Abstract
Scope
The incidence of cancer is significantly lower in regions where turmeric is heavily consumed. Whether lower cancer incidence is due to turmeric was investigated by examining its effects on tumor cell proliferation, on pro-inflammatory transcription factors NF-κB and STAT3, and on associated gene products.
Methods and results
Cell proliferation and cell cytotoxicity were measured by the MTT method, NF-κB activity by EMSA, protein expression by Western blot analysis, ROS generation by FACS analysis, and osteoclastogenesis by TRAP assay. Turmeric inhibited NF-κB activation and down-regulated NF-κB-regulated gene products linked to survival (Bcl-2, cFLIP, XIAP, and cIAP1), proliferation (cyclin D1 and c-Myc), and metastasis (CXCR4) of cancer cells. The spice suppressed the activation of STAT3, and induced the death receptors (DR)4 and DR5. Turmeric enhanced the production of ROS, and suppressed the growth of tumor cell lines. Furthermore, turmeric sensitized the tumor cells to chemotherapeutic agents capecitabine and taxol. Turmeric was found to be more potent than pure curcumin for cell growth inhibition. Turmeric also inhibited NF-κB activation induced by RANKL that correlated with the suppression of osteoclastogenesis.
Conclusion
Our results indicate that turmeric can effectively block the proliferation of tumor cells through the suppression of NF-κB and STAT3 pathways.
Keywords: Death receptor, NF-κB, Osteoclastogenesis, STAT3, Turmeric
1 Introduction
According to the World Health Organization, 80% of the Earth’s inhabitants (seven billion) rely upon the traditional medicine for their primary health-care needs, in part due to high cost of Western pharmaceuticals. Medicines derived from plants have played a pivotal role in the health care of both ancient and modern cultures [1-4]. One of the prime sources of plant-derived medicines is spices. Turmeric is one such spice that has been consumed over the years around the world. Derived from the rhizome of the plant Curcuma longa, turmeric has been used for centuries as a medicine to treat digestive disorders, liver problems, skin diseases, and wounds. Epidemiologic data indicate that some extremely common cancers in the Western world are much less prevalent in regions (Southeast Asia, for example) where turmeric is widely consumed in the diet (http:// wwwbotanicalcom/botanical/mgmh/t/turmer30html) [5, 6].
Although much more is known about curcumin, a component of turmeric, very little is known about turmeric itself [7]. A previous study demonstrated the anti-cancer potential of dietary turmeric in a 7,12-dimethylbenz(a)anthracene (DMBA)-induced carcinogenesis hamster model [8]. Turmeric contains over 300 different components including essential oil (2-7%), curcumin (3–5%), starch, acid glycans ukonan (A, B, and C), free arabinose (1%), fructose (12%), glucose (2%), and minerals [9]. Although dietary turmeric contains over 300 different components, only curcumin has been extensively investigated. Research over the past half century has indicated curcumin’s potential against various chronic diseases including cancer both by in vitro and in vivo studies [10, 11]. Turmeric oil has been shown to enhance the bioavailability of curcumin in vivo [12]. Other constituents of turmeric such as demethoxycurcumin (DMC), bisdemethoxycurcumin (BDMC), and tetrahydrocurcumin (THC) have also been reported to exert anti-cancer activity [13]. A recent study indicated that curcumin-free aqueous turmeric extract has the potential to suppress benzo[a]pyrene-induced tumorigenesis in mice [14]. In another study, curcumin-free turmeric inhibited DMBA-induced mammary tumorigenesis in rats [15]. These reports suggest that components other than curcumin may also contribute to the anticancer activities of turmeric.
While curcumin is a minor component of turmeric, it is the latter that is consumed everyday as a dietary spice. Therefore, the objective of the current study was to examine whether turmeric exhibits various in vitro activities similar to that of curcumin. The results to be described indicate that like curcumin, turmeric can also suppress pro-inflammatory transcription factors nuclear factor kappa B (NF-κB) and signal transducers and activators of transcription 3 (STAT3), inhibit tumor cell proliferation, and suppress bone loss.
2 Materials and methods
2.1 Materials
Turmeric used in our studies was a standardized preparation (Turmeric ForceTM) supplied by NewChapter (Brattleboro, VT, USA). Stock solutions of turmeric (100 mg/mL) were prepared in dimethyl sulfoxide (DMSO) and diluted as needed in media. Olive oil present in turmeric force was removed by dissolving in DMSO followed by centrifugation at 4°C for 10 min [16]. Bacteria-derived human tumor necrosis factor (TNF), purified to homogeneity at a specific activity of 5×107 U/mg, was kindly provided by Genentech (South San Francisco, CA, USA). Penicillin, streptomycin, RPMI 1640 medium, Iscove’s modified Dulbecco medium (IMDM), Dulbecco-modified essential medium (DMEM)/ F12 medium, and fetal bovine serum (FBS) were obtained from Invitrogen (Grand Island, NY, USA). The antibodies against cyclinD1, cellular inhibitor of apoptosis 1 (cIAP1), Bcl-2, Bax, p-STAT3 (Tyr705), STAT3, and β-actin were obtained from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Antibodies against cellular FLICE-inhibitory protein (c-FLIP) and X-linked IAP (XIAP) were purchased from Imgenex and BD Biosciences, respectively. Dichlorodihydrofluoresceindiacetate (DCF-DA) was purchased from Molecular Probes (Invitrogen, CA, USA).
2.2 Cell lines
Tumor cell lines KBM-5 (human myelogenous leukemia), HCT-116, HT-29 (human colon adenocarcinoma), Panc-28 (pancreatic cancer), MDA-MB-231 (human breast), U266 (human multiple myeloma) were obtained from the American Type Culture Collection (Manassas, VA, USA). The mouse macrophage cell line RAW 264.7 was kindly provided by Dr. Bryant Darnay. Human myeloid KBM-5 cells were cultured in IMDM with 15% FBS. HCT-116, HT-29, Panc-28, MDA-MB-231, CaCo-2, and A293 cells were cultured in DMEM. U266 cells were cultured in RPMI 1640 medium with 10% FBS. RAW 264.7 cells were cultured in DMEM/ F12 medium supplemented with 10% FBS. All media were supplemented with 100 U/mL penicillin and 100 μg/mL streptomycin. All cells were passaged at 80% confluency in 1 mM EDTA and 0.025% trypsin for 3–5 min.
2.3 Measurement of cell proliferation by the 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyl tetrazolium bromide (MTT) method
The effect of turmeric on the proliferation of cells was determined by measuring mitochondrial dehydrogenase activity, using MTT as the substrate. The assay relies on the fact that mitochondrial dehydrogenases of viable cells cleave the tetrazolium ring of MTT, yielding purple MTT formazan, which are measured spectrophotometrically at 570 nm.
2.4 Western blot analysis
Whole-cell extracts were prepared in lysis buffer (20 mM Tris (pH 7.4), 250 mM NaCl, 2 mM EDTA (pH 8.0), 0.1% Triton X-100, 0.01 μg/mL aprotinin, 0.005 μg/mL leupeptin, 0.4 M phenylmethylsulfonyl fluoride, and 4 mM Na3VO4) [17]. Lysates were centrifuged at 14 000 rpm for 10 min to remove insoluble material. The resulting supernatants were collected and kept at 80°C. Proteins were resolved by SDS-PAGE and electrotransferred to nitrocellulose membranes, blotted with relevant antibodies, and detected by incubation with an enhanced chemiluminescence reagent (GE Healthcare, Piscataway, NJ, USA).
2.5 Measurement of reactive oxygen species
Intracellular reactive oxygen species (ROS) were detected in cells pre-incubated with 20 μM DCF-DA for 15 min at 37°C before treatment with turmeric. After 30 min of incubation, the increase in fluorescence resulting from oxidation of DCF-DA to DCF was measured by fluorescence-activated cell sorting (FACS). The mean fluorescence intensity at 530 nm was calculated. Data were collected from at least 10 000 cells at a flow rate of 250-300 cells/s.
2.6 Osteoclast differentiation assay
To determine the effect of turmeric on receptor activator of NF-κB ligand (RANKL)-induced osteoclastogenesis [18, 19] we cultured RAW264.7 cells, which can be induced by RANKL to differentiate into osteoclasts in vitro. RAW 264.7 cells were cultured in 24-well dishes at a density of 1×104 cells/well and allowed to adhere overnight. The medium was then replaced, and the cells were co-incubated with different concentrations of turmeric and 5 nM RANKL. At Day 5, the cells were stained for tartrate-resistant acid phosphatase (TRAP) expression, as previously described, using an acid phosphatase kit (Sigma-Aldrich, St. Louis, MO, USA), and the TRAP-positive multi-nucleated osteoclasts (greater than three nuclei) were counted.
2.7 Electrophoretic mobility shift assay
To evaluate the effect of turmeric on NF-κB activation, we carried out electrophoretic mobility shift assay (EMSA) as described previously [20]. In brief, nuclear extracts were incubated with 32P-labeled 45-mer double-stranded NF-κB oligonucleotides (15 μg of protein with 16 fmol of DNA) from the human immunodeficiency virus long terminal repeat 50-TTGTTACAA GGGACTTTCC GCTG GGGACTTTCCAGGGAGGCGTGG-30 (boldface indicates NF-κB-binding sites) for 30 min at 37°C, and the DNA-protein complex formed was separated from free oligonucleotide on 6.6% native polyacrylamide gels. The dried gels were visualized, and the radioactive bands were quantitated using a Storm 820 phosphorimager equipped with ImageQuant software (Amersham, Piscataway, NJ, USA).
2.8 Measurement of cell viability by live/dead assay
To measure apoptosis, we used the live/deads viability assay kit (Invitrogen), which determines intracellular esterase activity and plasma membrane integrity. Calcein-AM is a nonfluorescent polyanionic dye retained by live cells, in which it produces intense green fluorescence through enzymatic (esterase) conversion. Ethidium homodimer enters cells with damaged membranes and binds to nucleic acids, producing bright red fluorescence in dead cells. In brief, treated or untreated cells were stained with the live/ dead reagent (5 μM ethidium homodimer and 5 μM calcein-AM) and incubated at 37°C for 30 min. Cells were analyzed under a fluorescence microscope (Labophot-2; Nikon, Melville, NY, USA).
2.9 NF-κB-dependent reporter gene expression assay
NF-κB-dependent reporter gene expression was assayed using NF-κB/SEAPorter™ assay kit (Imgenex, San Diego, CA, USA). HEK 293 cells were cultured in DMEM medium with 10% FBS and 500 μg/mL G418 antibiotic. Cells were cultured in 6-well dishes at a density of 0.5×106 cells/well and allowed to adhere overnight. The medium was then replaced, and the cells were treated with turmeric for 4 h. Cells were then treated with TNF (1 nM) for 24 h, and the culture medium was used for SEAP assay essentially following the manufacturer’s instructions and using Victor 3 microplate reader (Perkin Elmer Life and Analytical Sciences, Boston, MA, USA).
2.10 High-performance liquid chromatography analysis
Curcumin content in turmeric was determined by HPLC (Waters separation module Alliance 2695, and dual λ UV detector 2487; Waters, Milford, MA, USA). In brief, turmeric was dissolved in 100% DMSO (100 mg/mL), further diluted (100-fold) in a mobile phase and then 10 μL injected into HPLC column. The injected sample was then analyzed for curcumin content at 425 nm. Separation was performed on a silica-based C18 column and a mobile phase consisting of a mixture of ACN:tetrahydrofuran:water (containing 1% citric acid) in a ratio of 12:25:63, respectively. Amount of curcuminoids (curcumin, DMC, and BDMC) was calculated from area under curve (AUC) using pure curcuminoids as standards.
2.11 Statistical analysis
Experiments were repeated a minimum of three times, with consistent results. Data were given as the mean7 standard deviation (SD). Statistical analysis was carried out using a two-tailed unpaired Student’s t-test. Values of *p<0.05 and **p<0.01 were considered statistically significant.
3 Results
The objective of this study was to investigate whether turmeric has potential to modulate NF-κB and STAT3 pathways. We investigated the potential of turmeric in modulating gene products associated with tumor cell development. Whether turmeric can inhibit proliferation and survival of tumor cells and can suppress bone loss was also investigated. We used TNF-α as an inducer of NF-κB as NF-κB activation by this cytokine is relatively well known.
3.1 Turmeric contains curcuminoids
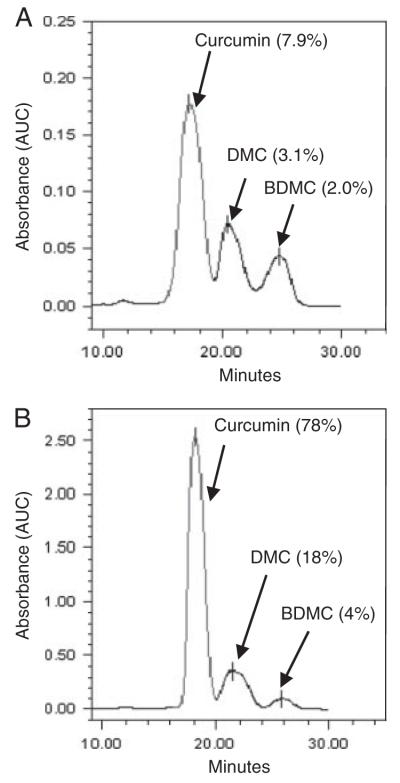
We analyzed the total amount of curcuminoids present in turmeric. The HPLC analysis indicated that turmeric contained 7.9% curcumin, 3.1% DMC, and 2% BDMC (Fig. 1A). These observations were based on the analysis of pure curcuminoids that represented curcumin (78%), DMC (18%), and BDMC (4%) as the major components (Fig. 1B).
Figure 1.
HPLC analysis of turmeric. (A)Turmeric was dissolved in 100% DMSO (100 mg/mL), further diluted (100-fold) in mobile phase and then 10 μL injected into HPLC column. Amount of curcuminoids (curcumin, DMC, and BDMC) were calculated from AUC using pure curcuminoids as standards (B).
3.2 Turmeric suppresses TNF-induced NF-κB activation
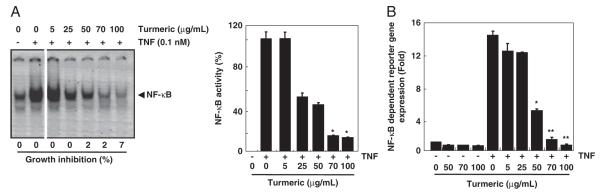
Because NF-κB plays a major role in tumor cell survival, proliferation, chemoresistance, metastasis, and in bone loss, we investigated whether turmeric has potential to inhibit NF-κB activation. We used chronic myeloid leukemia cells (KBM-5), treated them with different concentrations of turmeric and then with TNF-α for NF-κB activation. As evident from EMSA results, TNF-α induced NF-κB in KBM-5 cells. When the cells were treated with turmeric, the TNF-α-induced NF-κB activation was significantly reduced in a dose-dependent manner without affecting cell viability. We found that the inhibition in NF-κB activation was maximum at 70 and 100 μg/mL turmeric (Fig. 2A).
Figure 2.
Effect of turmeric on NF-κB activation. (A) Turmeric suppresses TNF-induced NF-κB activation in a dose-dependent manner. KBM-5 cells (1.5×106 cells/mL) were pre-incubated with turmeric (0, 5, 25, 50, 70, and 100 μg/mL) for 12 h and then treated with 0.1 nM TNF for 30 min. Nuclear extracts were prepared and assayed for NF-κB activation by EMSA (left panel). Values below the EMSA gel indicate percent growth inhibition. Percent inhibition of NF-κB by turmeric was calculated by quantitation of NF-κB bands using a Storm 820 phosphorimager equipped with ImageQuant software (Amersham) (right panel). Data represent the mean±SD of three measurements. *p<0.05, versus control. (B) Suppression of TNF-induced NF-κB-dependent reporter gene expression by turmeric. A293 cells stably transfected with an NF-κB-reporter plasmid were treated with turmeric (0, 5, 25, 50, 70, and 100 μg/mL) for 4 h and then with 1 nM TNF for 24 h. The cell supernatant was then used for SEAP assay. Determinations were made in triplicate. Data represent the mean±SD of three measurements *p<0.05; **p<0.01, significant with respect to control.
We also investigated whether turmeric has potential to inhibit constitutively active NF-κB in various kinds of cancer cells. We found that turmeric inhibited NF-κB activation in a variety of tumor cells such as breast, pancreas, and multiple myeloma (data not shown).
Using DNA-binding assays, we demonstrated that turmeric inhibits NF-κB activation. However, DNA binding alone is not always associated with NF-κB-dependent gene transcription. Therefore, we determined whether turmeric affects TNF-induced reporter gene expression. We stably transfected HEK293 cells with the NF-κB-regulated SEAP reporter construct and incubated the cells with different concentrations of turmeric before stimulation with TNF-α. We found that TNF-α induced almost 15-fold increase in NF-κB-regulated reporter gene expression and that turmeric suppressed the activity in a dose-dependent manner (Fig. 2B). These observations along with EMSA results confirmed the inhibitory effect of turmeric on NF-κB activation.
3.3 Turmeric suppresses STAT3 activation
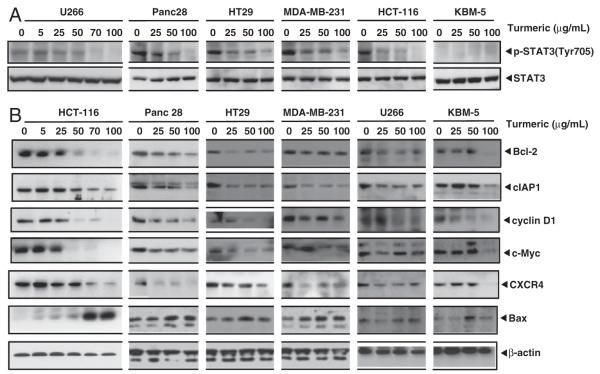
Next, we examined whether turmeric has potential to inhibit STAT3 activation, another pro-inflammatory transcription factor linked with tumor cell development. Activation of STAT3 requires the phosphorylation at Tyr705. Therefore, we examined the ability of turmeric to modulate phosphorylation at this site. We found that a variety of cancer cells expressed constitutive activation of STAT3. This included multiple myeloma (U266), pancreatic (Panc-28), colorectal (HCT-116 and HT29), and breast (MDA-MB-231) cancer cells. The chronic myeloid leukemia cells (KBM-5), however, did not express constitutive activation of STAT3. Turmeric inhibited the constitutive phosphorylation of STAT3 in most of these cells in a dose-dependent manner. Expression of total STAT3 protein was not affected under similar conditions (Fig. 3A).
Figure 3.
Suppression of STAT3 activation and NF-κB and STAT3-dependent gene expression by turmeric. (A) Turmeric suppresses STAT3 activation in a dose-dependent manner in cancer cells. U266, Panc28, HT29, MDA-MB-231, HCT-116, and KBM-5 cells were incubated with indicated concentrations of turmeric for 12 h. Whole cell extracts were prepared and assayed for STAT3 activation by Western blot analysis. (B) Turmeric suppresses expression of gene products involved in tumor cell survival, proliferation, and metastasis. Cells were treated with indicated concentrations of turmeric for 24 h. Whole cell protein extracts were prepared, separated by electrophoresis, and then transferred to the nitrocellulose membrane. The membrane was sliced according to molecular weight to probe with different antibodies. After stripping, the membrane was reprobed with β-actin to verify equal protein loading. Turmeric up-regulate the expression of Bax. Cells were treated with indicated concentrations of turmeric for 24 h, and whole cell protein extract was analyzed by western blotting using antibody against Bax.
3.4 Turmeric inhibits expression of proteins regulated by NF-κB and STAT3
Because turmeric inhibited NF-κB and STAT3 activation, we examined the effects of turmeric on the expression of proteins regulated by these transcription factors. We found that turmeric inhibited the expression of numerous gene products linked with tumor cell development in a dose-dependent manner in multiple myeloma, pancreatic, colorectal, and breast cancer cells in a dose-dependent manner (Fig. 3B). First, turmeric decreased survival-associated proteins (Bcl-2 and cIAP1). Second, the expression of proliferation-associated proteins (cyclin D1 and c-Myc) was down-regulated. Third, turmeric also inhibited the expression of CXC motif receptor 4 (CXCR4), a chemokine receptor regulated by NF-κB.
3.5 Turmeric up-regulates the expression of pro-apoptotic protein, Bax
We also examined whether turmeric modulates the expression of pro-apoptotic protein, Bax. We observed that this spice up-regulated the expression of Bax in various cancer cells in a dose-dependent manner (Fig. 3B).
3.6 Turmeric induces death receptors (DR)-4 and DR5
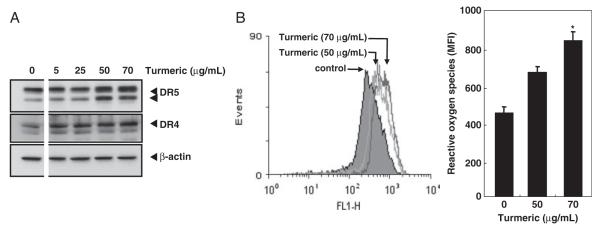
One of the ways for the induction of death in cancer cells is through up-regulation of death receptors, DR4 and DR5. Therefore, we examined whether turmeric can enhance the expression of DR4 and DR5 in colon cancer cells. The results indicated that turmeric induced both DR4 and DR5 in a dose-dependent manner. A maximum induction was observed at 50 and 70 μg/mL turmeric (Fig. 4A).
Figure 4.
Induction of DR5 and DR4 expression by turmeric. (A) HCT-116 cells (4×105 cells/well) were treated with turmeric (0, 5, 25, 50, and 70 μg/mL), whole cell extracts were prepared and analyzed for DR5 and DR4 expression by Western blot analysis. β-Actin was used as an internal control to show equal loading of proteins. (B) HCT-116 cells (3×105 cells/well) were incubated with DCF-DA for 30 min, treated with turmeric at the indicated doses for 3 h, and then examined for ROS production by FACS (left panel). The mean fluorescence intensity (MFI) was plotted against dose (right panel). Data represent the mean±SD of three measurements. *p<0.05, versus control.
The generation of ROS has been implicated in the induction of DR4 and DR5 by certain agents [21-25]. Therefore, we investigated whether induction of DR4 and DR5 by turmeric is through a similar mechanism. Consistent with the induction in death receptors, turmeric induced ROS production in a dose-dependent manner (Fig. 4B).
3.7 Turmeric potentiates the cytotoxic effects of chemotherapeutic drugs
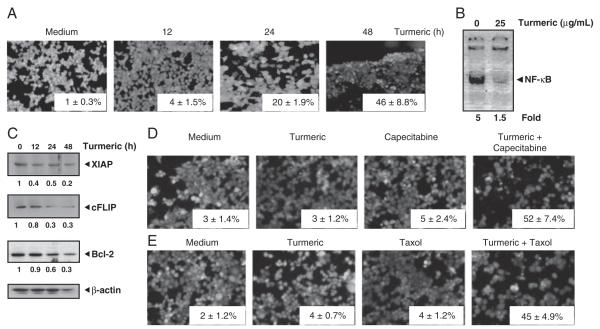
Whether turmeric affects the viability of HCT-116 cells was also examined. The results showed that turmeric alone can affect the viability of these cells in a time-dependent manner (Fig. 5A). We further found that in the same cells turmeric induced cytotoxicity correlated with the inhibition of NF-κB activation (Fig. 5B), and NF-κB-dependent cell survival proteins in a time-dependent manner (Fig. 5C).
Figure 5.
Enhancement of the cytotoxic effects of chemotherapeutic agents by turmeric. (A) HCT-116 cells treated with 25 μg/mL turmeric for 12, 24, and 48 h were analyzed for cell cytotoxicity using live/dead assay. Data represent the mean±SD of three field measurements. (B) Turmeric suppresses constitutive activation of NF-κB in HCT-116 cells. Cells (5×105 cells/mL) were incubated with 25 μg/mL turmeric for 12 h, nuclear extracts were prepared and assayed for NF-κB activation by EMSA. The values below the EMSA gel indicate fold decrease in NF-κB activity. (C) Turmeric suppresses expression of gene products involved in tumor cell survival. HCT-116 cells were incubated with turmeric (25 μg/mL) for 12, 24, and 48 h. Whole cell extracts were prepared and analyzed by Western blotting using the indicated antibodies. The same blots were stripped and reprobed with β-actin antibody to verify equal protein loading. (D, E) HCT-116 cells were treated with turmeric (25 μg/mL) for 12 h, washed with PBS to remove turmeric, and then treated with 20 mM capecitabine or 5 nM Taxol for 24 h. Cell cytotoxicity was determined by live/dead assay. Data represent the mean±SD of three field measurements.
Because NF-κB activation has been implicated in tumor cell chemoresistance [26, 27], we investigated whether turmeric can sensitize tumor cells to chemotherapeutic agents. We treated HCT-116 cells with turmeric (for 12 h) followed by treatment with capecitabine and taxol (for 24 h) and then assessed the membrane integrity using the live/ dead assay. Results indicated that turmeric, capecitabine, or taxol alone at the dose examined induced minimum death in cancer cells. However, when cells were pretreated with turmeric, capecitabine-induced apoptosis was enhanced from 5 to 52% (Fig. 5D) and that of taxol-induced apoptosis was enhanced from 4 to 45% (Fig. 5E). These results indicated synergistic interaction between turmeric and chemotherapeutic agents.
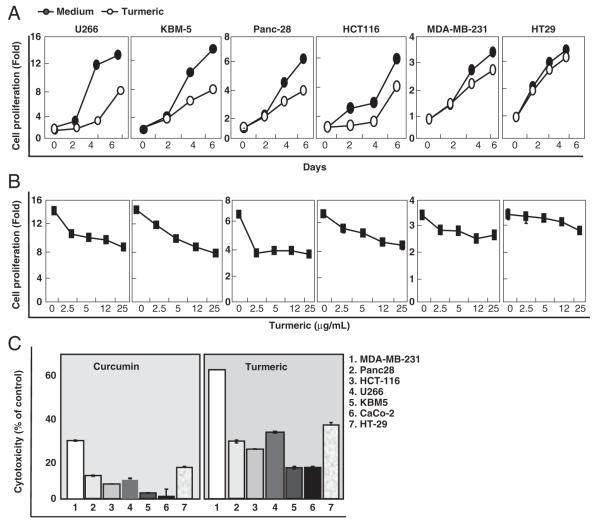
3.8 Turmeric inhibits cancer cell proliferation
Because NF-κB and STAT3 has been linked with cancer cell proliferation [26, 27] and turmeric inhibited activation of these transcription factors, we investigated turmeric’s effect on the proliferation of a series of cancer cells. The results indicate that turmeric inhibited the proliferation of tumor cells in a time- (Fig. 6A) and dose-dependent (Fig. 6B) manner.
Figure 6.
Effect of turmeric on tumor cell proliferation. (A) U266, KBM-5, Panc-28, HCT-116, MDA-MB-231, and HT-29 cells (5×103 cells/well) were seeded in triplicate in 96-well plates, treated with turmeric (25 μg/mL) for 0, 2, 4, and 6 days. (B) U266, KBM-5, Panc-28, HCT-116, MDA-MB-231, and HT-29 cells (5×103cells/well) were seeded in triplicate in 96-well plates, treated with turmeric (0, 2.5, 5, 12, and 25 μg/mL) for 72 h, and then assayed for cell viability by the MTT method. (C) Effect of turmeric and curcumin on tumor cell cytotoxicity. U266, KBM-5, Panc-28, HCT-116, MDA-MB-231, CaCo-2, and HT-29 cells (5×103 cells/well) were treated with turmeric (70 μg/mL) and curcumin (5 μg/mL) for 72 h and then cell viability was measured by the MTT method. Data represent the mean±SD of three measurements. *p<0.05; **p<0.01 with respect to control.
3.9 Turmeric is more potent than curcumin
Because curcumin is one of the active components present in turmeric, we compared the relative potency of turmeric with curcumin. Turmeric used in our studies contained 7.9% curcumin (Fig. 1). We treated cancer cells with 70 μg/ mL turmeric that corresponded to approximately 5 μg/mL curcumin. In a parallel set of experiments, we also treated tumor cells with 5 μg/mL curcumin. A comparison made between turmeric and curcumin indicated that turmeric was more potent in inducing inhibition of growth of various cell lines (Fig. 6C). For example, in breast cancer cells curcumin induced 33% growth inhibition, while 66% growth inhibition was observed by turmeric containing equivalent amount of curcumin. A similar trend was observed with other tumor cells. These observations indicate that components other than curcumin might contribute to the potency of turmeric.
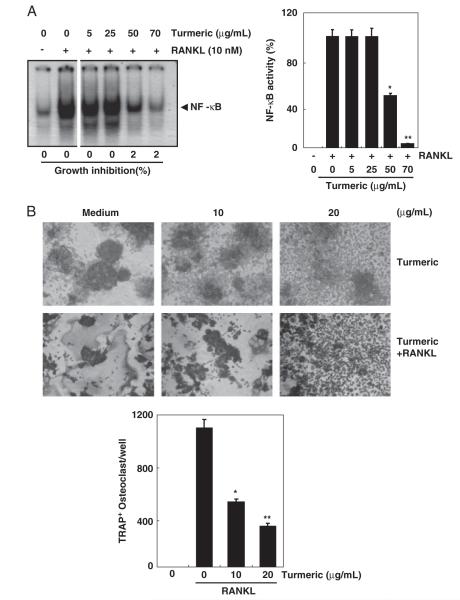
3.10 Turmeric suppresses RANKL-induced osteoclastogenesis
Because NF-κB is implicated in the process of osteoclast formation and bone destruction, we investigated whether turmeric can affect osteoclast formation. We used RANKL to induce NF-κB and osteoclastogenesis. Results indicated that RANKL induced NF-κB activation, while pretreatment with turmeric inhibited NF-κB activation in a dose-dependent manner (Fig. 7A). Consistent with these observations, turmeric also inhibited RANKL-induced osteoclast formation (Fig. 7B). Taken together, these results indicate that turmeric has potential to inhibit RANKL-induced NF-κB activation and osteoclastogenesis.
Figure 7.
Suppression of RANKL-induced osteoclastogenesis by tumeric. (A) Turmeric suppresses RANKL-induced NF-κB activation. RAW 264.7 cells (1×106 cells/well) were incubated either alone or in the presence of turmeric (0, 5, 25, 50, and 70 μg/mL) for 12 h and treated with RANKL (10 nM) for 30 min. Nuclear extracts were prepared and assayed for NF-κB activation by EMSA. Values below the EMSA gel indicate percent growth inhibition (left panel). Bands were quantitated using a Storm 820 phosphorimager equipped with ImageQuant software (Amersham). Data represent the mean 7SD of three measurements (right panel). *p<0.05; **p<0.01, versus control. (B) Turmeric suppresses RANKL-induced osteoclastogenesis. RAW 264.7 cells (1×104 cells/well) were incubated either alone or in the presence of RANKL (5 nM) and the indicated concentration of turmeric for 5 days and then stained for TRAP expression. TRAP-positive cells were photographed (original magnification, ×100). Data represent the mean 7SD of three measurements. *p<0.05; **p<0.01, versus control.
4 Discussion
Extensive research over the last three decades has made it clear that inflammation plays a major role in many chronic diseases including cancer [28-30]. It has also become apparent that as many as 90% of cancers are caused by lifestyle factors and thus may be preventable [31]. The golden spice turmeric has been used extensively in Southeast Asia in the diet and as an anti-inflammatory agent and is well described as ayurvedic medicine [7]. Curcumin, a minor component of turmeric (3-7%), has been linked to the regulation of inflammation and cancer [7, 32]. It is not known whether turmeric itself exhibit activities similar to curcumin. The present study was thus designed to address this question by investigating turmeric’s effects on NF-κB- and STAT3-activated inflammatory pathways, on associated inflammatory proteins, and on cellular responses activated by these pathways.
We found for the first time that turmeric inhibited constitutive and inducible NF-κB activation. Whether examined by DNA binding or reporter gene expression, turmeric inhibited NF-κB activation. This is in agreement with a previous study demonstrating dietary turmeric’s ability to modulate the DMBA-induced NF-κB pathway in a hamster model [8]. How turmeric inhibits NF-κB activation is not clear. However, previous studies have shown that curcumin has the ability to inhibit IKK activation, one of the major kinases involved in NF-κB activation pathway [33]. It is likely that turmeric is inhibiting NF-κB activation through a similar mechanism. We also found that turmeric inhibited STAT3 activity in tumor cells. Numerous mechanisms have been proposed for STAT3 activation including activation of JAKs [34], and down-regulation of phosphatases [35]. It is very likely that inhibitory effect of turmeric on STAT3 activity is through inhibition of one or more mechanisms.
We also found for the first time that turmeric inhibited expression of gene products involved in tumor cell survival, proliferation, and metastasis. The cell survival proteins Bcl-2 and cIAP1 are found overexpressed in tumor cells [36] and have been reported to be regulated by NF-κB and STAT3. The cell proliferation proteins cyclin D1 and c-Myc are other known downstream targets of NF-κB and STAT3 [36]. CXCR4 has been shown to be regulated by NF-κB as well [37]. That turmeric treatment was associated with down-regulation of expression of proteins involved in tumor cell survival, proliferation, and metastasis could be due to the observed inhibition of the activation of NF-κB and STAT3.
We found that turmeric has potential to induce proapoptotic protein Bax, thus indicating turmeric’s ability in inducing apoptosis in tumor cells. Cancer cells are known to undergo apoptosis chiefly through extrinsic and intrinsic pathways. While intrinsic pathway involves mitochondria, the extrinsic pathway is mediated through the death receptors. Turmeric’s potential in inducing death receptors suggests the involvement of extrinsic pathway of apoptosis. Tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) is one of the cytokines of TNF superfamily with potential to induce apoptosis selectively in cancer cells through induction of DR4 and DR5 [38]. However, acquired resistance to TRAIL owing to dysregulated expression of DR4 and DR5 represents one of the major hurdles in TRAIL-based therapeutics [39, 40]. That the turmeric was able to induce death receptor may offer an opportunity for its use in combination with TRAIL as cancer therapeutics. We found that turmeric induced ROS in colon cancer cells. Previous studies have suggested the role of ROS in inducing death receptor by certain agents [21-23, 25, 41, 42]. Therefore, it is likely that turmeric induced DRs through a similar mechanism.
We found that turmeric suppressed the proliferation of various tumor cells. This inhibition correlated with a reduction in the expression of cell proliferation proteins such as cyclin D1 and c-Myc. The dysregulation and overexpression of cyclin D1 [43] and c-Myc [44], which are observed in the majority of cancer patients, has been proposed to play a critical role in the pathogenesis of the disease. The downregulation of cyclin D1 and c-Myc expression are likely involved in turmeric’s ability to inhibit tumor growth.
We also found that turmeric has the potential to sensitize cancer cells to existing chemotherapeutic agents (capecitabine and taxol). The sensitization may be attributed to the inhibition of NF-κB activation pathway. These effects are similar to those reported previously for a specific inhibitor of NF-κB [45] and for curcumin [46]. The development of drug resistance has been one of the major hurdles for the success of cancer therapy. Thus, our observations provide an opportunity for the use of turmeric in combination with existing drugs to overcome chemoresistance. How cancer patients develop chemoresistance is not properly understood. However, cell survival proteins such as Bcl-2 [36] and cIAP1 [36] have been shown to play a role. Inhibition of cell survival proteins by turmeric as observed in the present study could sensitize cancer cells to the chemotherapeutic agents.
We also found that turmeric inhibited osteoclast differentiation and thus bone loss induced by RANKL. Inhibition in bone loss correlated with a decrease in NF-κB activation. An antibody against RANKL (Prolia) has been approved for the treatment of age- or cancer-induced bone loss. Osteoprotegrin (OPG) is another agent known to inhibit osteoclast differentiation by sequestering RANKL [47]. Our observations indicate that turmeric can antagonize RANKL through inhibition of NF-κB. In agreement with our observations, a previous study demonstrated the potential of dietary turmeric in preventing osteoclastogenesis and bone destruction in a rat model of rheumatoid arthritis [48]. Concomitant with inhibition in osteoclast formation in this model, turmeric also suppressed NF-κB activation and RANKL expression. Taken together, these observations indicate that both OPG and turmeric has similar activity although the mechanism may be different.
We found that turmeric was more potent when compared with curcumin, suggesting that components other than curcumin can also contribute to anti-cancer activities. Most of the activities reported for turmeric in this manuscript have already been reported for curcumin. Thus, our observations indicate that turmeric exhibits spectrum of activities similar to curcumin. Together, our findings indicate that turmeric can suppress inflammatory pathways and tumorigenesis, and can inhibit bone loss in vitro. These observations open up new possibilities with turmeric to conduct in vivo studies in animals followed by human clinical trials.
Acknowledgments
The authors thank Jude Richard for carefully proofreading the manuscript and providing valuable comments. Dr. Aggarwal is the Ransom Horne, Jr., Professor of Cancer Research. This work was supported by a grant (CA-16 672), and a program project grant (NIH CA-124787-01A2) from the National Institutes of Health and a grant from the Center for Targeted Therapy at M. D. Anderson Cancer Center.
Abbreviations
- BDMC
bisdemethoxycurcumin
- cIAP1
cellular inhibitor of apoptosis 1
- DCF-DA
dichlorodihydrofluoresceindiacetate
- DMC
demethoxycurcumin
- DR
death receptors
- EMSA
electrophoretic mobility shift assay
- FBS
fetal bovine serum
- NF-κB
nuclear factor kappa B
- RANKL
receptor activator of NF-κB ligand
- ROS
reactive oxygen species
- STAT3
signal transducers and activators of transcription 3
- TNF
tumor necrosis factor
- TRAIL
tumor necrosis factor-related apoptosis-inducing ligand
- TRAP
tartrate-resistant acid phosphatase
Footnotes
The authors have declared no conflict of interest.
5. References
- [1].Newman DJ, Cragg GM, Snader KM. Natural products as sources of new drugs over the period 1981-2002. J. Nat. Prod. 2003;66:1022–1037. doi: 10.1021/np030096l. [DOI] [PubMed] [Google Scholar]
- [2].Butler MS. The role of natural product chemistry in drug discovery. J. Nat. Prod. 2004;67:2141–2153. doi: 10.1021/np040106y. [DOI] [PubMed] [Google Scholar]
- [3].Balunas MJ, Kinghorn AD. Drug discovery from medicinal plants. Life Sci. 2005;78:431–441. doi: 10.1016/j.lfs.2005.09.012. [DOI] [PubMed] [Google Scholar]
- [4].Gurib-Fakim A. Medicinal plants: Traditions of yesterday and drugs of tomorrow. Mol. Asp. Med. 2006;27:1–93. doi: 10.1016/j.mam.2005.07.008. [DOI] [PubMed] [Google Scholar]
- [5].Grieve M. A modern herbal Leyel CF. Dover Publications, Inc.; New York: 1971. [Google Scholar]
- [6].Aggarwal BB, Shishodia S. Molecular targets of dietary agents for prevention and therapy of cancer. Biochem. Pharmacol. 2006;71:1397–1421. doi: 10.1016/j.bcp.2006.02.009. [DOI] [PubMed] [Google Scholar]
- [7].Prasad S, Aggarwal B. Turmeric, The Golden Spice: From Traditional Medicine to Modern Medicine. In: Benzie IFF, Wachtel-Galor S, editors. Herbal Medicine: Biomolecular and Clinical Aspects, Oxidative Stress & Disease Series. CRC Press; Boca Raton, FL: 2011. pp. 259–284. [PubMed] [Google Scholar]
- [8].Garg R, Ingle A, Maru G. Dietary turmeric modulates DMBA-induced p21ras, MAP kinases and AP-1/NF-kappaB pathway to alter cellular responses during hamster buccal pouch carcinogenesis. Toxicol. Appl. Pharmacol. 2008;232:428–439. doi: 10.1016/j.taap.2008.07.007. [DOI] [PubMed] [Google Scholar]
- [9].Li S, Yuan W, Deng G, Wang P, Yang P, Aggarwal BB. Chemical composition and product quality control of turmeric (Curcuma longa L.) Phytochemistry. 2011;2:28–54. [Google Scholar]
- [10].Aggarwal BB, Sung B. Pharmacological basis for the role of curcumin in chronic diseases: an age-old spice with modern targets. Trends Pharmacol. Sci. 2009;30:85–94. doi: 10.1016/j.tips.2008.11.002. [DOI] [PubMed] [Google Scholar]
- [11].Aggarwal BB. Targeting inflammation-induced obesity and metabolic diseases by curcumin and other nutraceuticals. Annu. Rev. Nutr. 2010;30:173–199. doi: 10.1146/annurev.nutr.012809.104755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Anand P, Kunnumakkara AB, Newman RA, Aggarwal BB. Bioavailability of curcumin: problems and promises. Mol. Pharm. 2007;4:807–818. doi: 10.1021/mp700113r. [DOI] [PubMed] [Google Scholar]
- [13].Sandur SK, Pandey MK, Sung B, Ahn KS, et al. Curcumin, demethoxycurcumin, bisdemethoxycurcumin, tetrahydrocurcumin and turmerones differentially regulate anti-inflammatory and anti-proliferative responses through a ROS-independent mechanism. Carcinogenesis. 2007;28:1765–1773. doi: 10.1093/carcin/bgm123. [DOI] [PubMed] [Google Scholar]
- [14].Deshpande SS, Ingle AD, Maru GB. Inhibitory effects of curcumin-free aqueous turmeric extract on benzo[a]pyrene-induced forestomach papillomas in mice. Cancer Lett. 1997;118:79–85. doi: 10.1016/s0304-3835(97)00238-3. [DOI] [PubMed] [Google Scholar]
- [15].Deshpande SS, Ingle AD, Maru GB. Chemopreventive efficacy of curcumin-free aqueous turmeric extract in 7,12-dimethylbenz[a]anthracene-induced rat mammary tumorigenesis. Cancer Lett. 1998;123:35–40. doi: 10.1016/s0304-3835(97)00400-x. [DOI] [PubMed] [Google Scholar]
- [16].Ramachandran C, Resek AP, Escalon E, Aviram A, Melnick SJ. Potentiation of gemcitabine by turmeric Force in pancreatic cancer cell lines. Oncol. Rep. 2010;23:1529–1535. doi: 10.3892/or_00000792. [DOI] [PubMed] [Google Scholar]
- [17].Chaturvedi MM, Mukhopadhyay A, Aggarwal BB. Assay for redox-sensitive transcription factor. Methods Enzymol. 2000;319:585–602. doi: 10.1016/s0076-6879(00)19055-x. [DOI] [PubMed] [Google Scholar]
- [18].Hsu H, Lacey DL, Dunstan CR, Solovyev I, et al. Tumor necrosis factor receptor family member RANK mediates osteoclast differentiation and activation induced by osteoprotegerin ligand. Proc. Natl. Acad. Sci. USA. 1999;96:3540–3545. doi: 10.1073/pnas.96.7.3540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Wei S, Teitelbaum SL, Wang MW, Ross FP. Receptor activator of nuclear factor-kappa b ligand activates nuclear factor-kappa b in osteoclast precursors. Endocrinology. 2001;142:1290–1295. doi: 10.1210/endo.142.3.8031. [DOI] [PubMed] [Google Scholar]
- [20].Chainy GB, Manna SK, Chaturvedi MM, Aggarwal BB. Anethole blocks both early and late cellular responses transduced by tumor necrosis factor: effect on NF-kappaB, AP-1, JNK, MAPKK and apoptosis. Oncogene. 2000;19:2943–2950. doi: 10.1038/sj.onc.1203614. [DOI] [PubMed] [Google Scholar]
- [21].Chen JJ, Chou CW, Chang YF, Chen CC. Proteasome inhibitors enhance TRAIL-induced apoptosis through the intronic regulation of DR5: involvement of NF-kappa B and reactive oxygen species-mediated p53 activation. J. Immunol. 2008;180:8030–8039. doi: 10.4049/jimmunol.180.12.8030. [DOI] [PubMed] [Google Scholar]
- [22].Jung EM, Lim JH, Lee TJ, Park JW, Choi KS, Kwon TK. Curcumin sensitizes tumor necrosis factor-related apoptosis-inducing ligand (TRAIL)-induced apoptosis through reactive oxygen species-mediated upregulation of death receptor 5 (DR5) Carcinogenesis. 2005;26:1905–1913. doi: 10.1093/carcin/bgi167. [DOI] [PubMed] [Google Scholar]
- [23].Kim H, Kim EH, Eom YW, Kim WH, et al. Sulforaphane sensitizes tumor necrosis factor-related apoptosis-inducing ligand (TRAIL)-resistant hepatoma cells to TRAIL-induced apoptosis through reactive oxygen species-mediated up-regulation of DR5. Cancer Res. 2006;66:1740–1750. doi: 10.1158/0008-5472.CAN-05-1568. [DOI] [PubMed] [Google Scholar]
- [24].Kim JY, Kim EH, Park SS, Lim JH, Kwon TK, Choi KS. Quercetin sensitizes human hepatoma cells to TRAIL-induced apoptosis via Sp1-mediated DR5 up-regulation and proteasome-mediated c-FLIPS down-regulation. J. Cell Biochem. 2008;105:1386–1398. doi: 10.1002/jcb.21958. [DOI] [PubMed] [Google Scholar]
- [25].Taniguchi H, Yoshida T, Horinaka M, Yasuda T, et al. Baicalein overcomes tumor necrosis factor-related apoptosis-inducing ligand resistance via two different cell-specific pathways in cancer cells but not in normal cells. Cancer Res. 2008;68:8918–8927. doi: 10.1158/0008-5472.CAN-08-1120. [DOI] [PubMed] [Google Scholar]
- [26].Van Antwerp DJ, Martin SJ, Kafri T, Green DR, Verma IM. Suppression of TNF-alpha-induced apoptosis by NF-kappaB. Science. 1996;274:787–789. doi: 10.1126/science.274.5288.787. [DOI] [PubMed] [Google Scholar]
- [27].Wang CY, Mayo MW, Baldwin AS., Jr. TNF- and cancer therapy-induced apoptosis: potentiation by inhibition of NF-kappaB. Science. 1996;274:784–787. doi: 10.1126/science.274.5288.784. [DOI] [PubMed] [Google Scholar]
- [28].Barnes PJ, Karin M. Nuclear factor-kappaB: A pivotal transcription factor in chronic inflammatory diseases. N. Engl. J. Med. 1997;336:1066–1071. doi: 10.1056/NEJM199704103361506. [DOI] [PubMed] [Google Scholar]
- [29].Aggarwal BB. Inflammation, a silent killer in cancer is not so silent! Curr. Opin. Pharmacol. 2009;9:347–350. doi: 10.1016/j.coph.2009.06.018. [DOI] [PubMed] [Google Scholar]
- [30].Aggarwal BB, Vijayalekshmi RV, Sung B. Targeting inflammatory pathways for prevention and therapy of cancer: short-term friend, long-term foe. Clin. Cancer Res. 2009;15:425–430. doi: 10.1158/1078-0432.CCR-08-0149. [DOI] [PubMed] [Google Scholar]
- [31].Anand P, Kunnumakkara AB, Sundaram C, Harikumar KB, et al. Cancer is a preventable disease that requires major lifestyle changes. Pharm. Res. 2008;25:2097–2116. doi: 10.1007/s11095-008-9661-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Ravindran PN, Nirmal Babu K, Sivaraman K. Turmeric: The Genus Curcuma. CRC Press; Boca Raton: 2007. [Google Scholar]
- [33].Bharti AC, Donato N, Singh S, Aggarwal BB. Curcumin (diferuloylmethane) down-regulates the constitutive activation of nuclear factor-kappa B and IkappaBalpha kinase in human multiple myeloma cells, leading to suppression of proliferation and induction of apoptosis. Blood. 2003;101:1053–1062. doi: 10.1182/blood-2002-05-1320. [DOI] [PubMed] [Google Scholar]
- [34].Bharti AC, Donato N, Aggarwal BB. Curcumin (diferuloylmethane) inhibits constitutive and IL-6-inducible STAT3 phosphorylation in human multiple myeloma cells. J. Immunol. 2003;171:3863–3871. doi: 10.4049/jimmunol.171.7.3863. [DOI] [PubMed] [Google Scholar]
- [35].Kannappan R, Yadav VR, Aggarwal BB. gamma-Tocotrienol but not gamma-tocopherol blocks STAT3 cell signaling pathway through induction of protein-tyrosine phosphatase SHP-1 and sensitizes tumor cells to chemotherapeutic agents. J. Biol. Chem. 2010;285:33520–33528. doi: 10.1074/jbc.M110.158378. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- [36].Gupta SC, Kim JH, Prasad S, Aggarwal BB. Regulation of survival, proliferation, invasion, angiogenesis, and metastasis of tumor cells through modulation of inflammatory pathways by nutraceuticals. Cancer Metas. Rev. 2010;29:405–434. doi: 10.1007/s10555-010-9235-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Helbig G, Christopherson KW, 2nd, Bhat-Nakshatri P, Kumar S, et al. NF-kappaB promotes breast cancer cell migration and metastasis by inducing the expression of the chemokine receptor CXCR4. J. Biol. Chem. 2003;278:21631–21638. doi: 10.1074/jbc.M300609200. [DOI] [PubMed] [Google Scholar]
- [38].Johnstone RW, Frew AJ, Smyth MJ. The TRAIL apoptotic pathway in cancer onset, progression and therapy. Nat. Rev. Cancer. 2008;8:782–798. doi: 10.1038/nrc2465. [DOI] [PubMed] [Google Scholar]
- [39].Lee SH, Shin MS, Kim HS, Lee HK, et al. Somatic mutations of TRAIL-receptor 1 and TRAIL-receptor 2 genes in non-Hodgkin’s lymphoma. Oncogene. 2001;20:399–403. doi: 10.1038/sj.onc.1204103. [DOI] [PubMed] [Google Scholar]
- [40].Shin MS, Kim HS, Lee SH, Park WS, et al. Mutations of tumor necrosis factor-related apoptosis-inducing ligand receptor 1 (TRAIL-R1) and receptor 2 (TRAIL-R2) genes in metastatic breast cancers. Cancer Res. 2001;61:4942–4946. [PubMed] [Google Scholar]
- [41].Kim S, Lee TJ, Leem J, Choi KS, Park JW, Kwon TK. Sanguinarine-induced apoptosis: generation of ROS, down-regulation of Bcl-2, c-FLIP, and synergy with TRAIL. J. Cell Biochem. 2008;104:895–907. doi: 10.1002/jcb.21672. [DOI] [PubMed] [Google Scholar]
- [42].Aggarwal BB. Nuclear factor-kappaB: The enemy within. Cancer Cell. 2004;6:203–208. doi: 10.1016/j.ccr.2004.09.003. [DOI] [PubMed] [Google Scholar]
- [43].Deshpande A, Sicinski P, Hinds PW. Cyclins and cdks in development and cancer: a perspective. Oncogene. 2005;24:2909–2915. doi: 10.1038/sj.onc.1208618. [DOI] [PubMed] [Google Scholar]
- [44].Soucek L, Whitfield J, Martins CP, Finch AJ, et al. Modelling Myc inhibition as a cancer therapy. Nature. 2008;455:679–683. doi: 10.1038/nature07260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Takada Y, Singh S, Aggarwal BB. Identification of a p65 peptide that selectively inhibits NF-kappa B activation induced by various inflammatory stimuli and its role in down-regulation of NF-kappaB-mediated gene expression and up-regulation of apoptosis. J. Biol. Chem. 2004;279:15096–15104. doi: 10.1074/jbc.M311192200. [DOI] [PubMed] [Google Scholar]
- [46].Aggarwal BB, Shishodia S, Takada Y, Banerjee S, et al. Curcumin suppresses the paclitaxel-induced nuclear factor-kappaB pathway in breast cancer cells and inhibits lung metastasis of human breast cancer in nude mice. Clin. Cancer Res. 2005;11:7490–7498. doi: 10.1158/1078-0432.CCR-05-1192. [DOI] [PubMed] [Google Scholar]
- [47].Udagawa N, Takahashi N, Yasuda H, Mizuno A, et al. Osteoprotegerin produced by osteoblasts is an important regulator in osteoclast development and function. Endocrinology. 2000;141:3478–3484. doi: 10.1210/endo.141.9.7634. [DOI] [PubMed] [Google Scholar]
- [48].Funk JL, Frye JB, Oyarzo JN, Kuscuoglu N, et al. Efficacy and mechanism of action of turmeric supplements in the treatment of experimental arthritis. Arthritis Rheum. 2006;54:3452–3464. doi: 10.1002/art.22180. [DOI] [PubMed] [Google Scholar]