Abstract
Purpose.
The current study examined if opioid-receptor-activation by morphine can improve retinal function and retinal ganglion cell (RGC) integrity in a chronic glaucoma rat model.
Methods.
IOP was raised in Brown Norway rats by injecting hypertonic saline into the limbal venous system. Rats were treated daily with 1 mg/kg morphine for 28 days at 24-hour intervals; animals were examined for changes in IOP by a TonoLab tonometer. Pattern-ERG (PERG) was obtained in response to contrast-reversal of patterned visual stimuli. RGCs were visualized by fluorogold retrograde-labeling. Changes in the expression pattern of TNF-α and caspases were measured by Western blotting.
Results.
A significant IOP elevation was seen as early as 7 days, and maintained for up to 8 weeks, after surgery. PERG amplitudes were significantly reduced in ocular-hypertensive eyes (15.84 ± 0.74 μvolts) when compared with normal eyes (19 ± 0.86 μvolts). PERG deficits in hypertensive eyes were reversed by morphine treatment (18.23 ± 0.78 μvolts; P < 0.05). In untreated rats, a 24% reduction in labeled RGCs was measured in the hypertensive eye compared with the normal eye. This reduction in RGC labeling was significantly ameliorated in the presence of morphine. In retinal samples, TNF-α, caspase-8, and caspase-3 expressions were significantly upregulated in ocular hypertensive eyes, but completely inhibited in the morphine-treated animals.
Conclusions.
These data provide evidence that activation of opioid receptors can provide significant improvement in PERG and RGC integrity against glaucomatous injury. Mechanistic data provide clues that activation of one or more opioid receptors can reduce glaucomatous-injury via suppression of TNF-α and caspase activation.
Morphine, a broad range opioid-receptors agonist, provides retina neuroprotection against glaucomatous injury in chronic experimental rat model. Morphine-induced retina neuroprotection in glaucoma model is mediated partly via inhibition of TNF-alpha production and caspase-3 and caspase-8 activation.
Introduction
Glaucoma is one of the world's leading causes of visual impairment and blindness. Nearly 67 million people worldwide are believed to have glaucoma, including an estimated 2.2 million in the United States.1 Clinically, glaucoma is characterized by “cupping” of the optic nerve head with a decline in visual field. These changes result from loss of retinal ganglion cell axons, combined with collapse and posterior bowing of their supporting connective tissue sheets, or the lamina cribrosa. It is believed that elevated IOP causes distortion or “bowing” of the extracellular matrix (ECM) plates, which in turn damages axon bundles by mechanical stress and eventually leads to retinal ganglion cell (RGC) death.2,3 Although a major risk factor for the development of glaucoma is elevated IOP, the pathophysiological mechanisms by which elevated IOP leads to optic nerve atrophy and retina degeneration are unknown.
Clinically, opioids are powerful analgesics; however, they are also potent modulators of immune, cardiovascular, gastrointestinal, and the central nervous systems. The effects of opioids are mediated through activation of three, opioid-receptor subtypes: δ-opioid, κ-opioid, and μ-opioid.4 The expression of opioid receptors has been shown in virtually all major organ systems, including the central nervous system,5 heart,6 skin,7 and retina.8 Endogenous opioids are key mediators and modulators of activity among the immuno-neuroendocrine systems, particularly in stress-related injury.9 In other systems, opioid-receptor activation by exogenous agonists (like endogenous opioid-induced preconditioning) has been shown to facilitate a protective effect against hypoxia, ischemia, cold, or an acidic environment.10–12 Recently, we published novel findings that morphine pretreatment can provide significant retinal neuroprotection against acute ischemic injury,8 and this neuroprotection is mediated in part, via inhibition of TNF-α production.13
TNF-α is a proinflammatory cytokine that is rapidly upregulated in several neurodegenerative disorders, such as multiple sclerosis, Parkinson disease, Alzheimer disease, and glaucoma.14,15 The levels of TNF-α and its receptor, TNF-R1, are also upregulated significantly in glaucoma.15,16 In the eye, TNF-α–mediated neurotoxicity has been linked to optic nerve degeneration in patients with glaucoma.16,17 In addition, studies have demonstrated that intravitreal injections of TNF-α induce axonal degeneration in the optic nerve of mouse,18 rat,19 and rabbit.20 TNF-α, via activating TNF-R1 receptors, triggers upregulation/activation of numerous cell death signaling molecules, including caspases. Caspases are a family of cysteine proteases that regulate apoptosis. Studies have shown that caspase-3 and -9 play pivotal roles in the apoptotic death of RGCs after axotomy.21–23
The current article describes potential participation of opioid receptors in a neuroprotective paradigm against glaucomatous injury in a chronic ocular-hypertensive rat model. Data presented herein provide evidence that opioid-receptor activation by the exogenous ligand, morphine, protects RGC function and integrity against glaucomatous injury. Mechanistic data provide clues that TNF-α, caspase-8, and caspase-3 are produced during the early phase of glaucoma, and protective actions of morphine are related to the suppression of these neuro-destructive signals.
Materials and Methods
Animals
Adult male or female Brown Norway rats (3–5 months of age; 150–225 grams; Harlan Laboratories, Inc., Indianapolis, IN) were used in this study. Rats were kept under a cycle of 12-hours light and 12-hours dark. Animal handling was performed in accordance with the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research; and the study protocol was approved by the Animal Care and Use Committee at the Medical University of South Carolina. Stock morphine (15 mg/mL) was diluted in normal saline (0.9%). Morphine (1 mg/kg) was injected intraperitoneally (IP) in Brown Norway rats daily for 28 days. Drug administration (100–150 μL) was performed daily at the same time between 9 AM and 11 AM. The control group was handled in a similar fashion except that normal saline was injected without morphine. Animals were examined for changes in IOP, mean arterial pressure (MAP), and body weight.
Development of the Glaucoma Model by Hypertonic Saline Injections
Brown Norway rats (150–225 g body weight) were housed under a standard cycle of 12-hours light:12-hours dark. Stable baseline IOP was determined before hypertonic saline injections. Rats were anesthetized with ketamine (75 mg/kg) and xylazine (8 mg/kg) and body temperature was maintained at 37°C with a heating pad. Topical proparacaine (0.5%) was applied to numb the cornea. A pulled-glass micropipette attached to a syringe by PE-50 tubing was inserted into a circumferential limbal vein near the cornea and approximately 50 μL of 2 M hypertonic saline was injected into the limbal venous system as described by Morrison et al.24 After surgery, an antibacterial ointment (neomycin) was applied at the injection site of each animal to prevent infection. IOP using a calibrated Tonolab tonometer (Colonial Medical Supply Co., Inc., Franconia, NH), was recorded as the average of six to eight consecutive measurements before surgery (baseline IOP) and followed by IOP measurement on a weekly basis after treatment, as described earlier.25 IOP exposure for each animal was calculated by performing separate integration of the IOP over days of exposure for the treated and control eyes as described by McKinnon et al.23 The control eye integral values were then subtracted from the treated eye integral, yielding the “IOP-integral difference”; values are expressed as mm Hg-days. As inclusion criteria, only animals with elevated IOP that was at least 25% over baseline IOP (18–20 mm Hg) were included in the study.
Pattern-ERG Recordings
Rats were anesthetized with ketamine (75 mg/kg) and xylazine (8 mg/kg) and body temperature was maintained at 37°C with a heating pad. One animal was excluded because IOP was not elevated significantly; 16 animals were used in this experiment. Pattern-ERG (PERG) recordings (without dark adaptation) were conducted in both eyes (sequentially) 3 days before IOP elevation by hypertonic saline injection, and then every 2 weeks after surgery, as described by Porciatti.26 The PERG electrode were placed on the corneal surface by means of a micromanipulator and positioned in such a way as to encircle the undilated pupil without limiting the field of view. A small drop of saline was applied to keep the cornea and lens moist during each recording. A visual stimulus generated by black and white alternating contrast reversing bars (mean luminance, 50 cd/m2; spatial frequency, 0.033 cycle/deg; contrast, 100%; and temporal frequency, 1 Hz) was aligned with the projection of the undilated pupil at an 11 cm distance using UTAS-2000 (LKC Technologies, Gaithersburg, MD). Each PERG was an average of 300 sweeps at an interval of 1 second. For the PERG amplitudes, measurements were made between a peak and adjacent trough of the waveform.
Retrograde Labeling of RGCs
Rats were deeply anesthetized with ketamine (75 mg/kg), xylazine (8 mg/kg), and body temperature was maintained at 37°C with a heating pad. Retrograde labeling of RGCs was performed as described by Zhang et al.27 Briefly, 3 μL of a 5% solution of fluorogold in PBS was injected into the superior colliculus of 11 anesthetized normal or ocular-hypertensive animals immobilized in a stereotaxic apparatus. Using a small drill, a 1/8-inch hole was made in the skull 6 mm from bregma and 2 mm from lambda. After making this hole in the skull, a Hamilton syringe was filled with fluorogold and the syringe needle gently inserted at the hole and going down 4 mm, whereupon the fluorogold was injected. The needle was left in the brain for 30 to 60 seconds, then slowly removed. The skull hole was filled with bone wax 903 (Lukens Cat # 2007–05). Seven days after injection, animals were euthanized and their eyes were enucleated and fixed in 4% paraformaldehyde for 24 hours at 4°C. After rinsing with PBS, each retina was detached from the eyecup and prepared as a flat-mount by mounting vitreous side up. RGCs were counted and averaged per six to eight microscopic fields of identical size (150 μm2; ×20 magnification) and located approximately the same distance from the optic disc, by using Scion software (National Institutes of Health, Bethesda, MD).
Measurement of MAP in Rats
Blood pressures were measured in 24 rats using a CODA-1 tail-cuff blood pressure monitoring system (Kent Scientific, Torrington, CT) using the procedure described by Feng et al.28 Rats were trained before the studies by restraint with 25 cycles of cuff inflation/deflation on 5 consecutive days. On subsequent days, measurements were made after 5 minutes of restraint with no external manipulation or stimulation.
Measurement of Morphine Levels in Retinal Extracts by ELISA
ELISA kits were used to measure the levels of morphine in retina extracts, per manufacturer direction (Bio-Quant, San Diego, CA). Sixteen rats were used in this experiment.
Western Blotting
Equivalent amounts of retina extracts (15 μg protein) were loaded onto 10% SDS-PAGE, proteins separated, and proteins transferred to nitrocellulose membranes as described earlier.8 The membranes were blocked with 5% nonfat dry milk followed by incubation for 12 hours at 4°C with appropriate primary antibodies (1:1000 dilution) selective for TNF-α, caspase-8, caspase-3, or β-actin. After washing, membranes were incubated for 1 hour at 20°C with appropriate secondary antibodies (horseradish peroxidase [HRP]-conjugated; dilution 1:3000). Prestained molecular weight markers were run in parallel to identify the molecular weight of proteins of interest. For chemiluminescent detection, the membranes were treated with enhanced chemiluminescent reagent, and the signal was monitored using a Biorad Versadoc imaging system (Biorad, Hercules, CA). A total of 34 rats were used to perform Western blot for TNF-α and caspase expression.
Statistical Analysis
Statistical comparisons were made using the Student's t test for paired data or ANOVA using the Dunnett posttest for multiple comparisons (GraphPad Software, Inc., San Diego, CA). P less than or equal to 0.05 was considered significant.
Results
Effects of Morphine on IOP, PERG, and RGC Survival in Chronic Ocular-Hypertensive Rats
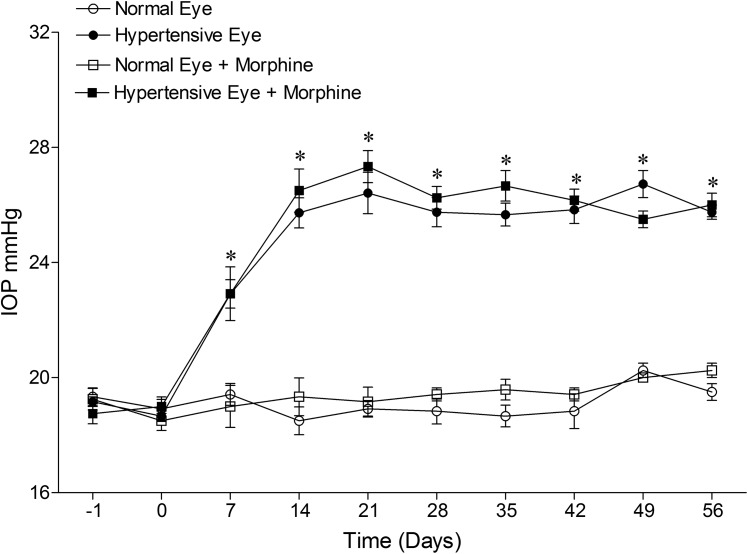
The injection of hypertonic saline (50 μL, 2 M) produced a significant elevation in IOP, which was seen as early as 7 days, and was maintained for up to 8 weeks. A single dose of 1 mg/kg morphine (IP) produced no significant effect on IOP in the hypertensive or normal eye when measured at 0, 2, 4, 6, 8, and 24 hours after administration (data not shown). Following chronic morphine administration (Fig. 1) neither the mean IOPs nor IOP-integrals over the 8-week test period were altered by morphine administration. The cumulative IOP-integral at 8 weeks for ocular hypertensive and morphine-treated ocular hypertensive groups were 438 ± 25 and 444 ± 30 mm Hg, respectively. In addition, we did not detect any significant change in MAP or body weight following chronic morphine administration (Table 1). Overall, these data support the idea that ocular responses induced by morphine administration were not mediated by changes in IOP or cardiovascular function.
Figure 1. .
IOP measurements in a hypertonic-saline-injected glaucoma rat model. Rats were divided into two groups: ocular-hypertensive group (n = 10); and morphine-treated ocular-hypertensive group (n = 12). IOP was elevated in one eye of Brown Norway rats by injecting approximately 50 μL of 2.0 M hypertonic saline; the remaining contralateral eye served as a control. Rats were maintained for up to 56 days after surgery. Data are mean ± SE; normal (○), ocular-hypertensive (●), morphine-treated normal (□), and morphine-treated ocular-hypertensive (▪) eyes. *P < 0.05; n = 10–12.
Table 1. .
IOP Was Raised Unilaterally by Injecting 50 μL of 2 M Hypertonic Saline into the Limbal Venous System Followed by 1 mg/kg Morphine Treatment for 28 Days, Once a Day
|
Groups |
Weight (g) |
MAP (mm Hg) |
IOP (mm Hg) |
Cumulative IOP (mm Hg) |
| Control group (n = 8) | 187 ± 4 | 92 ± 0.28 | 19 ± 0.5 | — |
| Ocular-hypertensive group (n = 8) | 182 ± 5 | 93 ± 3 | 26 ± 0.5 | 438 ± 25 |
| Ocular-hypertensive group + 1 mg/kg morphine (n = 8) | 191 ± 5 | 96 ± 1 | 29 ± 1 | 444 ± 30 |
Data shown in this table were collected at 8 weeks after injury. A total of 24 rats were used in this experiment.
To determine if morphine crosses the retinal blood barrier in sufficient concentration to activate retinal opioid receptors, retinas were collected at 1, 6, and 24 hours, after IP injection of 1 mg/kg morphine, and morphine levels were measured by ELISA. As shown in Table 2, morphine levels could be measured in retinal extracts as early as 1 hour, and up to 24 hours, after injection.
Table 2. .
Animals Were Treated with 1 mg/kg Morphine (IP) Followed by Retina Collection at Indicated Time Points
|
Untreated Eyes, Morphine (pg/mg protein) |
Morphine-Treated Eyes, Morphine (pg/mg protein) |
||
| 1–24 hours (n = 8) | 1 hour (n = 8) | 6 hours (n = 8) | 24 hours (n = 8) |
| Not detectable | 818,616 ± 62,387 | 454,504 ± 47,247 | 281,266 ± 28,249 |
Levels of morphine in retina samples were measured using an ELISA kit. Data are expressed as mean ± SE; n = 8. In each group, 4 rats (total 16 rats) were used in this experiment and each eye is represented as an individual observation (n = 8).
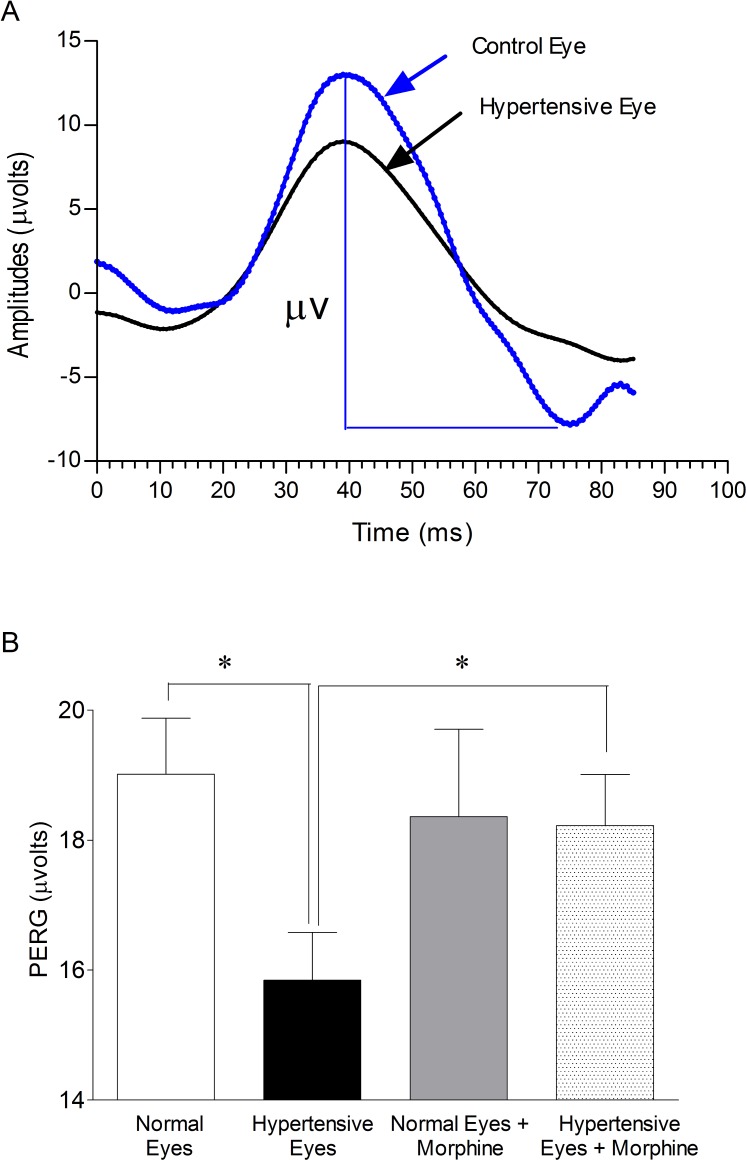
Figure 2A presents a typical signal array recorded from the normal and ocular-hypertensive rat eye. Each PERG corresponds to an average of 300 waveforms. Figure 2B presents the mean (±SEM) amplitudes of PERG in normal eyes, ocular-hypertensive eyes, from nontreated (control) and morphine-treated rats following 8 weeks of ocular hypertension. PERG amplitudes were significantly reduced in ocular-hypertensive eyes when compared with normal eyes (normal eyes, 19 ± 0.86 μvolts verses hypertensive eyes, 15.84 ± 0.74 μvolts; P < 0.05). PERG amplitudes in 1 mg/kg morphine (IP) treated hypertensive animals were significantly improved when compared with hypertensive eyes from nontreated animals (hypertensive eyes, 15.84 ± 0.74 μvolts versus hypertensive eyes + morphine, 18.23 ± 0.78 μvolts; P < 0.05). These data show a 17% reduction in PERG amplitudes when compared with normal control eyes. This loss in PERG was almost fully recovered in morphine-treated hypertensive eyes. Comparable losses in PERG were noted when animals were examined at 2 (17%), 4 (23%), and 6 (18%) weeks after injury. The losses in PERG amplitudes measured at all time points were almost fully recovered when animals were treated with morphine. In contrast, there was no change in b-wave amplitudes (scotopic-ERG) measured by conventional bright-flash ERG in dark-adapted normal eyes and hypertensive eyes (data not shown).
Figure 2. .
(A) Example of PERG recorded in normal and ocular-hypertensive rat eyes. The amplitude was measured from a positive peak to the following negative trough at week 8, post injury. Each waveform is a mean of 300 individual waveforms taken at an interval of 1 second. (B) Effects of morphine treatment on the PERG of normal and ocular-hypertensive rat eyes. Rats were divided into two groups: ocular-hypertensive group (n = 7); and morphine-treated ocular-hypertensive group (n = 8). Brown Norway rats were treated with 1 mg/kg morphine (IP) after hypertonic saline injection, and subsequently once each day for 28 days. PERG data shown in this figure were collected at week 8, post injury. Data are mean ±SE; *P < 0.05; n = 7–8.
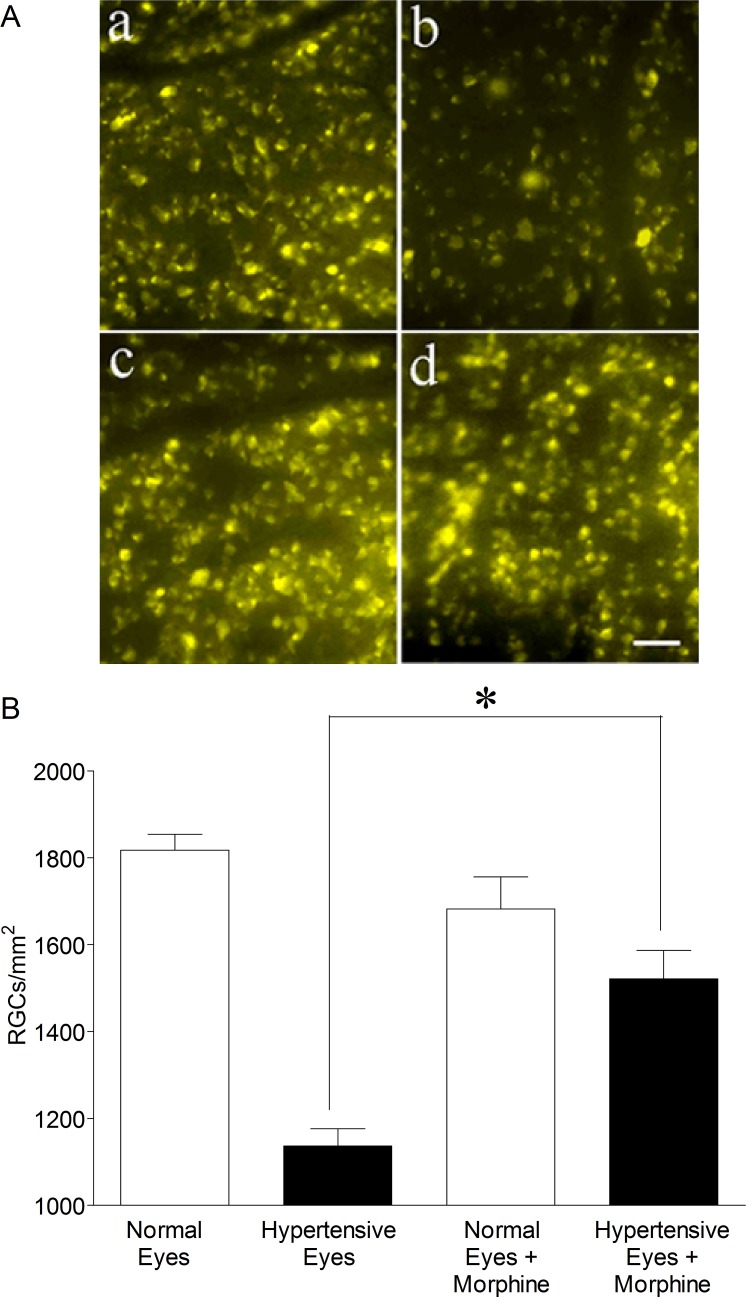
To confirm that declines in PERG amplitudes are due to RGC loss or dysfunction, RGCs were visualized by retrograde labeling with bilateral injections of fluorogold into the superior colliculus after 8 weeks of elevated IOP. Representative micrographs of fluorogold-labeled cells showed a clear loss of ganglion cells in the hypertensive eye as compared with the contralateral normal eye in untreated rats (Fig. 3A; a & b). In rats receiving morphine, the loss of RGCs appeared reduced when animals were treated with 1 mg/kg morphine (IP) each day for 28 days (Fig. 3A; c & d). Quantification of RGCs in normal eyes and ocular-hypertensive eyes, in untreated and morphine-treated rats revealed that morphine treatment significantly reduced the rate of cell loss in ocular hypertensive eyes. No changes in labeled RGCs were measured between the normotensive eyes of these animals.
Figure 3. .
(A) Fluorescence micrographs of flat-mounted retinas depicting fluorogold-labeled RGCs in normal (a), ocular-hypertensive, (b), morphine-treated normal (c), and morphine-treated ocular-hypertensive (d) eyes. After 7 weeks of ocular hypertension, 3 μL of a 5% solution of fluorogold was injected into the superior colliculus of anesthetized animals. Seven days post injection, animals were euthanized, eyes were enucleated and fixed in 4% paraformaldehyde for 24 hours at 4°C. Retinas were prepared as flat-mounts, with the vitreous side up. Fluorogold RGCs were visualized under Zeiss microscopy (Thornwood, NY). Bar = 20 μm. (B) Rats were divided into two groups: ocular-hypertensive group (n = 5); and morphine-treated ocular-hypertensive group (n = 6). RGCs were counted in 8 microscopic fields of identical size (150 μm2 area) for each eye using Scion image analysis software. *P < 0.05; n = 5–6.
Effects of Morphine Treatment on Cellular Proteins during Glaucomatous Injury
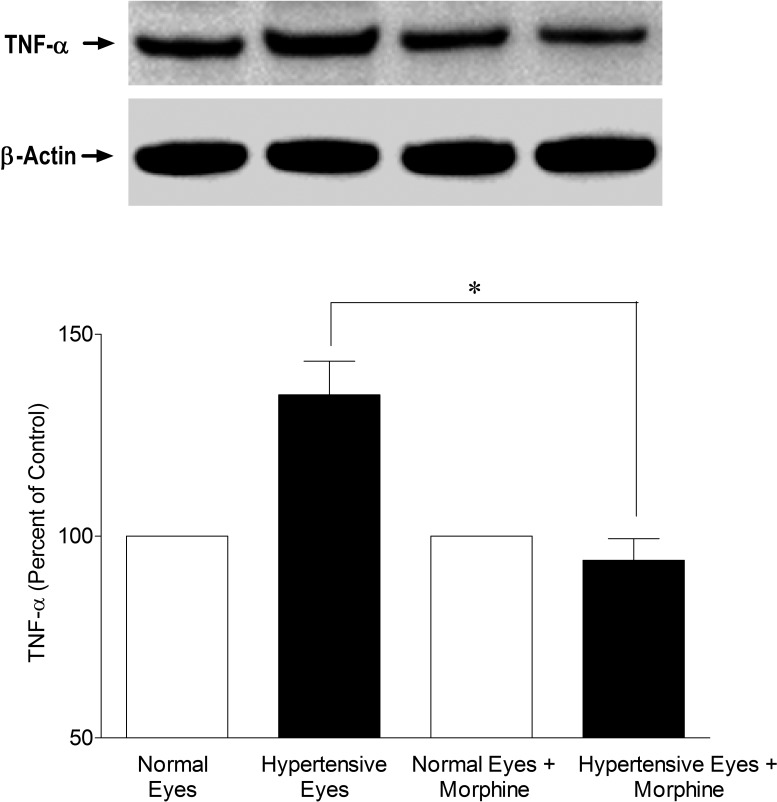
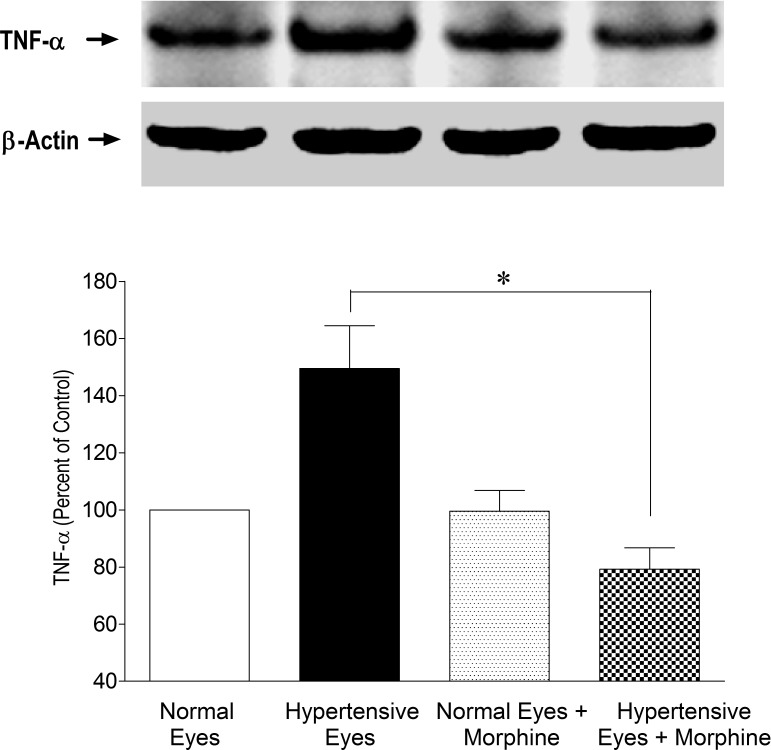
Studies have provided evidence that the cytokine TNF-α expression and caspase activation play a role in early glaucoma development. To determine if the structural and functional protection afforded by opioid-receptor-agonist, morphine, modulates TNF-α production and caspase activity, retinas from normal and ocular-hypertensive rats were analyzed for TNF-α expression. Retina extracts were analyzed for TNF-α production at 3 days post hypertonic saline injections when IOP was moderately elevated. As shown in Figure 4, there was a clear increase in the expression of TNF-α when measured 3 days after injury. To determine if TNF-α production is opposed by opioid-receptor activation, rats were treated with morphine (1 mg/kg; IP) once daily for 3 days. In these rats, TNF-α production was significantly inhibited in the presence of morphine (Fig. 4). Morphine by itself had no significant effects on TNF-α expression in normotensive eyes. Furthermore, we determined TNF-α production at 7 days after saline injection, a time point where an appreciable rise in IOP has been seen (Fig. 1). As shown in Figure 5, the level of TNF-α was significantly upregulated in ocular-hypertensive eyes, whereas this increase was also almost completely inhibited in morphine-treated animals. These data provide initial clues that TNF-α production under glaucomatous injury was indeed an early event, and remained significantly elevated during the initial phase of glaucomatous injury.
Figure 4. .
Inhibition of TNF-α expression in the presence of morphine (1 mg/kg; IP) 3 days post injury. Rats were divided into two groups: ocular-hypertensive group (n = 4); and morphine-treated ocular-hypertensive group (n = 4). Three days post hypertonic-saline injection into the limbal veins, animals were euthanized and retina extracts (15 μg retina protein) were analyzed by Western blotting using selective anti-TNF-α primary antibodies. The signal was captured using appropriate secondary antibodies (HRP-conjugated; dilution 1:3000), enhanced chemiluminescent reagent, and the Biorad Versadoc imaging system. Data shown are representative of four, independent experiments. Data are expressed as mean ± SE. *P < 0.05; n = 4.
Figure 5. .
Inhibition of TNF-α expression in the presence of morphine (1 mg/kg; IP) 7 days post injury. Rats were divided into two groups: ocular-hypertensive group (n = 4); and morphine-treated ocular-hypertensive group (n = 6). Seven days post hypertonic-saline injection into the limbal veins, animals were euthanized and retina extracts (15 μg retina protein) were analyzed by Western blotting using selective anti-TNF-α primary antibodies. The signal was captured using appropriate secondary antibodies (HRP-conjugated; dilution 1:3000), enhanced chemiluminescent reagent, and the Biorad Versadoc imaging system. Data shown are representative of 4 to 6 independent experiments. Data are expressed as mean ± SE. *P < 0.05; n = 4–6.
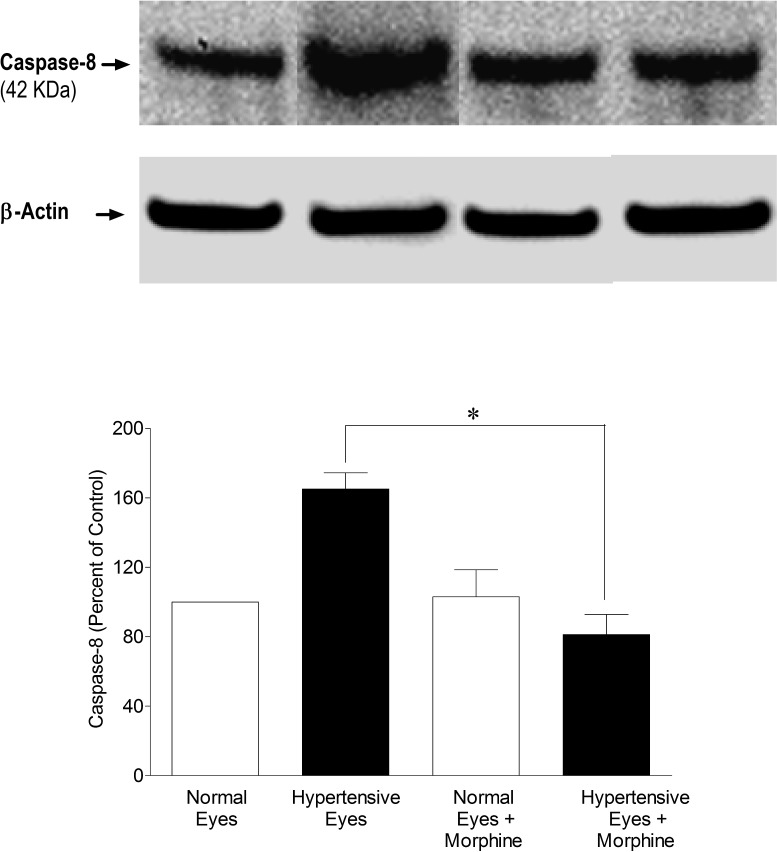
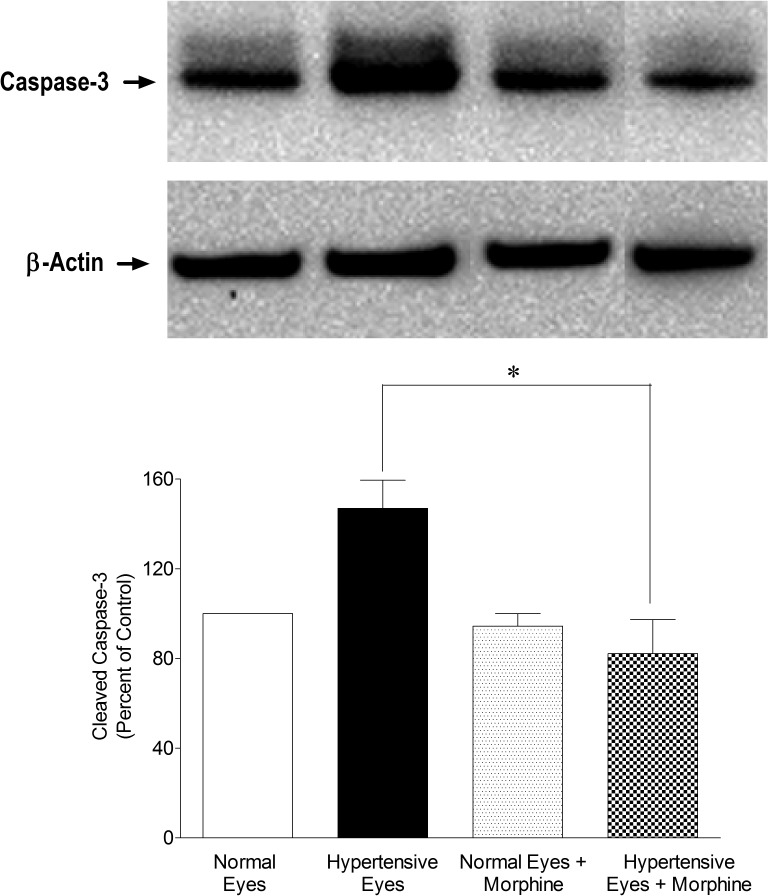
To determine the downstream signaling events, we analyzed retinal samples for caspases (e.g., caspase-8 and -3) expression in a hypertonic saline-injected rat glaucoma model on day 7 after injury. As shown in Figure 6, expression of caspase-8 (42 KDa) was upregulated in ocular hypertensive eyes, while it was inhibited in the presence of morphine. Subsequently, we also determined the expression of caspase-3, which is a downstream target of caspase-8. As shown in Figure 7, activated caspase-3 (17 kDa) was significantly upregulated in ocular-hypertensive eyes whereas it was completely inhibited in the presence of morphine, suggesting that caspase-8 and -3 are downstream targets whose expression may be regulated in a TNF-α-dependent manner.
Figure 6. .
Inhibition of caspase-8 in the presence of morphine (1 mg/kg; IP). Rats were divided into two groups: ocular-hypertensive group (n = 4); and morphine-treated ocular-hypertensive group (n = 4). Seven days post hypertonic-saline injection in limbal veins, animals were euthanized and retina extracts (15 μg retina protein) were analyzed by Western blotting using selective anti–caspase-8 primary antibodies. The signal was captured using appropriate secondary antibodies (HRP-conjugated; dilution 1:3000), enhanced chemiluminescent reagent, and the Biorad Versadoc imaging system. Data shown are representative of four independent experiments. Data are expressed as mean ±SE. *P < 0.05; n = 4.
Figure 7. .
Inhibition of activated, cleaved caspase-3 (17 kDa) in the presence of morphine (1 mg/kg; IP). Rats were divided into two groups: ocular-hypertensive group (n = 4); and morphine-treated ocular-hypertensive group (n = 4). Seven days post hypertonic-saline injection in limbal veins, animals were euthanized and retina extracts (15 μg retina protein) were analyzed by Western blotting using selective anti-caspase-3 primary antibodies. The signal was captured using appropriate secondary antibodies (HRP-conjugated; dilution 1:3000), enhanced chemiluminescent reagent, and the Biorad Versadoc imaging system. Data shown are representative of four independent experiments. Data are expressed as mean ±SE. *P < 0.05; n = 4.
Discussion
Glaucoma is a long-term optic neuropathy characterized by optic disc cupping, RGC death, and vision loss. Primary open-angle glaucoma is the most common type of glaucoma. Glaucoma pathogenesis is multifactorial; however, a major risk factor for the development of glaucoma is elevated IOP. The pathophysiological mechanisms by which elevated IOP leads to optic nerve atrophy and retinal degeneration are unknown. Current therapeutic management of glaucoma aims to halt or slow disease progression by reducing elevated IOP. Although IOP-lowering treatment can retard the disease progression in many glaucoma patients, it is not always sufficient to prevent disease progression. As a result, there is a need for therapeutic agents with neuroprotective activity, which can target the disease process manifested by the death of retinal ganglion cells and axonal loss, with irreversible loss of vision. Neuroprotectants may be used alone or in combination with IOP-reducing therapy (a treatment strategy called complete therapy). This article provides novel findings that opioid-receptor agonists, such as morphine treatment, provide significant retina neuroprotection against glaucomatous injury, suggesting that opioid-receptor agonists have a potential to be used as a therapeutic agent against glaucomatous injury.
Opioids have been used clinically for centuries as analgesics. Other biological effects induced by opioids include cytoprotection, immunomodulation, neuroendocrine regulation, and behavioral modification. Most of these biological responses are presumed to be manifested through the activation of G-protein-coupled (δ-, κ-, and μ-opioid) receptors. In the eye, activation of opioid-receptors has been implicated in the regulation of iris function, accommodative power, regulation of aqueous humor dynamics (e.g., IOP), corneal wound healing, retinal development,4 and retina neuroprotection.8 Functional evidence of opioid-receptor subtypes has been demonstrated in the anterior segment tissues as well as in the retina.8,29,30 Recently, we have demonstrated that opioid-receptor-activation is required for the development of ischemic preconditioning within the retina, and that the administration of morphine can reduce ischemic retina injury.8 Although the cellular mechanisms that are involved in opioid-mediated retina neuroprotection are poorly understood, we have shown that morphine-induced retina neuroprotection against ischemia-induced retina injury was mediated via, in part, inhibition of TNF-α production by glial cells.13 Riazi-Esfahani and colleagues30 have also shown that morphine protects the retina against ischemic injury. Additional studies have shown that the δ-opioid-receptor-antagonist, naltrindole, attenuated hypoxic-preconditioning–mediated upregulation of antioxidant proteins in the retina.29 In nonocular systems, activation of opioid receptors reduced infarct size in stroke and myocardial ischemia models.31,32 Moreover, chronic morphine treatment protected cultured human neurons against serum deprivation and staurosporin-induced cytotoxicity via downregulation of pro-apoptotic proteins.33 Based on the published research in ocular and nonocular systems, the opioid system has been shown to be neuroprotective against various injuries; however, the neuroprotective role(s) of opioids against glaucomatous injury remains fully unexplored.
The data presented in this article (Fig. 2) indicate that morphine administration acts to mitigate injurious events that lead to RGC death as determined by PERG. PERG was used to assess the functional deficits of RGCs in response to injury, which was obtained in response to contrast-reversal of patterned visual stimuli (gratings, checkerboards), rather than uniform flashes of light. PERG can reflect direct damage to the RGCs. Studies in humans, primates, and rodents have shown that PERG is an indicator of RGC function.26,34 In glaucomatous conditions, PERG overall response is reduced.35 In experimental primate models of optic nerve transection,36 and in glaucoma,37 the amount of PERG amplitude reduction is consistent with the degree of damage apparent by counting either RGCs or optic nerve fibers. In the same experimental animals, the a- and b-wave amplitudes of the conventional bright-flash ERG (scotopic ERG) were not affected. PERG, therefore, is a promising technique to measure early damage of RGCs in glaucoma. A significant reduction (17%) in PERG was seen in ocular-hypertensive eyes 8 weeks after injury, which was improved fully in the presence of morphine (Fig. 2). Interestingly, there were no reductions in the scotopic ERG (a- and b-wave amplitudes) when measured at 8 weeks after injury. Furthermore, the number of RGCs was reduced 24% in hypertensive eyes, and survival of RGCs (due to reduced rate of cell loss) was enhanced by 20% in morphine-treated ocular-hypertensive animals, as determined by fluorogold retrograde labeling (Fig. 3). However, morphine-induced improvements in PERG amplitudes, and reduction in retinal ganglion labeling, could be due to improved axonal transport that has been impaired in the ocular-hypertensive condition. In addition, the data shown in Figures 1–3 indicate that morphine-induced retina neuroprotection is IOP-independent, because the IOP was not reduced in morphine-treated ocular-hypertensive animals, whereas both PERG and RGC counts were significantly improved.
Although studies have shown that opioid-receptor agonists (e.g., morphine and bremazocine; 100 μg/2–4 kg body weight) lower IOP in rabbits when applied topically,38-41 it has remained unknown if opioid-agonists lower IOP in rodents. In rabbits, IOP-lowering effects of opioid-receptor agonists have been shown to be mediated via an increase in outflow facility, inhibition of cAMP production from the iris ciliary body, and activation of the endogenous NO/CO system and μ-opioid receptors. In contrast, we have not seen any decline in IOP when rats were treated with morphine daily intraperitoneally for 28 days. This difference could be due to the dosage of morphine, route of drug administration (e.g., topically verses intraperitoneally) or species differences.
In an acute ischemia model, we have shown that morphine-induced retina neuroprotection is reversed by the broad-range opioid-receptor antagonist, naloxone.8 Although naloxone is a widely used, nonselective, opioid-receptor antagonist, it also possesses nonreceptor, free-radical, scavenger properties. Considering the nonreceptor-related properties and long-term (28 days) treatment effects of naloxone, we decided not to use naloxone to reverse the morphine-induced retina neuroprotection in the current study. Alternatively, to reverse the morphine-induced retina neuroprotection, we stopped treating animals with morphine after 28 days, while the animals were still being measured for PERG at 6 and 8 weeks after injury. Interestingly, we have not seen any reversal in retina function (PERG amplitudes) at 6 and 8 weeks, suggesting that sustained morphine treatment is not required for the neuroprotection to continue. These data also suggest that morphine treatment influences signaling events/molecules (e.g., TNF-α) at an early phase of injury and blockage of such signaling events/molecules is sufficient to protect the retina for a prolonged period. It is important to emphasize that improvements in PERG amplitudes and RGC counts were seen after 8 weeks, whereas morphine treatment was stopped at 4 weeks and elevated IOP (Fig. 1) was maintained during the 8-week period. Further studies are under way to determine if periods shorter than 28 days of morphine treatment can provide comparable amounts of retina neuroprotection against glaucomatous injury.
Glaucoma is a complex disease and numerous retina proteins have been demonstrated to be upregulated during the pathogeneses of glaucoma, including TNF-α, TNF-R1, various protein kinases, and proteolytic caspases.15 It is widely accepted that chronic activation of glial cells and the accompanying increase in the production of proinflammatory cytokines, mainly including TNF-α, are hallmarks of inflammation/parainflammation in glaucomatous tissues, although a cause-effect relationship remains to be validated. Studies also have shown that RGCs die by apoptosis in rat, rabbit, and monkey glaucoma models,42,43 and in human glaucoma.44 A group of aspartate-specific proteases known as caspases play a central role in this apoptosis. Activated caspases kill cells by degrading structural elements, DNA, and by indirect activation of chromosomal endonucleases.45 To begin to dissect out early signaling targets in opioid-receptor–mediated retina neuroprotection, we have investigated the effects of morphine treatment on TNF-α, caspase-8, and caspase-3 production/activation in the rat retina after the induction of experimental glaucoma. As shown in Figures 4 and 5, TNF-α production was significantly upregulated after injury in the hypertonic saline-injected glaucoma model. The changes in TNF-α production were seen as early as 3 days (Fig. 4) after treatment, and remained significantly upregulated up to 7 days (Fig. 5). Interestingly, TNF-α production was completely inhibited in the presence of morphine. These data support the hypothesis that opioid-receptor activation opposes the production of TNF-α during the glaucomatous injury. Morphine treatment inhibited TNF-α production in the ocular-hypertensive eyes, but morphine may also have additional broader effects that have contributed to its neuroprotective activity. These data also demonstrate that production of TNF-α is an early event, which most likely initiates a downstream signaling cascade (e.g., activation of caspases) leading to RGC death under glaucomatous conditions. Furthermore, caspase-8 (Fig. 6), pro-caspase-3 (data not shown), and activated-caspase-3 (Fig. 7), expressions were significantly inhibited by morphine treatment. Although a causal relationship between TNF-α and caspases during glaucoma pathogenesis remains to be established, inhibition of TNF-α and caspase simultaneously appears to be an attractive approach for the neuroprotection of RGCs. Numerous caspases such as caspase-1, -3, -8, -9, -10, and -12 have been shown to be upregulated in human glaucoma.15 Further studies are warranted to determine the effects of morphine on other caspases. Studies are also under way in our laboratory to identify the opioid-receptor subtype(s) involved in the retina neuroprotection against glaucomatous injury.
In summary, our study provides evidence that opioids play key roles in retina neuroprotection against glaucomatous injury because (1) RGC function was preserved by exogenous morphine treatment in chronic ocular-hypertensive rat eyes, as determined by PERG; (2) loss of RGCs was reduced in morphine-treated ocular-hypertensive eyes, as determined by retrograde-labeling of RGCs; (3) TNF-α production in chronic ocular-hypertensive eyes was significantly inhibited in the presence of morphine; and (4) caspase-8 and caspase-3 activation in chronic ocular-hypertensive eyes was significantly inhibited in the presence of morphine. These studies also provide clues that production of TNF-α during glaucomatous injury is an early event, which may subsequently initiate downstream signaling events. Opioid-receptor-activation plays a central role in the suppression of TNF-α production and subsequently its downstream cascade of mechanisms such as caspase-8 and caspase-3 inactivation, which likely protects retina function. The findings presented herein support the concept that enhancement of opioidergic activity in the eye may present a viable neuroprotective strategy for the treatment of glaucoma.
Acknowledgments
The authors thankfully acknowledge Luanna Bartholomew (Medical University of South Carolina Storm Eye Institute), PhD, for critical review of the manuscript; and Mushfiqudding Khan (Department of Pediatrics, Medical University of South Carolina), PhD, and Nasrul Hoda (Department of Neurology, Georgia Health Sciences University, Augusta, GA), PhD, for their assistance in the measurement of rat blood pressures.
Footnotes
Supported in part by NIH/NEI Grants EY019081 (SH) and EY021368 (CEC), NIH Grant C06 RR015455 from the Extramural Research Facility Program of the National Center for Research Resources, and an unrestricted grant to the Medical University of South Carolina Storm Eye Institute from Research to Prevent Blindness, New York, New York.
Disclosure: S. Husain, None; Y. Abdul, None; C.E. Crosson, None
References
- 1. Quigley HA. Number of people with glaucoma worldwide. Br J Ophthalmol. 1996;80:389–393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hernandez MR, Pena JD. The optic nerve head in glaucomatous optic neuropathy. Arch Ophthalmol. 1997;115:389–395 [DOI] [PubMed] [Google Scholar]
- 3. Quigley HA. Experimental glaucoma damage mechanism. Arch Ophthalmol. 1983;101:1301–1302 [DOI] [PubMed] [Google Scholar]
- 4. Husain S, Potter DE. The opioidergic system: potential roles and therapeutic indications in the eye. J Ocul Pharmacol Ther. 2008;24:117–140 [DOI] [PubMed] [Google Scholar]
- 5. Minami M, Satoh M. Molecular biology of the opioid receptors: structures, functions and distributions. Neurosci Res. 1995;23:121–145 [DOI] [PubMed] [Google Scholar]
- 6. Howells RD, Kilpatrick DL, Bailey LC, Noe M, Udenfriend S. Proenkephalin mRNA in rat heart. Proc Natl Acad Sci U S A. 1986;83:1960–1963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Salemi S, Aeschlimann A, Reisch N, et al. Detection of kappa and delta opioid receptors in skin—outside the nervous system. Biochem Biophys Res Commun. 2005;338:1012–1017 [DOI] [PubMed] [Google Scholar]
- 8. Husain S, Potter DE, Crosson CE. Opioid-receptor-activation protects the retina from ischemic injury. Invest Ophthalmol Vis Sci. 2009;50:3853–3859 [DOI] [PubMed] [Google Scholar]
- 9. Chadzinska M, Savelkoul HF, Verburg-van Kemenade BM. Morphine affects the inflammatory response in carp by impairment of leukocyte migration. Dev Comp Immunol. 2009;33:88–96 [DOI] [PubMed] [Google Scholar]
- 10. Ferri S, Speroni E, Candeletti S, et al. Protection by opioids against gastric lesions caused by necrotizing agents. Pharmacology. 1988;36:140–144 [DOI] [PubMed] [Google Scholar]
- 11. Mayfield KP, D'Alecy LG. Delta-1 opioid agonist acutely increases hypoxic tolerance. J Pharmacol Exp Ther. 1994;268:683–688 [PubMed] [Google Scholar]
- 12. Schultz JE, Gross GJ. Opioids and cardioprotection. Pharmacol Ther. 2001;89:123–137 [DOI] [PubMed] [Google Scholar]
- 13. Husain S, Liou GI, Crosson CE. Opioid receptor activation: suppression of ischemia/reperfusion-induced production of TNF-alpha in the retina. Invest Ophthalmol Vis Sci. 2011;52:2577–2583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Shohami E, Ginis I, Hallenbeck JM. Dual role of tumor necrosis factor alpha in brain injury. Cytokine Growth Factor Rev. 1999;10:119–130 [DOI] [PubMed] [Google Scholar]
- 15. Yang X, Luo C, Cai J, et al. Neurodegenerative and inflammatory pathway components linked to TNF-alpha/TNFR1 signaling in the glaucomatous human retina. Invest Ophthalmol Vis Sci. 2011;52:8442–8454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Tezel G, Li LY, Patil RV, Wax MB. TNF-alpha and TNF-alpha receptor-1 in the retina of normal and glaucomatous eyes. Invest Ophthalmol Vis Sci. 2001;42:1787–1794 [PubMed] [Google Scholar]
- 17. Yuan L, Neufeld AH. Tumor necrosis factor-alpha: a potentially neurodestructive cytokine produced by glia in the human glaucomatous optic nerve head. Glia. 2000;32:42–50 [PubMed] [Google Scholar]
- 18. Jenkins HG, Ikeda H. Tumour necrosis factor causes an increase in axonal transport of protein and demyelination in the mouse optic nerve. J Neurol Sci. 1992;108:99–104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kitaoka Y, Kwong JM, Ross-Cisneros FN, et al. TNF-alpha-induced optic nerve degeneration and nuclear factor-kappaB p65. Invest Ophthalmol Vis Sci. 2006;47:1448–1457 [DOI] [PubMed] [Google Scholar]
- 20. Madigan MC, Sadun AA, Rao NS, Dugel PU, Tenhula WN, Gill PS. Tumor necrosis factor-alpha (TNF-alpha)-induced optic neuropathy in rabbits. Neurol Res. 1996;18:176–184 [DOI] [PubMed] [Google Scholar]
- 21. Cheung ZH, Chan YM, Siu FK, et al. Regulation of caspase activation in axotomized retinal ganglion cells. Mol Cell Neurosci. 2004;25:383–393 [DOI] [PubMed] [Google Scholar]
- 22. Levkovitch-Verbin H, Harizman N, Dardik R, Nisgav Y, Vander S, Melamed S. Regulation of cell death and survival pathways in experimental glaucoma. Exp Eye Res. 2007;85:250–258 [DOI] [PubMed] [Google Scholar]
- 23. McKinnon SJ, Lehman DM, Tahzib NG, et al. Baculoviral IAP repeat-containing-4 protects optic nerve axons in a rat glaucoma model. Mol Ther. 2002;5:780–787 [DOI] [PubMed] [Google Scholar]
- 24. Morrison JC, Moore CG, Deppmeier LM, Gold BG, Meshul CK, Johnson EC. A rat model of chronic pressure-induced optic nerve damage. Exp Eye Res. 1997;64:85–96 [DOI] [PubMed] [Google Scholar]
- 25. Husain S, Whitlock NA, Rice DS, Crosson CE. Effects of latanoprost on rodent intraocular pressure. Exp Eye Res. 2006;83:1453–1458 [DOI] [PubMed] [Google Scholar]
- 26. Porciatti V. The mouse pattern electroretinogram. Doc Ophthalmol. 2007;115:145–153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Zhang X, Cheng M, Chintala SK. Kainic acid-mediated upregulation of matrix metalloproteinase-9 promotes retinal degeneration. Invest Ophthalmol Vis Sci. 2004;45:2374–2383 [DOI] [PubMed] [Google Scholar]
- 28. Feng M, Whitesall S, Zhang Y, Beibel M, D'Alecy L, DiPetrillo K. Validation of volume-pressure recording tail-cuff blood pressure measurements. Am J Hypertens. 2008;21:1288–1291 [DOI] [PubMed] [Google Scholar]
- 29. Peng PH, Huang HS, Lee YJ, Chen YS, Ma MC. Novel role for the delta-opioid receptor in hypoxic preconditioning in rat retinas. J Neurochem. 2009;108:741–754 [DOI] [PubMed] [Google Scholar]
- 30. Riazi-Esfahani M, Kiumehr S, Asadi-Amoli F, Dehpour AR. Effects of intravitreal morphine administered at different time points after reperfusion in a rabbit model of ischemic retinopathy. Retina. 2009;29:262–268 [DOI] [PubMed] [Google Scholar]
- 31. Chen TY, Goyagi T, Toung TJ, et al. Prolonged opportunity for ischemic neuroprotection with selective kappa-opioid receptor agonist in rats. Stroke. 2004;35:1180–1185 [DOI] [PubMed] [Google Scholar]
- 32. Gross ER, Hsu AK, Gross GJ. Opioid-induced cardioprotection occurs via glycogen synthase kinase beta inhibition during reperfusion in intact rat hearts. Circ Res. 2004;94:960–966 [DOI] [PubMed] [Google Scholar]
- 33. Cui J, Chen Q, Yu LC, Zhang Y. Chronic morphine application is protective against cell death in primary human neurons. Neuroreport. 2008;19:1745–1749 [DOI] [PubMed] [Google Scholar]
- 34. Holder GE. Pattern electroretinography (PERG) and an integrated approach to visual pathway diagnosis. Prog Retin Eye Res. 2001;20:531–561 [DOI] [PubMed] [Google Scholar]
- 35. Wanger P, Persson HE. Pattern-reversal electroretinograms in unilateral glaucoma. Invest Ophthalmol Vis Sci. 1983;24:749–753 [PubMed] [Google Scholar]
- 36. Maffei L, Fiorentini A, Bisti S, Hollander H. Pattern ERG in the monkey after section of the optic nerve. Exp Brain Res. 1985;59:423–425 [DOI] [PubMed] [Google Scholar]
- 37. Johnson MA, Drum BA, Quigley HA, Sanchez RM, Dunkelberger GR. Pattern-evoked potentials and optic nerve fiber loss in monocular laser-induced glaucoma. Invest Ophthalmol Vis Sci. 1989;30:897–907 [PubMed] [Google Scholar]
- 38. Drago F, Aguglia E, Dal Bello A, et al. Ocular instillation of naloxone increases intraocular pressure in morphine-addicted patients: a possible test for detecting misuse of morphine. Experientia. 1985;41:266–267 [DOI] [PubMed] [Google Scholar]
- 39. Dortch-Carnes J, Potter DE. Bremazocine: a kappa-opioid agonist with potent analgesic and other pharmacologic properties. CNS Drug Rev. 2005;11:195–212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Dortch-Carnes J, Russell KR. Morphine-induced reduction of intraocular pressure and pupil diameter: role of nitric oxide. Pharmacology. 2006;77:17–24 [DOI] [PubMed] [Google Scholar]
- 41. Stagni E, Bucolo C, Motterlini R, Drago F. Morphine-induced ocular hypotension is modulated by nitric oxide and carbon monoxide: role of mu3 receptors. J Ocul Pharmacol Ther. 2010;26:31–35 [DOI] [PubMed] [Google Scholar]
- 42. Garcia-Valenzuela E, Shareef S, Walsh J, Sharma SC. Programmed cell death of retinal ganglion cells during experimental glaucoma. Exp Eye Res. 1995;61:33–44 [DOI] [PubMed] [Google Scholar]
- 43. Quigley HA. Ganglion cell death in glaucoma: pathology recapitulates ontogeny. Aust N Z J Ophthalmol. 1995;23:85–91 [DOI] [PubMed] [Google Scholar]
- 44. Kerrigan LA, Zack DJ, Quigley HA, Smith SD, Pease ME. TUNEL-positive ganglion cells in human primary open-angle glaucoma. Arch Ophthalmol. 1997;115:1031–1035 [DOI] [PubMed] [Google Scholar]
- 45. Enari M, Hug H, Nagata S. Involvement of an ICE-like protease in Fas-mediated apoptosis. Nature. 1995;375:78–81 [DOI] [PubMed] [Google Scholar]