Abstract
Trans-resveratrol, present in high concentration in the skin of red grapes and red wine, has a dose-dependent antiproliferative effect in vitro, prevents the formation of mammary tumors, and has been touted as a chemopreventive agent. Based upon in vitro studies demonstrating that trans-resveratrol downregulates the expression of 1) DNA methyltransferases and 2) the cancer promoting prostaglandin (PG)E2, we determined if trans-resveratrol had a dose-related effect on DNA methylation and prostaglandin expression in humans. Thirty-nine adult women at increased breast cancer risk were randomized in double-blind fashion to placebo, 5 or 50 mg trans-resveratrol twice daily for 12 wk. Methylation assessment of 4 cancer-related genes (p16, RASSF-1α, APC, CCND2) was performed on mammary ductoscopy specimens. The predominant resveratrol species in serum was the glucuronide metabolite. Total trans-resveratrol and glucuronide metabolite serum levels increased after consuming both trans-resveratrol doses (P < .001 for both). RASSF-1α methylation decreased with increasing levels of serum trans-resveratrol (P = .047). The change in RASSF-1α methylation was directly related to the change in PGE2 (P = .045). This work provides novel insights into the effects of trans-resveratrol on the breast of women at increased breast cancer risk, including a decrease in methylation of the tumor suppressor gene RASSF-1α. Because of the limited sample size, our findings should be validated in a larger study.
INTRODUCTION
Trans-resveratrol is a polyphenol present in foods that has been touted as an anticancer agent (1). The cis isomer is generally present at lower levels in foods and thought to have less potent anticancer properties (2). Trans-resveratrol is found in high concentration in the skin of red grapes and in red wine, with lesser amounts in mulberries and peanuts. High levels are also found in the rhizome of P. cuspidatum, and most trans-resveratrol dietary supplements contain P. cuspidatum. Trans-resveratrol inhibits human breast epithelial proliferation in a dose- and time-dependent manner (3), suggesting that the agent has chemopreventive effects. The agent inhibited the formation of mouse mammary preneoplastic lesions (4). It also reduced the incidence of mammary tumors by 45% and multiplicity by 55% when administered as part of the diet at a dose of 10 parts per million to DMBA-treated female Sprague Dawley rats compared to DMBA-treated rats that did not receive resveratrol (5).
A case-control study demonstrated a relative breast cancer risk of 0.39 among women with high levels and 0.50 for women with intermediate levels of total resveratrol intake compared to those with a low level of ingestion (6). Despite compelling preclinical and epidemiologic evidence for the agent’s chemopreventive effects, studies that address its mechanism of action in humans are limited and focus on resveratrol’s effect on proliferation.
CpG islands of many genes that are mostly unmethylated in normal tissue are hypermethylated to varying degrees in breast cancer (7). Hypermethylation can develop early in the carcinogenesis process. We demonstrated in the breast ductal epithelium of women at normal breast cancer risk that methylation changes occur in tumor suppressor genes after ingestion of the polyphenols genistein and daidzein that are present in soy (8). Methylation of CpG dinucleotides is known to be mediated by at least 3DNAmethyl transferases (DNMTs): DNMT1, DNMT3a, and DNMT3b (9,10). Decreased expression of DNMT1 and 3b in a colon cancer cell line led to reexpression of tumor suppressor genes (11). We previously reported that trans-resveratrol decreased the expression of DNTMT1 and 3b in breast cancer cells in a dose-dependent fashion (12). It is plausible that reduced DNA methyltransferase 1 and 3b expression induced by trans-resveratrol may lead to demethylation and reexpression of tumor suppressor genes.
Trans-resveratrol is efficiently absorbed after oral administration (13) and rapidly metabolized by glucuronidation and sulfation in the liver (14). This is similar to other botanicals such as soy, where the polyphenols genistein and daidzein (15) are rapidly metabolized. In the blood, trans-resveratrol binds both to albumin and low density lipoproteins (LDLs) (16), which assists in its delivery to the epithelial cell surface for cell membrane uptake, allowing intracellular biologic effect (16). The glucoronide can be enzymatically cleaved (17) or sulfated trans-resveratrol hydrolyzed (18) to its free form, such that after resveratrol administration intracellular levels in the kidney, for example, were predominantly in the free form, but in the urine the metabolites predominate (19). It appears that at least some resveratrol derivatives also have an antiproliferative effect (20).
Preclinical studies suggest that the chemopreventive properties of trans-resveratrol are due to the agent’s apoptotic, cell cycle arrest, kinase inhibition, and antiangiogenic properties (21). Based on our observation that trans-resveratrol downregulates DNMTs in estrogen receptor positive breast cancer (12), we conducted a double-blind, randomized, placebo-controlled clinical trial in women at increased breast cancer risk to determine the dose-dependent effects of trans-resveratrol on 4 genes, including 3 (CCND-2, p16, and RASSF-1α) that we previously demonstrated (22) were significantly more prevalent in tumor than in normal tissue specimens, and the APC gene, aberrant methylation of which is especially present in more aggressive forms of cancer such as inflammatory breast cancer (23). We also evaluated resveratrol’s effects on systemic and breast-specific prostaglandin (PG)E2 expression. Multiple lines of evidence support the tumor promotional properties of PGE2. PGE2 expression is directly related to cell proliferation (24), malignant breast tumors produce more PGE2 than benign breast tumors or normal breast tissue (25), and women with breast cancer who had PGE2 levels above 15 ng/g had a significantly worse survival rate than those with levels ≤15 ng/g (26). We correlated the changes in PGE2 and in gene methylation with resveratrol dose and with circulating levels of the agent and its metabolites.
The resveratrol doses were chosen based on what is commonly available in health food stores. The lower dose of trans-resveratrol is approximately twice the amount that a person receives when consuming 2 glasses of red wine (14). A preclinical study that administered a dose of resveratrol equivalent to approximately 14 mg/day resveratrol to a 70-kg human was found to significantly suppress mammary tumor formation (5). The higher dose is more than is generally found in food, but we wished to evaluate a higher dose of the agent promoted for consumption as a nutraceutical in health food stores (27).
MATERIALS AND METHODS
Subjects
To be eligible, women had to be at increased breast cancer risk, defined as having a first-degree relative with breast cancer, a Gail risk of > 1.66% of developing breast cancer in the next 5 yr, and/or a personal history of a breast biopsy demonstrating atypical hyperplasia, in situ, or invasive breast cancer (previously treated and currently free of disease). Each participant signed an Internal Review Board approved informed consent prior to enrollment. The participant was then randomized in double-blind fashion via the method of sealed envelopes and took a capsule twice daily for 12 weeks containing 1 of 3 preparations: placebo, P. cuspidatum with 5 mg trans-resveratrol, or P. cuspidatum with 50 mg of trans-resveratrol. The twice-daily regimen (rather than single-daily dosing) was chosen because of the rapid metabolism of the parent compound (27).
Women who were pregnant, lactating, or had nursed within 20 mo of study enrollment were excluded. Women could not have been on exogenous estrogens and/or progestins for the month prior to enrollment or during the study. They must have undergone a negative breast exam and, for women 40 yr or older, a benign mammogram within 6mo of enrollment. Subjects were asked not to consume red wine or eat mulberries and to limit peanuts/peanut product consumption to 1 oz. daily during the study. Customary consumption of macronutrients was assessed and maintained at constant levels throughout the study. Consumption of herbal medicines that contained or might contain hormonally active phytoestrogens was prohibited. Concentrated functional food supplements (e.g., capsules or powders) were not allowed. Also excluded were women who maintained a vegan or macrobiotic diet. Subjects taking tamoxifen, raloxifene, or an aromatase inhibitor were excluded.
Subjects were asked at the time of enrollment if they were taking nonsteroidal anti-inflammatory drugs (NSAIDS) and this information was recorded. Subjects taking NSAIDS were asked to maintain their normal practice of use. Subjects were given a calendar on which to record each capsule taken. Subjects deemed to daily be ingesting over 500 µg trans-resveratrol from all sources were considered not evaluable. Trans-resveratrol preparations were provided as a single lot by InterHealth Nutraceuticals (Benicia, CA). A placebo preparation consisting of microcrystalline cellulose was used. At return visits 4 and 12 wk after starting trans-resveratrol, participants were asked if there had been any medication-related side effects. Compliance was assessed by a capsule calendar and the collection of unused capsules.
Specimen Collection
Nipple aspirate fluid (NAF) and serum samples were collected at baseline, 4 and 12 wk after starting the agent. Mammary ductoscopy (MD) samples were collected at baseline and 12 wk as previously described (28,29).All samples were kept on ice until they were snap frozen in −80°C. NAF and serum were used to analyze PGE2. Serum was also used to measure total trans- and total cis-resveratrol and resveratrol metabolites (glucuronide and sulfate). MD samples were used to assess changes in methylation. For MD collection, a nipple grid was used to indicate the location of the duct cannulated at baseline. Attempts were made to cannulate the same duct before and after treatment. All specimen analyses were conducted in blinded fashion.
qMS-PCR
One µg salmon sperm carrier DNA was added to DNA extracted from each MD sample, which was then sodium bisulfite treated. A 2-step PCR strategy was employed for p16, RASSF-1α, APC, and CCND2. First-round PCR of MD DNA was carried out for the 4 genes using an AmpliTaq Gold PCR kit (Applied Biosystems, Foster City, CA), in which both methylated and unmethylated copies of a specific gene in the sample were amplified with a universal primer set. For second round SYBR green-based qMSP, diluted PCR products were amplified with specific primers of the 4 genes for both methylated and unmethylated DNA. The primer sets for qMSP have previously been reported (30,31). The percent of methylated DNA in each sample was calculated (32). DNA that was 100% methylated or 100% unmethylated was used to generate a standard curve to quantify the percent methylated DNA in each sample.
qMS-PCR Validation
To verify the specificity of second-round qMS-PCR products, selected amplicons for p16, RASSF-1α, APC, and CCND2 were subcloned using the TOPO-TA cloning system (Invitrogen, Carlsbad, CA). Plasmid DNA from 5–6 positive clones was isolated and sequenced using an ABI 3730 DNA Analyzer (Applied Biosystems, Foster City, CA).
Quantification of Resveratrol
Capsules
The botanical identity of the material provided by InterHealth Nutraceuticals was confirmed by high-performance liquid chromatography (HPLC) at baseline, and assurance that degradation had not occurred confirmed by analysis of random capsules yearly thereafter. Two capsules of each type (placebo, low-dose resveratrol, high-dose resveratrol) were evaluated yearly. The standard to calculate the standard curve was obtained from Sigma. Because in plant species both trans-resveratrol and cis-resveratrol can be present, both isomers were measured. The cis- form of the aglycone was obtained by UV exposure of the trans- isomer. Samples were analyzed for total resveratrol following enzymatic hydrolysis of the resveratrol beta-D-glucopyranosides by glucosidase (Sigma) followed by HPLC analysis with UV (306 nm) and fluorescence detection (ex 324 nm, em 370 nm).
Serum
We attempted to measure both free and conjugated trans- and cis-resveratrol, as well as resveratrol glucuronide and sulfate metabolites as described (33,34). Resveratrol isomers in 0.10 mL serum were incubated at 37°C overnight with no enzyme, β-D-glucuronidase/sulfatase and sulfatase (Sigma Aldrich, St. Louis, MO), acidified to pH 3.0 with acetic acid, vortexed, and centrifuged at 13,000 rpm for 5 min. Samples were then added to preconditioned C18 Sep-pak-vac columns (Waters). The columns were washed with water and trans- and cis-resveratrol eluted with methanol. The methanol eluant was taken to dryness, reconstituted, and analyzed using a 4-channel ESA CoulArray Model 5600 HPLC detection system along with an ESA isocratic HPLC pump connected to a Thermo Separation Products Spectrasystem AS3500 autosampler utilizing a Luna (250 mm × 4.6 mm) column (Phenomenex) with a mobile phase of methanol/50 mM sodium acetate (pH 4.8) (47:53, v/v). CoulArray settings were 200, 300, 450, and 600 mV. A standard curve was constructed using cis- and trans-resveratrol standards (Sigma). Because individual resveratrol sulfate and glucuronide metabolites were not readily available, free cis- and trans-resveratrol (no enzyme), total cis- and trans-resveratrol (β-D-glucuronidase/sulfatase), and total resveratrol sulfate (sulfatase) concentrations were determined. No free cis- nor trans-resveratrol was identified. Therefore, to determine total resveratrol glucuronide concentration, we subtracted the concentration of total resveratrol sulfate from the combined concentration of total trans- plus total cis-resveratrol. The extraction efficiency was > 90%–95%. Duplicate analysis was conducted for many of the samples, with consistent results.
PGE2
Each NAF and serum specimen, standard, and control were analyzed in duplicate. PGE2 was measured by immunoassay as previously described (35) according to the manufacturer’s instructions (Assay Design, Inc., Ann Arbor, MI). Briefly, samples were diluted in 100-µL assay buffer supplied by the manufacturer, pipetted into appropriate wells, incubated for 2 h at room temperature on a plate shaker, washed, substrate solution added, followed by 1-h incubation, and absorbance measured at 405 nm. A standard curve was prepared using serial dilutions of PGE2. A 4-parameter logistic curve was created from standards of known PGE2 concentration, and PGE2 concentrations of unknown samples fit to the standard curve regression equation, corrected for aliquot volume and expressed as nanograms of PGE2/mL of original sample. The goodness-of-fit of the standard curve, R2, was 0.9993. A QA standard was provided and used. The CV was < 10% within the standard range.
Statistical Analysis
Both within-group and between-group analyses were conducted. Serum resveratrol levels were logarithm transformed prior to analysis. A paired t-test was employed to detect differences between time points for resveratrol and PGE2 levels. A between group t-test was used to analyze differences between treatment groups in quantitative variables such as the fractions of methylated p16, RASSF-1α, APC, and CCND2. Linear regression was conducted to investigate relationships between quantitative variables such as change in resveratrol levels and changes in PGE2 and the fraction of methylated p16, RASSF-1α, APC, and CCND2. All analyses were carried out using SAS JMP software.
RESULTS
Subjects
Three hundred and six women were screened and 39 subjects enrolled. Reasons why women were not eligible included the fact that they did not meet high-risk criteria, and/or they were on a medication or food supplement not allowed in the exclusion criteria. Two women took their capsules as prescribed for at least 4 wk and 31 for the entire 12 wk of the study. Of the 8 who did not complete the study, 1 withdrew before baseline sample collection, 6 dropped out after baseline collection for personal reasons, and 1 individual withdrew prior to the 12-wk sample collection due to side effects. One of the 31 participants who finished the study was excluded from statistical analysis because the time from taking her last dose of medication to sample collection exceeded 12 h and the 12-wk trans-resveratrol concentration in her serum was <5% of the 4-wk concentration, whereas all other 4- and 12-wk comparisons of trans-resveratrol levels were similar. One third of participants in the placebo and low-dose resveratrol groups, and 22% of participants in the high-dose resveratrol group, indicated that they were taking nonsteroidal antiinflammatory medications at the time of study enrollment.NSAID use did not have a significant effect on PGE2 expression, nor did we find a significant interaction between NSAID use and either PGE2 or resveratrol levels.
We were able to analyze biomarkers at all time points in NAF in 22/30 (73%) and in serum in 28/30 (93%) women. Median age (61 yr vs. 59.5 yr vs. 54 yr) in the placebo vs. low vs. high dosage groups was similar. A median of 98.2% of the recommended capsules were consumed during the study. Overall, side effects were few and mild. The only individual who withdrew because of side effects had a history of irritable bowel syndrome and experienced CTC grade 1 diarrhea 9 wk into treatment.
Resveratrol Levels in Capsules and in Serum
At study start, capsules presumed to contain placebo, 5, and 50 mg contained on average 0 mg, 5.2 mg, and 52.5 mg trans-resveratrol, respectively. The CV for the measurements was < 10%. Trans-resveratrol content of the capsules was evaluated at yearly intervals thereafter, with a CV over time of < 10%. Mean and median times from last resveratrol dose to serum collection were 4.2 and 3.4 h, respectively, with a mean and median of 3.8 and 3.5 h at the 4-wk time point and 4.7 and 3.3 h at the 12-wk time point. Mean and median times were similar among the 3 groups, as was the range (up to 7.7, 8.6, and 10.9 h) in the low- and high-dose resveratrol and placebo groups, respectively.
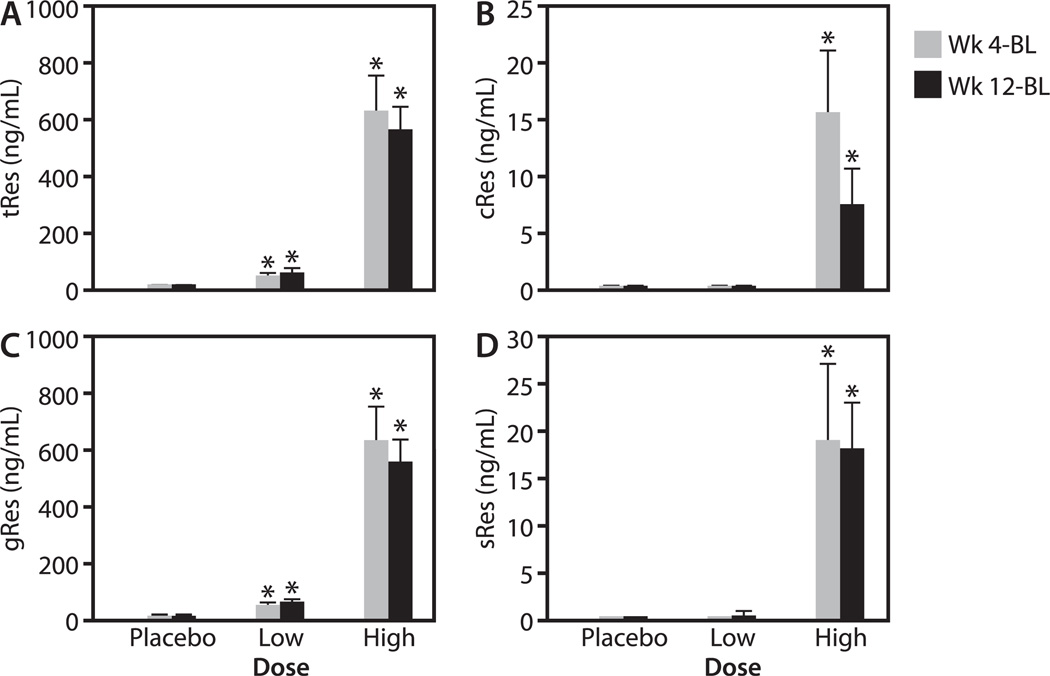
Total (free plus metabolites) trans-resveratrol levels were detectable in 6/30 (20%) serum samples at baseline. Levels significantly increased at 4 and 12 wk (Fig. 1A) after participants took either low- or high-dose resveratrol (P < .001 for all) but not after placebo treatment. Treatment with high-dose resveratrol resulted in higher levels at 4 and 12 wk than after low-dose treatment (P < .001 for both).
FIG. 1.
Serum (ng/mL) resveratrol concentrations after treatment. The change (compared to baseline-BL) in total trans (tRes; A) and cis (cRes; B) resveratrol, as well as resveratrol glucuronide (gRes; C) and sulfate (sRes; D) metabolites 4 and 12 wk after twice-daily administration of placebo, low- or high-dose resveratrol. Standard error bars are included. (*) above the bars indicates a significant change from baseline, P < 0.05. The number of individuals in each group was as follows: placebo, 9; low-dose resveratrol, 12; and high-dose resveratrol, 9.
Total cis-resveratrol (Fig. 1B) was not detectable in any (0/32) of the baseline serum samples, nor in samples at 4 and 12 wk in the placebo and low-dose resveratrol groups. Cis-resveratrol levels ranged from 0%–5.2% of total resveratrol in the high-dose group at 4 and 12 wk. We were not able to detect free resveratrol in any of the serum samples.
We also measured total resveratrol and total sulfate metabolites and thereby calculated total glucuronide metabolites in each sample. The glucuronide species (Fig. 1C) comprised 93%–100% of the metabolites, with the sulfate species (Fig. 1D) comprising the remainder. The glucuronide metabolites increase was similar to that of trans-resveratrol after treatment with both low- and high-dose resveratrol.
PGE2 Was Not Significantly Altered by Dose or Circulating Level of Resveratrol
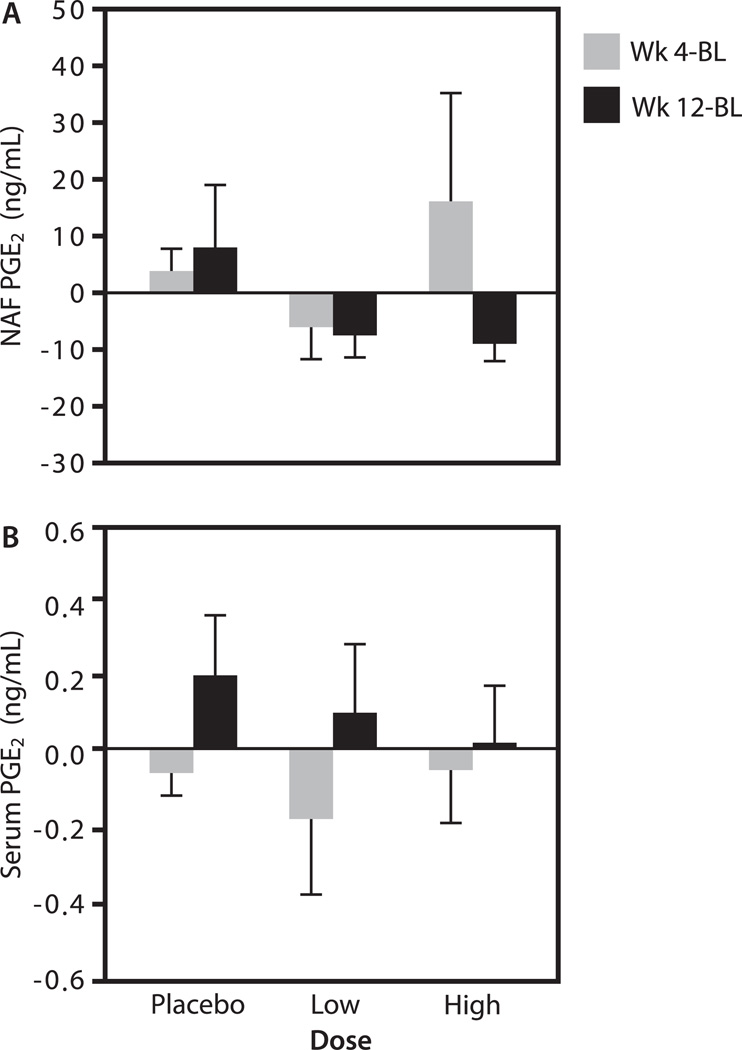
We evaluated PGE2 expression in both NAF (Fig. 2A) and serum (Fig. 2B) before and after resveratrol treatment. PGE2 levels did not significantly change after 4 or 12 wk of treatment with resveratrol in either NAF or serum.
FIG. 2.
PGE2 concentrations (ng/mL) after resveratrol treatment. The change [compared to baseline (BL)] in nipple aspirate fluid (NAF; A) and serum (B) levels of PGE2 4 and 12 wk after twice-daily administration of placebo, low- or high-dose resveratrol.
Change in DNA Methylation Is Related to Resveratrol Concentration
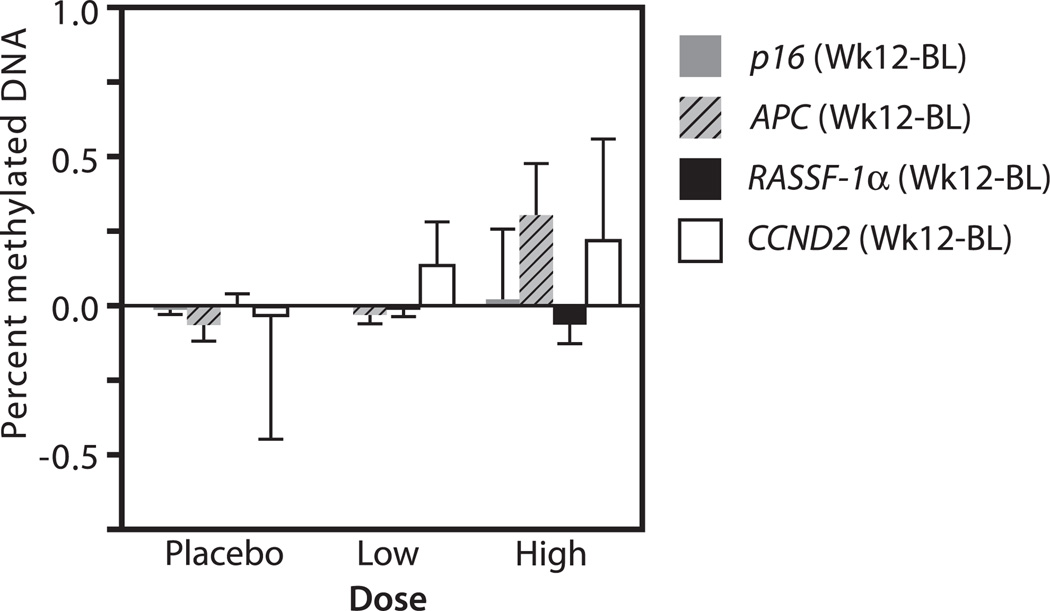
We evaluated the effect of resveratrol dose on change inDNA methylation (Fig. 3). We were able to measure DNA methylation in the 4 genes (p16, CCND2, APC, and RASSF-1a) at baseline and 12 wk in 22/30 (73%) women. Neither low- nor high-dose resveratrol had a significant effect on any of the 4 genes studied. Nonetheless, the fraction of methylated RASSF-1α DNA decreased, whereas that of APC increased for 3 out of 4 women after high-dose resveratrol compared to before treatment. Because of the variability in resveratrol concentration among women receiving similar doses, we next evaluated the change in methylation based on concentration of resveratrol in the circulation.
FIG. 3.
Percent methylated DNA in tumor suppressor genes after resveratrol treatment. The change (compared to baseline-BL) in percent methylation of p16, APC, RASSF-1a, and CCND2 12 wk after twice daily administration of placebo, low- or high-dose resveratrol.
Change in Both Serum Resveratrol and in NAF PGE2 Predict Change in RASSF-1α Methylation
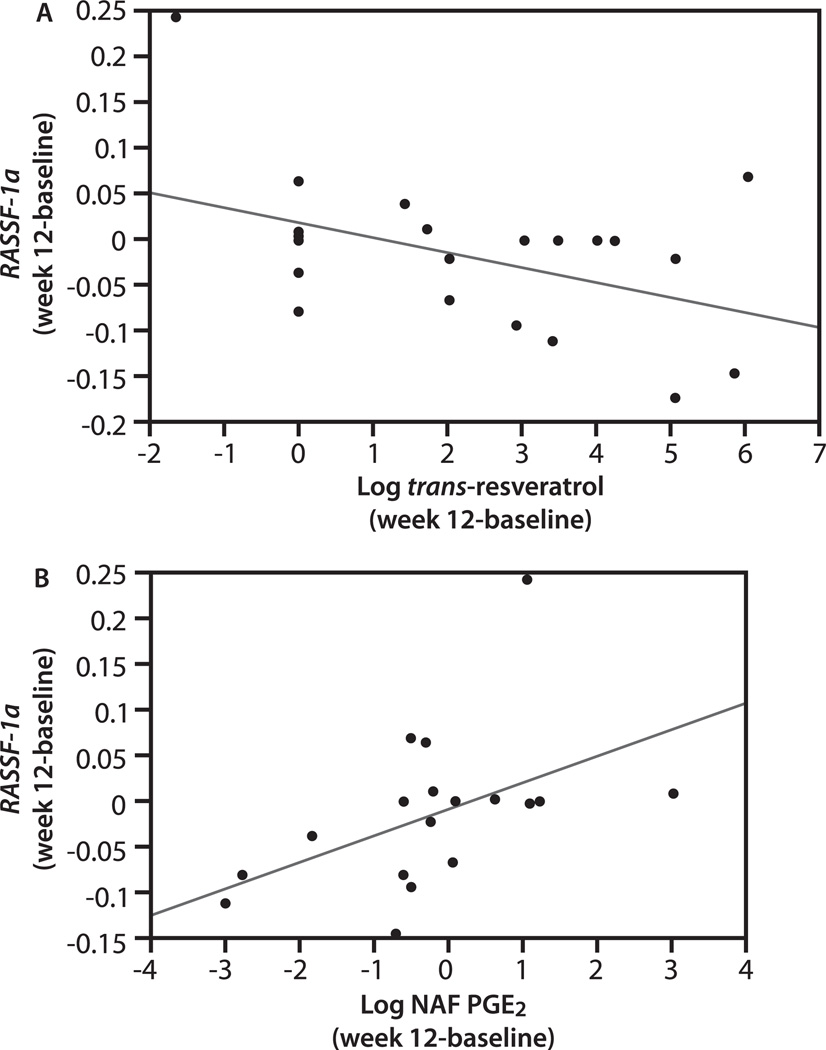
We determined how the level of trans-resveratrol and resveratrol-glucuronide in serum correlated with the change in the fraction of DNA that was methylated. The level of neither species correlated with the change in p16, APC, nor CCND2 methylation. On the other hand, higher concentration of both species correlated with a greater decrease in RASSF-1α methylation (P = 0.047) after 12 wk of treatment (Fig. 4A).
FIG. 4.
RASSF-1a methylation change vs. change in (A) trans-resveratrol and (B) NAF PGE2 concentration. Linear regression using least squares for model fitting was performed. RASSF-1a methylation significantly decreased as the concentration of total trans-resveratrol increased (P = 0.047, r2 = −0.14), and as PGE2 decreased (P = 0.045, r2 = 0.18).
We also evaluated the correlation between the change in NAF and serum PGE2 after treatment with the change in the level of methylated DNA for each gene studied. Change in serum PGE2 was not related to change in methylation for any of the 4 genes. Change in NAF PGE2 was not related to change in methylation of p16, APC, nor CCND2, whereas the change in the fraction of methylated RASSF-1a DNA after treatment (P = 0.045) positively correlated with the change in NAF PGE2 (Fig. 4B).
DISCUSSION
We observed that the predominant resveratrol species in the circulation was the glucuronide metabolite. Reports suggest that glucuronide and most sulfate metabolites have less biologic activity than the free compound (36).We designed the study based on evidence that free resveratrol was present within tissues, with resveratrol metabolites predominating in the circulation. MD and NAF samples did not provide sufficient material for resveratrol analyses. Because our investigation enrolled healthy individuals, tissue collection and analysis was not feasible. A recent report (37) that treated 20 individuals (10 per group) known to have colorectal cancer with 1 of 2 resveratrol doses for 8 days prior to surgical resection found that free resveratrol “accounted for a much larger portion of total resveratrol species in colorectal tissue than in plasma at an equivalent time point postdosing.” This report also demonstrated a significant decrease in proliferation in the colorectal tissue after treatment. The report demonstrates that although conjugation of free resveratrol after ingestion leads to the identification of metabolites as the predominant species in the circulation, the free form of resveratrol can still be found at significant levels within pathologically normal and malignant colorectal tissues (37). Since intestinal bacteria metabolize resveratrol (38), the fractional ratio of metabolites may vary between intestinal and other tissues.
We previously reported a dose-dependent effect of trans-resveratrol on DNA methytransferase gene expression (12) in breast cancer cells in vitro. In the present report, although we observed a nonsignificant trend toward lower RASSF-1a methylation based on dose, there was a significant decrease in methylation related to the circulating level of both total trans-resveratrol and its glucuronide metabolite (metabolite data not shown). In a prior report that evaluated the chemopreventive effects of the soy polyphenols genistein and daidzein (8), we observed that the circulating level of genistein was a better predictor of methylation change than dose. We believe this is true for treatment with the polyphenol resveratrol as well, likely because the rate at which the compound is metabolized and cleared varies from individual to individual.
In preclinical studies, resveratrol has been shown to decrease cyclooxygenase (COX)-1 and -2 (39), which leads to a decrease in PGE2 (40). Although we did not observe a decrease in PGE2 related to dose or circulating level of trans-resveratrol or the agent’s metabolites, there was a significant decrease in PGE2 expression in NAF in association with a decrease in RASSF-1a (but not APC, p16, or CCND2) methylation. We previously reported that RASSF1a methylation increases with disease progression from precancer to cancer in breast intraductal and tissue samples (22). The decrease in RASSF-1a methylation and association of methylation decrease with a decrease in PGE2 are consistent with the known chemopreventive effects of resveratrol.
We observed a dose-dependent increase in trans-resveratrol and the glucuronide metabolite after both 4 and 12 wk of treatment. Trans was the predominant resveratrol isomer in the circulation, with the cis isomer comprising 5.2% or less of the total detected. The predominant serum species, considering free resveratrol and the metabolites, was the glucuronide metabolite, comprising 93%–100% of the total present in each sample. This is similar to a prior report after short-term treatment in which the glucuronide metabolite comprised 95%–97% of the resveratrol species detected in the circulation. In that study, free resveratrol was not detected in the plasma but was readily detected in benign and malignant colorectal tissue in the same subjects (37). Both free resveratrol and the metabolites can get inside of cells in multiple tissues (41). We suspect that the glucuronide and sulfate metabolites are intracellularly cleaved by glucuronidases and sulfatases to their free form in the breast.
A strength of our study is that, to our knowledge, this is the first report assessing the dose-dependent effects of resveratrol on DNA methylation in a group of individuals at increased cancer risk. A second strength was the study design, which was prospective, double-blind, and placebo-controlled. Limitations of the study included our modest sample size and the fact that we did not exclude from enrollment individuals who were using NSAIDS. Although NSAID use did not appear to influence PGE2 or resveratrol, we cannot be sure that NSAID use did not limit our ability to detect an effect of resveratrol on PGE2 expression. We also cannot exclude the possibility that a higher resveratrol dose would have been more effective in lowering PGE2. A recent study indicated that doses of 500 and 1000 mg daily were well tolerated and had a significant antiproliferative effect on colorectal cancer tissue (34).
In conclusion, we demonstrate that the trans isomer and the glucuronide metabolite predominate in the circulation after chronic administration of trans-resveratrol, with a dose- dependent increase in both. RASSF-1a methylation decreased with increasing levels of trans-resveratrol and resveratrol-glucuronide in the circulation, and with decreasing PGE2 expression in the breast. Our preliminary observations suggest a novel mechanism for the chemopreventive effect of trans-resveratrol in the breast of high-risk women.
ACKNOWLEDGMENT
This study was funded in part by NIH grant CA124818.
Contributor Information
Weizhu Zhu, Departments of Surgery and Pathology, University of North Dakota School of Medicine and Health Sciences, Grand Forks, North Dakota, USA.
Wenyi Qin, Departments of Surgery and Pathology, University of North Dakota School of Medicine and Health Sciences, Grand Forks, North Dakota, USA.
Ke Zhang, Section of Biostatistics, University of North Dakota School of Medicine and Health Sciences, Grand Forks, North Dakota, USA.
George E. Rottinghaus, School of Veterinary Medicine, University of Missouri, Columbia, Missouri, USA
Yin-Chieh Chen, School of Veterinary Medicine, University of Missouri, Columbia, Missouri, USA.
Beth Kliethermes, Departments of Surgery and Pathology, University of North Dakota School of Medicine and Health Sciences, Grand Forks, North Dakota, USA.
Edward R. Sauter, Departments of Surgery and Pathology, University of North Dakota School of Medicine and Health Sciences, Grand Forks, North Dakota, USA
REFERENCES
- 1.Subbaramaiah K, Chung WJ, Michaluart P, Telang N, Tanabe T, et al. Resveratrol inhibits cyclooxygenase-2 transcription and activity in phorbol ester-treated human mammary epithelial cells. J Biol Chem. 1998;273:21875–21882. doi: 10.1074/jbc.273.34.21875. [DOI] [PubMed] [Google Scholar]
- 2.Roberti M, Pizzirani D, Simoni D, Rondanin R, Baruchello R, et al. Synthesis and biological evaluation of resveratrol and analogues as apoptosis-inducing agents. J Med Chem. 2003;46:3546–3554. doi: 10.1021/jm030785u. [DOI] [PubMed] [Google Scholar]
- 3.Mgbonyebi OP, Russo J, Russo IH. Antiproliferative effect of synthetic resveratrol on human breast epithelial cells. Int J Oncol. 1998;12:865–869. [PubMed] [Google Scholar]
- 4.Jang M, Cai L, Udeani GO, Slowing KV, Thomas CF, et al. Cancer chemopreventive activity of resveratrol, a natural product derived from grapes. Science. 1997;275:218–220. doi: 10.1126/science.275.5297.218. [DOI] [PubMed] [Google Scholar]
- 5.Banerjee S, Bueso-Ramos C, Aggarwal BB. Suppression of 7,12-dimethylbenz(a)anthracene-induced mammary carcinogenesis in rats by resveratrol: role of nuclear factor-kappaB, cyclooxygenase 2, and matrix metalloprotease 9. Cancer Res. 2002;62:4945–4954. [PubMed] [Google Scholar]
- 6.Levi F, Pasche C, Lucchini F, Ghidoni R, Ferraroni M, et al. Resveratrol and breast cancer risk. Eur J Cancer Prev. 2005;14:139–142. doi: 10.1097/00008469-200504000-00009. [DOI] [PubMed] [Google Scholar]
- 7.Widschwendter M, Jones PA. DNA methylation and breast carcinogenesis. Oncogene. 2002;21:5462–5482. doi: 10.1038/sj.onc.1205606. [DOI] [PubMed] [Google Scholar]
- 8.Qin W, Zhu W, Shi H, Hewett JE, Ruhlen RL, et al. Soy isoflavones have an antiestrogenic effect and alter mammary promoter hypermethylation in healthy premenopausal women. Nutr Cancer. 2009;61:238–244. doi: 10.1080/01635580802404196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bestor TH. The DNA methyltransferases of mammals. Hum Mol Genet. 2000;9:2395–2402. doi: 10.1093/hmg/9.16.2395. [DOI] [PubMed] [Google Scholar]
- 10.Leu YW, Rahmatpanah F, Shi H, Wei SH, Liu JC, et al. Double RNA interference of DNMT3b and DNMT1 enhances DNA demethylation and gene reactivation. Cancer Res. 2003;63:6110–6115. [PubMed] [Google Scholar]
- 11.Rhee I, Bachman KE, Park BH, Jair KW, Yen RW, et al. DNMT1 and DNMT3b cooperate to silence genes in human cancer cells. Nature. 2002;416(6880):552–556. doi: 10.1038/416552a. [DOI] [PubMed] [Google Scholar]
- 12.Qin W, Zhu W, Sauter ER. Resveratrol induced DNA methylation in ER+ breast cancer. Proc Am Assn Cancer Res. 2005;96:2750A. [Google Scholar]
- 13.Walle T, Hsieh F, DeLegge MH, Oatis JE, Jr, Walle UK. High absorption but very low bioavailability of oral resveratrol in humans. Drug Metab Dispos. 2004;32:1377–1382. doi: 10.1124/dmd.104.000885. [DOI] [PubMed] [Google Scholar]
- 14.de la Lastra CA, Villegas I. Resveratrol as an anti-inflammatory and anti-aging agent: mechanisms and clinical implications. Mol Nutr Food Res. 2005;49:405–430. doi: 10.1002/mnfr.200500022. [DOI] [PubMed] [Google Scholar]
- 15.Rowland I, Faughnan M, Hoey L, Wahala K, Williamson G, et al. Bioavailability of phyto-oestrogens. Br J Nutr. 2003;89(Suppl 1):S45–S58. doi: 10.1079/BJN2002796. [DOI] [PubMed] [Google Scholar]
- 16.Jannin B, Menzel M, Berlot JP, Delmas D, Lancon A, et al. Transport of resveratrol, a cancer chemopreventive agent, to cellular targets: plasmatic protein binding and cell uptake. Biochem Pharmacol. 2004;68:1113–1118. doi: 10.1016/j.bcp.2004.04.028. [DOI] [PubMed] [Google Scholar]
- 17.Wang LX, Heredia A, Song H, Zhang Z, Yu B, et al. Resveratrol glucuronides as the metabolites of resveratrol in humans: characterization, synthesis, and anti-HIV activity. J Pharm Sci. 2004;93:2448–2457. doi: 10.1002/jps.20156. [DOI] [PubMed] [Google Scholar]
- 18.Santner SJ, Feil PD, Santen RJ. In situ estrogen production via the estrone sulfatase pathway in breast tumors: relative importance versus the aromatase pathway. J Clin Endocrinol Metab. 1984;59:29–33. doi: 10.1210/jcem-59-1-29. [DOI] [PubMed] [Google Scholar]
- 19.Signorelli P, Ghidoni R. Resveratrol as an anticancer nutrient: molecular basis, open questions and promises. J Nutr Biochem. 2005;16:449–466. doi: 10.1016/j.jnutbio.2005.01.017. [DOI] [PubMed] [Google Scholar]
- 20.Colin D, Lancon A, Delmas D, Lizard G, Abrossinow J, et al. Antiproliferative activities of resveratrol and related compounds in human hepatocyte derived HepG2 cells are associated with biochemical cell disturbance revealed by fluorescence analyses. Biochimie. 2008;90:1674–1684. doi: 10.1016/j.biochi.2008.06.006. [DOI] [PubMed] [Google Scholar]
- 21.Delmas D, Lancon A, Colin D, Jannin B, Latruffe N. Resveratrol as a chemopreventive agent: a promising molecule for fighting cancer. Curr Drug Targets. 2006;7:423–442. doi: 10.2174/138945006776359331. [DOI] [PubMed] [Google Scholar]
- 22.Zhu W, Qin W, Hewett JE, Sauter ER. Quantitative evaluation of DNA hypermethylation in malignant and benign breast tissue and fluids. Int J Cancer. 2010;126:474–482. doi: 10.1002/ijc.24728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Van der Auwera I, Van Laere SJ, Van den Bosch SM, Van den Eynden GG, Trinh BX, et al. Aberrant methylation of the adenomatous polyposis coli (APC) gene promoter is associated with the inflammatory breast cancer phenotype. Br J Cancer. 2008;99:1735–1742. doi: 10.1038/sj.bjc.6604705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sheng H, Shao J, Morrow JD, Beauchamp RD, DuBois RN. Modulation of apoptosis and Bcl-2 expression by prostaglandin E2 in human colon cancer cells. Cancer Res. 1998;58:362–366. [PubMed] [Google Scholar]
- 25.Masferrer JL, Leahy KM, Koki AT, Zweifel BS, Settle SL, et al. Antiangiogenic and antitumor activities of cyclooxygenase-2 inhibitors. Cancer Res. 2000;60:1306–1311. [PubMed] [Google Scholar]
- 26.Fulton AM, Gimotty P, Alonsozana E, Dorsey R, Kundu N. Elevated prostaglandin E2 (PGE2) levels in human breast cancer are associated with poor long-term survival. Proc Am Assn Cancer Res. 2000;41:3660A. [Google Scholar]
- 27.Chachay VS, Kirkpatrick CM, Hickman IJ, Ferguson M, Prins JB, et al. Resveratrol: pills to replace a healthy diet? Br J Clin Pharmacol. 2011;72:27–38. doi: 10.1111/j.1365-2125.2011.03966.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sauter ER, Daly M, Linahan K, Ehya H, Engstrom PF, et al. Prostate-specific antigen levels in nipple aspirate fluid correlate with breast cancer risk. Cancer Epidemiol Biomarkers Prev. 1996;5:967–970. [PubMed] [Google Scholar]
- 29.Sauter ER, Ehya H, Klein-Szanto AJ, Wagner-Mann C, MacGibbon B. Fiberoptic ductoscopy findings in women with and without spontaneous nipple discharge. Cancer. 2005;103:914–921. doi: 10.1002/cncr.20865. [DOI] [PubMed] [Google Scholar]
- 30.Gustafson KS, Furth EE, Heitjan DF, Fansler ZB, Clark DP. DNA methylation profiling of cervical squamous intraepithelial lesions using liquid-based cytology specimens: an approach that utilizes receiver-operating characteristic analysis. Cancer. 2004;102:259–268. doi: 10.1002/cncr.20425. [DOI] [PubMed] [Google Scholar]
- 31.House MG, Guo M, Iacobuzio-Donahue C, Herman JG. Molecular progression of promoter methylation in intraductal papillarymucinous neoplasms (IPMN) of the pancreas. Carcinogenesis. 2003;24:193–198. doi: 10.1093/carcin/24.2.193. [DOI] [PubMed] [Google Scholar]
- 32.Fackler MJ, McVeigh M, Mehrotra J, Blum MA, Lange J, et al. Quantitative multiplex methylation-specific PCR assay for the detection of promoter hypermethylation in multiple genes in breast cancer. Cancer Res. 2004;64:4442–4452. doi: 10.1158/0008-5472.CAN-03-3341. [DOI] [PubMed] [Google Scholar]
- 33.Wang D, Hang T, Wu C, Liu W. Identification of the major metabolites of resveratrol in rat urine by HPLC-MS/MS. J Chromatogr B Analyt Technol Biomed Life Sci. 2005;829:97–106. doi: 10.1016/j.jchromb.2005.09.040. [DOI] [PubMed] [Google Scholar]
- 34.Yu C, Shin YG, Chow A, Li Y, Kosmeder JW, et al. Human, rat, and mouse metabolism of resveratrol. Pharm Res. 2002;19:1907–1914. doi: 10.1023/a:1021414129280. [DOI] [PubMed] [Google Scholar]
- 35.Sauter ER, Qin W, Schlatter L, Hewett JE, Flynn JT. Celecoxib decreases prostaglandin E2 concentrations in nipple aspirate fluid from high risk postmenopausal women and women with breast cancer. BMC Cancer. 2006 doi: 10.1186/1471-2407-6-248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Delmas D, Aires V, Limagne E, Dutartre P, Mazue F, et al. Transport, stability, and biological activity of resveratrol. Ann NY Acad Sci. 2011;1215:48–59. doi: 10.1111/j.1749-6632.2010.05871.x. [DOI] [PubMed] [Google Scholar]
- 37.Patel KR, Brown VA, Jones DJ, Britton RG, Hemingway D, et al. Clinical pharmacology of resveratrol and its metabolites in colorectal cancer patients. Cancer Res. 2010;70:7392–7399. doi: 10.1158/0008-5472.CAN-10-2027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rodrigo R, Miranda A, Vergara L. Modulation of endogenous antioxidant system by wine polyphenols in human disease. Clin Chim Acta. 2011;412:410–424. doi: 10.1016/j.cca.2010.11.034. [DOI] [PubMed] [Google Scholar]
- 39.Murias M, Handler N, Erker T, Pleban K, Ecker G, et al. Resveratrol analogues as selective cyclooxygenase-2 inhibitors: synthesis and structure-activity relationship. Bioorg Med Chem. 2004;12:5571–5578. doi: 10.1016/j.bmc.2004.08.008. [DOI] [PubMed] [Google Scholar]
- 40.Dave M, Attur M, Palmer G, Al-Mussawir HE, Kennish L, et al. The antioxidant resveratrol protects against chondrocyte apoptosis via effects on mitochondrial polarization and ATP production. Arthritis Rheum. 2008;58:2786–2797. doi: 10.1002/art.23799. [DOI] [PubMed] [Google Scholar]
- 41.Juan ME, Maijo M, Planas JM. Quantification of trans-resveratrol and its metabolites in rat plasma and tissues by HPLC. J Pharm Biomed Anal. 2010;51:391–398. doi: 10.1016/j.jpba.2009.03.026. [DOI] [PubMed] [Google Scholar]