Abstract
Chronic ethanol abuse results in hepatocyte injury and impairs hepatocyte replication. We have previously shown that ethanol metabolism results in cell cycle arrest at the G2/M transition, which is partially mediated by inhibitory phosphorylation of the cyclin-dependent kinase, Cdc2. To further delineate the mechanisms by which ethanol metabolism mediates this G2/M arrest, we investigated the involvement of upstream regulators of Cdc2 activity. Cdc2 is activated by the phosphatase Cdc25C. The activity of Cdc25C can, in turn, be regulated by the checkpoint kinase, Chk2, which is regulated by the kinase ataxia telangiectasia mutated (ATM). To investigate the involvement of these regulators of Cdc2 activity, VA-13 cells, which are Hep G2 cells modified to efficiently express alcohol dehydrogenase, were cultured in the presence or absence of 25 mM ethanol. Immunoblots were performed to determine the effects of ethanol metabolism on the activation of Cdc25C, Chk2, and ATM. Ethanol metabolism increased the active forms of ATM, and Chk2, as well as the phosphorylated form of Cdc25C. Additionally, inhibition of ATM resulted in approximately 50% of the cells being rescued from the G2/M cell cycle arrest, and ameliorated the inhibitory phosphorylation of Cdc2. Our findings demonstrate that ethanol metabolism activates ATM. ATM can activate the checkpoint kinase Chk2, resulting in phosphorylation of Cdc25C, and ultimately in the accumulation of inactive Cdc2. This may, in part, explain the ethanol metabolism-mediated impairment in hepatocyte replication, which may be important in the initiation and progression of alcoholic liver injury.
Keywords: Alcoholic Liver disease, Cell cycle arrest, Ethanol metabolism, Liver regeneration, Checkpoint kinase, ataxia telangiectasia mutated (ATM), Cdc2
Introduction
The liver is the primary site of ethanol metabolism and can result in hepatotoxicity. There is extensive evidence that ethanol metabolism delays or impairs the replication of hepatocytes and cultured hepatic cells (Clemens et al., 2003; Clemens et al., 2002; Koteish et al., 2002; Yang et al., 1998). Because of these facts, it appears that chronic ethanol consumption not only results in cell damage and death, but also alters the ability of the liver to respond appropriately to injury and replace damaged cells.
There are two mechanisms by which lost hepatocytes can be replaced (Diehl, 2005; Santoni-Rugiu et al., 2005). Replacement of damaged hepatocytes normally occurs by the replication of existing mature hepatocytes (Sell, 2001). If the replication of existing hepatocytes is inhibited, as is the case in many chronic liver diseases, replacement of damaged cells occurs by activation of a population of bipotential hepatic cells known as liver progenitor cells or oval cells (Kuwahara et al., 2008; Ohlson et al., 1998; Roskams et al., 2003; Yang et al., 2004). Because liver progenitor cells are bipotential and can differentiate into hepatocytes or bile ductular epithelium, it has been proposed that replacement of hepatocytes by liver progenitor cells leads not only to the replacement of hepatocytes, but also results in increased proliferation of ductals (Ray et al., 1993; Roskams et al., 2003). In patients suffering from alcoholic liver disease, it has been shown that increased expression of ductular epithelia correlates highly with portal inflammation, fibrosis, and cirrhosis (Ray et al., 1993). Thus, increased expression of ductular epithelia may be a precursor to the accumulation of fibrotic tissue in the liver. Furthermore, impairment of normal hepatocyte replication by ethanol metabolism may have a role in the fibrotic scarring associated with alcoholic liver disease.
We have previously shown that ethanol metabolism by recombinant Hep G2 cells, which have been engineered to efficiently metabolize ethanol, results in impaired cellular replication (Clemens et al., 2002; Donohue et al., 2006). This ethanol metabolism-meditated impaired cellular replication is, at least partially, the result of a cell cycle arrest at the G2/M transition of the cell cycle. Furthermore, this impairment is caused, at least in part, by an accumulation of the cyclin-dependent kinase, Cdc2, in the inactive, phosphorylated form (Clemens et al., 2003).
The activity of Cdc2 is required for the transition from the G2 to M-phase of the cell cycle (Nurse, 1990), and is regulated both positively and negatively by phosphorylation (Hunter, 1995). Cdc2 is maintained in an inactive state throughout the majority of the cell cycle. This is accomplished by phosphorylation at threonine 14 (Thr 14) and tyrosine 15 (Tyr 15), which renders Cdc2 incapable of binding ATP and thus inactive (Atherton-Fessler et al., 1993). The phosphorylation state of these two amino acid residues is regulated by a balance between the activities of the kinases Myt1 and Wee1, and the Cdc25 family of phosphatases (Liu et al., 1999; Liu et al., 1997; McGowan and Russell, 1993; Wells et al., 1999). In late G2, Cdc2 is activated by Cdc25C and mitosis progresses (Peng et al., 1997). Thus, the activity of Cdc25C is critical for the activation of Cdc2 and the occurrence of mitosis.
Cell cycle arrest can result from the activation of pathways known as cell cycle checkpoints. Induction of these pathways can be initiated by the activation of signal transduction pathways mediated by the kinase, ataxia telangiectasia mutated (ATM). ATM is activated by a number of stimuli including genotoxic assault, delayed or incomplete DNA replication, viral infection, and oxidative stress (Abraham, 2001; Marshall et al., 2005). Many of the downstream actions of this kinase are mediated through the actions of the serine/threonine kinase, checkpoint kinase 2 (Chk2) (Zhou and Elledge, 2000). Chk2 is activated by phosphorylation at threonine 68 by ATM (Ahn et al., 2000), and is able to phosphorylate Cdc25C at Ser 216, a site known to be involved in the negative regulation of this important cell cycle regulator (Abraham, 2001; Chaturvedi et al., 1999; Zhou et al., 2000). Phosphorylation of Cdc25C at Ser 216 creates a binding site for 14-3-3 proteins. Binding of Cdc25C to 14-3-3 proteins sequesters Cdc25C in the cytoplasm, effectively nullifying its ability to activate the nuclear protein Cdc2 (Peng et al., 1997). Thus, the checkpoint kinase Chk2 can impair progression through the G2/M transition of the cell cycle by phosphorylating Cdc25C.
Using the recombinant Hep G2 cell line VA-13, which efficiently metabolizes ethanol, we have investigated the involvement of the cell cycle checkpoints in the ethanol metabolism-mediated cell cycle arrest (Clemens et al., 2002). The results of these studies demonstrate that ethanol metabolism is associated with the activation of ATM and the checkpoint kinase Chk2. Additionally, we show that Ser 216-phosphorylated Cdc25C is increased. These findings provide an explanation for the increase in the phosphorylated form of Cdc2, which is observed in VA-13 cells actively metabolizing ethanol. Activation of the kinases involved in the cell cycle checkpoints may have a role in the impairment of normal hepatocyte replication that has been observed in the liver of individuals suffering from alcoholic liver disease.
Materials and Methods
Cell Culture
VA-13 cells are recombinant Hep G2 cells that have been stably transfected to express alcohol dehydrogenase. The construction and characterization of these cell lines has been previously described (Clemens et al., 2002). Cells were maintained in DMEM containing high glucose and supplemented with 10% fetal bovine serum, 2 mM glutamine, 400 μg/ml zeocin, 50 μg/ml gentamicin, 1,000 units/ml penicillin and 100 μg/ml streptomycin (Complete DMEM). For ethanol experiments, cells were cultured as above but 25 mM HEPES [N-(2-hydroxyethyl) piperazine-N-(2-ethanesulfonic acid)] was included in the growth media of all flasks and 25 mM ethanol was included in the growth media of cells exposed to ethanol. During ethanol experiments all flasks were tightly sealed to minimize evaporation of ethanol and acetaldehyde. ATM was inhibited by inclusion of caffeine at a concentration of 1 mM (Zhou et al., 2000). Alcohol dehydrogenase activity was inhibited by inclusion of 2 mM 4-methyl pyrazole.
Immunoblotting and Antibodies
Cells were removed from the flasks by trypsinization and collected by centrifugation. The cell pellets were lysed in RIPA buffer (50 mM Tris pH 7.4, 1% NP-40, 0.25% Na deoxycholate, 150 mM NaCl, 1 mM EDTA, 1 mM phenylmethylsulfonyl fluoride, 1 mM Na3VO2, 1 mM NaF). Complete mini protease cocktail was added to the buffer as recommended by the manufacturer (Roche, Indianapolis, IN). The protein concentrations of the lysates were determined using the Bio-Rad protein reagent (Bio-Rad Laboratories, Hercules, CA) with bovine serum albumin as a standard. Thirty micrograms of protein from each sample was separated by sodium dodecyl sulfate–polyacrylamide gel electrophoresis. Proteins were electroblotted onto polyvinyl difluoride (PVDF) membranes in 25 mM Tris and 192 mM glycine, at 100 volts for 60 min at 4 °C. The membranes were blocked in TBST (20 mM Tris pH 7.6, 136 mM NaCl, 0.1% Tween 20) containing 5% nonfat dry milk for 1 hour at room temperature, and incubated at 4°C overnight with the primary antibod y diluted in TBST/milk solution. The membranes were washed in TBST and incubated for 1 hour with a peroxidase-conjugated secondary antibody diluted in TBST/milk, washed, and the proteins visualized by chemiluminescence using the SuperSignal West Pico chemiluminescent substrate (Pierce, Rockford, IL). The resulting bands were quantified by densitometry using Quantity One software (Bio Rad). Antibodies used were anti-Cdc2, (Upstate Biotechnology, Lake Placid, NY), anti-phospho-Cdc2 (Tyr 15), anti-phospho-Cdc25C (Ser 216), anti-Chk2, anti-phospho-Chk2 (Thr 68), (Cell Signaling Beverly, MA), anti-Cdc25C (Santa Cruz Biotechnology, Santa Cruz, CA), anti-ATM (Gene Tex Inc, Irvine, CA) anti-phospho-ATM (Ser 1981) (Rockland Inc. Gilbertsville, PA) and anti-β-actin (Sigma, St Louis, MO).
Cell Cycle Analysis
Cells were cultured as described above, collected after typsinization, and their DNA content determined by staining with Vindelov’s solution as previously described (Clemens et al., 2003) using a FACaliber flow cytometer and analyzed using Modfit Software (Verity Software House, Topshan, ME).
Isolation of RNA and RT-PCR
RNA was isolated from cells cultured in the absence or presence of ethanol using a RNeasy Mini Kit essentially as described by the manufacturer (Qiagen, Valencia, CA), and cDNA was produced using a High Capacity cDNA Kit (Applied Biosystems, Foster City, CA). RT-PCR was performed with the Taqman system on a Applied Biosytems 7500 RT-PCR themocycler using primers specific for Chk2 and Cdc25C of human origin with 18S RNA as an internal control for each reaction. Differences between control and ethanol treated cells are expressed as fold changes of the target genes as determined by the 2−ΔΔCt method (Livak and Schmittgen, 2001).
Statistics
Statistical significance was determined by T-test. A probability of ≤ 0.05 was considered to be statistically significant.
Results
Effects of Ethanol Metabolism on Cdc25C
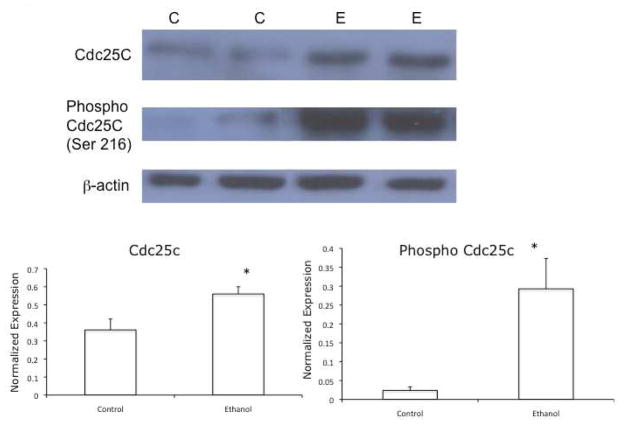
Phosphorylation of Cdc25c at Ser 216 can result in Cdc25c being unavailable to activate the mitotic cyclin-dependent kinase, Cdc2. Therefore, to determine the involvement of Cdc25C in the ethanol metabolism-mediated cell cycle arrest, we investigated the effects of ethanol metabolism on Ser 216 phosphorylation of Cdc25C. To accomplish this, VA-13 cells were cultured in the presence or the absence of 25 mM ethanol for 4 days. The cells were harvested and immunoblots performed using antiserum specific to Ser 216 phospho-Cdc25C. The results of these studies, shown in Figure 1, revealed that culturing VA-13 cells in the presence of 25 mM ethanol resulted in a 1.5-fold increase in Cdc25C, whereas the phosphorylated form of Cdc25C (Ser 216 phospho-Cdc25C) was increased 10-fold.
Figure 1.
Effects of ethanol metabolism on the expression of Cdc25C and Ser 216-phosphorylated Cdc25C. VA-13 cells were cultured in the presence or absence of 25 mM ethanol for 4 days, the cells were lysed, and analyzed by immunoblot for total and Ser 216-phosphorylated Cdc25C. Densitometry indicated approximately a 1.5-fold increase in Cdc25C and approximately a 10-fold increase in Ser 216-phosphorylated Cdc25C in cells cultured in the presence of ethanol, when normalized with b-actin. The blot is representative of at least three experiments. Error bars indicate the standard error of the mean *P ≤ 0.05. C: control, E: ethanol treated
Effects of Ethanol Metabolism on Chk2 Activation
In order to maintain the integrity of the genetic material, eukaryotic cells have developed mechanisms to delay cell cycle progression. The points of the cell cycle where these delays or arrests occur are known as checkpoints. These arrests, or checkpoints, are mediated by kinases known as checkpoint kinases (Chks). One of the major effector checkpoint kinases mediating arrests of the cell cycle is Chk2. Chk2 can phosphorylate Cdc25C at Ser 216, and by this mechanism mediate G2/M cell cycle arrest (Chaturvedi et al., 1999). Because we found that ethanol metabolism increased the amount of phosphorylated Cdc25C, we investigated if the active form of Chk2 was also increased.
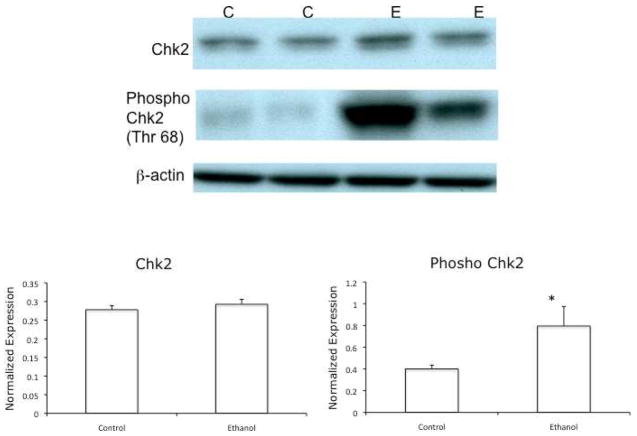
Chk2 is activated by phosphorylation at Thr 68 (Ahn et al., 2000). VA-13 cells were cultured as above, lysed, and immunoblots performed using antisera specific to the Thr 68-phosphorylated form of Chk2. The results of these experiments revealed that ethanol metabolism increased the active form of Chk2 approximately 2-fold in VA-13 cells cultured in the presence of ethanol (Figure 2).
Figure 2.
Effects of ethanol metabolism on the expression of Chk2 and Thr 68- phosphorylated Chk2. VA-13 cells were cultured in the presence or absence of 25 mM ethanol for 4 days, the cells were lysed, and analyzed by immunoblot for total and Thr 68-phosphorylated Chk2. Densitometry indicated no increase in total Chk2 and approximately a 2-fold increase in phosphorylated Chk2 in cells cultured in the presence of ethanol, when normalized to b-actin. The blot is representative of at least three experiments. Error bars indicate the standard error of the mean *P ≤ 0.05. C: control, E: ethanol treated
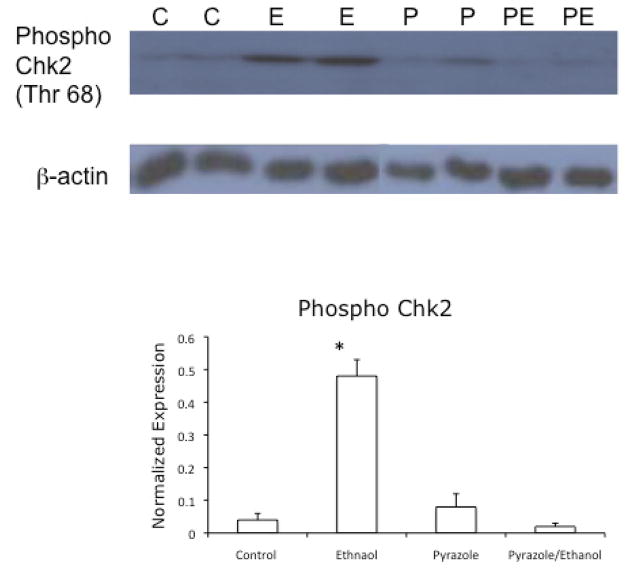
To ensure that the increase in Chk2 activation was a result of ethanol metabolism, and not the presence of ethanol itself, the above experiments were repeated using growth media containing 25 mM ethanol and the alcohol dehydrogenase inhibitor 4-methyl pyrazole (Osna et al., 2005). The results of these studies (Figure 3) showed that inhibition of ethanol metabolism abrogated the activation of Chk2, thus demonstrating a requirement for ethanol metabolism in the activation of Chk2.
Figure 3.
Effects of ethanol metabolism on the expression of Thr 68-phosphorylated Chk2. VA-13 cells were cultured in the presence or absence of 25 mM ethanol and the alcohol dehydrogenase inhibitor 4-methyl pyrazole for 4 days. The cells were lysed and analyzed by immunoblot for the presence of the active, phosphorylated form of Chk2. Densitometry revealed approximately a 10-fold increase in phosphorylated Chk2 in the cells cultured in the presence of ethanol when normalized to b-actin. No increase in phosphorylated Chk2 was detected in lysates from cells cultured in the presence of 4-methyl pyrazole or 4-methyl pyrazole and ethanol. The blot is representative of at least three experiments. Error bars indicate the standard error of the mean *P ≤ 0.05. C: control, E: ethanol, P: 4-methyl pyrazole, PE: ethanol and 4-methyl pyrazole.
Effects of Ethanol Metabolism on Transcription of Cell Cycle Components
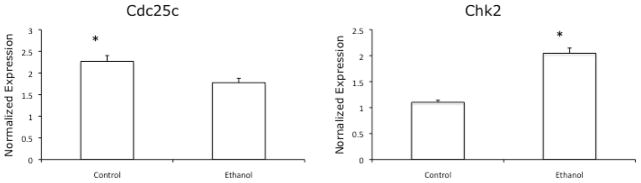
To determine if the increase in phosphorylated forms of the proteins was associated with an increase in their mRNA, RT-PCR was performed using primers specific to the Cdc25c and Chk2 transcripts. The results of these studies (Figure 4) indicated that culturing VA-13 cells in the presence of ethanol slightly decreased the abundance of Cdc25c transcripts, and increased the level of Chk2 transcripts approximately 2-fold.
Figure 4.
RT-PCR analysis of Cdc25c and Chk2 transcripts. VA-13 cells were cultured in the presence or absence of 25 mM ethanol for three days. RNA was isolated, reverse transcribed, and analyzed by RT-PCR. Analysis revealed that ethanol metabolism slightly decreased the abundance of Cdc25c transcripts and increased Chk2 transcripts 2-fold. Data is representative of at least three experiments. Error bars indicate the standard error of the mean *P ≤ 0.05.
Effects of Ethanol Metabolism on Ataxia Telangiectasia Mutated (ATM)
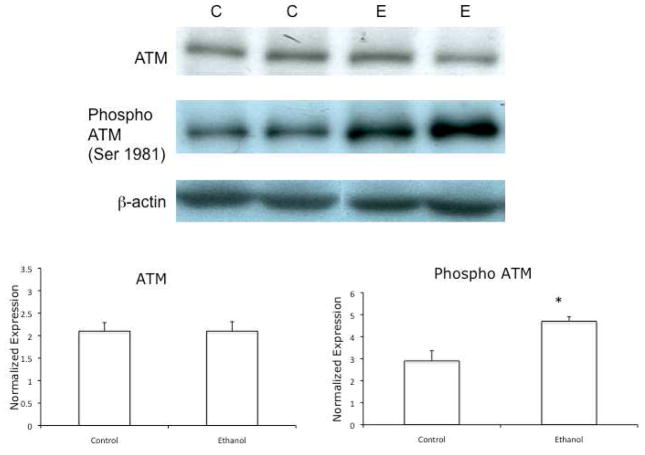
Because Chk2 is activated as a result of ethanol metabolism, we were interested in determining if the upstream kinase ATM, which is able to activate Chk2 (Chaturvedi et al., 1999), was affected by ethanol metabolism. ATM is activated by autophosphorylation at Ser 1981 (Bakkenist and Kastan, 2003). To determine the activation of ATM, VA-13 cells were cultured in the presence or absence of 25 mM ethanol for 4 days and immunoblots were performed using antisera specific to ATM and the Ser 1981-phosphorylated form of ATM. The results, shown in Figure 5, indicated that the level of active ATM was increased by approximately 1.6-fold in VA-13 cells cultured in the presence of ethanol.
Figure 5.
Effects of ethanol metabolism on the expression of total and Ser 1981- phosphorylated ATM. VA-13 cells were cultured in the presence or absence of 25 mM ethanol for 4 days, the cells were lysed, and analyzed by immunoblot for Ser 1981-phosphorylated ATM. Densitometry indicated no increase in total ATM and approximately a 1.6-fold increase in phosphorylated ATM in VA-13 cells cultured in the presence of ethanol, when normalized to b-actin. The blot is representative of at least three experiments. Error bars indicate the standard error of the mean *P ≤ 0.05. C: control, E: ethanol treated
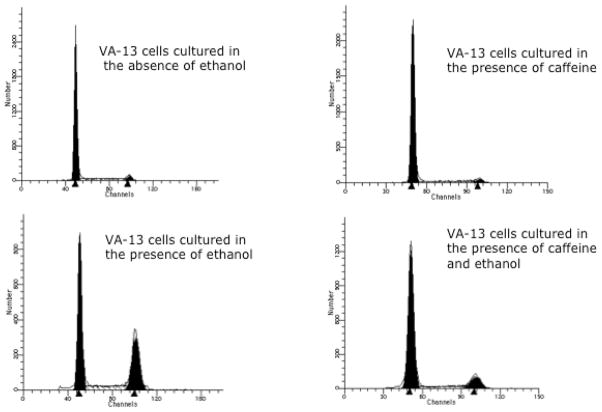
To further investigate the involvement of ATM in the ethanol-mediated cell cycle arrest, we investigated if caffeine, an inhibitor of ATM, would abrogate the ethanol metabolism-mediated cell cycle arrest (Zhou et al., 2000). To determine this, VA-13 cells were cultured as above, harvested, and the cell cycle progression was determined by flow cytometry. The results of this study, shown in Figure 6, revealed that approximately 4% of untreated controls and cells cultured in the presence of caffeine were in the G2/M transition of the cell cycle. In contrast, 33% of the cells cultured in the presence of ethanol were in G2/M. Inclusion of caffeine in the ethanol containing media dramatically reduced the percentage of cells in G2/M to approximately 12%. Additionally, caffeine treatment of VA-13 cells diminished the ethanol metabolism-mediated impairment in cell accumulation approximately a 50%.
Figure 6.
Cell cycle analysis. Cells were cultured in the presence or absence of 25 mM ethanol and 1 mM caffeine for 4 days, stained with Vindelov’s reagent, and analyzed by flow cytometry. Approximately 4% of untreated controls and cells cultured in the presence of caffeine were in the G2/M transition of the cell cycle. Whereas, approximately 33% of the cells cultured in the presence of ethanol were in G2/M. Inclusion of caffeine in the ethanol containing media dramatically reduced the percentage of cells in G2/M to approximately 12%.
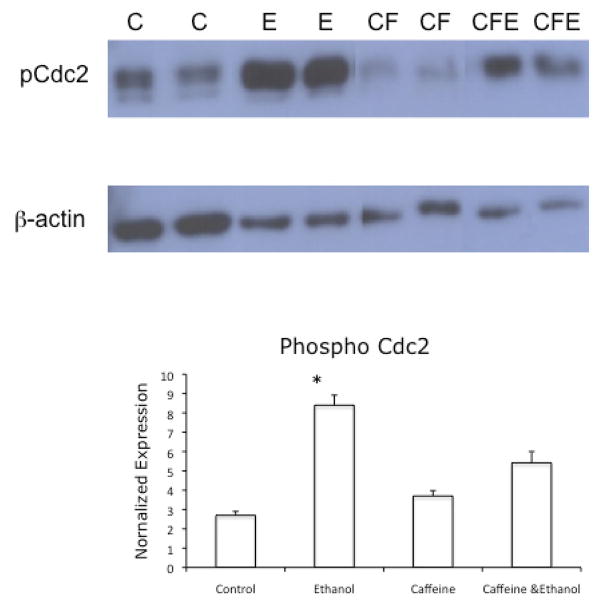
To investigate if inhibition of ATM with caffeine also affected the accumulation of the inactive, phosphorylated form of Cdc2, we repeated the above experiment and harvested the cells for immunoblotting. The results, depicted in Figure 7, show that caffeine treatment ameliorated the accumulation of the inactive, phosphorylated form of Cdc2, which is normally observed in cells cultured in the presence of ethanol.
Figure 7.
Effects of inhibition of ATM on the phosphorylation of Tyr 15 of Cdc2. VA-13 cells were cultured in the presence or absence of 25 mM ethanol and 1 mM caffeine for 4 days. The cells were lysed and analyzed by immunoblot for the presence of the inactive, phosphorylated form of Cdc2. Densitometry revealed approximately a 4-fold increase in phosphorylated Cdc2 (normalized with b-actin) in the cells cultured in the presence of ethanol, compared with control cells. No increase was observed in phosphorylated Cdc2 in lysates from cells cultured in the presence of caffeine or caffeine and ethanol. The blot is representative of at least three experiments. Error bars indicate the standard error of the mean. * is statistically significant (P ≤ 0.05) from all other treatment groups. C: control, E: ethanol, CF: caffeine, CFE: caffeine and ethanol
Discussion
Ethanol metabolism results in a number of physiologic changes in hepatocytes. Many of these changes can lead to hepatocyte death and the lost cells must be replaced. There are two pathways by which injured or lost hepatocytes can be replaced (Santoni-Rugiu et al., 2005; Van Hul et al., 2009). Normally, hepatocytes are replaced by existing mature hepatocytes. If existing hepatocytes are unable to replicate, as is the case during chronic liver diseases such as viral infection, fatty liver, chronic ethanol abuse, or other conditions that cause oxidative stress, a second regenerative pathway is activated (Santoni-Rugiu et al., 2005). The second regenerative pathway is associated with the activation of liver progenitor cells (also known as oval cells). These cells are bipotent, giving rise to both hepatocytes and billiary cells, and are normally located in the Canals of Hering, the junction between the hepatic plate and the bile ducts (Duncan et al., 2009; Theise et al., 1999).
Liver damage associated with chronic ethanol abuse is generally accompanied by the accumulation of liver progenitor cells (Diehl, 2005; Roskams et al., 2003). Activation of liver progenitor cells is associated with what is known as the ductular reaction (Richardson et al., 2007; Santoni-Rugiu et al., 2005; Theise and Kuwahara, 2007; Van Hul et al., 2009). The ductular reaction is a reactive lesion that is comprised of hepatic progenitors, as well as other cell types, which may include inflammatory, stromal, and endothelial cells (Roskams et al., 2004). The ductular reaction is also characterized by the activation of stellate cells, which give rise to myofibroblasts, the collagen producing cells that are thought to play a critical role in fibrotic liver disease (Friedman, 2008). This can result in the accumulation of periportal fibrotic matrix (Jung et al., 2008; Richardson et al., 2007). Hepatic progenitors proliferate in close proximity to stellate cells (Fausto, 2004). This may be because tissue and matrix remodeling is required for the efficient expansion of the cells (Van Hul et al., 2009). Thus, ethanol metabolism-mediated cell death, and the inability of normal mature hepatocytes to replicate and compensate for the loss of parenchymal cells, can potentially initiate the fibrotic scarring commonly observed as a result of alcoholic liver disease.
In order to investigate the molecular mechanisms by which the metabolism of ethanol can impair hepatocyte replication, we used the recombinant hepatic cell line VA-13. VA-13 cells express alcohol dehydrogenase and efficiently metabolize ethanol (Clemens et al., 2002). We have previously shown that ethanol metabolism by VA-13 cells results in cell cycle arrest at the G2/M transition of the cell cycle, and that this cell cycle arrest is mediated, at least partially, by accumulation of the cyclin-dependent kinase Cdc2 in the inactive, phosphorylated form (Clemens et al., 2003).
Cdc2 activity is required for mitosis to occur. Phosphorylation at Thr 14 and Tyr 15 of Cdc2 blocks the ATP binding site and renders Cdc2 inactive (Atherton-Fessler et al., 1993). Cdc2 is maintained in the inactive state throughout the majority of the cell cycle. After cellular DNA has been replicated, Cdc25C is transported to the nucleus where it activates Cdc2 by removing the inhibitory phosphorylation in late G2 phase of the cell cycle, allowing mitosis to proceed. Thus, Cdc25C must be present in the nucleus to activate Cdc2. If Cdc25C is phosphorylated at Ser 216 it becomes a substrate for 14-3-3 proteins, which leads to Cdc25C being sequestered in the cytoplasm, where it is effectively inactive. We have provided data that ethanol metabolism increases the accumulation of Ser 216-phosphorylated Cdc25C; this provides an explanation for the previously observed increase in the inactive form of Cdc2.
Cdc25C is a substrate of the checkpoint kinase Chk2. In fact, Ser 216 phosphorylation of Cdc25C is one of the major mechanisms by which this kinase mediates the G2/M cell cycle arrest. Investigation of Chk2 revealed that ethanol metabolism resulted in an increase in the active form of this kinase, thus providing an explanation for the increase in Ser 216-phosphorylated Cdc25C.
Ataxia telangiectasia mutated (ATM) can activate Chk2 and through this kinase mediate cell cycle arrest. ATM is a kinase that is activated in response to numerous physiologic stimuli that damage DNA or inhibit DNA replication (Abraham, 2001). Our data demonstrate that ethanol metabolism results in accumulation of activated ATM. This finding may, in part, explain the ethanol metabolism-mediated cell cycle arrest, and perhaps the inability of normal mature hepatocytes to replicate and replace hepatocytes damaged as a result of the toxic effects of ethanol metabolism or other toxic insults. To further demonstrate the involvement of ATM in the ethanol metabolism-mediated cell cycle arrest, we showed that inhibition of ATM activation partially ameliorated the ethanol metabolism-mediated cell cycle arrest. These data indicate that ATM has a role in the ethanol metabolism-mediated cell cycle arrest, but the fact that inhibition of ATM only partially ameliorated the cell cycle arrest and impairment in cell accumulation indicates that there may be other factors involved.
Activation of ATM and ATR (ATM and Rad3-related), the other major kinase involved in initiation of cell cycle checkpoints, may have a ubiquitous role in ethanol-mediated impairment of cellular replication. In animals fed ethanol, it has been shown that the regenerative capacity of the liver is impaired after partial hepatectomy (Diehl et al., 1988; Yang et al., 1998). This impairment has been shown to be caused by a delay in the G1/S transition of the cell cycle. Interestingly, ATM and ATR also mediate activation of this cell cycle checkpoint. Because of this, it may be of interest to investigate if ATM and/or ATR are activated in vivo as a result of chronic ethanol abuse, and other instances where normal hepatocyte replication is impaired and a ductular reaction is induced.
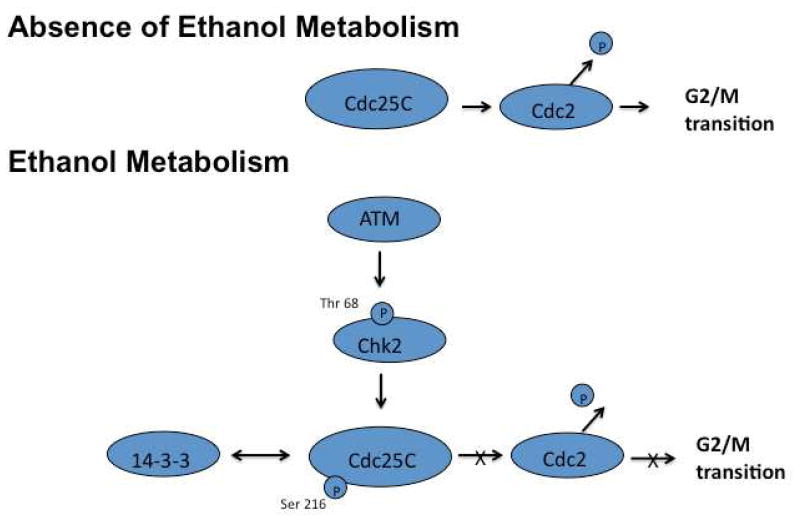
In conclusion, we have shown that ethanol metabolism results in the activation of ATM. Activation of this kinase results in the initiation of a signal transduction cascade that activates the checkpoint kinase Chk2. Activation of this kinases leads to increased phosphorylation of the phosphatase Cdc25C. Phosphorylation of Cdc25C results in decreased activation of the mitotic cyclin-dependent kinase Cdc2 and ultimately in G2/M cell cycle arrest (Figure 8). The fact that ethanol metabolism induces cell cycle arrest has serious pathophysiologic consequences. The inability of normal mature hepatocytes to replicate and replace lost cells may initiate the activation of hepatic progenitor cells. Activation and expansion of these hepatic progenitor cells is associated with the ductular reaction, which may result in portal fibrosis. Thus, the inability of normal mature hepatocytes to replicate may have a role in the initiation and progression of alcoholic liver disease, and may be important in the pathophysiology of other chronic liver diseases.
Figure 8.
Proposed model of ethanol metabolism-mediated G2/M cell cycle arrest. Ethanol metabolism activates ATM, which in turn activates the checkpoint kinase Chk2. Chk2 is able to phosphorylate Cdc25C at Ser 216 making it a substrate for 14-3-3 protein binding. Binding of Cdc25C to 14-3-3 proteins results in Cdc25C being sequestered in the cytoplasm and unable to activate Cdc2. This cascade results in cell cycle arrest at the G2/M transition of the cell cycle.
Acknowledgments
The Veterans Administration supported this work by a grant to DLC.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abraham RT. Cell cycle checkpoint signaling through the ATM and ATR kinases. Genes Dev. 2001;15:2177–2196. doi: 10.1101/gad.914401. [DOI] [PubMed] [Google Scholar]
- Ahn JY, Schwarz JK, Piwnica-Worms H, Canman CE. Threonine 68 phosphorylation by ataxia telangiectasia mutated is required for efficient activation of Chk2 in response to ionizing radiation. Cancer Res. 2000;60:5934–5936. [PubMed] [Google Scholar]
- Atherton-Fessler S, Parker LL, Geahlen RL, Piwnica-Worms H. Mechanisms of p34cdc2 regulation. Mol Cell Biol. 1993;13:1675–1685. doi: 10.1128/mcb.13.3.1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakkenist CJ, Kastan MB. DNA damage activates ATM through intermolecular autophosphorylation and dimer dissociation. Nature. 2003;421:499–506. doi: 10.1038/nature01368. [DOI] [PubMed] [Google Scholar]
- Chaturvedi P, Eng WK, Zhu Y, Mattern MR, Mishra R, Hurle MR, Zhang X, Annan RS, Lu Q, Faucette LF, et al. Mammalian Chk2 is a downstream effector of the ATM-dependent DNA damage checkpoint pathway. Oncogene. 1999;18:4047–4054. doi: 10.1038/sj.onc.1202925. [DOI] [PubMed] [Google Scholar]
- Clemens DL, Calisto LE, Sorrell MF, Tuma DJ. Ethanol metabolism results in a G2/M cell-cycle arrest in recombinant Hep G2 cells. Hepatology. 2003;38:385–393. doi: 10.1053/jhep.2003.50332. [DOI] [PubMed] [Google Scholar]
- Clemens DL, Forman A, Jerrells TR, Sorrell MF, Tuma DJ. Relationship between acetaldehyde levels and cell survival in ethanol-metabolizing hepatoma cells. Hepatology. 2002;35:1196–1204. doi: 10.1053/jhep.2002.32668. [DOI] [PubMed] [Google Scholar]
- Diehl AM. Recent events in alcoholic liver disease V. effects of ethanol on liver regeneration. Am J Physiol Gastrointest Liver Physiol. 2005;288:G1–6. doi: 10.1152/ajpgi.00376.2004. [DOI] [PubMed] [Google Scholar]
- Diehl AM, Chacon M, Wagner P. The effect of chronic ethanol feeding on ornithine decarboxylase activity and liver regeneration. Hepatology. 1988;8:237–242. doi: 10.1002/hep.1840080208. [DOI] [PubMed] [Google Scholar]
- Donohue TM, Osna NA, Clemens DL. Recombinant Hep G2 cells that express alcohol dehydrogenase and cytochrome P450 2E1 as a model of ethanol-elicited cytotoxicity. Int J Biochem Cell Biol. 2006;38:92–101. doi: 10.1016/j.biocel.2005.07.010. [DOI] [PubMed] [Google Scholar]
- Duncan AW, Dorrell C, Grompe M. Stem cells and liver regeneration. Gastroenterology. 2009;137:466–481. doi: 10.1053/j.gastro.2009.05.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fausto N. Liver regeneration and repair: hepatocytes, progenitor cells, and stem cells. Hepatology. 2004;39:1477–1487. doi: 10.1002/hep.20214. [DOI] [PubMed] [Google Scholar]
- Friedman SL. Mechanisms of hepatic fibrogenesis. Gastroenterology. 2008;134:1655–1669. doi: 10.1053/j.gastro.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter T. Protein kinases and phosphatases: the yin and yang of protein phosphorylation and signaling. Cell. 1995;80:225–236. doi: 10.1016/0092-8674(95)90405-0. [DOI] [PubMed] [Google Scholar]
- Jung Y, Brown KD, Witek RP, Omenetti A, Yang L, Vandongen M, Milton RJ, Hines IN, Rippe RA, Spahr L, et al. Accumulation of hedgehog-responsive progenitors parallels alcoholic liver disease severity in mice and humans. Gastroenterology. 2008;134:1532–1543. doi: 10.1053/j.gastro.2008.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koteish A, Yang S, Lin H, Huang J, Diehl AM. Ethanol induces redox-sensitive cell-cycle inhibitors and inhibits liver regeneration after partial hepatectomy. Alcohol Clin Exp Res. 2002;26:1710–1718. doi: 10.1097/01.ALC.0000036923.77613.59. [DOI] [PubMed] [Google Scholar]
- Kuwahara R, Kofman AV, Landis CS, Swenson ES, Barendswaard E, Theise ND. The hepatic stem cell niche: identification by label-retaining cell assay. Hepatology. 2008;47:1994–2002. doi: 10.1002/hep.22218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu F, Rothblum-Oviatt C, Ryan CE, Piwnica-Worms H. Overproduction of human Myt1 kinase induces a G2 cell cycle delay by interfering with the intracellular trafficking of Cdc2-cyclin B1 complexes. Mol Cell Biol. 1999;19:5113–5123. doi: 10.1128/mcb.19.7.5113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu F, Stanton JJ, Wu Z, Piwnica-Worms H. The human Myt1 kinase preferentially phosphorylates Cdc2 on threonine 14 and localizes to the endoplasmic reticulum and Golgi complex. Mol Cell Biol. 1997;17:571–583. doi: 10.1128/mcb.17.2.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Marshall A, Rushbrook S, Davies SE, Morris LS, Scott IS, Vowler SL, Coleman N, Alexander G. Relation between hepatocyte G1 arrest, impaired hepatic regeneration, and fibrosis in chronic hepatitis C virus infection. Gastroenterology. 2005;128:33–42. doi: 10.1053/j.gastro.2004.09.076. [DOI] [PubMed] [Google Scholar]
- McGowan CH, Russell P. Human Wee1 kinase inhibits cell division by phosphorylating p34cdc2 exclusively on Tyr15. Embo J. 1993;12:75–85. doi: 10.1002/j.1460-2075.1993.tb05633.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nurse P. Universal control mechanism regulating onset of M-phase. Nature. 1990;344:503–508. doi: 10.1038/344503a0. [DOI] [PubMed] [Google Scholar]
- Ohlson LC, Koroxenidou L, Hallstrom IP. Inhibition of in vivo rat liver regeneration by 2-acetylaminofluorene affects the regulation of cell cycle-related proteins. Hepatology. 1998;27:691–696. doi: 10.1002/hep.510270309. [DOI] [PubMed] [Google Scholar]
- Osna NA, Clemens DL, Donohue TM., Jr Ethanol metabolism alters interferon gamma signaling in recombinant HepG2 cells. Hepatology. 2005;42:1109–1117. doi: 10.1002/hep.20909. [DOI] [PubMed] [Google Scholar]
- Peng CY, Graves PR, Thoma RS, Wu Z, Shaw AS, Piwnica-Worms H. Mitotic and G2 checkpoint control: regulation of 14-3-3 protein binding by phosphorylation of Cdc25C on serine-216. Science. 1997;277:1501–1505. doi: 10.1126/science.277.5331.1501. [DOI] [PubMed] [Google Scholar]
- Ray MB, Mendenhall CL, French SW, Gartside PS. Bile duct changes in alcoholic liver disease. The Veterans Administration Cooperative Study Group. Liver. 1993;13:36–45. doi: 10.1111/j.1600-0676.1993.tb00603.x. [DOI] [PubMed] [Google Scholar]
- Richardson MM, Jonsson JR, Powell EE, Brunt EM, Neuschwander-Tetri BA, Bhathal PS, Dixon JB, Weltman MD, Tilg H, Moschen AR, et al. Progressive fibrosis in nonalcoholic steatohepatitis: association with altered regeneration and a ductular reaction. Gastroenterology. 2007;133:80–90. doi: 10.1053/j.gastro.2007.05.012. [DOI] [PubMed] [Google Scholar]
- Roskams T, Yang SQ, Koteish A, Durnez A, DeVos R, Huang X, Achten R, Verslype C, Diehl AM. Oxidative stress and oval cell accumulation in mice and humans with alcoholic and nonalcoholic fatty liver disease. Am J Pathol. 2003;163:1301–1311. doi: 10.1016/S0002-9440(10)63489-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roskams TA, Theise ND, Balabaud C, Bhagat G, Bhathal PS, Bioulac-Sage P, Brunt EM, Crawford JM, Crosby HA, Desmet V, et al. Nomenclature of the finer branches of the biliary tree: canals, ductules, and ductular reactions in human livers. Hepatology. 2004;39:1739–1745. doi: 10.1002/hep.20130. [DOI] [PubMed] [Google Scholar]
- Santoni-Rugiu E, Jelnes P, Thorgeirsson SS, Bisgaard HC. Progenitor cells in liver regeneration: molecular responses controlling their activation and expansion. APMIS. 2005;113:876–902. doi: 10.1111/j.1600-0463.2005.apm_386.x. [DOI] [PubMed] [Google Scholar]
- Sell S. Heterogeneity and plasticity of hepatocyte lineage cells. Hepatology. 2001;33:738–750. doi: 10.1053/jhep.2001.21900. [DOI] [PubMed] [Google Scholar]
- Theise ND, Kuwahara R. The tissue biology of ductular reactions in human chronic liver disease. Gastroenterology. 2007;133:350–352. doi: 10.1053/j.gastro.2007.05.040. [DOI] [PubMed] [Google Scholar]
- Theise ND, Saxena R, Portmann BC, Thung SN, Yee H, Chiriboga L, Kumar A, Crawford JM. The canals of Hering and hepatic stem cells in humans. Hepatology. 1999;30:1425–1433. doi: 10.1002/hep.510300614. [DOI] [PubMed] [Google Scholar]
- Van Hul NK, Abarca-Quinones J, Sempoux C, Horsmans Y, Leclercq IA. Relation between liver progenitor cell expansion and extracellular matrix deposition in a CDE-induced murine model of chronic liver injury. Hepatology. 2009;49:1625–1635. doi: 10.1002/hep.22820. [DOI] [PubMed] [Google Scholar]
- Wells NJ, Watanabe N, Tokusumi T, Jiang W, Verdecia MA, Hunter T. The C-terminal domain of the Cdc2 inhibitory kinase Myt1 interacts with Cdc2 complexes and is required for inhibition of G(2)/M progression. J Cell Sci. 1999;112:3361–3371. doi: 10.1242/jcs.112.19.3361. [DOI] [PubMed] [Google Scholar]
- Yang S, Koteish A, Lin H, Huang J, Roskams T, Dawson V, Diehl AM. Oval cells compensate for damage and replicative senescence of mature hepatocytes in mice with fatty liver disease. Hepatology. 2004;39:403–411. doi: 10.1002/hep.20082. [DOI] [PubMed] [Google Scholar]
- Yang SQ, Lin HZ, Yin M, Albrecht JH, Diehl AM. Effects of chronic ethanol consumption on cytokine regulation of liver regeneration. Am J Physiol. 1998;275:G696–704. doi: 10.1152/ajpgi.1998.275.4.G696. [DOI] [PubMed] [Google Scholar]
- Zhou BB, Chaturvedi P, Spring K, Scott SP, Johanson RA, Mishra R, Mattern MR, Winkler JD, Khanna KK. Caffeine abolishes the mammalian G(2)/M DNA damage checkpoint by inhibiting ataxia-telangiectasia-mutated kinase activity. J Biol Chem. 2000;275:10342–10348. doi: 10.1074/jbc.275.14.10342. [DOI] [PubMed] [Google Scholar]
- Zhou BB, Elledge SJ. The DNA damage response: putting checkpoints in perspective. Nature. 2000;408:433–439. doi: 10.1038/35044005. [DOI] [PubMed] [Google Scholar]