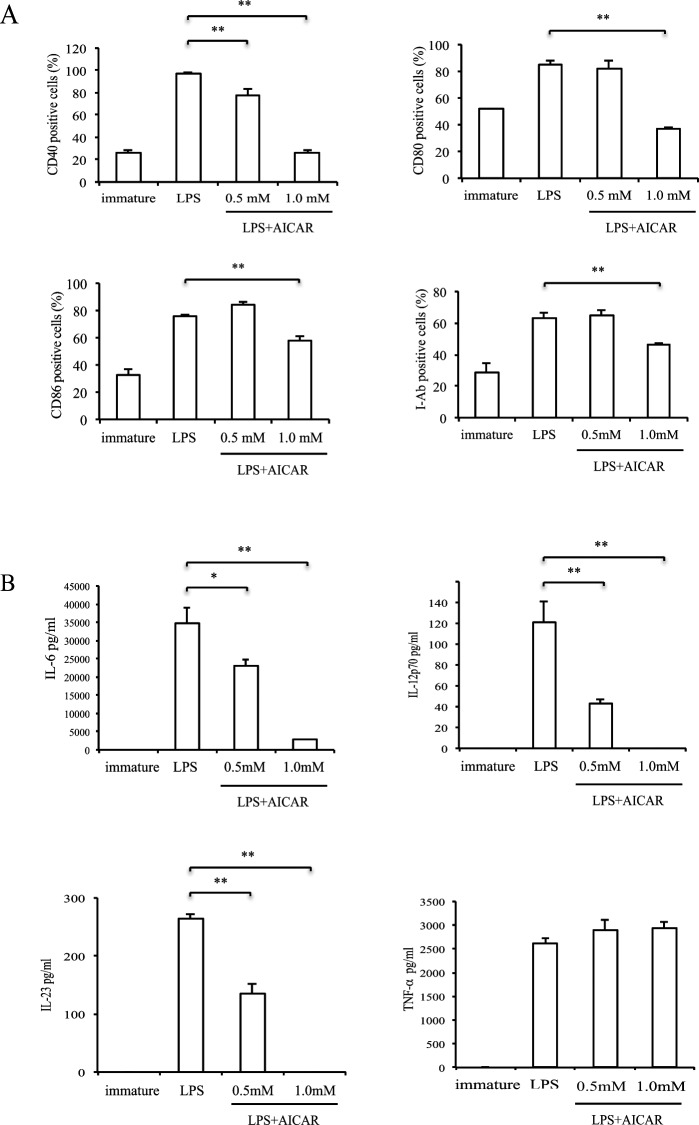
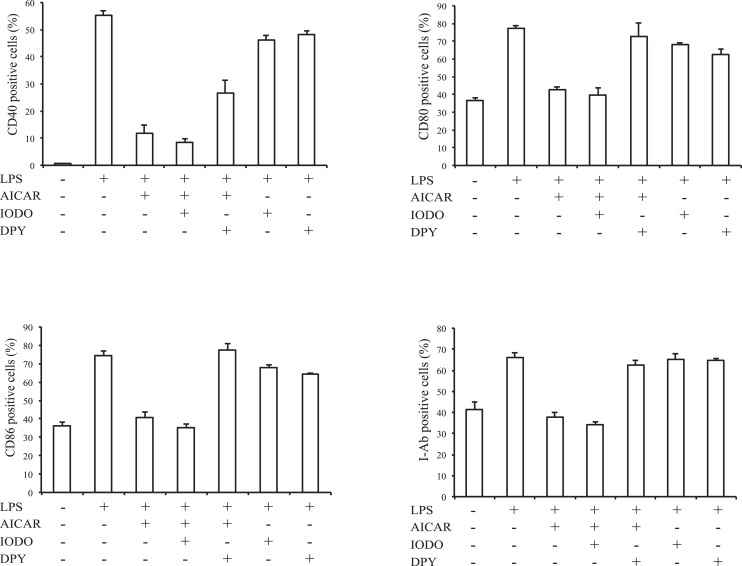
Since BMDCs expressed only AMPKα1 (see Supplementary Material and Supplementary Fig. S3, http://www.iovs.org/content/53/7/4158/suppl/DC1), we used BMDCs of AMPKα1 KO mouse to examine whether AMPK is involved in AICAR's suppressive effect. Similar to WT BMDCs, AICAR suppressed co-stimulatory molecule expression and cytokine production in BMDCs after LPS stimulation (Fig. 8). To further study AICAR's suppressive effect, we next used an adenosine kinase inhibitor (IODO) to inhibit AICAR conversion to ZMP, and an inhibitor of nucleoside transporter (DPY) to block AICAR translocation into cells. Co-stimulatory molecule expression downregulated by AICAR was not affected by IODO but was restored by DPY (Fig. 9). As a negative control, IODO and DPY treatment alone did not affect LPS stimulation.
Figure 8. .
Effect of AMPKα1 knock down and AICAR on BMDC maturation. BMDCs derived from AMPKα1KO mice were stimulated with LPS with or without AICAR (1 mM). (A) CD11c-gated CD40, CD80, CD86, and I-Ab positive cell numbers were measured by FACS. Bars represent mean ± SD from three independent experiments. (B) IL-6, IL-12 p70, IL-23, and TNF-α production were measured by ELISA. Data are expressed as mean ± SD and are representative of two independent experiments. *P < 0.05, **P < 0.01.
Figure 9. .
Effects of nucleoside transporter or adenosine kinase inhibitors on BMDC maturation in setting of AICAR treatment. WT BMDCs were stimulated with LPS and 1 mM AICAR in the presence or absence of 0.1 mM IODO or 1 μM DPY. CD11c-gated CD40, CD80, CD86, and I-Ab positive cell numbers were measured by FACS. Bars represent mean ± SD from three independent experiments.
Discussion
We previously published the effect of AICAR on LPS-induced uveitis through down-regulation of monocyte susceptibility.24 In this report, we show that AICAR also has an anti-inflammatory effect on EAU by clinical and histologic findings and intraocular inflammatory cytokine production. The inflammation in these two models, EIU and EAU, is generated by two distinct mechanisms, innate and adaptive immunity, respectively. In EAU, the Th1 and Th17 cell responses against retinal antigen lead to intraocular inflammation characterized by posterior uveitis such as disc edema, retinal exudates, vasculitis, and retinal detachment.5,30 Therefore, we focused on the T-cell response and the interaction between T cells and antigen-presenting cells to investigate AICAR's effect on EAU.
We examined the LN cell response and cytokine production by stimulation with IRBP. In contrast to nonspecific T-cell stimulation (anti-CD3), antigen-specific T-cell proliferation was significantly suppressed with AICAR. Moreover, mRNA expression of T-bet and RoRγt in CD4 T cells was decreased, and cytokine production of Th1 and Th17 after IRBP stimulation was suppressed in AICAR-treated mice. AICAR also suppressed already-developed IRBP-reactive T-cell proliferation and production of IFN-γ and IL-17 when it was added to the in vitro culture. Consistent with this result, AICAR treatment beginning during the effector phase also suppressed EAU. These results suggest that AICAR could have therapeutic potential for ongoing human uveitis.
As a result of Th1 and Th17 cell suppression, a shifting towards a Th2 response and an increase in regulatory cytokine production and Treg population has been previously demonstrated in EAU.31,32 It has been reported that this Th2 response is related to resolution of EAU,33 and the Th2 response increases in AICAR-treated EAE mice in the late phase.23 In the current experiment, during the inflammatory stage, a Th2 response was not detected and could not be induced by in vitro treatment with AICAR. Moreover, production of IL-10, one of the regulatory cytokines produced by Type-1 T regulatory (Tr1) cell34 and Treg cells was decreased in AICAR-treated mice. We next examined the number of Treg cells by counting CD4+FoxP3+ cells; however, AICAR treatment did not increase the Treg population. These results suggest that AICAR might affect naïve T-cell induction and development and, additionally, effector T-cell differentiation and proliferation independent of any effects on Th2, Tr1, and Treg cells.
These findings led us to speculate that AICAR might impair the interaction between T cells and APCs. To address this issue, we examined the function of APCs, especially focusing on DCs, also known as a professional APCs. It has been reported that AMPK negatively regulates DC maturation by affecting their energy production pathway.18 We first examined the co-stimulatory molecule expression on DCs in the spleens of EAU mice. Compared with naïve mice, the expression of CD80 and I-Ab was elevated, whereas CD40 and CD86 were not changed after IRBP immunization. There was no difference between the AICAR-treated and the nontreated groups. It is possible that we missed the effects of AICAR on DCs because they are a heterogeneous population in vivo.35 Thus, we next examined the DC phenotype by using cultured BMDCs from unimmunized mice. Stimulation with LPS elevated the expression of co-stimulatory molecules during DC maturation, and AICAR significantly suppressed this upregulation. Production of IL-6, IL-12 p70, IL-23, and TNF-α, which are key cytokines to the development of Th1 and Th17,36–38 was also decreased by AICAR. Moreover, IFN-γ and IL-17 production from IRBP-specific CD4 T cells was suppressed when co-cultured with AICAR-treated DC. Although we did not examine the effect of AICAR on AMPK in T cells in this study, it has been reported that AMPK is related to metabolic stress but not to the immunologic response in T cells.39 Maciver et al.40 reported that AMPK regulates CD8 T-cell activation but not CD4 T-cell activation and cytokine production. Together with these results, we conclude that AICAR suppressed EAU development by impairing Th1 and Th17 development, and this could be at least in part through insufficient DC maturation and cytokine production. Because AICAR was administered systemically, our treatment has systemic consequences with potentially diminished expression of many co-stimulating molecules in all dendritic cells; thus immunologic response by DC to antigens other than the one used in this study may be suppressed in vivo. However, because these experiments on DC were done in vitro and with LPS stimulation, the exact role of AICAR on DC maturation in EAU suppression remains unclear.
AMPK is a heterotrimeric complex, and the catalytic subunit of AMPKα consists of α1 and α2 and regulatory β and γ subunits.41 Similar to previous reports using lymphocytes and macrophages,17,42 BMDCs also expressed only the AMPKα1 subunit. Of interest, the suppression of BMDC maturation by AICAR was also observed in AMPKα1 KO BMDCs. Moreover, this suppression was reversed by DPY blockade of AICAR transport into the cell, suggesting that the effects of AICAR are mediated via intracellular pathways. On the other hand, inhibition of AICAR conversion into the AMP analog ZMP (the direct activator of AMPK) did not reverse AICAR's inhibitory effects, suggesting that AICAR suppressed DC maturation mainly through an AMPK-independent pathway. Other AICAR effects have also been reported to be independent of AMPK activation.43–46 Further investigations are needed to clarify the mechanism of AICAR's effect on immune cells.
In summary, we have demonstrated that AICAR diminishes ocular inflammation by inhibiting pathogenic T-cell expansion and cytokine production in EAU. AICAR's safety has already been proven in human trials of myocardial infarction,47 and glucose metabolism.48 If further study confirms its efficacy in combating ocular inflammation, AICAR may have therapeutic potential in the treatment of human uveitis.
Supplementary Material
Footnotes
Disclosure: J. Suzuki, None; T. Yoshimura, None; M. Simeonova, None; K. Takeuchi, None; Y. Murakami, None; Y. Morizane, None; J.W. Miller, None; L. Sobrin, None; D.G. Vavvas, None
Supported in part by National Eye Institute Grant EY014104 (Massachusetts Eye and Ear Infirmary core grant).
References
- 1. Yang P, Fang W, Meng Q, Ren Y, Xing L, Kijlstra A. Clinical features of Chinese patients with Behcet's disease. Ophthalmology. 2008;115:312–318 [DOI] [PubMed] [Google Scholar]
- 2. Al-Kharashi AS, Aldibhi H, Al-Fraykh H, Kangave D. Abu El-Asrar AM. Prognostic factors in Vogt-Koyanagi-Harada disease. Int Ophthalmol. 2007;27:201–210 [DOI] [PubMed] [Google Scholar]
- 3. Durrani OM, Tehrani NN, Marr JE, Moradi P, Stavrou P, Murray PI. Degree, duration, and causes of visual loss in uveitis. Br J Ophthalmol. 2004;88:1159–1162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lobo A, Barton K, Minassian D, du Bois RM, Lightman S. Visual loss in sarcoid-related uveitis. Clin Exp Ophthalmol. 2003;31:310–316 [DOI] [PubMed] [Google Scholar]
- 5. Luger D, Caspi RR. New perspectives on effector mechanisms in uveitis. Semin Immunopathol. 2008;30:135–143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Rizzo LV, Silver P, Wiggert B, et al. Establishment and characterization of a murine CD4+ T cell line and clone that induce experimental autoimmune uveoretinitis in B10.A mice. J Immunol. 1996;156:1654–1660 [PubMed] [Google Scholar]
- 7. Sanui H, Redmond TM, Kotake S, et al. Identification of an immunodominant and highly immunopathogenic determinant in the retinal interphotoreceptor retinoid-binding protein (IRBP). J Exp Med. 1989;169:1947–1960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Yoshimura T, Sonoda KH, Miyazaki Y, et al. Differential roles for IFN-gamma and IL-17 in experimental autoimmune uveoretinitis. Int Immunol. 2008;20:209–214 [DOI] [PubMed] [Google Scholar]
- 9. Amadi-Obi A, Yu CR, Liu X, et al. TH17 cells contribute to uveitis and scleritis and are expanded by IL-2 and inhibited by IL-27/STAT1. Nat Med. 2007;13:711–718 [DOI] [PubMed] [Google Scholar]
- 10. Jenkins MK. The ups and downs of T cell costimulation. Immunity. 1994;1:443–446 [DOI] [PubMed] [Google Scholar]
- 11. Janeway CA, Jr, Bottomly K. Signals and signs for lymphocyte responses. Cell. 1994;76:275–285 [DOI] [PubMed] [Google Scholar]
- 12. Bagenstose LM, Agarwal RK, Silver PB, et al. Disruption of CD40/CD40-ligand interactions in a retinal autoimmunity model results in protection without tolerance. J Immunol. 2005;175:124–130 [DOI] [PubMed] [Google Scholar]
- 13. Namba K, Ogasawara K, Kitaichi N, et al. Amelioration of experimental autoimmune uveoretinitis by pretreatment with a pathogenic peptide in liposome and anti-CD40 ligand monoclonal antibody. J Immunol. 2000;165:2962–2969 [DOI] [PubMed] [Google Scholar]
- 14. Fukai T, Okada AA, Sakai J, et al. The role of costimulatory molecules B7-1 and B7-2 in mice with experimental autoimmune uveoretinitis. Graefes Arch Clin Exp Ophthalmol. 1999;237:928–933 [DOI] [PubMed] [Google Scholar]
- 15. Hardie DG. The AMP-activated protein kinase pathway—new players upstream and downstream. J Cell Sci. 2004;117:5479–5487 [DOI] [PubMed] [Google Scholar]
- 16. Winder WW, Hardie DG. AMP-activated protein kinase, a metabolic master switch: possible roles in type 2 diabetes. Am J Physiol. 1999;277:E1–10 [DOI] [PubMed] [Google Scholar]
- 17. Sag D, Carling D, Stout RD, Suttles J. Adenosine 5′-monophosphate-activated protein kinase promotes macrophage polarization to an anti-inflammatory functional phenotype. J Immunol. 2008;181:8633–8641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Krawczyk CM, Holowka T, Sun J, et al. Toll-like receptor-induced changes in glycolytic metabolism regulate dendritic cell activation. Blood. 2010;115:4742–4749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Henin N, Vincent MF, Van den Berghe G. Stimulation of rat liver AMP-activated protein kinase by AMP analogues. Biochim Biophys Acta. 1996;1290:197–203 [DOI] [PubMed] [Google Scholar]
- 20. Giri S, Nath N, Smith B, Viollet B, Singh AK, Singh I. 5-aminoimidazole-4-carboxamide-1-beta-4-ribofuranoside inhibits proinflammatory response in glial cells: a possible role of AMP-activated protein kinase. J Neurosci. 2004;24:479–487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Zhao X, Zmijewski JW, Lorne E, et al. Activation of AMPK attenuates neutrophil proinflammatory activity and decreases the severity of acute lung injury. Am J Physiol Lung Cell Mol Physiol. 2008;295:L497–504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Prasad R, Giri S, Nath N, Singh I, Singh AK. 5-aminoimidazole-4-carboxamide-1-beta-4-ribofuranoside attenuates experimental autoimmune encephalomyelitis via modulation of endothelial-monocyte interaction. J Neurosci Res. 2006;84:614–625 [DOI] [PubMed] [Google Scholar]
- 23. Nath N, Giri S, Prasad R, Salem ML, Singh AK, Singh I. 5-aminoimidazole-4-carboxamide ribonucleoside: a novel immunomodulator with therapeutic efficacy in experimental autoimmune encephalomyelitis. J Immunol. 2005;175:566–574 [DOI] [PubMed] [Google Scholar]
- 24. Suzuki J, Manola A, Murakami Y, et al. Inhibitory effect of aminoimidazole carboxamide ribonucleotide (AICAR) on endotoxin-induced uveitis in rats. Invest Ophthalmol Vis Sci. 2011;52:6565–6571 [DOI] [PubMed] [Google Scholar]
- 25. Jorgensen SB, Viollet B, Andreelli F, et al. Knockout of the alpha2 but not alpha1 5′-AMP-activated protein kinase isoform abolishes 5-aminoimidazole-4-carboxamide-1-beta-4-ribofuranoside but not contraction-induced glucose uptake in skeletal muscle. J Biol Chem. 2004;279:1070–1079 [DOI] [PubMed] [Google Scholar]
- 26. Pouvreau I, Zech JC, Thillaye-Goldenberg B, Naud MC, Van Rooijen N, de Kozak Y. Effect of macrophage depletion by liposomes containing dichloromethylene-diphosphonate on endotoxin-induced uveitis. J Neuroimmunol. 1998;86:171–181 [DOI] [PubMed] [Google Scholar]
- 27. Thurau SR, Chan CC, Nussenblatt RB, Caspi RR. Oral tolerance in a murine model of relapsing experimental autoimmune uveoretinitis (EAU): induction of protective tolerance in primed animals. Clin Exp Immunol. 1997;109:370–376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Caspi RR, Roberge FG, Chan CC, et al. A new model of autoimmune disease. Experimental autoimmune uveoretinitis induced in mice with two different retinal antigens. J Immunol. 1988;140:1490–1495 [PubMed] [Google Scholar]
- 29. Wang X, Uto T, Sato K, et al. Potent activation of antigen-specific T cells by antigen-loaded nanospheres. Immunol Lett. 2005;98:123–130 [DOI] [PubMed] [Google Scholar]
- 30. Caspi R. Autoimmunity in the immune privileged eye: pathogenic and regulatory T cells. Immunol Res. 2008;42:41–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sun M, Yang P, Du L, Zhou H, Ren X, Kijlstra A. Contribution of CD4+CD25+ T cells to the regression phase of experimental autoimmune uveoretinitis. Invest Ophthalmol Vis Sci. 2010;51:383–389 [DOI] [PubMed] [Google Scholar]
- 32. Keino H, Takeuchi M, Usui Y, et al. Supplementation of CD4+CD25+ regulatory T cells suppresses experimental autoimmune uveoretinitis. Br J Ophthalmol. 2007;91:105–110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Takeuchi M, Yokoi H, Tsukahara R, Sakai J, Usui M. Differentiation of Th1 and Th2 cells in lymph nodes and spleens of mice during experimental autoimmune uveoretinitis. Jpn J Ophthalmol. 2001;45:463–469 [DOI] [PubMed] [Google Scholar]
- 34. Pot C, Apetoh L, Kuchroo VK. Type 1 regulatory T cells (Tr1) in autoimmunity. Semin Immunol. 2011;23:202–208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kushwah R, Hu J. Complexity of dendritic cell subsets and their function in the host immune system. Immunology. 2011;133:409–419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Bettelli E, Oukka M, Kuchroo VK. T(H)-17 cells in the circle of immunity and autoimmunity. Nat Immunol. 2007;8:345–350 [DOI] [PubMed] [Google Scholar]
- 37. Sutton C, Brereton C, Keogh B, Mills KH, Lavelle EC. A crucial role for interleukin (IL)-1 in the induction of IL-17-producing T cells that mediate autoimmune encephalomyelitis. J Exp Med. 2006;203:1685–1691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Macatonia SE, Hosken NA, Litton M, et al. Dendritic cells produce IL-12 and direct the development of Th1 cells from naive CD4+ T cells. J Immunol. 1995;154:5071–5079 [PubMed] [Google Scholar]
- 39. Mayer A, Denanglaire S, Viollet B, Leo O, Andris F. AMP-activated protein kinase regulates lymphocyte responses to metabolic stress but is largely dispensable for immune cell development and function. Eur J Immunol. 2008;38:948–956 [DOI] [PubMed] [Google Scholar]
- 40. Maciver NJ, Blagih J, Saucillo DC, et al. The liver kinase b1 is a central regulator of T cell development, activation, and metabolism. J Immunol. 2011;187:4187–4198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Hardie DG, Scott JW, Pan DA, Hudson ER. Management of cellular energy by the AMP-activated protein kinase system. FEBS Lett. 2003;546:113–120 [DOI] [PubMed] [Google Scholar]
- 42. Tamas P, Hawley SA, Clarke RG, et al. Regulation of the energy sensor AMP-activated protein kinase by antigen receptor and Ca2+ in T lymphocytes. J Exp Med. 2006;203:1665–1670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Labuzek K, Liber S, Gabryel B, Okopien B. AICAR. (5-aminoimidazole-4-carboxamide-1-beta-4-ribofuranoside) increases the production of toxic molecules and affects the profile of cytokines release in LPS-stimulated rat primary microglial cultures. Neurotoxicology. 2010;31:134–146 [DOI] [PubMed] [Google Scholar]
- 44. Qin S, Ni M, De Vries GW. Implication of S-adenosylhomocysteine hydrolase in inhibition of TNF-alpha- and IL-1beta-induced expression of inflammatory mediators by AICAR in RPE cells. Invest Ophthalmol Vis Sci. 2008;49:1274–1281 [DOI] [PubMed] [Google Scholar]
- 45. Kuo CL, Ho FM, Chang MY, Prakash E, Lin WW. Inhibition of lipopolysaccharide-induced inducible nitric oxide synthase and cyclooxygenase-2 gene expression by 5-aminoimidazole-4-carboxamide riboside is independent of AMP-activated protein kinase. J Cell Biochem. 2008;103:931–940 [DOI] [PubMed] [Google Scholar]
- 46. Jhun BS, Jin Q, Oh YT, et al. 5-Aminoimidazole-4-carboxamide riboside suppresses lipopolysaccharide-induced TNF-alpha production through inhibition of phosphatidylinositol 3-kinase/Akt activation in RAW 264.7 murine macrophages. Biochem Biophys Res Commun. 2004;318:372–380 [DOI] [PubMed] [Google Scholar]
- 47. Mangano DT, Miao Y, Tudor IC, Dietzel C. Post-reperfusion myocardial infarction: long-term survival improvement using adenosine regulation with acadesine. J Am Coll Cardiol. 2006;48:206–214 [DOI] [PubMed] [Google Scholar]
- 48. Cuthbertson DJ, Babraj JA, Mustard KJ, et al. 5-aminoimidazole-4-carboxamide 1-beta-D-ribofuranoside acutely stimulates skeletal muscle 2-deoxyglucose uptake in healthy men. Diabetes. 2007;56:2078–2084 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.