Abstract
Methicillin-resistant Staphylococcus aureus (MRSA) pneumonia-induced sepsis is a common cause of morbidity in the intensive care unit. Although pneumonia is initiated in the lungs, extrapulmonary manifestations occur commonly. In light of the key role the intestine plays in the pathophysiology of sepsis, we sought to determine whether MRSA pneumonia induces intestinal injury. FVB/N mice were subjected to MRSA or sham pneumonia and sacrificed 24 hours later. Septic animals had a marked increase in intestinal epithelial apoptosis by both H&E and active caspase-3 staining. MRSA-induced intestinal apoptosis was associated with an increase in the expression of the pro-apoptotic proteins Bid and Bax and the anti-apoptotic protein Bcl-xL in the mitochondrial pathway. In the receptor-mediated pathway, MRSA pneumonia induced an increase in Fas-ligand but decreased protein levels of Fas, FADD, pFADD, TNF-R1 and TRADD. To assess the functional significance of these changes, MRSA pneumonia was induced in mice with genetic manipulations in proteins in either the mitochondrial or receptor-mediated pathways. Both Bid−/− mice and animals with intestine specific overexpression of Bcl-2 had decreased intestinal apoptosis compared to wild type animals. In contrast, Fas-ligand−/− mice had no alterations in apoptosis. To determine if these findings were organism-specific, similar experiments were performed in mice subjected to Pseudomonas aeruginosa pneumonia. P. aeruginosa induced gut apoptosis, but unlike MRSA, this was associated with increased Bcl-2 and TNF-R1 and decreased Fas. MRSA pneumonia thus induces organism-specific changes in intestinal apoptosis via changes in both the mitochondrial and receptor-mediated pathways although the former may be more functionally significant.
Keywords: Sepsis, gut, cell death, Pseudomonas aeruginosa, Bid, Bax, Bcl-2, Fas Ligand
INTRODUCTION
More than 50,000 patients die of pneumonia in the United States each year, despite the vast majority receiving antibiotics targeted against the initiating microorganism (1). The most common organism causing pneumonia in the intensive care unit is Staphylococcus aureus, accounting for a quarter of all new cases (2). Regardless of the site of infection, methicillin-resistant Staphylococcus aureus (MRSA) infection is becoming increasingly common in both the hospital setting and the community setting, and nearly 100,000 patients are diagnosed with invasive MRSA annually (3). Unfortunately, compared to methicillin-sensitive Staphylococcus aureus, MRSA is associated with increased length of stay, hospital costs, and mortality.
Essentially all patients with pneumonia requiring intensive care unit admission meet the SCCM/ACCP diagnostic criteria for sepsis (4), with the systemic inflammatory response syndrome (SIRS) criteria in the setting of infection. Of these, a significant proportion of patients with pneumonia-induced sepsis develop organ dysfunction remote from the lungs.
The intestine has been long been characterized as the “motor” of SIRS (5) and has been hypothesized to play a central role in the pathophysiology of sepsis. Intestinal integrity is abnormal in sepsis induced by pneumonia (caused by bacteria other than MRSA), with increased intestinal epithelial apoptosis, decreased crypt proliferation, and intestinal hyperpermeability (6–9). Preventing sepsis-induced apoptosis leads to improvement in survival following P. aeruginosa pneumonia although the pathways responsible for this are unknown. It is unclear whether pneumonia changes intestinal integrity via a generic host response (10;11) or via an organism-specific response (12;13).
Despite the increasing prevalence of MRSA pneumonia in intensive care unit patients, there are few studies examining the extrapulmonary effects of this virulent organism. We sought to determine whether MRSA pneumonia induces a secondary injury in the intestinal epithelium and if so, to identify whether the mechanisms responsible for these alterations are distinct from P. aeruginosa pneumonia.
MATERIALS AND METHODS
Animals
Six to fifteen week old mice were used for experiments. All mice were on an FVB/N genetic background unless otherwise indicated. Transgenic mice containing nucleotides -596 to +21 of a rat fatty acid binding protein (Fabpl) linked to human Bcl-2 were generated as previously described (14). Fabpl-Bcl-2 mice have intestine-specific overexpression of Bcl-2 and appear phenotypically identical to wild type (WT) littermates. Bid−/− mice on a C57Bl/6 background were a generous gift from Dr. Richard Hotchkiss (Washington University, St. Louis, MO). Fas-ligand−/− mice (Fas-Lgld) and control C57BL/6 mice were purchased from Jackson Laboratory (Bar Harbor, ME). All mice received water and mouse chow ad libitum and were kept on a 12 hour light-dark schedule. All studies were approved by the Animal Studies Committees of both Emory University and Washington University in accordance with National Institutes of Health laboratory animal use guidelines.
MRSA and P. aeruginosa Preparation
MRSA strain 313 was isolated from a patient in the BJC HealthCare system (St. Louis, MO) (15). This strain is Panton-Valentine leukocidin negative by PCR analysis, multilocus sequence type (MLST) 5 and staphylococcal cassette chromosome (SCCmec) type II. Experiments using P. aeruginosa used the ATCC 27853 strain (16). Bacteria were grown overnight in trypticase soy broth (Becton Dickinson, Sparks, MD) at 37 °C, centrifuged at 6,000 g, washed in sterile 0.9% NaCl twice and resuspended in sterile 0.9% NaCl. MRSA was brought to a final density of 0.5 at 600nm, which corresponds to approximately 5×108 CFU/ml, while P. aeruginosa final density ranged between 5 × 108 and 1 × 109 CFU/mL.
Pneumonia Models
Animals underwent isoflurane anesthesia followed by midline cervical incision and blunt dissection down to the trachea. Septic animal received either 40μl (2×107 CFU) of MRSA inocula or 20μl (2–4×106 CFU) P. aeruginosa inocula via intratracheal injection. Of note, we have previously demonstrated that all animals subjected to P. aeruginosa pneumonia are bacteremic 24 hours after the induction of sepsis (9). Sham operated mice were treated identically except they received an injection of the same volume of 0.9% NaCl. Mice received a single 1 ml subcutaneous injection of 0.9% NaCl for fluid resuscitation following incision closure and were held vertically for 10 seconds to enhance the delivery of bacteria into the lungs. Mice were sacrificed 24 hours later (6;7;12).
Apoptosis
Intestinal epithelial apoptosis was quantified in crypts of 100 well-oriented crypt-villus units using both morphological and functional techniques by an investigator blinded to sample identity (17;18). Hematoxylin and Eosin (H&E)-stained sections were evaluated for morphologic changes characteristic of apoptosis including nuclear fragmentation (karyorrhexis) and cell shrinkage with condensed nuclei (pyknosis). Functional assessment of apoptosis was assessed by active caspase-3 staining. Paraffin-embedded sections were deparaffinized and rehydrated. Endogenous peroxidase activity was blocked by incubation in 30% H2O2 in methanol for 10 minutes, and slides were then heated in antigen decloaker solution (Biocare Medical, Concord, CA) for 45 minutes. Sections were then blocked with 20% goat serum (Vector Laboratories, Burlingame, CA) for 30 minutes and incubated overnight at 4 °C with rabbit polyclonal anti-active caspase-3 antibody (1:100 diluted in PBS; Cell Signaling Technology, Danvers, MA). The next day, sections were incubated with goat anti-rabbit biotinylated secondary antibody (1:200 diluted in PBS; Vector Laboratories) for 30 minutes at room temperature followed by incubation with Vectastain Elite avidin-biotin-peroxidase complex reagent (Vector Laboratories) for 30 minutes. Sections were developed with diaminobenzidine and counterstained with hematoxylin. Splenic apoptosis was quantified in a similar manner in 5 random high power fields.
Western Blot Analysis
Frozen segments of jejunum were homogenized in 5x volume of ice-cold homogenization buffer and centrifuged at 10,000 rpm at 4 °C for 5 minutes (19). The supernatant was collected, and the Bradford protein assay was used to determine total protein concentration. Protein samples (40μg) and equal volume of 2x Laemmli buffer were heated at 95°C for 5 minutes. Samples were run on polyacrylamide gels (Bio-Rad, Hercules, CA) for 45 minutes at 180V and then transferred to Immuno-Blot polyvinylidene difluoride membrane (Bio-Rad) for 2 hours at 80V. Membranes were then blocked in 5% nonfat milk in Tris-buffered saline with 0.1% Tween 20 (Sigma, St. Louis, MO) at room temperature for 60 minutes. Membranes were incubated overnight with primary antibody in 4°C. The following primary antibodies were used: rabbit anti-β-actin; rabbit anti-Bax, rabbit anti-Bcl-xL, rabbit anti-Bcl-2, rabbit anti-Bid, rabbit anti-TRADD, rabbit anti-pFADD (Cell Signaling Technology) rabbit anti-Fas-ligand, mouse anti-TNF-R1, rabbit anti-Fas, and rabbit anti-FADD (Santa Cruz Biotechnology, Santa Cruz, CA). The following day, membranes were washed and incubated for 60 minutes at room temperature with horseradish peroxidase-conjugated goat anti-rabbit or horse anti-mouse immunoglobulin G (Cell Signaling Technology, Santa Cruz Biotechnology). Membranes were developed with chemiluminescent system (Pierce, Rockford, IL) and proteins were detected following exposure to x-ray film.
Villus Length
Villus length was measured in micrometers on H&E-stained sections of proximal jejunum by measuring the distance from the villus tip to the crypt neck using MetaMorph Version 7.1.2.0 (Downingtown, PA). Twelve well-oriented crypt-villus units were measured in each animal.
Crypt Proliferation
Intestinal proliferation was assessed by quantifying S-phase cells in the crypts of 100 well-oriented crypt-villus units in a blinded fashion. Mice were injected with 5-bromo-2′deoxyuridine (BrdU, Sigma) 90 minutes prior to sacrifice using a dose of 5mg/ml diluted in 0.9% NaCl. Following sacrifice, the intestine was removed, fixed, paraffin embedded and sectioned. BrdU was detected in jejunal sections via immunohistochemistry using a commercially available kit (BD PharMingen, San Diego, CA).
Intestinal Permeability
Mice were gavaged with 0.5 ml of 22 mg/ml fluorescein isothiocyanate-dextran (FD4, molecular mass 4.4 kDa, Sigma) in sterile PBS using a 20 gauge feeding needle 19 hours after MRSA inoculation (20). Five hours later, animals were sacrificed and blood was immediately collected. Plasma was isolated by centrifugation at 3,000 rpm for 20 minutes. The concentration of FD4 in the plasma was measured and compared to a standard using fluorospectrometry (NanoDrop 3300, Thermo Scientific, Wilmington, DE).
Cultures
BAL and blood samples were taken from mice immediately after sacrifice, diluted serially, and plated on blood agar plates. Twenty four hours after incubation at 37 °C, plates were examined for colony counts.
Statistical Analysis
After determining the groups did not have Gaussian distributions, data were compared using the Mann-Whitney test. All data were analyzed using the statistical program Prism 4.0 (GraphPad Software, San Diego, CA) and are presented as mean ± SEM. A p value of less than 0.05 was considered to be statistically significant.
RESULTS
MRSA pneumonia increases intestinal epithelial cell apoptosis
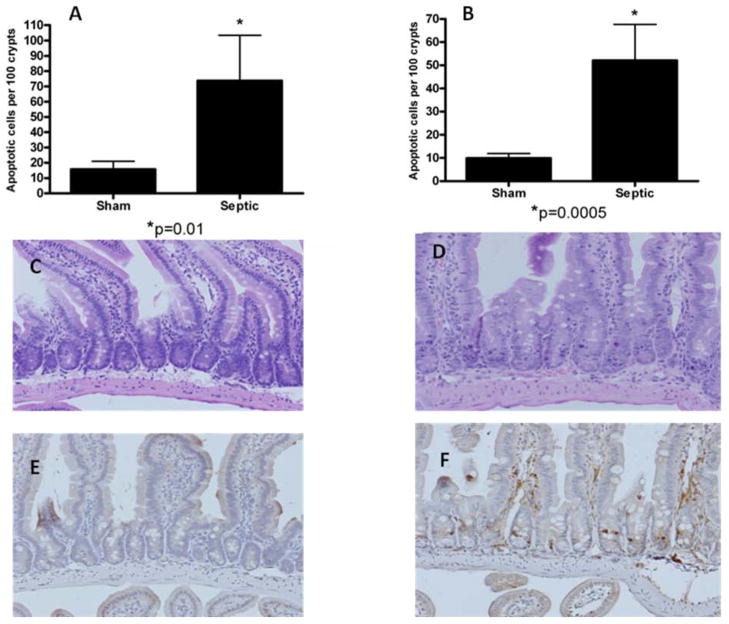
FVB/N mice that received intratracheal MRSA and sham mice that received intratracheal 0.9% NaCl were compared for intestinal epithelial apoptosis 24 hours postoperatively. Mice with MRSA pneumonia had a marked increase in intestinal apoptosis (Fig. 1) whether assayed by H&E staining (p=0.01) or active caspase 3 staining (p=0.0005).
FIG. 1. MRSA pneumonia and intestinal epithelial cell apoptosis.
Apoptosis was quantified in 100 crypts in sham and septic mice (n=9–13) by both H&E (A) and active caspase-3 staining (B). MRSA pneumonia causes increased intestinal epithelial apoptosis. Representative micrographs are shown for sham (C,E) and septic mice (D,F) stained for H&E (C,D) and active caspase-3 (E,F).
MRSA pneumonia alters expression of apoptotic molecules in both the mitochondrial and receptor-mediated pathways
In order to determine possible mechanisms through which MRSA pneumonia increased intestinal epithelial apoptosis, Western blots were performed on intestinal isolates from mice receiving sham pneumonia or MRSA pneumonia assaying both the mitochondrial andreceptor-mediated pathways of apoptosis.
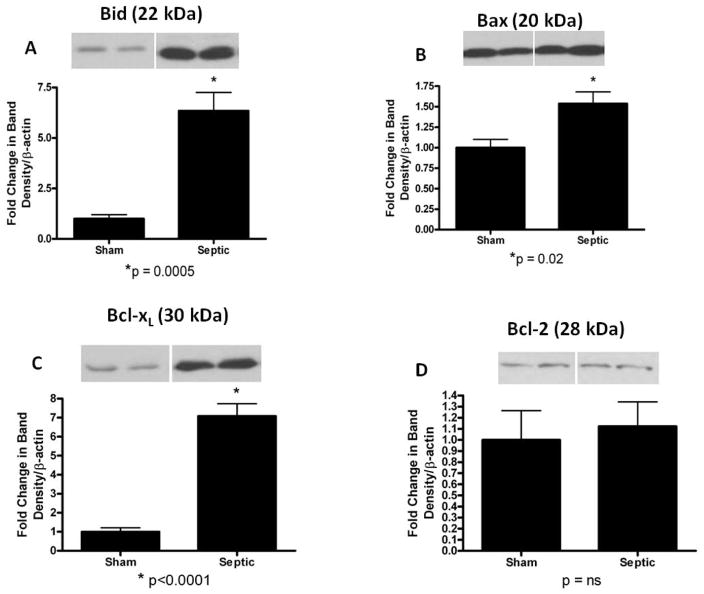
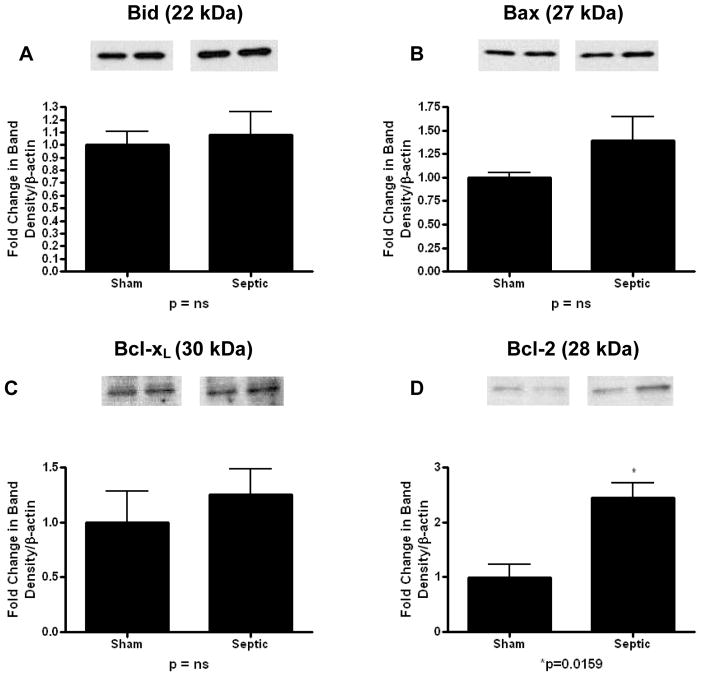
In the mitochondrial pathway (Fig. 2), pro-apoptotic proteins Bid and Bax were both increased in mice with MRSA pneumonia (Bid: 6.3 ± 0.9 vs. 1 ± 0.2, p=0.0005; Bax: 1.5 ± 0.1 vs. 1 ± 0.1, p=0.02; Fig. 2A, B). Anti-apoptotic Bcl-xL was also markedly increased in septic mice (7.1 ± 0.6 vs. 1 ± 0.2, p<0.0001; Fig. 2C), while there was no change in expression of the anti-apoptotic protein Bcl-2 (1.1 ± 0.2 vs. 1 ± 0.3, p=ns; Fig. 2D).
FIG. 2. Apoptotic mediators in the mitochondrial pathway in the gut epithelium of mice subjected to MRSA pneumonia.
Expression of pro-apoptotic mediators Bid (A) and Bax (B) were both increased in septic animals compared to shams. Expression of anti-apoptotic protein Bcl-xL (C) was also increased in septic animals compared to shams while Bcl-2 (D) was unchanged. Expression data comes from experiments with 5–7 mice/group. Protein densities were normalized to β-actin and are reported as fold difference from sham mice (assigned the value of 1 in arbitrary units). Representative western blots from 2 out of the 5–7 mice in each group are shown for each mediator.
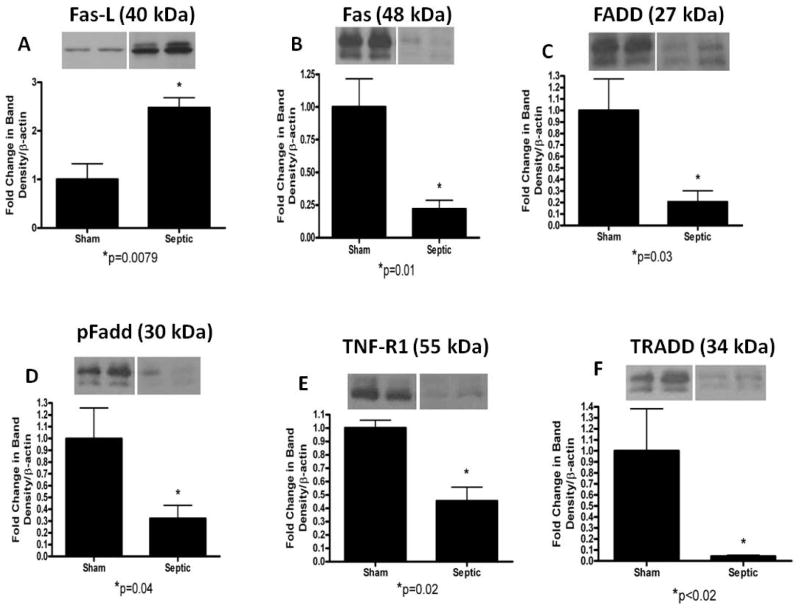
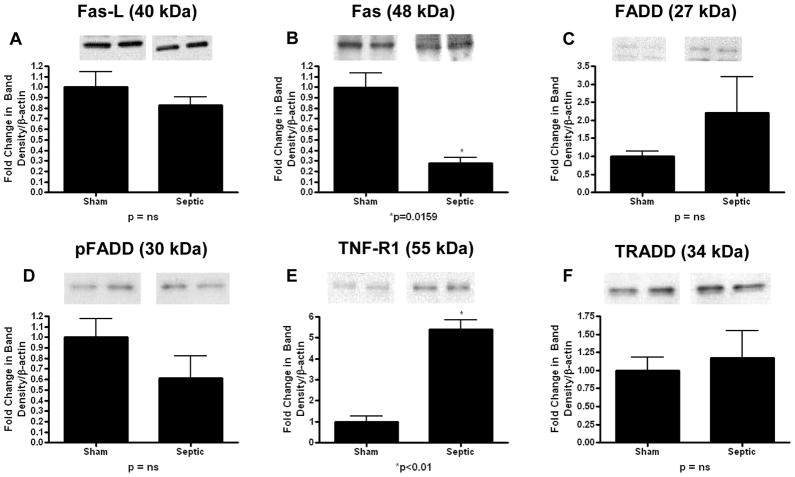
In the receptor-mediated pathway (Fig. 3), Fas-ligand expression was increased in mice with MRSA pneumonia (2.5 ± 0.2 vs. 1 ± 0.3, p=0.0079; Fig. 3A). In contrast, Fas, FADD, pFADD, TNF-R1, TRADD were all decreased in mice with MRSA pneumonia compared to sham mice (Fas: 0.2 ± 0.1 vs. 1 ± 0.2, p=0.01; FADD: 0.2 ± 0.1 vs. 1 ± 0.3, p=0.03; pFADD: 0.3 ± 0.1 vs. 1 ± 0.3, p=0.04; TNF-R1: 0.4 ± 0.1 vs. 1 ± 0.1, p=0.02; TRADD: 0.04 ± 0.01 vs. 1 ± 0.4, p=0.02; Fig. 3B–F).
FIG. 3. Apoptotic mediators in the receptor-mediated pathway in the gut epithelium of mice subjected to MRSA pneumonia.
Expression of Fas-ligand expression was increased in septic animals (A), while Fas (B), FADD (C), pFADD (D), TNF-R1 (E), and TRADD (F) were all decreased compared to sham animals. Expression data comes from experiments with 5–7 mice/group. Protein densities were normalized to β-actin and are reported as fold difference from sham mice (assigned the value of 1 in arbitrary units). Representative western blots from 2 out of the 5–7 mice in each group are shown for each mediator.
Genetic manipulation of the mitochondrial pathway prevents MRSA-induced intestinal epithelial apoptosis
In order to determine the functional significance of the altered expression of apoptotic mediators in the mitochondrial pathway, MRSA pneumonia was given to animals that had two distinct manipulations of this pathway.
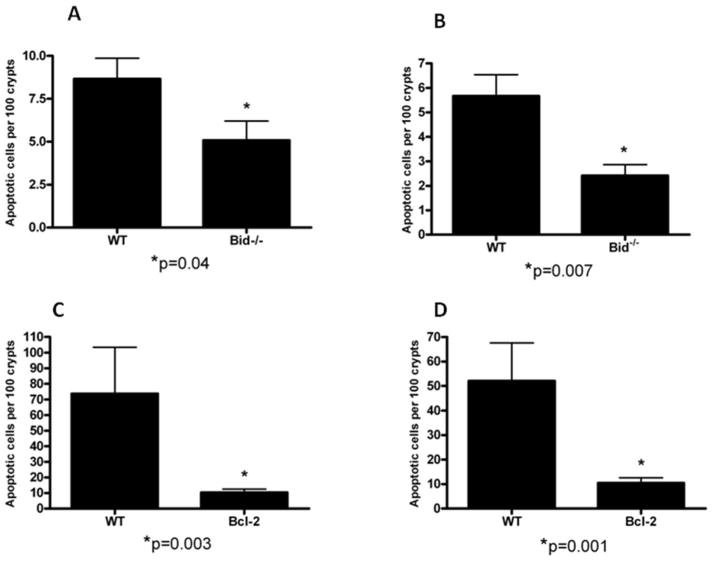
First, mice with a genetic deficiency of the pro-apoptotic protein Bid were compared to WT C57Bl/6 control animals. Intestinal epithelial apoptosis was decreased in Bid−/− mice subjected to MRSA pneumonia compared to WT mice subjected to the same insult whether assayed by H&E (Fig. 4A, p=0.04) or active caspase-3 (Fig. 4B, p=0.007).
FIG. 4. Effect of genetic manipulation of the mitochondrial pathway of apoptosis.
Apoptotic cells/100 crypts were compared between Bid−/− mice and C57Bl/6 mice (A, B, n=12 mice/group) and Fabpl-Bcl-2 mice and FVB/N mice (C,D, n=9–13 mice/group). All animals were subjected to MRSA pneumonia. Both Bid−/− mice and Fabpl-Bcl-2 mice have less apoptotic cells than WT mice whether assayed by either H&E (A,C) or active caspase-3 staining (B,D).
Next, mice with intestine specific overexpression of the anti-apoptotic protein Bcl-2 (Fabpl-Bcl-2 mice) were compared to FVB/N littermates. Intestinal epithelial apoptosis was decreased in Fabpl-Bcl-2 mice subjected to MRSA pneumonia compared to WT mice subjected to the same insult whether assayed by H&E (Fig. 4C, p=0.003) or active caspase-3 (Fig. 4D, p=0.001). Of note, MRSA induced a lower level of apoptosis in C57Bl/6 WT mice than FVB/N WT mice given the same dose of bacteria (compare Fig. 4A and B to C and D).
WT C57Bl/6, Bid−/−, WT FVB/N and Fabpl-Bcl-2 mice all had MRSA detectable in both their blood and BAL fluid 24 hours after injection of MRSA. There were no differences in bacterial levels between WT and transgenic or knockout mice on the same genetic background. Consistent with higher levels of gut apoptosis, BAL levels of MRSA were higher in WT FVB/N and Fabpl-Bcl-2 mice than in WT C57Bl/6 and Bid−/− mice (data not shown).
Genetic manipulation of the receptor-mediated pathway does not prevent MRSA-induced intestinal epithelial apoptosis
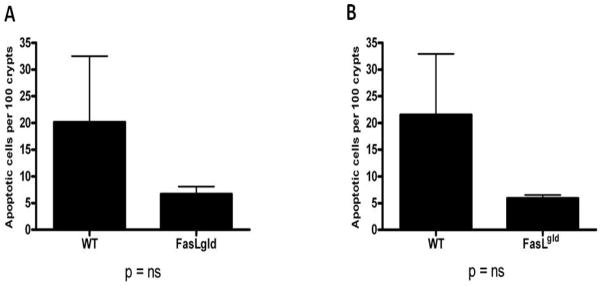
In light of the fact that the only pro-apoptotic member of the receptor-mediated pathway that MRSA pneumonia increased in the intestine was Fas-ligand expression, the functional significance of this was assayed by comparing mice with a genetic deficiency in Fas-ligand (Fas-Lgld) to WT C57Bl/6 control animals. No statistically significant difference in intestinal epithelial apoptosis was detected in Fas-Lgld mice subjected to MRSA pneumonia compared to WT mice subjected to the same insult whether assayed by H&E (Fig. 5A) or active caspase-3 (Fig. 5B).
FIG. 5. Effect of genetic manipulation of the receptor-mediated pathway of apoptosis.
Apoptotic cells/100 crypts were compared between septic Fas-Lgld mice and C57Bl/6 mice (n=8–10) by H&E (A) and active caspase-3 staining (B). No statistically significant difference in apoptosis was detected between the groups.
Pathways of gut epithelial apoptosis differ between MRSA pneumonia and P. aeruginosa pneumonias
To determine whether MRSA pneumonia induced gut apoptosis via organism-specific or organism-independent pathways, a different cohort of mice was subjected to P. aeruginosa pneumonia, and Western blots were performed on intestinal isolates as above. Of note, even though this model has previously been shown to cause significant seven-day mortality (9, 12), all mice receiving P. aeruginosa pneumonia survived for 24 hours after induction of sepsis, at which time they were sacrificed for gut harvest.
In the mitochondrial pathway (Fig. 6), there was no change in the expression of pro-apoptotic Bid and Bax or anti-apoptotic Bcl-xL with P. aeruginosa pneumonia. Despite the fact that P. aeruginosa pneumonia increases gut epithelial apoptosis (6;9;17), the only difference detected in the mitochondrial pathway was a paradoxical increase in the anti-apoptotic mediator Bcl-2 (2.4 ± 0.3 vs. 1 ± 0.2, p= 0.016).
FIG 6. Apoptotic mediators in the mitochondrial pathway in the gut epithelium of mice subjected to P. aeruginosa pneumonia.
Expression of pro-apoptotic Bid (A) and Bax (B) and anti-apoptotic Bcl-xL (C) were unchanged. Expression of Bcl-2 (D) was increased in septic animals compared to sham animals. Expression data comes from experiments with 5–7 mice/group. Protein densities were normalized to β-actin and are reported as fold difference from sham mice (assigned the value of 1 in arbitrary units). Representative western blots from 2 out of the 5–7 mice in each group are shown for each mediator.
In the receptor-mediated pathway (Fig. 7), TNF-R1 was increased in mice with P. aeruginosa pneumonia (5.4 ± 0.4 vs. 1 ± 0.3, p<0.01, p<0.01) while Fas was decreased in septic mice (0.3 ± 0.05 vs. 1 ± 0.1, p= 0.016). No changes were detected in Fas-ligand, FADD, pFADD or TRADD expression in mice given P. aeruginosa pneumonia compared to sham animals. Pathways of gut epithelial apoptosis were therefore significantly different in both the mitochondrial pathway (compare Fig. 2 to Fig. 6) and the receptor mediated pathway (compare Fig. 3 to Fig. 7) for animals subjected to either MRSA or P. aeruginosa pneumonia.
FIG 7. Apoptotic mediators in the receptor-mediated pathway in the gut epithelium of mice subjected to Pseudomonas aeruginosa pneumonia.
Expression of Fas-ligand (A), FADD (C), pFADD (D), and TRADD (F) were unchanged following septic insult. Expression of Fas (B) was decreased in septic animals, while expression of TNF-R1 (E) was increased in septic animals compared to sham animals. Note the figure is laid out in identical orientation to figure 3 to facilitate direct comparison of apoptotic pathways in the intestine between MRSA pneumonia and P. aeruginosa pneumonia. Expression data comes from experiments with 5–7 mice/group. Protein densities were normalized to β-actin and are reported as fold difference from sham mice (assigned the value of 1 in arbitrary units). Representative western blots from 2 out of the 5–7 mice in each group are shown for each mediator.
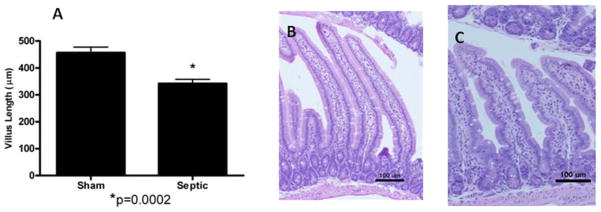
MRSA pneumonia decreases intestinal villus length and crypt proliferation but does not affect intestinal permeability
To determine whether the intestinal manifestations of MRSA pneumonia were limited to increased epithelial apoptosis, intestinal proliferation, villus length and permeability were assayed.
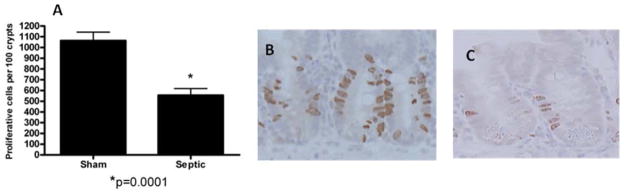
MRSA pneumonia decreased proliferation in septic mice compared to animals subjected to sham pneumonia as measured by labeling S-phase cells with BrdU (Fig. 8, p=0.0001). This was associated with decreased villus length in mice with MRSA pneumonia compared to WT animals (Fig. 9, p=0.0002). Neither crypt proliferation nor villus length was affected by preventing apoptosis as there were no statistically significant differences in either when septic Fabpl-Bcl-2 mice were compared to septic FVB/N littermates (data not shown).
FIG. 8. MRSA pneumonia and crypt proliferation.
S-phase cells were quantified in 100 crypts in sham and septic mice (n=7–10, A). Septic mice had decreased proliferation compared to sham mice. Representative micrographs are shown for both sham (B) and septic (C) mice.
FIG. 9. MRSA pneumonia and villus length.
Villus length was quantified in 12 well oriented crypt-villus units in H&E-stained jejunal sections in sham and septic mice (n=10–13, A). Septic mice had shorter villi compared to sham mice. Representative micrographs are shown for both sham (B) and septic (C) mice.
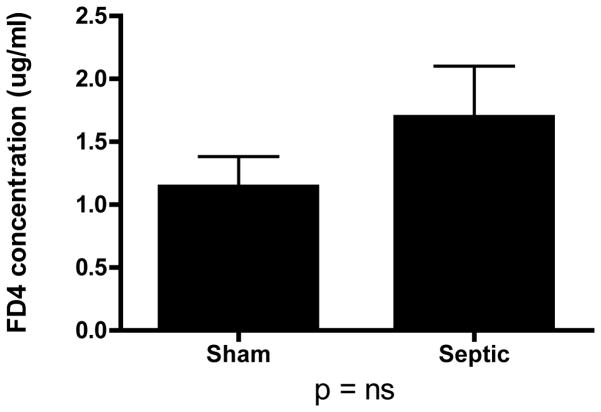
MRSA pneumonia did not alter intestinal permeability in septic mice compared to shams animals as measured by the appearance of FD-4 in the bloodstream (Fig. 10). Consistent with these findings, there were no differences in intestinal protein expressions of tight junction proteins occludin or claudin 3 (data not shown).
FIG. 10. MRSA pneumonia and intestinal permeability.
Intestinal permeability was measured in vivo by measuring the concentration of FD4 in the plasma (n=21–24). No statistically significant difference in permeability was observed between sham and septic animals.
DISCUSSION
This study demonstrates that MRSA pneumonia-induced sepsis alters intestinal integrity as manifested by increased intestinal epithelial apoptosis, decreased crypt proliferation and decreased villus length. Further, the mechanisms by which MRSA pneumonia induces intestinal apoptosis do not appear to be part of a common host response as pathways of gut apoptosis differ markedly between MRSA and P. aeruginosa pneumonia.
Although it is known that both the mitochondrial and receptor-mediated pathways contribute to sepsis-induced lymphocyte apoptosis (21;22), this study is the first to examine pathways of sepsis-induced intestinal epithelial apoptosis in detail. Western blot analysis demonstrated that both the mitochondrial and receptor-mediated pathways in the intestine are altered by MRSA pneumonia. However, the types and significance of these alterations appear to be different between the two pathways.
In the mitochondrial pathway, the Bcl-2 family contains both pro- and anti-apoptotic proteins, and the balance between these plays a critical role in cell survival (23). This study indicates that both pro-apoptotic (Bid and Bax) and anti-apoptotic (Bcl-xL) proteins are increased by MRSA pneumonia-induced sepsis. This is consistent with findings that these three mediators are elevated in the intestinal epithelium following cecal ligation and puncture (19;24). Bcl-xL inhibits apoptosis by binding to pro-apoptotic proteins (25), and the ratio of Bax/Bcl-xL or Bax/Bcl-2 is often used as a rheostat for cellular survival (26). These ratios are also correlated with mortality in septic patients (27). However, these ratios do not explain the increased apoptosis in septic mice compared to sham mice following MRSA pneumonia since the relative increase in Bcl-xL was much greater than the relative increase in Bax (and Bcl-2 was unaffected). The truncated form of Bid translocates to the mitochondria to activate Bax and Bak (28). This suggests that MRSA may induce intestinal epithelial apoptosis by inducing Bid which secondarily induces Bak as its primary downstream mediator.
The functional significance of the mitochondrial pathway is reinforced by data demonstrating that Bid−/− mice and Fabpl-Bcl-2 mice are both able to inhibit sepsis-induced intestinal apoptosis. Although intestinal Bcl-2 levels are unaffected by MRSA pneumonia, either knocking out Bid or overexpressing Bcl-2 should alter the Bax/Bcl-xL or Bax/Bcl-2 ratios directly or indirectly, leading to the phenotype seen. The ability to genetically manipulate the mitochondrial pathway could theoretically have therapeutic benefit in more lethal models of sepsis where elevated gut apoptosis in associated with increased mortality.
The results from the receptor mediated pathway were more complicated. Ayala and colleagues have previously shown that preventing signaling through Fas/Fas-ligand prevents apoptosis in a wide variety of cell types following either cecal ligation and puncture or a two hit model of critical illness (29;30). Based upon these results and previous work from our lab showing that both FADD and pFADD are elevated following cecal ligation and puncture (19), it was reasonable to assume that multiple mediators within the receptor-mediated pathway would be increased following MRSA pneumonia, consistent with the increase in intestinal epithelial apoptosis. However, while MRSA increased Fas-ligand levels, the other 5 mediators examined (Fas, FADD, pFADD, TNF-R1 and TRADD ) were all decreased.
While the data suggest that the mitochondrial pathway plays a critical role in pneumonia-induced apoptosis, the importance of the receptor-mediated pathway is less clear. First, while a single mediator (Fas-ligand) was increased by MRSA pneumonia, the other five mediators were decreased, and preventing signaling through the only pro-apoptotic mediator upregulated by MRSA failed to prevent pneumonia-induced intestinal epithelial apoptosis. There are a number of possible explanations for this. First, the changes in apoptotic mediators in the receptor pathway may cancel themselves out, rendering the pathway unimportant in the final live/die decision a cell faces. Next, this may be explained by the fact that Western blot analysis was performed on whole bowel homogenates and may represent an artifact of immune cells. While Fas is expressed in intestinal epithelial cells, the expression of Fas-ligand in these cells is more controversial (31). It is therefore possible that the Western blot analysis was measuring Fas-ligand in the lamina propria, although even if that were the case, Fas-ligand in this location has been demonstrated to activate Fas on intestinal epithelial cells resulting in apoptosis (31). It is also possible that elevated Fas-ligand is a significant cause of MRSA-induced apoptosis, and the fact that no difference was seen in Fas-Lgld mice relates to the fact that either they were studied at a single timepoint or the fact that there is a large variance seen in WT C57Bl/6 subjected to MRSA at 24 hours (note error bars in Fig. 5) and our findings represent a type II error.
The comparison between mice given MRSA pneumonia and P. aeruginosa pneumonia demonstrate that the host response in the intestinal epithelium to these septic insults is not uniform. Depending on which component of the host response studied, the literature contains evidence of both a common host response independent of the initiating microorganism (10;11) and an organism-specific response (12;13;32). The differences between apoptotic pathways between MRSA and CLP outlined above can potentially be attributed to different anatomic locations of initiating infection (intrathoracic vs. intraabdominal) and to complexity of infection (polymicrobial vs. monomicrobial). To control for this, we examined two models of monomicrobial pneumonia and examined pathways of gut apoptosis. The results demonstrated a striking difference in both the mitochondrial and receptor-mediated pathways. Of the four mitochondrial mediators examined, three were altered in MRSA pneumonia (Bid, Bax, Bcl-XL) while one was altered in P. aeruginosa pneumonia, without any overlap detected. The differences were nearly as pronounced for the receptor-mediated pathway where overlap was noted in a single mediator (decreased expression of Fas in both) with differing results noted in all other mediators examined (FasL, TNF-R1, FADD, pFADD, TRADD).
An additional surprising result of this study was that intestinal permeability was not altered by MRSA pneumonia. Although intestinal apoptosis, proliferation, length and permeability have autonomous control, they are often linked, and alterations in one aspect of intestinal integrity frequently affect another. In addition, multiple models of critical illness including P. aeruginosa pneumonia-induced sepsis cause intestinal hyperpermeability (8). It is unclear if the fact that intestinal permeability was unaffected when all other components of gut integrity were altered is secondary to the initiating organism involved (MRSA vs. P. aeruginosa) or differences in study design between our data and published literature.
This study has a number of limitations. The MRSA model used in this study was non-lethal. While MRSA is a virulent pathogen in patients, it does not have the same degree of virulence in mice, which are highly resistant to infections with Staphylococcus aureus compared to humans secondary to the microorganism’s preferential ability to take iron needed to proliferate from host hemoglobin in humans (33). This is consistent with recent studies from our group demonstrated that multiple isolates of Staphylococcus aureus taken from patient samples (including MRSA, MSSA, PVL positive, PVL negative) caused essentially no mortality in multiple strains of inbred and outbred mice (15;34). Thus even though preventing intestinal epithelial apoptosis has been associated with improved survival following multiple models of sepsis, it is difficult to know if the findings presented herein translate to more lethal models of sepsis. It is possible that the differences in apoptosis pathways between MRSA and P. aeruginosa were related to differences in mortality as opposed to differences in initiating microorganism. The study also did not examine the truncated form of Bid which mediates crosstalk between the mitochondrial and receptor-mediated pathways of apoptosis and also did not examine levels of Bak in the intestinal epithelium. Further, since Bid−/− mice have a germline deletion, there is no way of knowing whether the decreases in MRSA-induced gut epithelial apoptosis in Bid−/− mice are due to direct effects on the intestinal epithelium or are secondary to other factors initiated outside of the intestine, although it should also be noted that these mice have alterations in lymphocyte apoptosis following CLP. Along these lines, this study focused specifically on the intestine, while not examining other organ systems in any degree of detail. In addition, based upon prior studies from our laboratory using either CLP or P. aeruginosa pneumonia which showed maximal apoptosis at 24 hours (7;17;35), all endpoints were measured at this timepoint in this study. However, it is important to note that gram positive bacteremia may have a much more indolent timecourse than polymicrobial or gram negative bacteremia, so important changes that may have occurred earlier or later would not have been determined in our study design.
Despite these limitations, this is the first description of how the most common type of pneumonia in the intensive care unit affects the intestinal epithelium. Since extrapulmonary effects of pneumonia may account for some of its morbidity, understanding the mechanisms through which MRSA pneumonia affects the host response may yield targets for future therapeutic intervention.
Acknowledgments
This work was supported by funding from the National Institutes of Health (GM66202, GM072808, GM008795, GM082008, P30 DK52574).
Reference List
- 1.Heron M, Hoyert DL, Murphy SL, Xu J, Kochanek KD, Tejada-Vera B. Deaths: final data for 2006. Natl Vital Stat Rep. 2009;57(14):1–134. [PubMed] [Google Scholar]
- 2.Hidron AI, Edwards JR, Patel J, Horan TC, Sievert DM, Pollock DA, Fridkin SK. NHSN annual update: antimicrobial-resistant pathogens associated with healthcare-associated infections: annual summary of data reported to the National Healthcare Safety Network at the Centers for Disease Control and Prevention, 2006–2007. Infect Control Hosp Epidemiol. 2008;29(11):996–1011. doi: 10.1086/591861. [DOI] [PubMed] [Google Scholar]
- 3.Klevens RM, Morrison MA, Nadle J, Petit S, Gershman K, Ray S, Harrison LH, Lynfield R, Dumyati G, Townes JM, Craig AS, Zell ER, Fosheim GE, McDougal LK, Carey RB, Fridkin SK. Invasive methicillin-resistant Staphylococcus aureus infections in the United States. JAMA. 2007;298(15):1763–1771. doi: 10.1001/jama.298.15.1763. [DOI] [PubMed] [Google Scholar]
- 4.American College of Chest Physicians/Society of Critical Care Medicine Consensus Conference: definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. Crit Care Med. 1992;20(6):864–874. [PubMed] [Google Scholar]
- 5.Clark JA, Coopersmith CM. Intestinal crosstalk: a new paradigm for understanding the gut as the “motor” of critical illness. Shock. 2007;28(4):384–393. doi: 10.1097/shk.0b013e31805569df. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Coopersmith CM, Stromberg PE, Dunne WM, Davis CG, Amiot DM, Buchman TG, Karl IE, Hotchkiss RS. Inhibition of intestinal epithelial apoptosis and survival in a murine model of pneumonia-induced sepsis. JAMA. 2002;287:1716–1721. doi: 10.1001/jama.287.13.1716. [DOI] [PubMed] [Google Scholar]
- 7.Coopersmith CM, Stromberg PE, Davis CG, Dunne WM, Amiot DM, Karl IE, Hotchkiss RS, Buchman TG. Sepsis from Pseudomonas aeruginosa pneumonia decreases intestinal proliferation and induces gut epithelial cell cycle arrest. Crit Care Med. 2003;1(6):1630–1637. doi: 10.1097/01.CCM.0000055385.29232.11. [DOI] [PubMed] [Google Scholar]
- 8.Yu P, Martin CM. Increased gut permeability and bacterial translocation in Pseudomonas pneumonia-induced sepsis. Crit Care Med. 2000;28(7):2573–2577. doi: 10.1097/00003246-200007000-00065. [DOI] [PubMed] [Google Scholar]
- 9.Dominguez JA, Vithayathil PJ, Khailova L, Lawrance CP, Samocha AJ, Jung E, Leathersich AM, Dunne WM, Coopersmith CM. Epidermal Growth Factor Improves Survival and Prevents Intestinal Injury in a Murine Model of Pseudomonas Aeruginosa Pneumonia. Shock. 2011;36(4):381–389. doi: 10.1097/SHK.0b013e31822793c4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fry DE. The generic septic response. Crit Care Med. 2008;36(4):1369–1370. doi: 10.1097/CCM.0b013e31816a11e9. [DOI] [PubMed] [Google Scholar]
- 11.Tang BM, McLean AS, Dawes IW, Huang SJ, Lin RC. Gene-expression profiling of peripheral blood mononuclear cells in sepsis. Crit Care Med. 2009;37(3):882–888. doi: 10.1097/CCM.0b013e31819b52fd. [DOI] [PubMed] [Google Scholar]
- 12.McConnell KW, McDunn JE, Clark AT, Dunne WM, Dixon DJ, Turnbull IR, Dipasco PJ, Osberghaus WF, Sherman B, Martin JR, Walter MJ, Cobb JP, Buchman TG, Hotchkiss RS, Coopersmith CM. Streptococcus pneumoniae and Pseudomonas aeruginosa pneumonia induce distinct host responses. Crit Care Med. 2010;1:223–241. doi: 10.1097/CCM.0b013e3181b4a76b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sousse LE, Jonkam CC, Traber DL, Hawkins HK, Rehberg SW, Traber LD, Herndon DN, Enkhbaatar P. Pseudomonas aeruginosa Is Associated With Increased Lung Cytokines and Asymmetric Dimethylarginine Compared With Methicillin-Resistant Staphylococcus aureus. Shock. 2011;36(5):466–470. doi: 10.1097/SHK.0b013e3182336b45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Coopersmith CM, O’Donnell D, Gordon JI. Bcl-2 inhibits ischemia-reperfusion-induced apoptosis in the intestinal epithelium of transgenic mice. Am J Physiol. 1999;276(3 Pt 1):G677–G686. doi: 10.1152/ajpgi.1999.276.3.G677. [DOI] [PubMed] [Google Scholar]
- 15.Robertson CM, Perrone EE, McConnell KW, Dunne WM, Boody B, Brahmbhatt T, Julia DM, Van RN, Hogue LA, Cannon CL, Buchman TG, Hotchkiss RS, Coopersmith CM. Neutrophil Depletion Causes a Fatal Defect in Murine Pulmonary Staphylococcus aureus clearance. J Surg Res. 2008;150(2):278–285. doi: 10.1016/j.jss.2008.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fox AC, Breed ER, Liang Z, Clark AT, Zee-Cheng BR, Chang KC, Dominguez JA, Jung E, Dunne WM, Burd EM, Farris AB, Linehan DC, Coopersmith CM. Prevention of Lymphocyte Apoptosis in Septic Mice with Cancer Increases Mortality. J Immunol. 2011;187(4):1950–1956. doi: 10.4049/jimmunol.1003391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vyas D, Robertson CM, Stromberg PE, Martin JR, Dunne WM, Houchen CW, Barrett TA, Ayala A, Perl M, Buchman TG, Coopersmith CM. Epithelial apoptosis in mechanistically distinct methods of injury in the murine small intestine. Histol Histopathol. 2007;22(6):623–630. doi: 10.14670/hh-22.623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fox AC, Robertson CM, Belt B, Clark AT, Chang KC, Leathersich AM, Dominguez JA, Perrone EE, Dunne WM, Hotchkiss RS, Buchman TG, Linehan DC, Coopersmith CM. Cancer causes increased mortality and is associated with altered apoptosis in murine sepsis. Crit Care Med. 2010;38(3):886–893. doi: 10.1097/CCM.0b013e3181c8fdb1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Clark JA, Clark AT, Hotchkiss RS, Buchman TG, Coopersmith CM. Epidermal growth factor treatment decreases mortality and is associated with improved gut integrity in sepsis. Shock. 2008;30(1):36–42. doi: 10.1097/shk.0b013e31815D0820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Clark JA, Gan H, Samocha AJ, Fox AC, Buchman TG, Coopersmith CM. Enterocyte-specific epidermal growth factor prevents barrier dysfunction and improves mortality in murine peritonitis. Am J Physiol Gastrointest Liver Physiol. 2009;297(3):G471–G479. doi: 10.1152/ajpgi.00012.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chang KC, Unsinger J, Davis CG, Schwulst SJ, Muenzer JT, Strasser A, Hotchkiss RS. Multiple triggers of cell death in sepsis: death receptor and mitochondrial-mediated apoptosis. FASEB J. 2007;21(3):708–719. doi: 10.1096/fj.06-6805com. [DOI] [PubMed] [Google Scholar]
- 22.Hotchkiss RS, Osmon SB, Chang KC, Wagner TH, Coopersmith CM, Karl IE. Accelerated lymphocyte death in sepsis occurs by both the death receptor and mitochondrial pathways. J Immunol. 2005;174(8):5110–5118. doi: 10.4049/jimmunol.174.8.5110. [DOI] [PubMed] [Google Scholar]
- 23.Youle RJ, Strasser A. The BCL-2 protein family: opposing activities that mediate cell death. Nat Rev Mol Cell Biol. 2008;9(1):47–59. doi: 10.1038/nrm2308. [DOI] [PubMed] [Google Scholar]
- 24.Stromberg PE, Woolsey CA, Clark AT, Clark JA, Turnbull IR, McConnell KW, Chang KC, Chung CS, Ayala A, Buchman TG, Hotchkiss RS, Coopersmith CM. CD4+ lymphocytes control gut epithelial apoptosis and mediate survival in sepsis. FASEB J. 2009;23(6):1817–1825. doi: 10.1096/fj.08-119024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Billen LP, Kokoski CL, Lovell JF, Leber B, Andrews DW. Bcl-XL inhibits membrane permeabilization by competing with Bax. PLoS Biol. 2008;6(6):e147. doi: 10.1371/journal.pbio.0060147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.George NM, Evans JJ, Luo X. A three-helix homo-oligomerization domain containing BH3 and BH1 is responsible for the apoptotic activity of Bax. Genes Dev. 2007;1(15):1937–1948. doi: 10.1101/gad.1553607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bilbault P, Lavaux T, Launoy A, Gaub MP, Meyer N, Oudet P, Pottecher T, Jaeger A, Schneider F. Influence of drotrecogin alpha (activated) infusion on the variation of Bax/Bcl-2 and Bax/Bcl-xl ratios in circulating mononuclear cells: a cohort study in septic shock patients. Crit Care Med. 2007;35(1):69–75. doi: 10.1097/01.CCM.0000251133.26979.F4. [DOI] [PubMed] [Google Scholar]
- 28.Wei MC, Zong WX, Cheng EH, Lindsten T, Panoutsakopoulou V, Ross AJ, Roth KA, MacGregor GR, Thompson CB, Korsmeyer SJ. Proapoptotic BAX and BAK: a requisite gateway to mitochondrial dysfunction and death. Science. 2001;292(5517):727–730. doi: 10.1126/science.1059108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wesche-Soldato DE, Chung CS, Gregory SH, Salazar-Mather TP, Ayala CA, Ayala A. CD8+ T cells promote inflammation and apoptosis in the liver after sepsis: role of Fas-FasL. Am J Pathol. 2007;171(1):87–96. doi: 10.2353/ajpath.2007.061099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Perl M, Chung CS, Lomas-Neira J, Rachel TM, Biffl WL, Cioffi WG, Ayala A. Silencing of Fas, but not caspase-8, in lung epithelial cells ameliorates pulmonary apoptosis, inflammation, and neutrophil influx after hemorrhagic shock and sepsis. Am J Pathol. 2005;167(6):1545–1559. doi: 10.1016/S0002-9440(10)61240-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Strater J, Moller P. CD95 (Fas/APO-1)/CD95L in the gastrointestinal tract: fictions and facts. Virchows Arch. 2003;442:218–225. doi: 10.1007/s00428-003-0760-z. [DOI] [PubMed] [Google Scholar]
- 32.Feezor RJ, Oberholzer C, Baker HV, Novick D, Rubinstein M, Moldawer LL, Pribble J, Souza S, Dinarello CA, Ertel W, Oberholzer A. Molecular characterization of the acute inflammatory response to infections with gram-negative versus gram-positive bacteria. Infect Immun. 2003;71(10):5803–5813. doi: 10.1128/IAI.71.10.5803-5813.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pishchany G, McCoy AL, Torres VJ, Krause JC, Crowe JE, Jr, Fabry ME, Skaar EP. Specificity for human hemoglobin enhances Staphylococcus aureus infection. Cell Host Microbe. 2010;8(6):544–550. doi: 10.1016/j.chom.2010.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jung E, Perrone EE, Liang Z, Breed ER, Dominguez JA, Clark AT, Fox AC, Dunne WM, Burd EM, Farris AB, Hotchkiss RS, Coopersmith CM. Cecal Ligation and Puncture Followed by MRSA Pneumonia Increases Mortality in Mice and Blunts Production of Local and Systemic Cytokines. Shock. 2012;37(1):85–94. doi: 10.1097/SHK.0b013e3182360faf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Coopersmith CM, Chang KC, Swanson PE, Tinsley KW, Stromberg PE, Buchman TG, Karl IE, Hotchkiss RS. Overexpression of Bcl-2 in the intestinal epithelium improves survival in septic mice. Crit Care Med. 2002;30(1):195–201. doi: 10.1097/00003246-200201000-00028. [DOI] [PubMed] [Google Scholar]