Abstract
The African Swine Fever Virus (ASFV) encodes a single Nudix enzyme in its genome, termed the g5R protein (g5Rp). Nudix phosphohydrolases cleave a variety of substrates, such as nucleotides and diphosphoinositol polyphosphates. Previously, ASFV g5Rp was shown to hydrolyze diphosphoinositol polyphosphates and GTP, but was unable to cleave methylated mRNA cap analogues. In vaccinia virus (VACV), a distant relative of ASFV, the D9 and D10 Nudix enzymes were shown to cleave the mRNA cap, but only when the cap was attached to an RNA body. Here, we show that recombinant ASFV g5Rp hydrolyzes the mRNA cap when tethered to an RNA moiety, liberating m7GDP as product. Mutations in the Nudix motif abolished mRNA decapping activity, confirming that g5Rp was responsible for cap cleavage. The decapping activity of g5Rp was potently inhibited by excess uncapped RNA but not by methylated cap analogues, suggesting that substrate recognition occurs by RNA binding.
Keywords: mRNA decapping, Nudix enzyme, African Swine Fever Virus, g5R protein
Introduction
The Nudix hydrolase motif is a signature sequence characteristic of a diverse group of phosphohydrolases found in viruses, prokaryotes, and eukaryotes (reviewed in McLennan, 2006). Nudix enzymes cleave a broad group of substrates that are generally comprised of a nucleoside diphosphate linked to another moiety, X (Koonin, 1993; Bessman et al., 1996). Interestingly, the Nudix enzymes found in viruses are restricted almost exclusively to the five viral families that belong to the monophyletic lineage of large nucleocytoplasmic DNA viruses, comprised of poxviruses, asfarviruses, iridoviruses, phycodnaviruses and mimiviruses, suggesting possible overlapping functions for these proteins (Iyer et al., 2001; Iyer et al., 2006).
The D9 and D10 proteins of vaccinia virus (VACV), the prototypic poxvirus, are Nudix hydrolases that share 25% sequence identity to each other (Shors et al., 1999). D9 and D10 are expressed at different times during virus infection; D9 is expressed early whereas D10 is expressed during the late phase of viral infection (Lee-Chen and Niles, 1988; Parrish and Moss, 2006). Previous genetic studies demonstrated that over-expression of the D9R (VACV-WR_114) or the D10R (VACV-WR_115) gene resulted in enhanced turnover of mRNA molecules containing a 5′ m7GpppNm cap, a stabilizing component of both VACV and cellular transcripts (Shors et al., 1999). Moreover, deletion of the D10R gene from the VACV genome resulted in the persistence of cellular and viral transcripts and a delay in the shutoff of host protein synthesis (Parrish and Moss, 2006). These two genetic observations led to the hypothesis that D9 and D10 cleave the mRNA cap, thereby accelerating viral and cellular mRNA turnover and promoting the sequential cascade of viral gene expression and the shutoff of host protein synthesis. In support of this hypothesis, Dcp2, a Nudix enzyme conserved from yeasts to mammals, has been shown to be an mRNA decapping enzyme (Wang et al., 2002; Van Dijk et al., 2002; Steiger et al., 2003; Cohen et al., 2005; Xu et al., 2006).
More recent biochemical studies confirmed that both VACV D9 and D10 contain intrinsic mRNA decapping activity, releasing m7GDP as a reaction product (Parrish et al., 2007; Parrish and Moss, 2007). Similar to eukaryotic Dcp2, D9 and D10 were unable to efficiently cleave a free methylated cap analogue (m7GpppNm); robust decapping activity was only observed when the methylated cap structure was tethered to an RNA moiety (Wang et al., 2002; Van Dijk et al., 2002; Piccirillo et al., 2003; Steiger et al., 2003; Cohen et al., 2005; Parrish et al., 2007; Parrish and Moss, 2007). In accord with this observation, uncapped RNA inhibited D9 and D10 decapping activity, suggesting RNA binding is required for these proteins to locate and cleave the cap structure (Parrish et al., 2007; Parrish and Moss, 2007). In addition, free methylated cap derivatives inhibited cap cleavage by D9 and D10, indicating that these proteins may also interact with the cap structure during substrate recognition (Parrish et al., 2007; Parrish and Moss, 2007).
African Swine Fever Virus (ASFV), the lone representative of the Asfarviridae virus family, encodes a single Nudix enzyme in its genome, denoted as the g5R protein (g5Rp) (NCBI ID: NP_042795) (Cartwright et al., 2002). Intriguingly, ASFV g5Rp shares greater sequence similarity to the Schizosaccharomyces pombe Dcp2 mRNA decapping enzyme than either VACV D9 or D10 (McLennan, 2007). Previous biochemical studies demonstrated that g5Rp hydrolyzes a broad range of substrates, most efficiently cleaving diphosphoinositol polyphosphates but also hydrolyzing nucleotide substrates such as GTP (Cartwright et al., 2002). Despite its broad substrate range, g5Rp was unable to efficiently cleave free methylated cap analogues, which led to the conclusion that g5Rp was not an mRNA decapping enzyme (Cartwright et al., 2002). However, in light of the recent observations that cap attachment to an mRNA body is required for Nudix-mediated mRNA decapping, the role of g5Rp in this process needs to be reevaluated (Wang et al., 2002; Van Dijk et al., 2002; Piccirillo et al., 2003; Steiger et al., 2003; Cohen et al., 2005; Parrish et al., 2007; Parrish and Moss, 2007).
To examine if ASFV g5Rp possesses mRNA decapping activity, a g5Rp fusion protein was expressed in bacteria and subsequently purified by affinity chromatography. In contrast with the limited activity of g5Rp on free methylated cap analogues, g5Rp was able to robustly cleave a cap structure attached to an mRNA moiety in a manner dependent on the Nudix motif, releasing m7GDP as product. g5Rp-decapping activity was inhibited by uncapped RNA but not methylated cap analogue derivatives, suggesting that g5Rp recognizes the RNA moiety to find target substrates.
Results
Recombinant ASFV g5Rp decaps mRNA
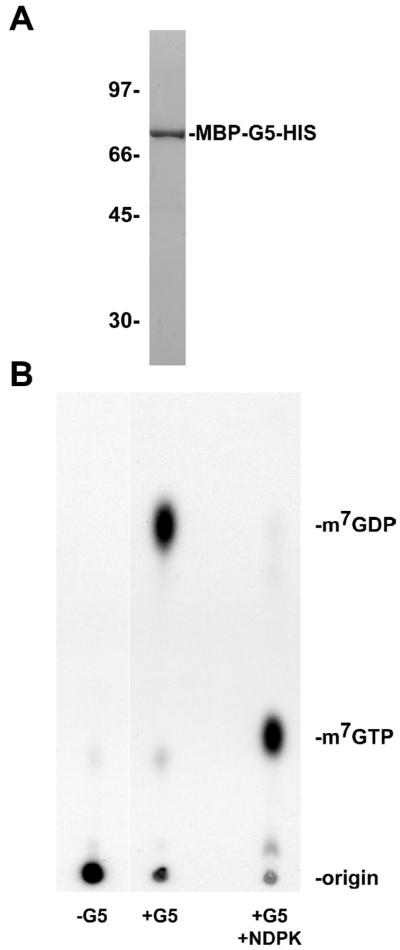
Although it was previously shown that ASFV g5Rp cannot cleave a free methylated cap structure, recent studies demonstrated that mRNA decapping mediated by Nudix enzymes is dependent on the methylated cap structure being tethered to an mRNA body (Wang et al., 2002; Van Dijk et al., 2002; Piccirillo et al., 2003; Steiger et al., 2003; Cohen et al., 2005; Parrish et al., 2007; Parrish and Moss, 2007). To determine if the ASFV g5Rp can cleave a cap structure on an intact mRNA, a maltose binding protein (MBP)-g5R fusion protein containing a C-terminal His10 tag (MBP-g5R-HIS) was expressed in Escherichia coli and purified by affinity chromatography through amylose and nickel-nitrilotriacetic acid columns. Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS/PAGE) analysis of the purified protein fractions revealed a single ∼75-kDa band corresponding to the predicted mass of MBP-g5R-HIS (Fig. 1A).
Fig. 1.
Recombinant ASFV g5Rp catalyzes RNA cap cleavage. (A) ASFV g5Rp was expressed as an MBP-g5R fusion protein appended with a C-terminal His10 tag in Escherichia coli and then purified over amylose and nickel-nitrilotriacetic acid columns. Purified recombinant g5Rp was resolved by SDS/PAGE and detected by staining with Coomassie blue. Protein mass standards (in kDa) are labeled on the left whereas the ∼75-kDa recombinant MBP-g5R-HIS band is indicated on the right. (B) 75 ng of recombinant ASFV g5Rp was incubated with 0.02 pmol of 32P-cap-labeled actin RNA in decapping buffer for 30 min at 37°C. A portion of this reaction was treated with 2 units of nucleoside diphosphate kinase (NDPK) and 1 mM ATP for an additional 30 min at 37°C. NDPK adds a phosphate group exclusively to nucleoside diphosphates, resulting in the production of nucleoside triphosphates. Reaction products were resolved by PEI-cellulose TLC and detected by autoradiography. Unlabeled nucleotide standards were visualized by UV shadowing and are designated on the right.
Next, the recombinant g5Rp was incubated with a 309-nt 32P-cap-labeled RNA substrate and the products of the reaction were resolved by polyethyleneimine (PEI)-cellulose thin layer chromatography (TLC) and detected by autoradiography. Unlabeled nucleotide standards were visualized by UV shadowing. In the absence of recombinant g5Rp, the 32P-cap-labeled RNA substrate remained at the origin of the plate (Fig. 1B). However, inclusion of recombinant g5Rp in the decapping reaction resulted in the release of a product that co-migrated with an unlabeled m7GDP standard (Fig. 1B). To confirm the identity of the released m7GDP product, a portion of the decapping reaction was incubated with nucleoside diphosphate kinase (NDPK), an enzyme that specifically adds a phosphate group to nucleoside diphosphate substrates, thereby producing nucleoside triphosphate products. Following treatment with NDPK and resolution by PEI-cellulose TLC, the m7GDP product shifted to co-migrate with the unlabeled m7GTP standard, verifying that the product originally released by g5Rp was m7GDP (Fig. 1B). The amount of product liberated by g5Rp cap cleavage increased with increasing enzyme concentration and incubation time (Fig. 2A and 2B, respectively).
Fig. 2.
Effects of enzyme concentration and time on ASFV g5Rp cap cleavage activity. (A) Increasing amounts of recombinant g5Rp were added to 0.02 pmol of 32P-cap-labeled actin RNA in decapping buffer for 30 min at 37° C. Following resolution of the products by PEI-cellulose TLC, the percentage of m7GDP product released was calculated using a PhosphorImager. (B) 50 ng of recombinant g5Rp was assessed for mRNA decapping activity as in Panel A, except that incubation times varied as indicated on the graph.
The Nudix motif of ASFV g5Rp is required for mRNA decapping
The Nudix hydrolase motif consists of the highly conserved amino acid sequence GX5EX5[UA]XREX2EEXGU where U represents an aliphatic, hydrophobic residue and X represents any amino acid (Koonin, 1993; Bessman et al., 1996). For several Nudix hydrolases, the glutamic acid residues in the EX2EE sequence have been shown to be essential for catalytic activity, coordinating divalent cation binding and nucleophilic attack of the phosphate bond (reviewed in Mildvan et al., 2005). To demonstrate that recombinant g5Rp was solely responsible for cap cleavage in a manner dependent on the Nudix motif, mutations were introduced in the critical EX2EE residues of this sequence. Specifically, one g5R mutant protein was synthesized in which the glutamic acid at residue 147 was converted into a glutamine (E147Q). A second g5R mutant protein was created in which the two glutamic acid residues at positions 150 and 151 were changed to glutamine residues (E150Q/E151Q). These two mutant proteins were expressed and purified concomitantly with wild-type recombinant g5Rp and resolved by SDS/PAGE (Fig. 3A). As expected, incubation of the 32P-cap-labeled RNA substrate with wild-type g5Rp resulted in cap cleavage, as observed by m7GDP release (Fig. 3B). When equivalent amounts of the two mutant versions of the g5Rp were included in the decapping reaction, m7GDP was not released, verifying that g5Rp was the protein responsible for mRNA decapping and that this activity was dependent on the Nudix hydrolase motif (Fig. 3B).
Fig. 3.
The Nudix motif is required for ASFV g5rp mRNA decapping activity. (A) Two mutated versions of g5Rp, E147Q and E150Q/E151Q, were created through site-directed mutagenesis. The E147Q mutant contains a point mutation at position 147 that changes a glutamic acid residue to a glutamine residue. Likewise, E150Q/E151Q contains two point mutations in which the glutamic acid residues at positions 150 and 151 have both been transformed to glutamine residues. The E147Q and E150Q/E151Q mutant proteins were expressed in Escherichia coli and purified concurrently with wild-type recombinant g5Rp as described in Fig.1A. The purified proteins were electrophoretically separated by SDS/PAGE and visualized by Coomassie blue staining. Masses of protein markers (in kDa) are indicated on the left. (B) Equal quantities (100 ng) of recombinant g5Rp and the two mutated proteins (E147Q and E150Q/E151Q) were included in separate decapping reactions and assayed for cap hydrolysis as described in Fig. 1B.
The ASFV g5Rp recognizes the RNA body
The finding that g5Rp requires an RNA moiety to mediate mRNA decapping suggests that this protein binds mRNA to locate its substrate. To investigate if g5Rp interacts with mRNA during substrate recognition, increasing amounts of uncapped RNA substrate were included in the decapping reaction and the effect on g5Rp decapping activity was calculated. Inclusion of even a modest 1-fold molar excess of uncapped RNA reduced g5R decapping activity by greater than ∼81%, compared to the ∼17% reduction of decapping activity observed for VACV D10 (Fig. 4A). In fact, the significant inhibition of g5Rp decapping activity by such minute amounts of uncapped RNA hindered efforts to determine kinetic constants for the g5R enzyme. Each cap-labeled RNA substrate preparation contains a proportion of uncapped RNA. Kinetic parameters are calculated by increasing the amount of substrate while maintaining a constant enzyme concentration; hence as the amount of substrate was increased, the subsequent rise in uncapped RNA competitor abolished g5Rp-decapping activity.
Fig. 4.
ASFV g5Rp recognizes the RNA moiety. (A) Uncapped, unlabeled 309-nt actin RNA was added in increasing quantities to decapping reactions containing either 80 ng of recombinant ASFV g5Rp or VACV D10. The reaction products were separated by PEI-cellulose TLC and quantified by PhosphorImager analysis. (B) Recombinant ASFVg5Rp and uniformly 32P-labeled uncapped actin RNA were incubated in electrophoretic gel shift assay buffer. After 15 min on ice, the reactions were separated on a 6% native polyacrylamide gel and visualized using a PhosphorImager. mDAZL, a characterized RNA binding protein, was used as a positive control for RNA binding (Jiao et al, 2002). MBP, which should not bind RNA, was utilized as a negative control for the assay.
To directly determine if ASFV g5Rp is capable of binding RNA, an electrophoretic gel mobility assay was performed in which non-denaturing PAGE was employed to resolve a uniformly 32P-labeled 309-nt uncapped RNA. As a positive control, the RNA binding protein mDAZL was added to the labeled RNA, resulting in a shift in the migration of the RNA through the gel (Fig. 4B) (Jiao et al., 2002). Conversely, MBP alone, which should not bind RNA, did not shift the mobility of the RNA (Fig. 4B). Upon addition of the recombinant ASFV g5Rp, a shift in mobility was observed for a portion of the RNA, suggesting that g5Rp bound to the RNA and reduced its mobility through the gel matrix (Fig. 4B). The incomplete RNA shift exerted by g5R may reflect a transient interaction between g5R and RNA in the absence of a cap structure; alternatively, the RNA-protein complex may have partially dissociated upon electrophoresis.
The mRNA decapping activity of ASFV g5rp is not inhibited by methylated nucleotides
The mRNA decapping activity of the VACV D9 and D10 proteins was inhibited by m7GpppG, m7GTP, and m7GDP, with D10 mRNA decapping activity being more hindered by these methylated cap structures than D9 (Parrish et al., 2007; Parrish and Moss, 2007). To determine if ASFV g5rp activity was also inhibited by addition of methylated nucleotides, increasing amounts of m7GpppG or m7GTP, or the unmethylated versions of these nucleotides, were added to the decapping reactions and the amount of product released was calculated. The addition of either m7GpppG or m7GTP had no effect on g5R cap cleavage, suggesting that this protein may not recognize the cap structure to locate its substrate (Fig. 5A and B). Interestingly, g5R decapping activity was not inhibited by GTP or GpppG, despite the observation that g5R was capable of cleaving GTP and GpppG in vitro (Cartwright et al., 2002). The ability of the recombinant g5R to cleave GTP and GpppG was reconfirmed in our studies (data not shown).
Fig. 5.
ASFV g5Rp decapping activity is not affected by cap analogue or methylated nucleotides. (A) 80 ng of recombinant ASFV g5Rp or VACV D10 and 0.02 pmol of 32P-cap-labeled actin RNA were incubated with increasing amounts of m7GpppG or GpppG and the products of the reaction were resolved by PEI-cellulose TLC. The percentage of m7GDP released was quantified using PhosphorImager analysis. (B) Increasing amounts of m7GTP or GTP were added to the decapping reaction and quantified as in Panel A.
Discussion
The nearly exclusive restriction of viral Nudix enzymes to the ancestral lineage of the large nucleocytoplasmic DNA viruses suggests that these proteins may have analogous functions. In VACV, the D9 and D10 Nudix enzymes were shown to hydrolyze the mRNA cap structure, thereby accelerating mRNA turnover and allowing the virus to manipulate host and viral gene expression (Parrish et al., 2007; Parrish and Moss, 2007). ASFV, another member of the large nucleocytoplasmic DNA virus lineage, encodes a single Nudix enzyme in its genome, g5Rp, which shares greater sequence similarity to VACV D10 than D9. Previous studies suggested that ASFV g5Rp was not an mRNA decapping enzyme, given that the enzyme did not cleave free cap analogues in vitro (Cartwright et al., 2002). However, in the present study, recombinant ASFV g5Rp was shown to possess decapping activity, efficiently hydrolyzing the cap structure only when attached to an mRNA molecule, mimicking VACV D9 and D10 and eukaryotic Dcp2 substrate requirements (Wang et al., 2002; Van Dijk et al., 2002; Piccirillo et al., 2003; Steiger et al., 2003; Cohen et al., 2005; Parrish et al., 2007; Parrish and Moss, 2007). Indeed, ASFV g5Rp shares greater sequence similarity to eukaryotic Dcp2 than either VACV D9 or D10 (McLennan, 2007), further implicating g5Rp as an mRNA decapping enzyme. The product released by g5Rp cleavage of the mRNA cap was confirmed to be m7GDP, distinguishing g5Rp activity from DcpS, a eukaryotic enzyme that cleaves free mRNA caps to release m7GMP as a reaction product (Wang and Kiledjian, 2001). Importantly, g5Rp-decapping activity was abolished by mutations in critical Nudix motif residues, confirming that catalytic activity was dependent on the Nudix motif and that g5Rp was the protein directly responsible for the mRNA decapping activity.
Like VACV D9 and D10, as well as eukaryotic Dcp2, ASFV g5Rp mRNA decapping activity was strongly inhibited by uncapped mRNA, further supporting the requirement of the mRNA moiety for substrate recognition (Piccirillo et al., 2003; Cohen et al., 2005; Parrish et al., 2007; Parrish and Moss, 2007; Gunawardana et al., 2008). The effect of uncapped mRNA was much more striking for ASFV g5Rp than VACV D9 or D10 (g5Rp>D9>D10), suggesting g5Rp may have a stronger affinity for RNA than the VACV enzymes (Parrish et al., 2007; Parrish and Moss, 2007). Moreover, g5Rp was shown to directly bind mRNA through an electrophoretic gel shift assay. A complete shift of the RNA by g5Rp was not observed, suggesting that the cap structure may be required to stabilize the g5Rp-RNA interaction. Alternatively, g5Rp may only have strong affinity for target RNA molecules that possess a particular secondary structure. For example, human Dcp2 has been shown to preferentially bind mRNA targets containing a specific 5′ stem loop structure (Li et al., 2008; and Li et al., 2009). Furthermore, eukaryotic Dcp2 contains two additional domains that modulate enzyme function: Box A, a region thought to be involved in protein-protein interactions and cleavage specificity, and Box B, which is proposed to bind RNA (Wang et al., 2002; Piccirillo et al., 2003). Only weak sequence similarity can be detected between these regions of Dcp2 and the VACV and ASFV enzymes; future structure/function analyses need to be performed to define the RNA binding domains in the viral decapping enzymes. In contrast to RNA, free methylated cap derivatives had no effect on ASFV g5Rp decapping, whereas they potently inhibited VACV D10 decapping and had a modest effect on D9 decapping (D10>D9>g5Rp) (Parrish et al., 2007; Parrish and Moss, 2007). In this respect, ASFV g5Rp more closely resembles eukaryotic Dcp2, which is not affected by the addition of methylated cap derivatives unattached to RNA (Van Dijk et al., 2002; Piccirillo et al., 2003; Cohen et al., 2005).
Similar to VACV, ASFV mRNA transcripts are capped by a virally encoded capping enzyme and expressed in a programmed temporal cascade (Salas et al., 1981; Salas et al., 1986; Carvalho and Rodrigues-Pousada, 1986; Sanataren and Vinuela, 1986). Moreover, ASFV infection induces a shutoff of host protein synthesis, potentially allowing the virus preferential access to translation machinery and macromolecular building blocks, while also hindering synthesis of immunomodulatory proteins (Tabares et al., 1980; Rodriguez et al., 2001). Hence, g5Rp mediated mRNA decapping may provide the virus with a mechanism to regulate the transitions between viral gene expression and promote the shutoff of host protein synthesis. In addition to the mRNA cap, ASFV g5Rp has also been shown to efficiently hydrolyze diphosphoinositol polyphosphates and GTP in vitro, raising the possibility that g5Rp have multiple in vivo functions, not unusual for viral enzymes (Cartwright et al., 2002). Future studies examining the effects of deletion or over-expression of the g5R gene should help clarify the in vivo function(s) of ASFV g5Rp.
Materials and Methods
Plasmid construction
An Escherichia coli codon optimized version of the ASFV g5R gene appended with a C-terminal 10X histidine tag was synthesized by GENEART (Regensburg, Germany) and used as template for amplification by the polymerase chain reaction (PCR) using the oligonucleotide primers 5′-ATG GAT ACC GCC ATG CAG CTG AAA ACC AGC and 5′-GCG CAA GCT TTT AAT GGT GAT GGT GAT GAT GGT GGT GAT G. The gel purified PCR product was cloned into the pMal-c2x protein expression vector (New England Biolabs, Ipswich, MA) immediately downstream of the malE gene, creating the pMAL-c2x-malE-g5R-his plasmid that encodes the MBP-g5R-10X histidine (MBP-g5R-HIS) recombinant protein. Mutated versions of MBP-g5R-HIS were generated through use of the Quikchange site-directed mutagenesis kit (Stratagene, La Jolla, CA).
Expression and purification of recombinant ASFV g5R protein
Escherichia coli strain BL21 (EMD Biosciences, San Diego, CA) was transformed with wild-type or mutated versions of pMAL-c2x-malE-g5R-his and propagated in LB broth containing 50 μg/ml carbenicillin and 0.2% (w/v) glucose. Recombinant protein synthesis was induced with 0.3 mM isopropyl β-D-1 thiogalactopyranoside at 30 °C. After 4 h, the cells were lysed in B-per detergent (Pierce, Rockford, IL) and the recombinant protein was purified through an amylose column (New England Biolabs) followed by a nickel nitrilotriacetic acid column (Qiagen, Valencia, CA). The purified protein was then dialyzed into 10 mM Tris-HCl pH 7.5, 100 mM NaCl, 10% glycerol, 1 mM DTT, and 2 mM Mg acetate (Piccirillo, et al., 2003). Recombinant MBP-D10 was synthesized and purified as described by Parrish et al. (Parrish et al., 2007).
RNA substrate synthesis
The pTRI-β-actin-human template (Ambion, Austin, TX) was in vitro transcribed using the MEGAshortscript kit (Ambion) and the resulting 309-nt actin RNA was cap-labeled using recombinant VACV guanylyltransferase/guanine-7-methyltransferase (Martin and Moss, 1975) acquired from Epicentre Biotechnologies (Madison, WI) in the presence of 0.132 μM [α32P] GTP, capping buffer (50 mM Tris-HCl pH 8.0, 6 mM KCl, 1.25 mM DTT, 1.25 MgCl2) and 0.1 mM S-adenosylmethionine. Unincorporated nucleotides were removed from the cap-labeled RNA through use of ProbeQuant G-50 gel filtration columns (GE Healthcare, Piscataway, NJ).
RNA decapping assays
Reaction mixtures (15 μl) containing decapping buffer (100 mM K acetate, 10 mM Tris-HCl pH 7.5, 2 mM MgCl2, 0.5 mM MnCl2, and 2 mM DTT), 0.02 pmol of cap-labeled RNA, and approximately 1.05 pmol (80 ng) purified recombinant MBP-g5R-HIS protein (unless specified otherwise) were incubated for 30 min at 37°C (Piccirillo et al., 2003). The products of the reaction (2 μl) were spotted on a polyethyleneimine-cellulose thin layer chromatography plate (Alltech Associates, Inc., Columbia, MD) and developed in 0.75 M LiCl. The radioactive signals were visualized by autoradiography or PhosphorImager analysis (Molecular Dynamics, Sunnyvale, CA) and unlabeled TLC standards were detected by UV shadowing.
Electrophoretic Mobility Shift Assay
Increasing amounts of recombinant ASFVg5Rp (0.28 μg, 0.56 μg, and 1.1 μg) were incubated with uniformly 32P-labeled uncapped actin RNA in electrophoretic mobility shift assay buffer (10 mM Tris HCl, pH 7.5, 50 mM KCl, 1.5 mM MgCl2) on ice for 15 min. 1.1 μg of mDAZL, an RNA binding protein, was utilized as a positive control for RNA binding (Jiao et al., 2002). As a negative control, 2 μg of recombinant MBP, which should not possess mRNA binding activity, was incubated with the 32P-labeled RNA substrate. Protein-RNA complexes were resolved on a 6% native polyacrylamide gel that was subsequently visualized by PhosphorImager analysis.
Acknowledgments
We thank Wolfgang Resch, George Katsafanas, and Teri Shors for helpful discussions. This research was supported by the Intramural Research Program of the National Institute of Allergy and Infectious Diseases/ National Institutes of Health and a McDaniel College Faculty Development Grant.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bessman MJ, Frick DN, O’Handley SF. The MutT proteins or “Nudix” hydrolases, a family of versatile, widely distributed, “housecleaning” enzymes. J. Biol. Chem. 1996;271:25059–25062. doi: 10.1074/jbc.271.41.25059. [DOI] [PubMed] [Google Scholar]
- Cartwright JL, Safrany ST, Dixon LK, Darzynkiewicz E, Stepinski J, Burke R, McLennan AG. The g5R (D250) gene of African swine fever virus encodes a Nudix hydrolase that preferentially degrades diphosphoinositol polyphosphates. J. Virol. 2002;76:1415–1421. doi: 10.1128/JVI.76.3.1415-1421.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvalho ZG, Rodrigues-Pousada C. African swine fever virus gene expression in infected Vero cells. J. Gen. Virol. 1986;67(Pt 7):1343–1350. doi: 10.1099/0022-1317-67-7-1343. [DOI] [PubMed] [Google Scholar]
- Cohen LS, Mikhli C, Jiao X, Kiledjian M, Kunkel G, Davis RE. Dcp2 Decaps m2,2,7GpppN-capped RNAs, and its activity is sequence and context dependent. Mol. Cell Biol. 2005;25:8779–8791. doi: 10.1128/MCB.25.20.8779-8791.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunawardana D, Cheng HC, Gayler KR. Identification of functional domains in Arabidopsis thaliana mRNA decapping enzyme (AtDcp2) Nucleic Acids Res. 2008;36:203–216. doi: 10.1093/nar/gkm1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iyer LM, Aravind L, Koonin EV. Common origin of four diverse families of large eukaryotic DNA viruses. J. Virol. 2001;75:11720–11734. doi: 10.1128/JVI.75.23.11720-11734.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iyer LM, Balaji S, Koonin EV, Aravind L. Evolutionary genomics of nucleocytoplasmic large DNA viruses. Virus Res. 2006;117:156–184. doi: 10.1016/j.virusres.2006.01.009. [DOI] [PubMed] [Google Scholar]
- Jiao X, Trifillis P, Kiledjian M. Identification of target messenger RNA substrates for the murine deleted in azoospermia-like RNA-binding protein. Biol. Reprod. 2002;66:475–485. doi: 10.1095/biolreprod66.2.475. [DOI] [PubMed] [Google Scholar]
- Koonin EV. A highly conserved sequence motif defining the family of MutT-related proteins from eubacteria, eukaryotes and viruses. Nucleic Acids Res. 1993;21:4847. doi: 10.1093/nar/21.20.4847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee-Chen GJ, Niles EG. Transcription and translation mapping of the 13 genes in the vaccinia virus HindIII D fragment. Virology. 1988;163:52–63. doi: 10.1016/0042-6822(88)90233-4. [DOI] [PubMed] [Google Scholar]
- Li Y, Song MG, Kiledjian M. Transcript-specific decapping and regulated stability by the human Dcp2 decapping protein. Mol. Cell Biol. 2008;28:939–948. doi: 10.1128/MCB.01727-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Ho ES, Gunderson SI, Kiledjian M. Mutational analysis of a Dcp2-binding element reveals general enhancement of decapping by 5′-end stem-loop structures. Nucleic Acids Res. 2009;37:2227–2237. doi: 10.1093/nar/gkp087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin SA, Moss B. Modification of RNA by mRNA guanylyltransferase and mRNA (guanine-7-)methyltransferase from vaccinia virions. J. Biol. Chem. 1975;250:9330–9335. [PubMed] [Google Scholar]
- McLennan AG. The Nudix hydrolase superfamily. Cell Mol. Life. Sci. 2006;63:123–143. doi: 10.1007/s00018-005-5386-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLennan AG. Decapitation: Poxvirus makes RNA lose its head. Trends Biochem. Sci. 2007;32:297–299. doi: 10.1016/j.tibs.2007.05.001. [DOI] [PubMed] [Google Scholar]
- Mildvan AS, Xia Z, Azurmendi HF, Saraswat V, Legler PM, Massiah MA, Gabelli SB, Bianchet MA, Kang LW, Amzel LM. Structures and mechanisms of Nudix hydrolases. Arch. Biochem. Biophys. 2005;433:129–143. doi: 10.1016/j.abb.2004.08.017. [DOI] [PubMed] [Google Scholar]
- Parrish S, Moss B. Characterization of a vaccinia virus mutant with a deletion of the D10R gene encoding a putative negative regulator of gene expression. J .Virol. 2006;80:553–561. doi: 10.1128/JVI.80.2.553-561.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parrish S, Resch W, Moss B. Vaccinia virus D10 protein has mRNA decapping activity, providing a mechanism for control of host and viral gene expression. Proc. Natl. Acad. Sci. U. S. A. 2007;104:2139–2144. doi: 10.1073/pnas.0611685104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parrish S, Moss B. Characterization of a second vaccinia virus mRNA-decapping enzyme conserved in poxviruses. J. Virol. 2007;81:12973–12978. doi: 10.1128/JVI.01668-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piccirillo C, Khanna R, Kiledjian M. Functional characterization of the mammalian mRNA decapping enzyme hDcp2. RNA. 2003;9:1138–1147. doi: 10.1261/rna.5690503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez JM, Salas ML, Santaren JF. African swine fever virus-induced polypeptides in porcine alveolar macrophages and in Vero cells: two-dimensional gel analysis. Proteomics. 2001;1:1447–1456. doi: 10.1002/1615-9861(200111)1:11<1447::AID-PROT1447>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- Salas ML, Kuznar J, Vinuela E. Polyadenylation, methylation, and capping of the RNA synthesized in vitro by African swine fever virus. Virology. 1981;113:484–491. doi: 10.1016/0042-6822(81)90176-8. [DOI] [PubMed] [Google Scholar]
- Salas ML, Rey-Campos J, Almendral JM, Talavera A, Vinuela E. Transcription and translation maps of African swine fever virus. Virology. 1986;152:228–240. doi: 10.1016/0042-6822(86)90387-9. [DOI] [PubMed] [Google Scholar]
- Santaren JF, Vinuela E. African swine fever virus-induced polypeptides in Vero cells. Virus Res. 1986;5:391–405. doi: 10.1016/0168-1702(86)90031-6. [DOI] [PubMed] [Google Scholar]
- Shors T, Keck JG, Moss B. Down regulation of gene expression by the vaccinia virus D10 protein. J. Virol. 1999;73:791–796. doi: 10.1128/jvi.73.1.791-796.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiger M, Carr-Schmid A, Schwartz DC, Kiledjian M, Parker R. Analysis of recombinant yeast decapping enzyme. RNA. 2003;9:231–238. doi: 10.1261/rna.2151403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabares E, Martinez J, Ruiz Gonzalvo F, Sanchez-Botija C. Proteins specified by African swine fever virus. II. Analysis of proteins in infected cells and antigenic properties. Arch. Virol. 1980;66:119–132. doi: 10.1007/BF01314980. [DOI] [PubMed] [Google Scholar]
- van Dijk E, Cougot N, Meyer S, Babajko S, Wahle E, Seraphin B. Human Dcp2: a catalytically active mRNA decapping enzyme located in specific cytoplasmic structures. EMBO J. 2002;21:6915–6924. doi: 10.1093/emboj/cdf678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Kiledjian M. Functional link between the mammalian exosome and mRNA decapping. Cell. 2001;107:751–762. doi: 10.1016/s0092-8674(01)00592-x. [DOI] [PubMed] [Google Scholar]
- Wang Z, Jiao X, Carr-Schmid A, Kiledjian M. The hDcp2 protein is a mammalian mRNA decapping enzyme. Proc. Natl. Acad. Sci. U. S. A. 2002;99:12663–12668. doi: 10.1073/pnas.192445599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Yang JY, Niu QW, Chua NH. Arabidopsis DCP2, DCP1, and VARICOSE form a decapping complex required for postembryonic development. Plant Cell. 2006;18:3386–3398. doi: 10.1105/tpc.106.047605. [DOI] [PMC free article] [PubMed] [Google Scholar]