Abstract
The triple curve pattern (three lateral curvatures of equal severity) has been recognized as a distinct and unique clinical subtype of scoliosis. As part of a large study of familial idiopathic scoliosis (FIS), a subset of five families with a triple curve pattern (at least one member of each family having a triple curve) was evaluated to determine if this curve pattern was linked to any of the markers previously genotyped as part of the STRP-based previous linkage screen. Model independent linkage analysis (SIBPAL, v4.5) of the initial genomic screen identified candidate regions on chromosomes 6 and 10 when FIS was analyzed both as qualitative and quantitative traits in single- and multipoint linkage analyses. Additional fine mapping analyses of this subgroup with SNPs corroborated the findings in these regions (P <0.001). These regions have been previously linked to FIS, however, this is the first time these regions have been implicated in a clinically well-defined subgroup and may suggest a unique genetic etiology for the formation of a triple curve.
Keywords: idiopathic scoliosis, triple curve, genomic, chromosomes, familial, loci, screen, identification, spine, genes
INTRODUCTION
Familial idiopathic scoliosis (FIS) is a structural lateral curvature of the spine that has its onset during puberty in otherwise normal children [Weinstein, 1994]. The condition occurs in approximately 2–3% of children, with the most severe curves occurring in 0.2–0.5% of (predominantly female) children [Kane, 1977; Weinstein and Ponseti, 1983]. Clinical and genetic studies support the hypothesis of genetic factors influential in its pathogenesis [Garland, 1934; Filho and Thompson, 1971; Bonati et al., 1976; Czeizel et al., 1978; Carr, 1990; Kesling and Reinker, 1997]. However, the mode of inheritance is still unclear. Studies to date have suggested autosomal dominant, multifactorial, or X-linked inheritance [Wynne-Davies, 1968; Cowell et al., 1972; Riseborough and Wynne-Davies, 1973; Axenovich et al., 1999]. A review of the recent literature suggests several candidate regions, including chromosome 6p, distal 10q, and 18q [Wise et al., 2000]; 17p11.2 [Salehi et al., 2002]; 19p13.3 and 2q [Chan et al., 2002]; Xq23–26 [Justice et al., 2003]; 6p25–22, 6q14–16, 9q32–34, 16q11–q12 and 17p11–q11 [Miller et al., 2005]; 8q12 [Gao et al., 2007]; and 9q31.2–q34.2 and 17q25.3-qtel [Ocaka et al., 2008]. It is possible that several loci may play a role in the manifestation of the disorder and that the loci responsible may also vary from family to family, resulting in substantial clinical and genetic heterogeneity. Careful characterization of phenotypes within a study group is required to identify genetically homogenous groups of families that would enable us to delineate the complexity of this disorder.
Historically, a variety of classification systems for curve assessment have been developed in an effort to standardize patient groups to aid in clinical treatment, and predict potential outcomes [Schulthess, 1905; Cobb, 1948; Ponseti and Friedman, 1950; Stagnara and Queneau, 1953; James, 1954; Lange, 1956; Nash and Moe, 1969; Moe and Kettleson, 1970; Goldstein and Waugh, 1973; Travaglini, 1975; King et al., 1983; Asher, 1988; Bunch and Patwardhan, 1989; Cruickshank et al., 1989; Conrad et al., 1998; Lenke et al., 2002]. The first widely recognized classification of scoliosis was identified by Ponseti and Friedman in 1950 and is based on a five-curve classification. King et al. [1983] developed a principal means for classification of thoracic curves to select patients for surgery and vertebrae for optimal fusion. Both of these systems however, do not delineate a triple curve. The most recent method of classification, developed by Lenke et al. [2002], utilized a three-component assessment of the curve that includes curve type, a lumbar spine modifier, and a sagittal thoracic modifier. This classification system yields 42 possible types of curves and includes a triple curve category. Structural curvatures are described by their position in the spinal column, and their lack of flexibility. One or more than one of the curves may be considered major or primary in nature (the highest Cobb angle measurement on an anterioposterior standing spinal radiograph). The smaller curve(s) may then be considered minor; however, all of the curves lack flexibility, as determined through bending radiographs (Fig. 1).
FIG. 1.

Anterioposterior radiograph of one individual with a triple scoliotic lateral curvature of the spine.
The curves predominantly reported in the literature are single and double lateral curvatures. Triple curves, which involve three distinct structural lateral curves in the cervical, thoracic and lumbar vertebrae, are rare in comparison [Davidson et al., 2003]. Previous studies of classification systems and surgical outcomes reported that between 1.4 and 3% of cases examined have triple curves [Stokes et al., 1988; Conrad et al., 1998; Lenke et al., 2002; Davidson et al., 2003]. Cruickshank et al. [1989] reported that 27% of the patients in their study sample had a triple curve. In that study however, the assessment of curvatures examined addresses characterization of the upper thoracic region of the spine and indicated that many triple curves could have been diagnosed as double major curves if the small upper thoracic curvature was not considered.
The triple curve phenotype represents a small proportion of individuals with idiopathic scoliosis. Their scarcity makes the study of the natural history of these curvatures difficult. To date, studies suggest that when compared to other curve types, their course is generally benign and rarely progress to surgical intervention. In this study we focus on the triple curve phenotype and attempt to identify genetic variations that contribute to the overall process of scoliosis development and spinal stability.
MATERIALS AND METHODS
Sample and Ascertainment
In the original study, 202 families with at least two individuals with idiopathic scoliosis were ascertained and examined by a single orthopedic surgeon [Justice et al., 2003; Miller et al., 2005]. Written informed consent was obtained from all study participants, in accordance with a protocol approved by the Johns Hopkins School of Medicine Institutional Review Board. In the original study the criteria for a diagnosis of scoliosis were history and physical examination consistent with a spinal curvature in the coronal plane, standing anteroposterior spinal radiographs exhibiting ≥10° curvature by the Cobb method with pedicle rotation and no congenital deformity [Shands and Eisberg, 1955; Kane, 1977; Armstrong et al., 1982]. If there was any historical evidence within the family of other genetic conditions, regardless of whether that member did or did not have scoliosis, the family was excluded from the current study sample [Shapiro et al., 1989; Boileau et al., 1993]. Additionally, individuals and families with secondary causes of idiopathic scoliosis were excluded from the study. The sample was consistent with previous epidemiological studies with respect to gender, curve type, and curve size as measured by the Cobb angle [Miller et al., 2001].
Triple Curves
In the present study, a subset of 5 families with 26 individuals was identified from the original study group (202 families) based on the identification of a triple curve phenotype. The triple curve pattern is three distinct scoliotic curves each measuring ≥10°, which are structural in nature, that is, curves that lack flexibility. Side bending AP radiographs were taken to determine the lack of flexibility of the curvatures, thus supporting the diagnosis of a triple curve pattern. Of these 26 individuals, 17 had scoliosis and 5 were classified as having a triple curve. To allow for the possibility of variable expressivity, any individual with scoliosis (regardless of type) was classified as affected for the purposes of this study. If an individual had more than one curve, the largest curve was used for curve measurement. The average curvature of all affected individuals was 32.7°and ranged from 11–60°.
Genomic Screen
Blood samples were obtained from all participants and genomic DNA was extracted using standard purification protocols [Ausubel, 1987; Moore and Dowhan, 2002; Fritsch et al., 1989]. A genomic screen for the 202 families was performed at the Center for Inherited Disease Research with a modified CHLC v.9 marker set consisting of 391 short tandem repeat markers [Miller et al., 2005].
Fine Mapping Short Tandem Repeat Polymorphisms
In the current study, additional short tandem repeat polymorphisms (STRPs) were genotyped in candidate regions suggestive of linkage. Database sources for STRPs (tetranucleotide and trinucleotides) included Marshfield Center for Medical Genetics (Marshfield, WI), the Ensembl Genome Browser, and the Centre d’Etude du Polymorphisme Humain (Paris, France). The markers were obtained as labeled map pairs (Research Genetics, Huntsville, AL). Polymerase chain reaction (PCR) amplification was performed in a 10 μl reaction mix containing 40 ng of genomic DNA, a final concentration of 1 × PCR buffer (Invitrogen, Carlsbad, CA), 1.5 mM MgCl2 (Invitrogen), 0.25 mM dNTPs (Invitrogen), 0.24 μM of forward primer and reverse primer (Research Genetics), and 1 U of Taq polymerase (Invitrogen). All primers were optimized for annealing temperature prior to amplification of samples. Annealing temperatures ranged from 55 to 65°C. PCR conditions were 35 cycles at 95°C for 30 sec, the optimized annealing temperature for 1 min, and 72°C for 1 min, followed by one cycle at 72°C for 5 min.
Single Nucleotide Polymorphisms—Restriction Fragment Length Polymorphisms
All genotyped single nucleotide polymorphisms (SNPs) were iden-tified through the National Center for Biotechnology Information (NCBI) dbSNP database (www.ncbi.nlm.nih.gov/SNP). SNPs were selected based on their location between markers d6s1043 and d6s501 on chromosome 6, with a reported minor allele frequency in dbSNP of ≥0.2; the SNP location being within a restriction enzyme cut site, and the number and size of fragments produced by a restriction enzyme. Eighteen SNPs formed the SNP map (1 SNP/ 150 kb) and PCR primers were chosen by the program Primer 3 [Rozen and Skaletsky, 2000]. PCR was performed in a 100 μl reaction mix containing 120 ng of genomic DNA, a final concentration of 1× PCR buffer (Invitrogen), 0.25 mM dNTPs (Invitrogen), 1.5 mM MgCl2 (Invitrogen), 0.24 μM of forward primer and reverse primer (Johns Hopkins University Genetics Resources CORE facility, Baltimore, MD), and 2 U of HotstarTaq® DNA polymerase (Qiagen, Valencia, CA). All primers were optimized for annealing temperature prior to amplification by PCR. Annealing temperatures for the primers ranged from 55 to 65°C. PCR conditions were one cycle of 95°C for 15 min followed by 35 cycles of 95°C for 30 sec, the optimal annealing temperature for 1 min, and 72°C for 1 min, followed by one cycle at 72°C for 5 min. The PCR product was concentrated to 20 μl using dry centrifugation. A restriction enzyme digestion was prepared in a 15 μl reaction mix containing 5 μl of the concentrated PCR product and a final concentration of 1 U of restriction enzyme (New England BioLabs, Beverly, MA), 1× digestion buffer (New England BioLabs) and incubated according to the manufacturer’s specifications. The digested product was separated out on a 2.5% Nusieve® 3:1 agarose gel, post-stained with 50 mg ethidium bromide. A 50-bp ladder (Invitrogen) was used to identify the different length fragments.
Single Nucleotide Polymorphisms—Allelic Discrimination With Taqman
Allelic discrimination was performed with the ABI Taqman method (Applied Biosystems, Inc., FosterCity, CA). A 5-μl reaction mixture [20 ng of genomic DNA, 2.5 μl of Taqman Universal PCR master mix (Applied Biosystems, Inc.), and 0.25 μl of 20 × Assays-on-Demand SNP mix (Applied Biosystems, Inc.)] was prepared according to the manufacturer’s specifications. PCR conditions were 95°C for 20 min, followed by 40 cycles of 92°C for 15 sec and 60°C for 1 min. The PCR product then was analyzed for fluorescence with the ABI PRISM 7900 Sequence Detection System (Applied Bio-systems, Inc.).
Statistical Analyses
Allele frequencies for the 391 genomic screen markers were determined using FREQ [S.A.G.E., v4.5]. Familial relationships were verified using RELCHECK, which infers the most likely relationship between pairs of relatives by use of identity-by-descent sharing estimates [Boehnke and Cox, 1997; Broman and Weber, 1998]. Mendelian inconsistencies were determined using PEDCHECK [O’Connell and Weeks, 1995]. Non-systematic inconsistencies were removed from the data. Genetic and physical map distances for the STRPs were obtained from the University of California-Santa Cruz Human Genome browser (March 2004 release). Physical map distances for the SNPs were obtained from the NCBI dbSNP database (human build 35). Monomorphic SNPs and those with a P-value from an exact test for Hardy–Weinberg equilibrium less than 0.001 were discarded prior to linkage analysis.
Model-independent sib-pair linkage analysis was used to screen for linkage between the trait and each marker [SIBPAL, S.A.G.E., v4.5] on the subgroup of triples families (5 families, 27 individuals) for all autosomal markers. Sib-pair analyses were performed on 16 sib-pairs, of which 3 were discordant and 13 were concordantly affected. FIS was analyzed as a qualitative trait, where the threshold for affection was ≥10°, and as a quantitative trait, where the trait analyzed was the degree of lateral curvature. Skewness and kurtosis of the distribution of lateral curves were 0.41 and −1.08. The degree of lateral curvature was log transformed in an attempt to normalize the data. To corroborate these analyses, two additional linkage tests, the Whittemore and Halpern [1994] nonparametric linkage statistic (NPL), and the Kong and Cox [1997] exponential model, were used to analyze the qualitative trait data, as implemented in the program MERLIN [Abecasis et al., 2002].
Prior to multipoint linkage analysis, pairs of SNPs were assessed for linkage disequilibrium (LD) using Haploview (version 3.2) [Barret, 2005]. A pair of SNPs were defined as being in high LD if D′ = 1.0 and r2 ≥ 0.4. The most informative SNPs were retained from each cluster of SNPs in LD. This decreased the number of SNPs from 66 to 33 for chromosome 6 and from 34 to 16 for chromosome 10.
RESULTS
Genomic Screen
In this study, for the analyses of the STRP makers, a chromosomal region was considered significant if two or more adjacent markers had P-values less than 0.05. Five candidate regions were identified in the triples subgroup (chromosomes 6, 8, 10, 11, and 22, Table I). The regions on chromosomes 6, 8, and 11 were previously reported by Miller et al. [2005] in the original set of 202 families (1,198 individuals). In the triple curves subset, the most significant P-values were observed in regions on chromosome 6, D6S1056 (P <2 × 10−10) and chromosome 10, D10S677 (P <5 × 10−7). These regions span approximately 35 and 100 Mb, respectively. These regions were fine mapped with additional STRPs and SNPs. The areas on chromosomes 8 and 11 identified in this study are approximately 11 and 35 Mb upstream, respectively, from the regions reported by Miller et al. [2005]. The regions on chromosomes 10 and 22 were not reported as candidate regions in the previous analysis of our original population.
TABLE I.
Qualitative Model-Independent Linkage Analysis Results of Genomic Screen and Fine mapping STRP Markers
| Marker | Mba | Singlepoint P-values |
|---|---|---|
| d6slO31 | 77.518 | 0.02888 |
| d6slO45 | 85.64 | 0.00015 |
| d6slO43b | 92.507 | 0.01774 |
| d6slO56 | 94.154 | <0.00001 |
| d6s501 | 97.938 | 0.00058 |
| d6s475 | 100.711 | 0.00001 |
| gatal64h01b | 102.53 | 0.00048 |
| d6slO21 | 104.781 | 0.00002 |
| d6s474 | 112.986 | 0.00116 |
| d8sl132 | 107.398 | 0.00029 |
| d8s592 | 118.525 | 0.03001 |
| d8sll28 | 128.664 | 0.04268 |
| dl0sl435 | 2.233 | 0.00015 |
| dl0sl89 | 6.762 | 0.31750 |
| dl0sl412 | 9.304 | 0.49435 |
| dl0s2325 | 12.833 | 0.00077 |
| dl0s570b | 12.965 | 0.20341 |
| dl0s1653b | 15.718 | 0.01959 |
| dl0sl423 | 19.478 | 0.00738 |
| dl0sl225 | 64.425 | 0.08311 |
| dl0sl432 | 74.329 | 0.05106 |
| dl0sl658 | 85.765 | 0.26886 |
| dl0sl765 | 89.592 | 0.01023 |
| dl0s2470 | 92.355 | 0.00070 |
| dl0sl85b | 95.178 | 0.00660 |
| dl0s677 | 95.954 | <0.00001 |
| dl0sl239 | 103.186 | 0.01582 |
| d11sl999 | 10.676 | 0.00001 |
| d11sl981 | 17.042 | 0.01058 |
| d22s689 | 27.181 | 0.00003 |
| d22s685 | 32.92 | 0.02182 |
| d22s683 | 34.838 | 0.04198 |
| d22s44S | 35.891 | 0.05376 |
Map positions from www.genome.ucse.edu.
Fine mapping STRP markers.
Fine-Mapping STRPs
Two additional STRPs within the chromosome 6 region and 4 additional STRPS within the chromosome 10 region were genotyped on all 27 individuals of the triples subgroup. Model-independent linkage analysis corroborated the regions initially identified by the genomic screen (Table I). The region on chromosome 6 in the triples subset was effectively narrowed to approximately 27 Mb. Linkage analysis on chromosome 10 revealed two possible regions (D10S2325-D10S1423 and D10S1765-D10S1239), each approximately 15 Mb in length. The distance between the two regions is approximately 70 Mb and includes the centromere. Based on the level of significance, the second region on chromosome 10 was considered a higher priority.
Fine-Mapping SNPs
Sixty-six SNPs between markers D6S1043 and D6S474 were genotyped on chromosome 6 (5 families, 27 individuals). Eighteen of the 66 SNPs on chromosome 6 were genotyped by RFLP-PCR. Of these, 6 out of 576 genotypes (1%) could not be determined. The remaining 48 SNPs were genotyped by the ABI Taqman method. Thirty-three of these SNPs were retained for analysis after running
Tagger on Haploview, version 3.2 [Barret, 2005]. Model independent linkage analysis revealed multiple consecutive SNPs significant at the 0.001 level (Fig. 2 and Table II). The NPL and Kong and Cox analyses corroborated these findings with multiple significant SNPs in the same region (Table II). When comparing the log of the inverse of the P-values obtained from all the SNPs and STRPs genotyped on chromosome 6, the SIBPAL, NPL, and Kong and Cox results were highly correlated with all the pairwise correlations being greater than 0.82 (Table III). The SNPs that are most significant in the qualitative Haseman–Elston analyses are the most significant SNPs for the NPL and Kung and Cox analyses.
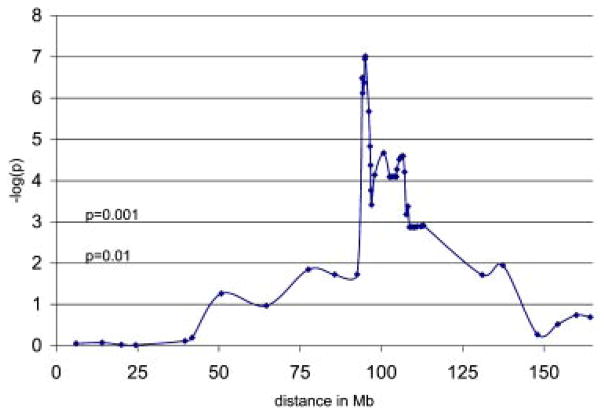
FIG. 2.
P-plot of model-independent multipoint linkage analysis results for microsatellite markers and SNPs on chromosome 6. In this plot, the inverse of the log of the P-value is plotted against the location of each marker. The log scale gives more emphasis to highly significant results and less to results with a significance level closer to 1.0. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
TABLE II.
Linkage Analysis of STRPs and SNPs on Chromosome 6
| Marker | Mba | Qualitative P-values Haseman–Elston
|
Quantitative P-values Haseman–Elston
|
Qualitative P-values
|
|||
|---|---|---|---|---|---|---|---|
| Singlepoint | Multipoint | Singlepoint | Multipoint | NPL multipoint | Kong and Cox multipoint | ||
| d6s1043 | 92.51 | 0.01774 | 0.01871 | 0.01401 | 0.01532 | 0.0004 | 0.0002 |
| rs164545 | 94.13 | <0.00001 | <0.00001 | 0.0001 | <0.00001 | 0.0003 | 0.0002 |
| d6s1056 | 94.15 | <0.00001 | <0.00001 | <0.00001 | 0.00001 | 0.0003 | 0.0002 |
| rs549965 | 94.16 | 0.00391 | <0.00001 | 0.00798 | <0.00001 | 0.0004 | 0.0002 |
| rs599005 | 94.25 | 0.15837 | <0.00001 | 0.21231 | 0.00001 | 0.0006 | 0.0005 |
| rs1386916 | 94.69 | 0.00203 | <0.00001 | 0.00457 | <0.00001 | 0.0006 | 0.0005 |
| rs1750604 | 94.96 | 0.00001 | <0.00001 | <0.00001 | <0.00001 | 0.0006 | 0.0003 |
| rs220969 | 95.04 | 0.04236 | <0.00001 | 0.01632 | <0.00001 | 0.0006 | 0.0003 |
| rs9320497 | 96.13 | <0.00001 | <0.00001 | <0.00001 | 0.00001 | 0.0008 | 0.0003 |
| rs2799642 | 96.47 | 0.00149 | 0.00001 | 0.00949 | 0.00002 | 0.0010 | 0.0007 |
| rs7752510 | 96.66 | 0.01096 | 0.00004 | 0.01563 | 0.00004 | 0.0012 | 0.0014 |
| rs9322788 | 96.81 | 0.22669 | 0.00017 | 0.16313 | 0.00008 | 0.0012 | 0.0014 |
| rs2205754 | 96.95 | 0.00004 | 0.00039 | 0.00001 | 0.00010 | 0.0010 | 0.0006 |
| d6s501 | 97.94 | 0.00058 | 0.00007 | 0.00068 | 0.00004 | 0.0002 | 0.0001 |
| d6s475 | 100.72 | <0.00001 | 0.00002 | 0.00005 | 0.00005 | 0.0005 | 0.0003 |
| gata164h01 | 102.53 | 0.00048 | 0.00008 | 0.00171 | 0.00017 | 0.0009 | 0.0007 |
| rs2518271 | 102.55 | 0.44689 | 0.00008 | 0.39512 | 0.00017 | 0.0009 | 0.0007 |
| rs2399802 | 103.00 | 0.12162 | 0.00008 | 0.13523 | 0.00017 | 0.0009 | 0.0007 |
| rs537534 | 103.02 | 0.00027 | 0.00008 | 0.00087 | 0.00017 | 0.0009 | 0.0007 |
| rs1041927 | 103.54 | 0.00002 | 0.00008 | 0.00042 | 0.00016 | 0.0009 | 0.0007 |
| rs9377547 | 104.18 | 0.00179 | 0.00008 | 0.00321 | 0.00016 | 0.0008 | 0.0006 |
| rs926275 | 104.56 | 0.00001 | 0.00008 | 0.00004 | 0.00016 | 0.0009 | 0.0007 |
| rs6571154 | 104.57 | 0.00096 | 0.00008 | 0.00103 | 0.00016 | 0.0009 | 0.0007 |
| d6s1021 | 104.78 | 0.00002 | 0.00005 | 0.00002 | 0.00011 | 0.0008 | 0.0006 |
| rs167539 | 105.52 | 0.00186 | 0.00003 | 0.00473 | 0.00006 | 0.0007 | 0.0006 |
| rs9399916 | 106.08 | 0.00025 | 0.00003 | 0.00017 | 0.00005 | 0.0007 | 0.0005 |
| rs1984224 | 106.64 | 0.01722 | 0.00003 | 0.03614 | 0.00005 | 0.0006 | 0.0005 |
| rs11153009 | 107.13 | 0.00001 | 0.00006 | 0.00004 | 0.00008 | 0.0040 | 0.0010 |
| rs7754744 | 107.66 | 0.02521 | 0.00066 | 0.02696 | 0.00061 | 0.0200 | 0.0300 |
| rs1625630 | 108.14 | 0.33741 | 0.00042 | 0.29866 | 0.00046 | 0.0400 | 0.0500 |
| rs217529 | 108.61 | 0.02583 | 0.0013 | 0.03316 | 0.00165 | 0.0500 | 0.0700 |
| rs2802290 | 109.01 | 0.00184 | 0.00133 | 0.00386 | 0.00165 | 0.0500 | 0.0700 |
| rs9398182 | 109.37 | <0.00001 | 0.00133 | <0.00001 | 0.00165 | 0.0500 | 0.0700 |
| rs1358997 | 109.81 | 0.00013 | 0.00134 | 0.00013 | 0.00166 | 0.0500 | 0.0700 |
| rs4072342 | 110.23 | 0.38271 | 0.00135 | 0.46401 | 0.00167 | 0.0500 | 0.0700 |
| rs9374172 | 110.84 | 0.45326 | 0.0013 | 0.52152 | 0.00162 | 0.0500 | 0.0700 |
| rs1543990 | 111.04 | 0.01483 | 0.00129 | 0.02553 | 0.0016 | 0.0500 | 0.0700 |
| rs20719626 | 112.12 | 0.00173 | 0.00128 | 0.00015 | 0.00159 | 0.0600 | 0.0700 |
| rs1050348 | 112.60 | 0.48402 | 0.00125 | 0.56811 | 0.00156 | 0.0600 | 0.0700 |
| d6s474 | 112.99 | 0.00116 | 0.00123 | 0.00198 | 0.00154 | 0.0600 | 0.0700 |
P-values <0.01 shown in bold.
Map positions from www.genome.use.edu.
TABLE III.
Pairwise Pearson’s Correlation Coefficients of Minus the Log of the P-Values for SNPs and STRPs on Chromosome 6
| SIBPAL qualitative | SIBPAL quantitative | NPL | Kong and Cox | |
|---|---|---|---|---|
| SIBPAL qualitative | 1.0000 | 0.9881 | 0.8362 | 0.8057 |
| <0.0001 | <0.0001 | <0.0001 | ||
| SIBPAL quantitative | 1.0000 | 0.8457 | 0.8265 | |
| <0.0001 | <0.0001 | |||
| NPL | 1.0000 | 0.9201 | ||
| <0.0001 | ||||
| Kong and Cox | 1.0000 |
r2 and significance of the r2 are listed.
Thirty-four SNPs between markers D10S1765 and D10S1239 were genotyped on chromosome 10 (5 families, 27 individuals). All 34 SNPs were genotyped using the ABI Taqman method. Sixteen SNPs were retained for analysis after running Tagger on Haploview, version 3.2 [Barret, 2005]. Model independent linkage analysis revealed multiple consecutive SNPs significant at P <0.001 (Fig. 3 and Table IV). Linkage results obtained from the NPL and Kong and Cox analyses again corroborated these findings in the same region on chromosome 10 (Table IV). Pearson’s correlation tests between the P-values obtained with the qualitative model and quantitative models as implemented in SIBPAL and the P-values obtained using NPL linkage and the Kong and Cox exponential model were again correlated (Table V), although not as highly correlated as the results on chromosome 6, with all the pairwise correlations being at least 0.61.
FIG. 3.

P-plot of model-independent multipoint linkage analysis results for microsatellite markers and SNPs on chromosome 10. The p-plot is representative of the data as in Figure 2. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
TABLE IV.
Linkage Analysis Results of STRPs and SNPs on Chromosome 10
| Marker | Mba | Qualitative P-values Haseman–Elston
|
Qualitative P-values Haseman–Elston
|
Quantitative P-values
|
|||
|---|---|---|---|---|---|---|---|
| Singlepoint | Multipoint | Singlepoint | Multipoint | NPL multipoint | Kong and Cox multipoint | ||
| d10s1765 | 89.59 | 0.01023 | 0.01056 | 0.01796 | 0.01817 | 0.02000 | 0.02000 |
| rs2296545 | 90.33 | 0.03429 | 0.00359 | 0.01248 | 0.00523 | 0.02000 | 0.01300 |
| rs2274878 | 91.09 | 0.02727 | 0.00133 | 0.01844 | 0.00143 | 0.01500 | 0.01100 |
| rs962524 | 91.52 | <0.00001 | 0.0054 | <0.00001 | 0.0061V | 0.00700 | 0.00500 |
| d10s2470 | 92.33 | 0.0007 | 0.00032 | 0.00065 | 0.00023 | 0.00300 | 0.00300 |
| rs12412496 | 92.54 | <0.00001 | 0.00032 | <0.00001 | 0.00023 | 0.00200 | 0.00300 |
| rs7913826 | 93.57 | 0.03252 | 0.00032 | 0.05268 | 0.00022 | 0.00200 | 0.00200 |
| rs10748585 | 94.67 | 0.03112 | 0.00032 | 0.06278 | 0.00022 | 0.00200 | 0.00200 |
| 10s185 | 95.18 | 0.00660 | 0.00320 | 0.01660 | 0.00022 | 0.00200 | 0.00200 |
| rs1555870 | 95.51 | 0.05395 | 0.00032 | 0.04093 | 0.00022 | 0.00150 | 0.00200 |
| d10s677 | 95.95 | <0.00001 | 0.00032 | <0.00001 | 0.00022 | 0.00150 | 0.00200 |
| rs1408S20 | 96.00 | 0.35869 | 0.00032 | 0.32229 | 0.00022 | 0.00150 | 0.00200 |
| rs7898759 | 96.79 | 0.02461 | 0.00032 | 0.02664 | 0.00022 | 0.00200 | 0.00200 |
| rs732102 | 97.18 | 0.00017 | 0.00032 | 0.00285 | 0.00022 | 0.00200 | 0.00200 |
| rs12571884 | 98.37 | 0.35603 | 0.00033 | 0.31990 | 0.00022 | 0.00200 | 0.00200 |
| rs7899632 | 99.99 | <0.00001 | 0.0003 | <0.000001 | 0.00021 | 0.00200 | 0.00200 |
| rs7094763 | 100.49 | 0.13853 | 0.03256 | 0.03882 | 0.02112 | 0.00003 | 0.00013 |
| rs4919438 | 102.00 | <0.00001 | 0.00247 | <0.00001 | 0.00059 | 0.00003 | 0.00012 |
| rs1361265 | 102.86 | 0.44611 | 0.00267 | 0.39126 | 0.00065 | 0.00003 | 0.00012 |
| rs946327 | 102.88 | 0.0015 | 0.00268 | 0.00045 | 0.00065 | 0.00003 | 0.00012 |
| d10s1239 | 103.19 | 0.01582 | 0.00291 | 0.03520 | 0.00073 | 0.00004 | 0.00012 |
| d10s1237 | 116.11 | 0.31373 | 0.34653 | 0.31313 | 0.31709 | 0.03000 | 0.04000 |
P-values <0.01 shown in bold.
Map positions from www.genome.ucse.edu.
TABLE V.
Pairwise Pearson’s Correlation Coefficients of Minus the Log of the Inverse of the P-Values for SNPs and STRPs on Chromosome 10
| SIBPAL qualitative | SIBPAL quantitative | NPL | Kong and Cox | |
|---|---|---|---|---|
| SIBPAL qualitative | 1.0000 | 0.9844 | 0.6109 | 0.6564 |
| <0.0001 | 0.0001 | <0.0001 | ||
| SIBPAL quantitative | 1.0000 | 0.6906 | 0.7363 | |
| <0.0001 | <0.0001 | |||
| NPL | 1.0000 | 0.9886 | ||
| <0.0001 | ||||
| Kong & Cox | 1.0000 |
r2 and significance of the r2 are listed.
DISCUSSION
In this study, we identified a subgroup of families based on a triple curve phenotype from a large study group of FIS families. Genome-wide linkage analysis followed by SNP mapping of significant areas has identified two distinct chromosomal regions. These regions on chromosomes 6 and 10 were effectively narrowed to 1 and 7 Mb, respectively. In the original analysis (set of 202 families), linkage analysis identified the same region on chromosome 6. The area on chromosome 10 was unique to this phenotype in the current study group. Wise et al. [2000] identified regions on 6p, distal 10q, and 18q from a genome-wide linkage study in one pedigree. However, both the chromosomes 6 and 10 regions identified by Wise et al. [2000] are a considerable distance away from the areas we report in the current study.
Classification systems used for identification of curvature types are used in the determination of specific treatment modalities (e.g., bracing and/or surgery), because some curve types are amenable to specific brace types [Montgomery and Willner, 1989; Katz et al., 1997; Janicki et al., 2007]. The primary utilization of current classification schemes are the ability to identify which levels to be fused at surgery, and to be able to compare more specifically clinical study groups in relation to surgical intervention. While these classification systems have been very useful in the clinical domain, their relationship to the genetic factors believed to be contributing to this disorder is unknown. In an attempt to further minimize the genetic heterogeneity within this study group of FIS families, we identified and evaluated different curve phenotypes including kyphoscoliosis, left thoracic, and triple curves for their statistical significance.
Data from the triple curves subset corroborated the original finding on chromosome 6 and identified an additional region on chromosome 10. Due to the high significance of these areas within this phenotype, additional genotyping studies were performed. As with any statistical study, the results reported could be due to chance. Replication of these findings in an independent sample will be key to determining if this subgroup represents a distinct and homogenous clinical entity. However, the scarcity of families including individuals with triple curves will make independent replication difficult.
The clinical deformity of scoliosis is a lateral rotatory curvature of the spine, which appears in otherwise normal individuals. Studies involving the pathophysiology of the bone itself and surrounding soft tissue structures have not yet identified an element or factor that could be designated as causative. Avenues of investigation now include multiple biological pathways as potentially related to this disease process including the central and peripheral nervous system, endocrine and hormonal influences, bone development and maintenance, skeletal muscle and signaling pathways, embryological signaling, and growth plate dysfunction [Miller, 2007; Miller et al., 2006; Pourquie, 2008]. Several genes in the candidate regions (Table VI) have been identified that may have relevance to the etiology of idiopathic scoliosis.
TABLE VI.
Candidate Genes on Chromosomes 6 and 10
| Candidate gene | Chromosome | Description |
|---|---|---|
| EPHA7 | 6 | Ligand involved in the repulsion of axonal growth cones and migrating cells |
| FUT9 | 6 | Synthesizes the Lewis X oligosaccharide is expressed in organ buds during embryogenesis and is developmentally regulated. Functions in cell to cell interaction during neuronal development |
| MANEA | 6 | Secretory pathway glycosylation enzyme found in mitochondria |
| CHUK | 10 | Essential for NFKB activation during limb and craniofacial development |
| CRTAC1 | 10 | An extracellular matrix protein expressed in bone, cartilage, and cultured chondrocytes |
| CSPG6 | 10 | Chondroitin sulfate proteoglycan |
| FBXW4 | 10 | Involved in signaling pathways crucial for normal limb development/split-hand/foot malformation |
| FER1L3 | 10 | Connected to muscular dystrophies |
| HABP2 | 10 | Glycosaminoglycan that is present in the extracellular matrix, connective tissue, cartilage, bone marrow, and synovial fluid |
| HTR7 | 10 | Serotonin receptor, regulatory effects on osteoclast development |
| KAZALD1 | 10 | Promotes proliferation of osteoblastic cells |
| LBX1 | 10 | Expressed during embryogenesis, expression restricted to developing CNS and muscles |
| LOXL4 | 10 | Cross-linking of collagen fibrils and insoluble elastic fibers in ECM, expressed in hypertrophic/calcified chondrocytes/growth plates |
| NFKB2 | 10 | Bone development/knockout mouse model of osteopetrosis due to a defect in osteoclast differentiation |
| NRAP | 10 | Giant myofibrillar proteins found within the sarcomeres of skeletal muscle |
| PLCE1 | 10 | Initiate a cascade of intracellular responses that result in cell growth and differentiation and gene expression |
| SEMA4G | 10 | Belongs to the semaphorin family, axonal guidance, and development in the A/P axis between the doral and ventral margins of the trunk and tail (zebrafish) |
| SFRP5 | 10 | Member of “frizzled” family of proteins/function as receptors for WNT genes |
| SL1T1 | 10 | Play a critical role in central nervous system midline formation |
| SUFU | 10 | Part of the Sonic Hedgehog signaling pathway/neural tube development |
| TLL2 | 10 | Structurally related to BMP-I/functions in ECM formation |
| WNT8 | 10 | WNT genes encode intercellular signaling glycoproteins that play important roles in key processes of embryonic development such as mesoderm induction, specification of the embryonic axis, and patterning of the central nervous system, spinal cord, and limbs |
On chromosome 6, a more confined region than that on chromosome 10, Ephrin Receptor (EPHA7) and Fucosyltransferase 9 (FUT9) are of particular interest due to their involvement in the embryological and migrational aspects of the nervous system. Multiple studies have indicated the potential involvement of the nervous system as an instigating factor that ultimately results in the loss of spinal stability resulting in the lateral rotatory curvature. Several genes in the candidate regions (Table VI) have been identified that may have relevance to the etiology of idiopathic scoliosis. Examples include lysl oxidase-like 4 gene (LOXL4), an enzyme that is involved in the cross-linking of collagen fibrils and insoluble elastic fibers within the extra cellular matrix, and 5-hydroxytryptamine receptor 7 (HTR7) gene, which regulates effects on osteoclast development and proliferation. Additional genes are involved in early embryonic signaling and development such as suppressor of fused (SUFU) and wingless type 8 (WNT8). SUFU maintains a critical part in neuronal tube development as part of the sonic hedgehog-signaling pathway. WNT8, as part of the WNT gene family, is involved in embryonic processes related to the spinal/ neuronal axis, and patterning of the early spinal cord. Despite the temporal disparity of the known action of these genes in embryonic development with that of the appearance of idiopathic scoliosis, underlying subtle aberrations in early regulatory processes hypothetically may lead to a later growth abnormality with hormonal signaling.
In conclusion, we have identified and narrowed candidate regions in a small subgroup of families with triple curves. Further analyses of these regions and sequencing of candidate genes will be required in order to identify genetic variants that may be associated with this disorder. Idiopathic scoliosis is the most common spinal deformity that is diagnosed in the skeletally immature individual. The definition of genetic markers associated with FIS will eventually allow the identification of those individuals with severely progressive disease. The immediate implication of the identification of an FIS gene is the development of a test for disease susceptibility and curve progression, with an ultimate goal of a test for accurate and early diagnosis. Once early diagnosis is available, more attention can be given to the development of non-surgical techniques that target spinal growth and development, with attention to responsiveness at the cellular level.
Acknowledgments
The Intramural Research Program of the National Human Genome Research Institute, National Institutes of Health, supported this research, in part. The genome-wide linkage screen was performed at the Center for Inherited Disease Research (CIDR). CIDR was funded through Federal contract N01-HG-65403 from the National Institutes of Health to the Johns Hopkins University. Some of the results were obtained with the program S.A.G.E., which is supported by grant 1 P41 RR03655 from the National Center for Research Resources. The Scoliosis Research Society, the National Scoliosis Foundation, Scoliosis Association, Inc., the Institute de France Foundation Yves Cotrel, the Orthopaedic Pediatric Society of North America provided additional grant funding, and the Orthopaedic Research and Education Foundation, and NIH grant 1-R01-AR048862-01A1. The authors would like to thank Leslie Barnes-Berry for her editorial assistance. All persons involved in this study gave their consent prior to their inclusion in the study.
Grant sponsor: Funding provided in part by International Research Program of the National Human Genome, Research Institute; Grant sponsor: Federal contract N01-HG-65403 from the National Institutes of Health to the Johns Hopkins University; Grant sponsor: National Center for Research Resources; Grant number: 1 P41 RR03655; Grant sponsor: The Scoliosis Research Society; Grant sponsor: National Scoliosis Foundation; Grant sponsor: Scoliosis Association, Inc.; Grant sponsor: Institute de France Foundation Yves Cotrel; Grant sponsor: Orthopaedic Pediatric Society of North America; Grant sponsor: Orthopaedic Research and Education Foundation; Grant sponsor: NIH; Grant number: 1-R01-AR048862-01A1.
References
- Abecasis GR, Cherny SS, Cookson WO, Cardon LR. Merlin-rapid analysis of dense genetic maps using sparse gene flow trees. Nat Genet. 2002;30:97–101. doi: 10.1038/ng786. [DOI] [PubMed] [Google Scholar]
- Armstrong GW, Gordon WD, Norman B, Livermore NB, Suzuki N. Nonstandard vertebral rotation in scoliosis screening patients: Its prevalence and relation to the clinical deformity. Spine. 1982;7:50–54. doi: 10.1097/00007632-198200710-00006. [DOI] [PubMed] [Google Scholar]
- Asher MA. Scoliosis evaluation. Orthop Clin North Am. 1988;19:805–814. [PubMed] [Google Scholar]
- Ausubel FM, editor. Current protocols in molecular biology. New York: Greene Publishing Associates and Wiley Interscience; 1987. [Google Scholar]
- Axenovich TI, Zaidman AM, Zorkolseve IV, Tregubora IL, Boroden PM. Segregation analysis of idiopathic scoliosis: Evidence for a major-gene effect. Am J Med Genet. 1999;86:389–394. [PubMed] [Google Scholar]
- Barret JC. Haploview: Analysis and visualization of LD and haplotype maps. Bioinformatics. 2005;21:263–265. doi: 10.1093/bioinformatics/bth457. [DOI] [PubMed] [Google Scholar]
- Boehnke M, Cox NJ. Accurate inference of relationships in sib-pair linkage studies. Am J Hum Genet. 1997;61:423–429. doi: 10.1086/514862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boileau C, Jondeau G, Babron MC, Coulon M, Alexandre JA, Sakai L, Melki J, Delmore G, Dubourg O, Bonaiti-Pellie C. Autosomal dominant Marfan-like connective-tissue disorder with aortic dilation and skeletal anomalies not linked to the fibrillin genes. Am J Hum Genet. 1993;53:46–54. [PMC free article] [PubMed] [Google Scholar]
- Bonati C, Feingold J, Briard M, Lapeyre F, Rigault P, Guivarch J. Genetics of idiopathic scoliosis. Helv Paediatr Acta. 1976;31:229–240. [PubMed] [Google Scholar]
- Broman KW, Weber JL. Estimation of pairwise relationships in the presence of genotyping errors. Am J Hum Genet. 1998;63:1563–1564. doi: 10.1086/302112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunch WH, Patwardhan AG. Recognizing patterns: Classification of scoliosis. In: Bunch WH, Patwardhan G, editors. Scoliosis, making clinical decisions. St. Louis: C.V. Mosby; 1989. pp. 21–149. [Google Scholar]
- Carr AJ. Adolescent idiopathic scoliosis in identical twins. J Bone Joint Surg. 1990;72-B:1077. doi: 10.1302/0301-620X.72B6.2246294. [DOI] [PubMed] [Google Scholar]
- Chan V, Fong GCY, Luk KDK, Yip B, Lee MK, Wong MS, Lu DDS, Chan TK. A genetic locus for adolescent idiopathic scoliosis linked to chromosome 19p13.3. Am J Hum Genet. 2002;71:401–4406. doi: 10.1086/341607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cobb JR. Outline for the study of scoliosis. Am Acad Orthop Surg Instr Course Lect. 1948;5:261–275. [Google Scholar]
- Conrad RW, Murrell GAC, Motley G, Lytle E, Hey LA. A logical coronal pattern classification of 2,000 consecutive idiopathic scoliosis cases based on the scoliosis research society defined apical vertebra. Spine. 1998;23:1380–1391. doi: 10.1097/00007632-199806150-00016. [DOI] [PubMed] [Google Scholar]
- Cowell HR, Hall JN, MacEwen GD. Genetic aspects of idiopathic scoliosis. A Nicholas Andry Award essay. Clin Orthop. 1972;86:121–131. doi: 10.1097/00003086-197207000-00018. [DOI] [PubMed] [Google Scholar]
- Cruickshank JL, Koike M, Dickson RA. Curve patterns in idiopathic scoliosis. J Bone Joint Surg. 1989;71-B:259–262. doi: 10.1302/0301-620X.71B2.2925744. [DOI] [PubMed] [Google Scholar]
- Czeizel A, Bellyei A, Barta O, Magda T, Molnar L. Genetics of adolescent idiopathic scoliosis. J Med Genet. 1978;15:424–427. doi: 10.1136/jmg.15.6.424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson D, Letts M, Jarvis J. Triple major curves in children. Can J Surg. 2003;46:193–198. [PMC free article] [PubMed] [Google Scholar]
- Filho NA, Thompson MW. Genetic studies in scoliosis. J Bone Joint Surg. 1971;53-A:199. [Google Scholar]
- Fritsch EF, Maniatis T, Sambrook J. Molecular cloning: A laboratory manual. Cold Spring Harbor, New York: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- Gao X, Gordon D, Zhang D, Browne R, Helms C, Gillum J, Weber S, Devrov S, Swaney S, Dobbs M, Morcuende J, Sheffield V, Lovett M, Bowcock A, Herring J, Wise C. CHD7 gene polymorphisms are associated with susceptibility to idiopathic scoliosis. Am J Hum Genet. 2007;80:957–965. doi: 10.1086/513571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garland HG. Hereditary scoliosis. Br Med J. 1934;1:328. doi: 10.1136/bmj.1.3816.328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein LA, Waugh TR. Classification and terminology of scoliosis. Clin Orthop. 1973;93:10–122. doi: 10.1097/00003086-197306000-00003. [DOI] [PubMed] [Google Scholar]
- James JL. Idiopathic scoliosis; the prognosis, diagnosis, and operative indications related to curve patterns and the age at onset. J Bone Joint Surg Br. 1954;36-B:36–49. doi: 10.1302/0301-620X.36B1.36. [DOI] [PubMed] [Google Scholar]
- Janicki JA, Peo-Dochert C, Armstrong DG, Thompson GH. A comparison of the thoracolumbosacral orthoses and providence orthosis in the treatment of adolescent idiopathic scoliosis: Results using the new SRS inclusion and assessment criteria for bracing studies. J Pediatr Orthop. 2007;27:369–374. doi: 10.1097/01.bpb.0000271331.71857.9a. [DOI] [PubMed] [Google Scholar]
- Justice CM, Miller NH, Marosy B, Zhang J, Wilson AF. Familial idiopathic scoliosis evidence of an X-linked susceptibility locus. Spine. 2003;28:589–594. doi: 10.1097/01.BRS.0000049940.39801.E6. [DOI] [PubMed] [Google Scholar]
- Kane WJ. Scoliosis prevalence: A call for a statement of terms. Clin Ortho. 1977;126:43–46. [PubMed] [Google Scholar]
- Katz DE, Richards BS, Browns RH, Herring JA. A comparison between the Boston brace and the Charleston beding brace in adolescent idiopathic scoliosis. Spine. 1997;22:1302–1313. doi: 10.1097/00007632-199706150-00005. [DOI] [PubMed] [Google Scholar]
- Kesling KL, Reinker KA. Scoliosis in twins: A meta-analysis of the literature and report of six cases. Spine. 1997;22:2009–2015. doi: 10.1097/00007632-199709010-00014. [DOI] [PubMed] [Google Scholar]
- King HA, Moe JH, Bradford DS, Winter RB. The selection of fusion levels in thoracic idiopathic scoliosis. J Bone Joint Surg. 1983;65-A:1302–1313. [PubMed] [Google Scholar]
- Kong A, Cox NJ. Allele-sharing models. Am J Hum Genet. 1997;61:1179–1188. doi: 10.1086/301592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lange M. Die operative behandlung des skoliose. Zeitschrift Orthopadie. 1956;88:41–65. [PubMed] [Google Scholar]
- Lenke LG, Betz RR, Clements D, Merola A, Haher T, Lowe T, Newton P, Bridwell KH, Blanke K. Curve prevalence of a new classification of operative adolescent idiopathic scoliosis. Does classification correlate with treatment? Spine. 2002;27:604–611. doi: 10.1097/00007632-200203150-00008. [DOI] [PubMed] [Google Scholar]
- Miller NH. Genetics of familial idiopathic scoliosis. Clin Orthop Relat Res. 2007;462:6–10. doi: 10.1097/BLO.0b013e318126c062. [DOI] [PubMed] [Google Scholar]
- Miller NH, Schwab DL, Sponseller PD, Shugert E, Bell J, Maestri N. Characterization of idiopathic scoliosis in a clinically well-defined population. Clin Orthop. 2001;392:349–357. doi: 10.1097/00003086-200111000-00045. [DOI] [PubMed] [Google Scholar]
- Miller NH, Justice CM, Marosy B, Doheny KF, Pugh E, Zhang J, Dietz HC, Wilson AF. Identification of candidate regions for familial idiopathic scoliosis. Spine. 2005;30:1181–1187. doi: 10.1097/01.brs.0000162282.46160.0a. [DOI] [PubMed] [Google Scholar]
- Miller NH, Marosy B, Justice CM, Novak SM, Tang EY, Boyce P, Pettenqil J, Dohenv KF, Pugh EW, Wislon AF. Linkage analysis of genetic loci for kyphoscoliosis on chromosomes 5p13, 13q13.3 and 13q32. Am J Med Genet Part A. 2006;140A:1059–1068. doi: 10.1002/ajmg.a.31211. [DOI] [PubMed] [Google Scholar]
- Moe JH, Kettleson DN. Idiopathic scoliosis. Analysis of curve patterns and the preliminary results of Milwaukee-brace treatment in 169 patients. J Bone Joint Surg. 1970;52-A:1509–1533. [PubMed] [Google Scholar]
- Montgomery F, Willner S. Prognosis of brace treated scoliosis. Comparison of Boston and Milwaukee methods in 244 girls. Acta Orthop Scand. 1989;60:383–385. doi: 10.3109/17453678909149302. [DOI] [PubMed] [Google Scholar]
- Moore D, Dowhan D. Purification and concentration of DNA from aqueous solutions. In: Ausubel FM, Brent R, Kingston RE, Moore DD, Seidman JG, Smith JA, Struhl K, editors. Current protocols in molecular biology. New York: John Wiley & Sons; 2002. pp. 2.1.1–2.1.10. [Google Scholar]
- Nash CL, Jr, Moe JH. A study of vertebral rotation. J Bone Joint Surg. 1969;51-A:223–229. [PubMed] [Google Scholar]
- O’Connell JR, Weeks DE. The VITESSE algorithm for rapid exact multilocus linkage analysis via genotype set-recording and fuzzy inheritance. Nat Genet. 1995;11:402–408. doi: 10.1038/ng1295-402. [DOI] [PubMed] [Google Scholar]
- Ocaka L, Zhao C, Reed JA, Ebenezer ND, Brice G, Morley T, Mehta M, O’Dowd J, Weber JL, Hardcastle AJ, Child AH. Assignment of two loci for autosomal dominant adolescent idiopathic scoliosis to chromosomes 9q31.2-q34.2 and 17q25.3-qtel. J Med Genet. 2008;45:87–92. doi: 10.1136/jmg.2007.051896. [DOI] [PubMed] [Google Scholar]
- Ponseti IV, Friedman B. Prognosis in idiopathic scoliosis. J Bone Joint Surg. 1950;32-A:381–395. [PubMed] [Google Scholar]
- Pourquie O. Building the spine: The vertebrate segmentation clock. Cold Spring Harb Symp Quant Biol. 2008;72:445–449. doi: 10.1101/sqb.2007.72.016. [DOI] [PubMed] [Google Scholar]
- Riseborough EJ, Wynne-Davies R. A genetic survey of idiopathic scoliosis in Boston, Massachusetts. J Bone Joint Surg. 1973;55-A:974–982. [PubMed] [Google Scholar]
- Rozen S, Skaletsky H. Primer3 on the WWW for general users and for biologist programmers. In: Misener S, Krawetz SA, editors. Bioinformatics methods and protocols. Totowa, NJ: Humana Press; 2000. pp. 365–386. [DOI] [PubMed] [Google Scholar]
- Salehi LB, Mangino M, De Serio S, Decicco D, Capon F, Semprini S, Pizzuti A. Assignment of a locus for autosomal dominant idiopathic scoliosis (IS) to human chromosome 17p11. Hum Genet. 2002;111:401–404. doi: 10.1007/s00439-002-0785-4. [DOI] [PubMed] [Google Scholar]
- Schulthess W. Die Pathologie und Therapie der Ruckgratsverkrummungen. Joachimsthal:Handbuch der Orthopadischen Chirurgie, BD. 1,Abt. 2. Jena, Gustav Fischer. 1905:1905–1907. [Google Scholar]
- Shands AR, Jr, Eisberg HB. The incidence of scoliosis in the State of Delaware: A study of 50,000 films of the chest made during a survey for tuberculosis. J Bone Joint Surg. 1955;37-A:1243–1247. [PubMed] [Google Scholar]
- Shapiro JR, Burn VE, Chipman SD, Velis KP, Bansal M. Osteoporosis and familial idiopathic scoliosis: Association with an abnormal alpha 2(I) collagen. Conn Tiss Res. 1989;21:117–123. doi: 10.3109/03008208909050002. [DOI] [PubMed] [Google Scholar]
- Stagnara P, Queneau P. Scolioises evolutives en periode de croissance. Rev Orthop. 1953;39:378–449. [PubMed] [Google Scholar]
- S.A.G.E., Statistical Analysis for Genetic Epidemiology. [Software package] Version 4.5. Department of Epidemiology and Biostatistics, Case Western University; Cleveland, Ohio: [Google Scholar]
- Stokes IAF, Armstrong JG, Moreland MS. Spinal deformity and back surface asymmetry in idiopathic scoliosis. J Ortho Res. 1988;6:129–137. doi: 10.1002/jor.1100060117. [DOI] [PubMed] [Google Scholar]
- Travaglini F. Multiple primary idiopathic scoliosis. Ital J Orthop Traumatol. 1975;1:1167–1180. [PubMed] [Google Scholar]
- Weinstein SL. Adolescent idiopathic scoliosis: Prevalence and natural history. In: Weinstein SL, editor. The pediatric spine: Principles and practice. New York: Raven Press, Ltd; 1994. pp. 463–478. [Google Scholar]
- Weinstein SL, Ponseti IV. Curve progression in idiopathic scoliosis. J Bone Joint Surg. 1983;65-A:447–455. [PubMed] [Google Scholar]
- Whittemore AS, Halpern J. A class of tests for linkage using affected pedigree members. Biometrics. 1994;50:118–127. [PubMed] [Google Scholar]
- Wise CA, Barnes R, Gillum J, Herring JA, Bowcock AM, Lovett M. Localization of susceptibility to familial idiopathic scoliosis. Spine. 2000;25:2372–2380. doi: 10.1097/00007632-200009150-00017. [DOI] [PubMed] [Google Scholar]
- Wynne-Davies R. Familial (idiopathic) scoliosis. A family survey. J Bone Joint Surg. 1968;50-B:24–30. [PubMed] [Google Scholar]