Abstract
An enantioselective arylation–cyclization cascade has been accomplished using a combination of diaryliodonium salts and asymmetric copper catalysis. These mild catalytic conditions provide a new strategy for the enantioselective construction of pyrroloindolines, an important alkaloid structural motif that is commonly found among biologically active natural products.
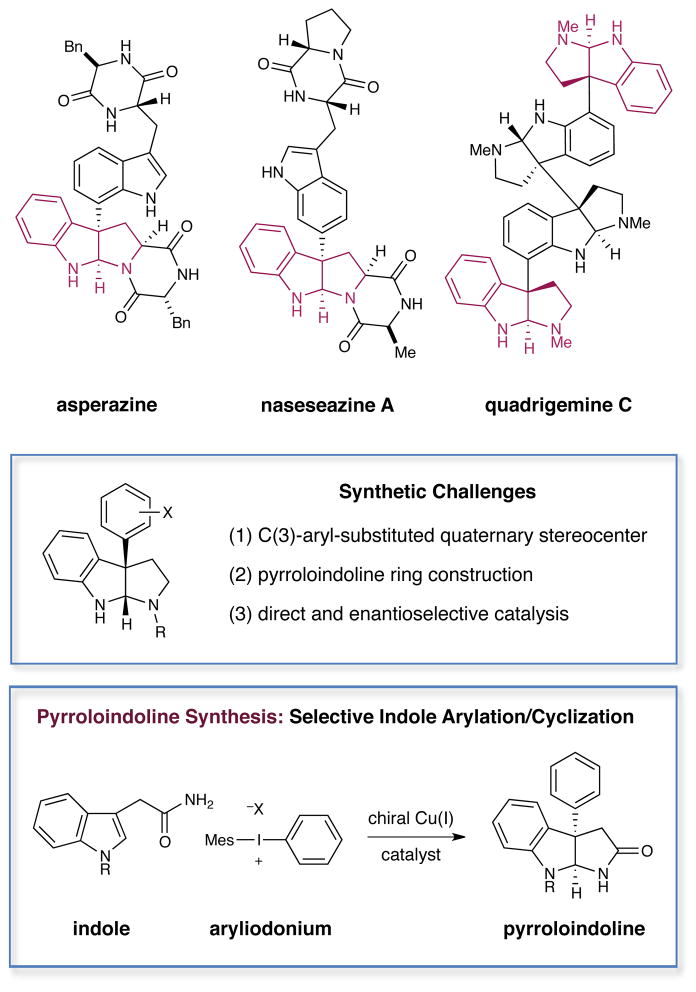
The pyrroloindolines and poly-pyrroloindolines represent a diverse family of structurally complex polyindoline alkaloids that have been isolated from a widespread series of natural sources, including amphibians, plants and marine algae.1 An important structural subclass, the C(3)-aryl pyrroloindoline unit (Figure 1) is incorporated in a range of natural products that have been shown to be cytotoxic against both lymphocytic leukemia2a and lymphoblastoma2b cell lines. Moreover, the structurally related pyrroloindoline-thiodiketopiperazine family display nematicidal activity against pathogenic fungi such as P. ultimum and R. solani,2c while the C(3)-aryl-containing hodgkinsine (not shown) has been found to exhibit antinociceptive properties that are similar to morphine.2d, 3 The structural complexity of the C(3)-aryl pyrroloindolines makes them a particularly elusive and at the same time appealing target for total synthetic efforts.4 In this context, both Overman and Movassaghi have made seminal contributions in the design of new reaction methods that allow for the rapid construction of many of these complex alkaloids. The Overman group has focused on the development of a Heck strategy for the enantioselective construction of oxindoles that were elegantly converted into quadrigemine C, psycholeine, asperazine and idiospermuline.4d–g In a complementary approach, the Movassaghi group has employed Friedel–Crafts additions to enantiopure tryptophan derivatives in the synthesis of naseseazines A and B.4a–b, 5
Figure 1.
Representative Pyrroloindolines and Arylation Strategy
Recently our laboratory reported the enantioselective α-arylation of carbonyls using copper bisoxazoline catalysis and iodonium salts.6 As a thematic extension, we postulated that this Cu(III)-aryl strategy could serve as a platform for pyrroloindoline construction via an enantioselective arylation–cyclization cascade process using indole-based nucleophiles. Herein we present the successful execution of these ideas and describe an operationally trivial, asymmetric catalytic approach that allows the formation of a diverse range of C(3)-aryl pyrroloindoline architecture in only one step. We expect this new enantioselective catalysis method should be broadly applicable to natural product and medicinal agent synthesis.
Design Plan
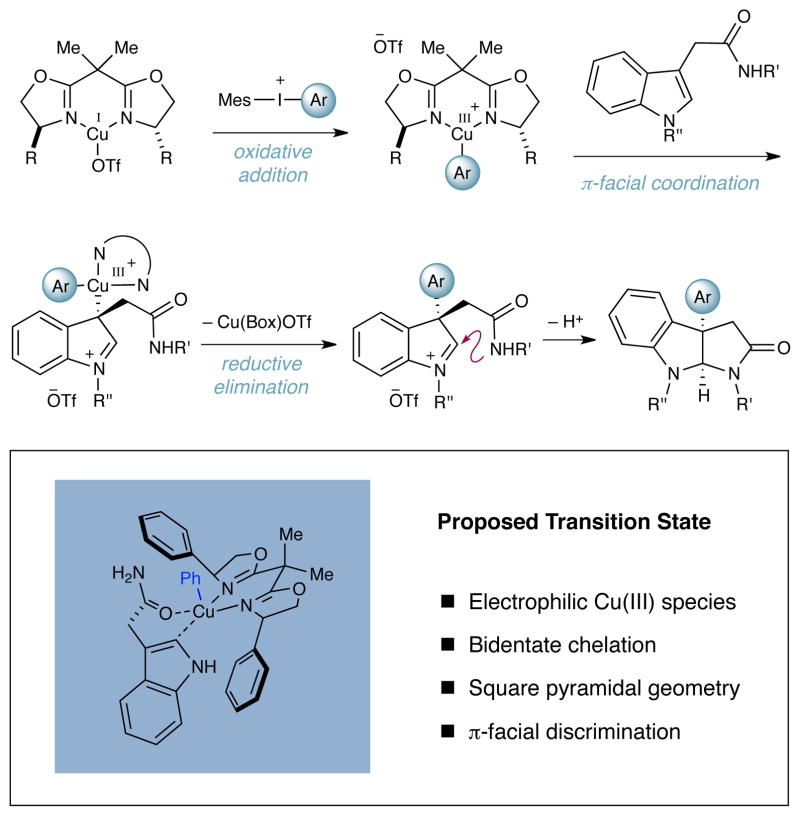
As outlined in Scheme 1, we proposed that oxidative insertion of a ligand-bound Cu(I) complex into a suitable diaryliodonium salt7,8 would result in a highly electrophilic chiral Cu(III) species.9,10 Subsequent addition of the indole nucleophile followed by reductive elimination and amine–iminium cyclization would then yield the desired enantioenriched pyrroloindoline product while reconstituting the Cu(I) catalyst. As in our previous studies, we recognized that substrate-catalyst bidentate coordination should be important, and as such, we sought to incorporate a pendent carbonyl on the tryptamine nucleophile unit to facilitate formation of a square pyramidal Cu(III) complex.11 Given the architectural constraints of the ligand framework, this would impose a significant bias for enantiofacial indole Si-face coordination (as shown), thereby enabling the required enantioselective addition and cyclization steps.
Scheme 1.
Mechanism of Asymmetric Arylation–Cyclization
The feasibility of the proposed arylation–cyclization cascade was first examined using the indole acetamide 4, diphenyliodonium triflate and a series of copper catalysts. As shown in Table 1, the absence of catalyst resulted in no detectable product formation. In contrast, when 5 mol% of (CuOTf)2•PhMe was employed, complete consumption of the iodonium was observed; however, only the undesired product of C(2)-indole arylation was observed (entry 2, 69% yield). We next turned our attention to ligated copper catalysts in the hope that this would allow the Cu(III) aryl species to more rapidly participate in the reductive elimination step (thereby circumventing a deleterious C(3) to C(2) migration step). Indeed, implementation of both the t-butyl- and isopropyl- substituted bisoxazoline (Box)12 ligands with copper yielded an improved level of the desired C(3)-aryl adduct albeit with modest enantiocontrol (entries 3 and 4, 8–61% ee). Fortunately, when the phenyl-substituted bisoxazoline ligand was employed, a dramatic increase in regio- and enantioselectivity was observed (entry 5, 90% yield, 98% ee). Moreover, further optimization of temperature and the iodonium counterion afforded the desired C(3)-aryl pyrroloindoline in 96% yield and >99% ee as essentially a single regioisomer (entries 6–8).
Table 1.
Evaluation of CuBox Catalysts and Iodonium Counterions
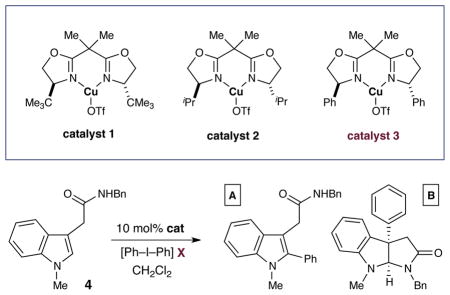
| ||||||
|---|---|---|---|---|---|---|
| entry | catalyst | X | temp. (°C) | A:B | yield (%)a | ee (%)b |
| 1 | None | OTf | 23 | -- | 0 | -- |
| 2 | (CuOTf)2•PhMe | OTf | 23 | 29:1 | 69 | -- |
| 3 | 1 | OTf | 23 | 4:1 | 20 | 8 |
| 4 | 2 | OTf | 23 | 3:1 | 71 | 61 |
| 5 | 3 | OTf | 23 | 1:10 | 90 | 98 |
| 6 | 3 | PF6 | 23 | 1:16 | 99 | 98 |
| 7 | 3 | AsF6 | 23 | 1:17 | 99 | 98 |
| 8 | 3 | AsF6 | −20 | 1:40 | 96 | >99 |
Isolated yield.
Determined by chiral HPLC analysis; absolute configuration determined by chemical correlation or by analogy.
With these optimized conditions in hand, we next turned our attention to the scope of the aryl or heteroaryl coupling partner in this new pyrroloindoline forming reaction (Table 2). While symmetrical diaryliodonium salts can be successfully employed in this context, the approach pioneered by Gaunt in which aryl-mesityl reagents are used to generate Ar-Cu(III) intermediates is preferred for reasons of practicality.9b Importantly, both electron-rich (entries 1, 2 and 11, 82–92% yield, ≥98% ee) and electron-deficient arenes (entries 4–8 and 10, 55–89% yield, 91–98% ee) were found to be suitable coupling partners in this new protocol. Moreover, a broad range of ortho-, meta- and para-substituted aryl rings with diverse steric and electronic properties can be readily exploited (entries 2, 4, 5, 7, 8, 10 and 11, 55–92% yield, 91–99% ee). Notably, halogen-substituted aryl rings are tolerated in this Cu(I)-catalyzed transformation, a critical consideration for further elaboration of these pharmacophores in medicinal chemistry or natural product studies (entry 5, 83% yield, 97% ee).
Table 2.
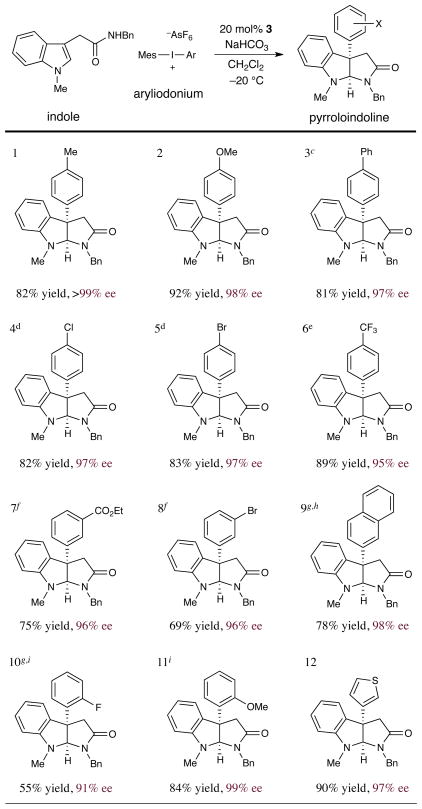
|
Absolute configuration assigned by chemical correlation or by analogy.
Enantiomeric excess determined by chiral HPLC analysis of the isolated product.
Reaction performed at −15 °C.
Reaction performed at 0 °C.
Symmetrical diaryliodonium salt was used.
Reaction performed at −5 °C.
Using PF6 counterion.
Reaction performed at −10 °C.
Reaction performed at rt.
As revealed in Tables 3 and 4, this enantioselective arylation–cyclization technology is tolerant to a wide range of substituents on the indole component. Initial examination of the possible substitution on the indolic nitrogen13 demonstrates that a range of alkyl protecting groups are compatible; e.g. N-methyl, N-benzyl, and N-allyl substituted indole acetamides undergo addition–cyclization in >92% yield and near perfect enantiocontrol (Table 3, entries 1–3, 8, 92–96% yield, 97–99% ee). Moreover, unsubstituted indolic nitrogens were tolerated with little or no effect (entries 4–6, 80–98% yield, 90–95% ee). Examination of the substituent patterns on the nucleophile framework revealed that this protocol is amenable to both electron-rich (Table 4, entries 3 and 5, 91–96% yield, 97–99% ee) and electron-poor indoles (Table 4, entries 1, 2, 4, 80–93% yield, ≥99% ee). As was the case for the aryl coupling partner, this addition–cyclization sequence proceeds with perfect chemoselectivity for oxidative addition into the iodonium C–I bond in the presence of halogens on the nucleophilic substrate (entry 1, 88% yield, >99% ee). Finally, we were delighted to find that this mechanism can be translated to the formation of six-membered piperidinyl-indolines with excellent enantiocontrol using an indole propionamide substrate (entry 6, 67% yield, 97% ee). This result suggests that a variety of alkaloid pharmacophores might be readily generated using this new asymmetric arylation strategy.
Table 3.
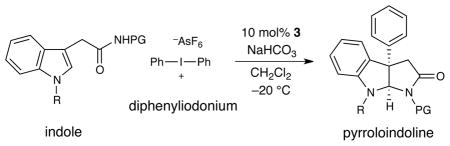
| ||||
|---|---|---|---|---|
| entry | R | PG | yield (%) | ee (%) |
| 1 | Me | Bn | 96 | > 99 |
| 2 | Bn | Bn | 96 | > 99 |
| 3c | Allyl | Bn | 92 | > 99 |
| 4 | H | Bn | 80 | 90 |
| 5 | H | Me | 98 | 93 |
| 6 | H | H | 86 | 95 |
| 7 | Me | H | 92 | > 99 |
| 8 | Me | Me | 96 | 97 |
Absolute configuration assigned by chemical correlation or analogy.
Enantiomeric excess determined by chiral HPLC analysis.
Reaction performed at rt.
Table 4.
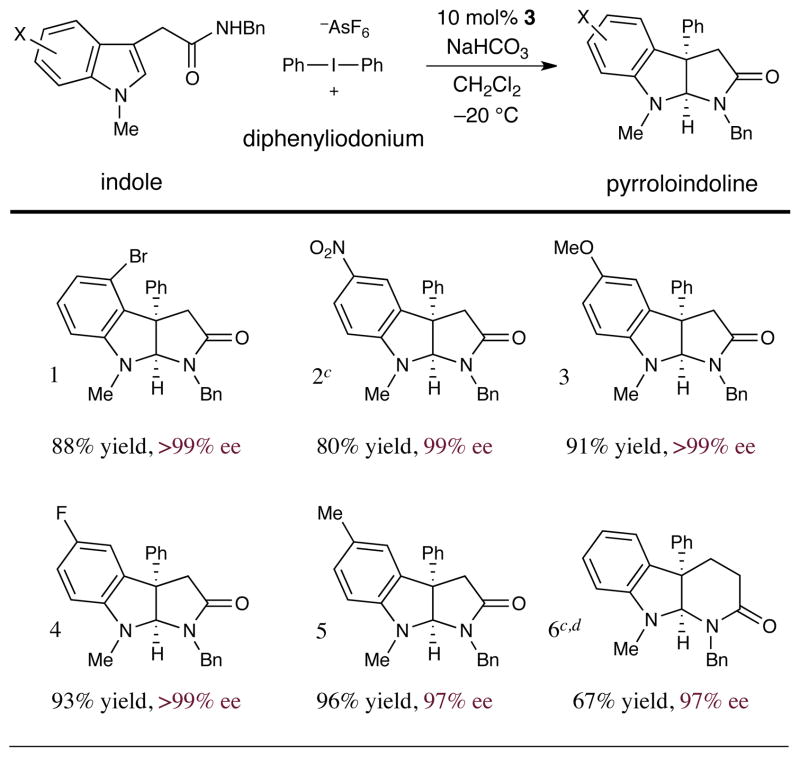
|
Absolute configuration assigned by chemical correlation or analogy.
Enantiomeric excess determined by chiral HPLC analysis.
Using 30 mol% catalyst.
Using PF6 counterion at 0 °C.
In conclusion, we have developed a new copper-catalyzed cascade protocol that allows the rapid and enantioselective construction of C(3)-aryl pyrroloindoline architecture. Further investigations into the mechanistic details of this transformation including models for asymmetric induction are currently underway.
Supplementary Material
Acknowledgments
Financial support was provided by the NIHGMS (R01 GM103558-01) and kind gifts from Merck, Amgen and Abbott. S. Z. thanks the Shanghai Institute of Organic Chemistry for a postdoctoral fellowship.
Footnotes
Supporting Information Available. Experimental procedures and spectral data are provided. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.For reviews on pyrroloindoline natural products, see: Steven A, Overman LE. Angew Chem Int Ed. 2007;46:5488. doi: 10.1002/anie.200700612.Ruiz-Sanchis P, Savina SA, Albericio F, Álvarez M. Chem Eur J. 2011;17:1388. doi: 10.1002/chem.201001451.Crich D, Banerjee A. Acc Chem Res. 2007;40:151. doi: 10.1021/ar050175j.
- 2.(a) Usami Y, Yamaguchi J, Numata A. Heterocycles. 2004;63:1123. [Google Scholar]; (b) Yanagihara M, Sasaki-Takahashi N, Sugahara T, Yamamoto S, Shinomi M, Yamashita I, Hayashida M, Yamanoha B, Numata A, Yamori T, Andoh T. Cancer Sci. 2005;96:816. doi: 10.1111/j.1349-7006.2005.00117.x. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Dong JY, He HP, Shen YM, Zhang KQ. J Nat Prod. 2005;68:1510. doi: 10.1021/np0502241. [DOI] [PubMed] [Google Scholar]; (d) Amador TA, Verotta L, Nunes DS, Elisabetsky E. Planta Med. 2000;66:770. doi: 10.1055/s-2000-9604. [DOI] [PubMed] [Google Scholar]
- 3.Additionally, the bionectin family of C(3)-aryl pyrroloindolines has been shown to possess anti-MRSA activity, see: Zheng C-J, Kim C-J, Bae KS, Kim Y-H, Kim W-G. J Nat Prod. 2006;69:1816. doi: 10.1021/np060348t.
- 4.(a) Kim J, Movassaghi M. J Am Chem Soc. 2011;133:14940. doi: 10.1021/ja206743v. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Boyer N, Movassaghi M. Chem Sci. 2012;3:1798. doi: 10.1039/C2SC20270K. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Furst L, Narayanam JMR, Stephenson CRJ. Angew Chem Int Ed. 2011;50:9655. doi: 10.1002/anie.201103145. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Kodanko JJ, Overman LE. Angew Chem Int Ed. 2003;42:2528. doi: 10.1002/anie.200351261. [DOI] [PubMed] [Google Scholar]; (e) Govek SP, Overman LE. Tetrahedron. 2007;63:8499. [Google Scholar]; (f) Overman LE, Peterson EA. Angew Chem Int Ed. 2003;42:2525. doi: 10.1002/anie.200351260. [DOI] [PubMed] [Google Scholar]; (g) Lebsack AD, Link JT, Overman LE, Stearns BA. J Am Chem Soc. 2002;124:9008. doi: 10.1021/ja0267425. [DOI] [PubMed] [Google Scholar]; (h) DeLorbe JE, Jabri SY, Mennen SM, Overman LE, Zhang FL. J Am Chem Soc. 2011;133:6549. doi: 10.1021/ja201789v. [DOI] [PMC free article] [PubMed] [Google Scholar]; (i) Snell RH, Woodward RL, Willis MC. Angew Chem Int Ed. 2011;50:9116. doi: 10.1002/anie.201103864. [DOI] [PubMed] [Google Scholar]
- 5.For additional strategies to construct C(3)-aryl pyrroloindolines involving visible-light photoredox catalysis, palladium-catalyzed cross-coupling, and a Steglich-type rearrangement, respectively, see: references 4c, 4i, and 4h.
- 6.Harvey JS, Simonovich SP, Jamison CR, MacMillan DWC. J Am Chem Soc. 2011;133:13782. doi: 10.1021/ja206050b.The Gaunt group at Cambridge University was involved in similar α-arylation studies and graciously agreed to publish their results in a back-to-back format with our own results: Bigot A, Williamson AE, Gaunt MJ. J Am Chem Soc. 2011;133:13778. doi: 10.1021/ja206047h.
- 7.For reviews on diaryliodonium salts, see: Merritt EA, Olofsson B. Angew Chem Int Ed. 2009;48:9052. doi: 10.1002/anie.200904689.Varvoglis A. The Organic Chemistry of Polycoordinated Iodine. VCH Publishers; New York: 1992. Grushin VV. Chem Soc Rev. 2000;29:315.
- 8.For convenient one-pot syntheses of diaryliodonium salts, see: Bielawski M, Olofsson B. Chem Commun. 2007:2521. doi: 10.1039/b701864a.Bielawski M, Aili D, Olofsson B. J Org Chem. 2008;73:4602. doi: 10.1021/jo8004974.
- 9.For examples of copper-catalyzed arylation reactions, see: Phipps RJ, Grimster NP, Gaunt MJ. J Am Chem Soc. 2008;130:8172. doi: 10.1021/ja801767s.Phipps RJ, Gaunt MJ. Science. 2009;323:1593. doi: 10.1126/science.1169975.Ryan JH, Stang PJ. Tetrahedron Lett. 1997;38:5061.
- 10.For studies on the mechanism of copper-catalyzed arylation reactions, see: Lockhart TP. J Am Chem Soc. 1983;105:1940.Beringer FM, Geering EJ, Kuntz I, Mausner M. J Phys Chem. 1956;60:141.Chen B, Hou XL, Li YX, Wu YD. J Am Chem Soc. 2011;133:7668. doi: 10.1021/ja201425e.Huang Z, Hartwig JF. Angew Chem Int Ed. 2012;51:1028. doi: 10.1002/anie.201106719.
- 11.For studies on the structure of copper(III) aryl species, see: Casitas A, King AE, Parella T, Costas M, Stahl SS, Ribas X. Chem Sci. 2010;1:326.Huffman LM, Stahl SS. Dalton Trans. 2011;40:8959. doi: 10.1039/c1dt10463b.Huffman LM, Stahl S. J Am Chem Soc. 2008;130:9196. doi: 10.1021/ja802123p.Ribas X, Jackson DA, Donnadieu B, Mahía J, Parella T, Xifra R, Hedman B, Hodgson KO, Llobet A, Stack TDP. Angew Chem Int Ed. 2002;41:2991. doi: 10.1002/1521-3773(20020816)41:16<2991::AID-ANIE2991>3.0.CO;2-6.
- 12.(a) Johnson JS, Evans DA. Acc Chem Res. 2000;33:325. doi: 10.1021/ar960062n. [DOI] [PubMed] [Google Scholar]; (b) Evans DA, Scheidt KA, Johnston JN, Willis MC. J Am Chem Soc. 2001;123:4480. doi: 10.1021/ja010302g. [DOI] [PubMed] [Google Scholar]; (c) Evans DA, Willis MC, Johnston JN. Org Lett. 1999;1:865. doi: 10.1021/ol9901570. [DOI] [PubMed] [Google Scholar]; (d) Evans DA, Johnson DS. Org Lett. 1999;1:595. doi: 10.1021/ol990113r. [DOI] [PubMed] [Google Scholar]
- 13.The use of carbamate protecting groups, such as BOC or CBz on the indolic or acetamidyl nitrogens results in complete suppression of the desired reaction under the conditions outlined.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.