Abstract
Adenovirus invades host cells by first binding to host receptors through a trimeric fiber, which contains three domains: a receptor-binding knob domain, a long flexible shaft domain and a penton base-attachment tail domain. Although the structure of the knob domain associated with a portion of the shaft has been solved by X-ray crystallography, the in situ structure of the fiber in the virion is not known; thus it remains a mystery how the trimeric fiber attaches to its underlying pentameric penton base. By high-resolution cryo-electron microscopy (cryoEM), we have determined the structure of the human adenovirus type 5 (Ad5) to 3.6Å resolution and have reported the full atomic models for its capsid proteins, but not for the fiber whose density cannot be directly interpreted due to symmetry mismatch with the penton base. Here we report the determination of the Ad5 fiber structure and its mode of attachment to the pentameric penton base by using an integrative approach of multi-resolution filtering, homology modeling, computational simulation of mismatched symmetries and fitting of atomic models into cryoEM density maps. Our structure reveals that the interactions between the trimeric fiber and the pentameric penton base are mediated by a hydrophobic ring on the top surface of the penton base and three flexible tails inserted into three of the five available grooves formed by neighboring subunits of penton base. These interaction sites provide the molecular basis for the symmetry mismatch and can be targeted for optimizing adenovirus for gene therapy applications.
Keywords: adenovirus, fiber, penton base, symmetry mismatch, cryoEM
Introduction
Human adenovirus (Ad) causes acute respiratory, gastrointestinal diseases, especially among children and immuno-compromised individuals. Engineered adenovirus particles are used as gene therapy vectors for transferring genes into mammalian cells.1–5 Ad is one of the largest (~900. diameter, excluding the fiber) and most complex (~150MD) non-enveloped, double-stranded (ds) DNA viruses. Its icosahedral capsid shell contains three major proteins (hexon, penton base and fiber) and four “minor” proteins (IIIa, VI, VIII and IX).6–9 Each virion contains 240 hexon trimers (hexons) and the 12 penton-base pentomers (penton base), each of which is bound with one fiber trimer (fiber) (Fig. 1a,b). Hexons and pentons are joined together by three minor proteins: IX, IIIa and VIII. 240 copies of minor protein IX are located at the outer surface of the capsid, while 60 copies of minor protein IIIa, and 120 copies of minor protein VIII are both at the inner surface of capsid.10 These minor proteins form exquisite interaction networks, which stabilize two kinds of building blocks – groups of nine hexons (GONs) and groups of six capsomers (GOS) (a penton base and its five surrounding hexons) - into the perfect adenovirus capsid shell.11
Fig. 1.
Overall structure of Ad5 and the penton-base protein. (a–b) Overall structure of Ad5 capsid filtered at 4Å (a) and 10Å (b), respectively, centered on a two-fold axis of icosahedron, showing the fiber (magenta) associating with the penton base (yellow) locate at the vertices of icosahedron. The remaining capsid proteins are colored blue. (c) Superposition of the cryoEM density map (semi-transparent) and the backbone of the atomic model (magenta sticks) of penton-base protein, including the newly resolved ‘N-arm’ region (blue sticks). (d) Representative enlargement of a β sheet [boxed region in (c)] showing separation of strands (left). Stick model of one strand superimposed on its density map (mesh) showing a representative carboxyl (red arrow) density (middle) and is rotated to show side chains (labeled) that are perpendicular to the β sheet (right).
In the virion, each fiber attaches to a penton base and plays a central role in host cell attachment and entry.12–14 The fiber monomer consists of three domains: an N-terminal tail, a central shaft with variable lengths and a C-terminal knob (or head). CryoEM structures of intact adenovirus9, 15, 16 and of a recombinant, dodecahedral particle consisting of pentons 17 have revealed that the trimeric fibers attach to the pentameric penton bases. X-ray crystallography of the human adenovirus type 2 (Ad2) penton base in complex with an N-terminal polypeptide of the fiber protein has shown that a 10-amino-acid-long N-terminal polypeptide (aa10–19) binds to the groove formed by two adjacent penton-base monomers.18 The extended fiber-shaft domain contains “triple β-spiral” repeat motifs, 19 each consisting of 15–20 residues (i.e., 15-residue pseudo-repeat).20 The length of the shaft domain can vary and is determined by the total number of this repeat in the shaft. For example, Ad2 and Ad5 fiber shafts have a similar length of about 300Å with 21 repeats, but the Ad3 fiber shaft is about 100Å with only 6 repeats.21 Previous results have shown that short fiber shafts, such as those of Ad3, are straight and rigid;16 but long fiber shafts, such as those of Ad2 and Ad5, are sufficiently flexible to allow interactions between the penton base and its cellular receptor.22–24 The C-terminal globular knob contains three eight-stranded β-barrels, one in each subunit, and has a central depression with three valleys. 25, 26
However, in the absence of in situ high-resolution structural information of the fiber in the context of the entire virion, many questions remain unaddressed. First, only some segments of fiber protein are known to atomic detail. Second, although a 10-amino-acid long N-terminal fiber polypeptide (aa10–19) has been resolved in the crystal structure of Ad2 penton base, how the 10 aa-long fiber tails connect with the fiber shaft in the virion is unknown. Third, how the trimeric fiber attaches to its underlying pentameric penton base remains a dilemma. In this study, we use an integrative approach of multi-resolution filtering, homology modeling, computational simulation of mismatched symmetries and fitting of atomic models into cryoEM density maps to establish the structure of the Ad5 trimeric fiber and its attachment to the pentameric penton base. An atomic model for the N-terminal tail is built based on the cryoEM density map, and then combined with homology models of the shaft and the knob domains to construct a pseudo-atomic model for the full fiber. We show that the bottom of the fiber shaft interacts with a hydrophobic ring of the penton base and that the three fiber tails of each fiber act as stay-cables, residing inside three of the five grooves between neighboring penton-base monomers. These observations provide the molecular basis for the symmetry mismatch between the trimeric fiber and the pentameric penton base.
Results
Observations of the N-terminal tail of the Ad5 fiber interacting with the penton base
The 3D structure of the Ad5 virion has been reconstructed to 3.6Å resolution.11 Based on this structure, we have built the full atom model for its capsid proteins, including major proteins penton-base protein and hexon protein, as well as minor proteins IIIa, VIII and IX. However, on the one hand, because icosahedral symmetry was imposed in this reconstruction, the densities for the trimeric fiber above each icosahedral 5-fold axis are smeared out and thus cannot be directly interpreted. On the other hand, because the mass of the trimeric fiber is only 2% of that of the icosahedrally related proteins in the virion, attempts to reconstruct Ad without using icosahedral symmetry have been unsuccessful.16 Here, to obtain structural information about the fiber protein, we first filtered the 3.6Å icosahedral density map to different resolutions (see details in Materials and Methods). Figure 1a,b shows the overall structure (density map) of Ad5 filtered at 4Å and 10Å resolutions, respectively. Each of the 12 fiber densities (magenta) attaches to the penton bases (yellow), occupying one of the 12 vertices of the icosahedral virion. Only the first 45Å length of the fiber is reconstructed due to the use of a radial mask to improve signal to noise ratio. When a larger radial mask was used, longer fiber densities were observed but the densities at larger radius gradually decrease, implying more flexibility/deviation from 5-fold axial position. The atomic model of the penton-base protein of Ad5 (Fig 1c; Supplementary Movie 1) derived from our 3.6Å cryoEM density11 is in excellent agreement with the crystal structure of penton-base protein of Ad2.18 Moreover, we resolved additional amino acids (‘N-arm’ in Fig 1c) in the N terminus of the penton-base protein. Representative densities of β strands, carboxyl groups and amino acid side chains (Fig. 1d) are indicative of the high resolving power of our map, which has allowed us to identify amino acid segments of the fiber protein interacting with the penton base.
It is known that, in the virion, the trimeric fiber interacts with the pentameric penton base in a specific manner, involving side chain hydrogen bonds and a salt bridge.18 Therefore, it is expected that the parts of the three monomers in each fiber participating in these interactions are also arranged with a five-fold symmetry relationship (i.e., related by a 1–5 times of 72° rotation). (Because each fiber only has three monomers to interact with the five available sites on the pentameric penton base, two of these 5-fold symmetry related sites would be left unoccupied.) When we filtered the 3.6Å density map to 4Å resolution (Fig. 1a), we observe five extra crescent shape densities (magenta) on the top surface of the penton base (Fig. 2a,b; Supplementary Movie 2), each having a length of about 30Å. Because five-fold symmetry is imposed during 3D reconstruction, five densities are expected to show up, but at a reduced density level (Fig. 2b). Indeed, the average value of these extra densities is about 40% less as compared to the average density of the penton-base monomer, consistent with two unoccupied sites per penton base. There is a pore of ~15Å diameter at the center of the penton base, which is covered by the fiber shaft (Fig. 2a and c). Structural features, such as distinct grooves and side-chain densities of an α helix (Fig. 2d) from the top surface of the penton base, are consistent with the 4Å resolution of the map.
Fig. 2.
CryoEM map of the fiber tail and its interaction with the penton base. (a) Top view of the individual penton complex cut from the 4Å density map of Ad5 (Fig. 1a) showing the fiber (magenta) binding to penton base by its tails. The five penton-base monomers are shown in alternating colors cyan, yellow and green. (b) Side view of the fiber density segmented from (a). Five tail densities are visible due to the imposition of five-fold symmetry (left), but two positions (transparent) should be left unoccupied (right). (c) Penton complex showing five fiber-tail densities (magenta) attached to the penton-base pentamer. The fiber shaft density is removed for clarity. Lower inset: Enlargement of the black boxed region showing one fiber tail lying in the groove of two adjacent penton-base monomers. (d) Stereo view of the atomic model (sticks) of an α helix from the top surface of penton-base protein superimposed on its density (mesh). (e) Stereo view of our atomic model (sticks) of the fiber tail (aa7–19) superimposed on its densities (mesh). Three large and distinct side chains (Phe11, Tyr15 and Tyr17) are used as ‘landmarks’ for accurate registration of amino acids. (f) Stereo view of the fiber tail (sticks) inside the groove between two adjacent penton-base monomers (ribbon models, colored yellow and cyan respectively). The interactions between the fiber tail and the penton base include many hydrophobic interactions [side chains Met227, Pro228 and Tyr292 from one penton-base monomer and Leu193, Phe499 and His494 form the other monomer], two probable hydrogen bonds [dashed lines, between Glu203 (penton base) and Asn12 (fiber) and between His495 (penton base) and Tyr15 (fiber)] and one probable salt bridge [dashed lines, between Lys387 (penton base) and Asp18 (fiber)].
The extra density, lying in the groove between two adjacent penton-base monomers (Fig. 2c, lower inset), is confirmed to be one part of the fiber tail by atomic model building (Fig. 2e). Three large and distinct side chains (Phe11, Tyr15 and Tyr17) are resolved and used as ‘landmarks’ to accomplish an accurate registration of amino acids (Fig. 2e). Within this model, the structure of aa10–19 is identical to that revealed in the crystal structure of the penton base in complex with the N terminal fiber polypeptide, 18 but our structure (aa7–19) contains additional residues. The three N-terminal tails of each fiber could attach to three of the five available grooves in only two geometric configurations: either all three tails occupying three adjacent grooves or two of them sit in the neighboring grooves with the third separated by an unoccupied groove (i.e., 144° away) (right panel in Fig. 2b). The second configuration, as also suggested previously, 18 is both more energetically favorable (less total deviation from the 120° separation of the tails in its trimeric state) and more geometrically feasible to maintain a radial protrusion of the fiber from the virion as observed in cryoEM images. The interactions between fiber tail and penton-base proteins (Fig. 2f) include hydrophobic interactions (hydrophobic side chains of the two adjacent penton-base monomers colored green in Fig. 2f), probable hydrogen bonds and a salt bridge (both denoted by black lines in Fig. 2f). These in situ interactions confirm those revealed in the crystal structure of Ad2 penton base in complex with fiber polypeptides.18
Full model of the Ad5 fiber from cryoEM and comparative homology modeling
Using an integrative approach, we construct a pseudo-atomic model for the entire fiber (Fig. 3). Among the three domains of adenovirus fiber, the structures of the fiber-knob domain of Ad5 and Ad2 were confirmed by X-ray crystallography to be very similar except for some of the flexible loops on the top surface.25, 26 The Ad2 fiber-shaft domain contains a total of 21 pseudo-repeats of a 15-residue segment.20 The structure of the last four repeats together with its knob domain (Fig. 3a) was solved by X-ray crystallography as well.19 The sequence alignment of the 21 pseudo-repeat of Ad5 fiber-shaft domain showing the pattern of hydrophobic residues (colored in Fig. 3b) is similar to that of Ad2 fiber-shaft domain.19 Within the 15 residues in each repeat (positions a – o in Fig. 3b), three positions (c, e and k) correspond to hydrophobic residues with their side chains pointing towards the central three-fold axis of the fiber in the Ad2 structure.19 Amino acids at positions g and m are also hydrophobic and that at position j corresponds to conserved glycines and prolines. Insertions of residue segments beyond 15 residues in some repeats (Fig. 3b), e.g., 3nd and 19th, might introduce interruption to the shaft leading to flexibilities of the long fiber shaft. The high sequence identity (67%) and the identical pattern of their pseudo-repeats between shaft domains of Ad2 and Ad5 encouraged us to build homology model for the Ad5 fiber shaft using the crystal structure19 of the Ad2 fiber shaft as a template. In both models, the residues from position b to h in every repeat form an extended β-strand which runs parallel to the fiber axis, and the residues from position k to n organize into another β-strand which runs anti-parallel to the shaft axis. We built a pseudo-atomic model of the entire Ad5 fiber (Fig. 3c) by merging the fiber tail structures from our cryoEM density, the homology model of the shaft and the crystal structure of Ad5 fiber knob25 (see the Materials and Methods).
Fig. 3.
Fiber model. (a) Ribbon model of the fiber protein (aa319–582) of Ad2 comprises the knob domain and four 15-residue pseudo-repeats in the shaft domain.17 (b) Sequence alignment and secondary structure prediction for the fiber shaft domain of Ad5. The symbols and colors follow Figure 2 in ref.17: the italic types (a–o) indicate the 15-repeat positions; β indicates β-strands; The conserved glycines or prolines in the repeats, residues involved in the hydrophobic and residues possible forming the peripheral hydrophobic patches are filled by purple, yellow and green, respectively. (c) Full space-filling model of Ad5 fiber comprising a global knob, a long shaft and three short N-terminal tails. Three subunits are colored by red, green and blue, respectively.
Interpreting the fiber density in the cryoEM reconstruction by simulation and fitting
Having obtained a pseudo-atomic model of the fiber, we attempted to simulate the effect of imposing 5-fold symmetry on our trimeric fiber model (Fig. 4a) and compared the symmetrized densities with our cryoEM map. As shown in Figure 4a, all the structural features of the tail are preserved after imposing 5-fold symmetry around the fiber axis although the three tails in our fiber model now become five (Fig. 4a, right panel). This is because the three tails are related by 72° or 144° rotations. The symmetrization also made the density value in the tail to become weaker, consistent with the weaker density of the fiber tail in our cryoEM reconstruction (Fig. 2a–b).
Fig. 4.
Interpreting the fiber density in the cryoEM reconstruction by simulation and fitting. (a) Left: top view of simulated density map of a trimeric fiber. Only three of the 21 repeats of the fiber shaft (same region as the black box in Fig. 3c) are shown. Right: Imposing five-fold symmetry leads to the appearance of five fiber tails and smearing of the fiber shaft. (b) Left: Side view of the first three 15-residue repeats of fiber-shaft model [same as (a), but its tails are removed computationally for clarity]. Right: Surface view of the top slab after being rotated by 90° to show the top view. (c) Same as (b), but after imposing five-fold symmetry. The right panel shows a top slab superimposed by the fiber density before symmetrization (color). (d) CryoEM density map (left) of fiber shaft extracted from the 4Å reconstruction, showing the layered structure (each layer is about 13Å in height, see left panel), and the gear-shaped top slab (middle panel). The right panel shows a superposition of a top slab with the copies of fiber densities before symmetrization (red, green and blue).
In contrast, 5-fold symmetrization made the fiber shaft (Fig. 4b) to become stacked gears with 10 characteristic radial spikes (Fig. 4c). The gears are stacked with a periodicity of about 13Å, which is the same as the height of one pseudo-repeat (13.2Å length).21 The first 45Å length of the fiber density corresponds to about three pseudo-repeats (Fig. 4d, left panel), and its features, including the stacked gear shape and the characteristic protrusions around the shaft (Fig. 4d) agree with those in the simulated density obtained by imposing 5-fold symmetry (Fig. 4c). Match of these structural features further supports our interpretation of the trimeric fiber and its binding to the penton base in our cryoEM density map.
Attachment mechanism of the trimeric fiber to the pentameric penton base
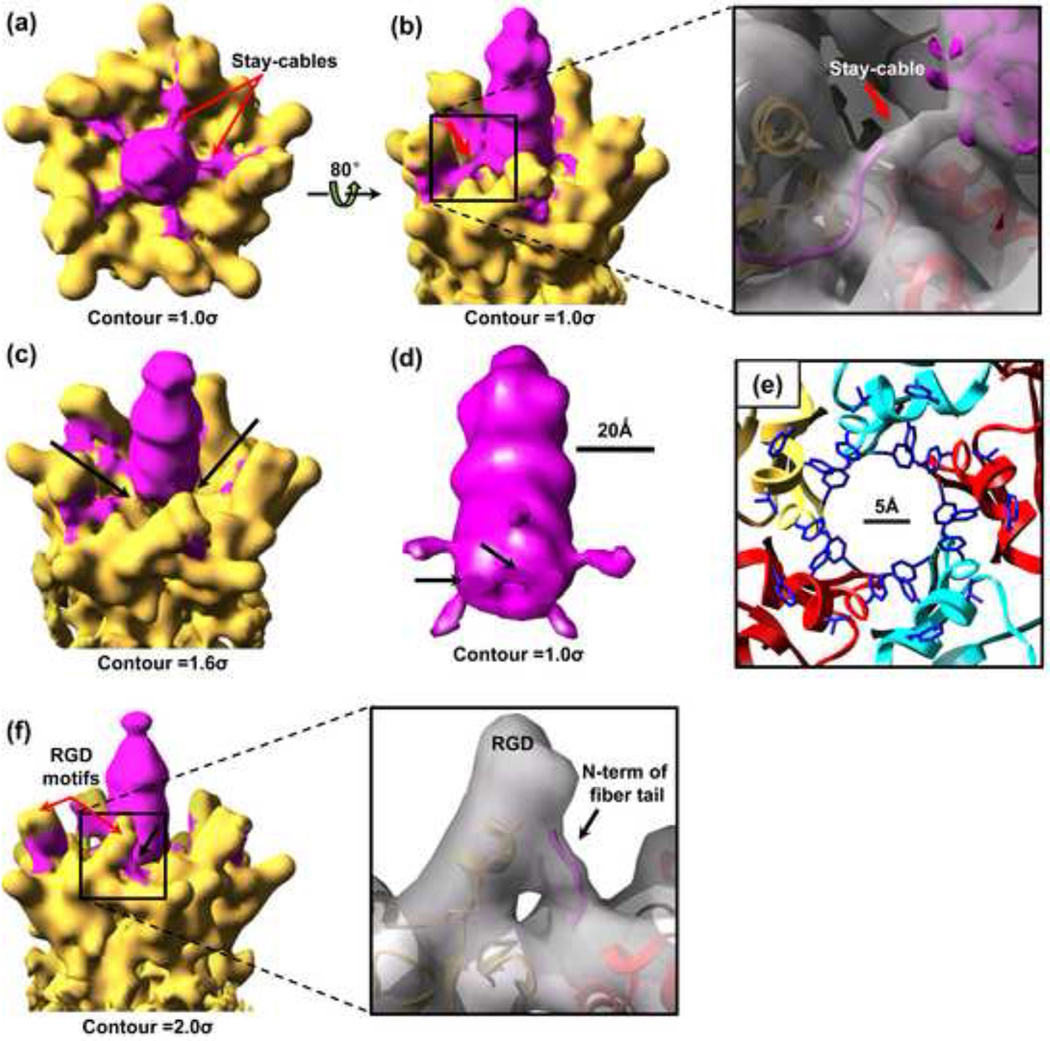
The match of the atomic models derived from the cryoEM density map (Fig. 2e) of the fiber tail and those from X-ray crystallography18 encourages us to further identify other structures of the fiber and to suggests a possible mechanism of attachment of the fiber to the penton base. As shown in the top and side views of the penton complex (Fig. 5a,b; Supplementary Movie 3) cut from the 10Å resolution structure (Fig. 1b), five cable-like densities (‘stay-cables’ in Fig. 5a,b) of about 10Å length surround the fiber shaft and connect it to the penton base. Fitting the atomic models of the penton-base monomers (yellow and red ribbon models in the inset of Fig. 5b) and fiber tail (magenta) to the 10Å cryoEM density map (semitransparent) (Fig. 5b, inset) indicates that these ‘stay-cables’ are part of the fiber tails extending further from the last resolved amino acid residue 19 of the fiber tail (Fig. 2e). Filtering the map to 10Å resolution has made these ‘stay-cables’ to become continuously visible connecting the fiber tail to the fiber shaft. These connecting densities are discontinuous in our high-resolution structure (Fig. 2b), indicating that they are not related by an exact 72° rotation due to the symmetry mismatch between fiber and penton base.
Fig. 5.
Attachment of the fiber to the penton base revealed at different contour levels. (a–b) Top (a) and side view (b) of the penton complex cut form the whole 10Å map (Fig. 1b), showing the five cable-like densities (‘stay-cables’) connecting the fiber shaft (magenta) to the penton base (yellow). Inset in (b): enlargement of the black box region, showing the fitting of the ribbon models of two penton-base monomers (yellow and red) and a fiber tail (magenta) to the 10Å map (semi-transparent). Also fitting the density map of fiber shaft (magenta) extracted from the 4Å reconstruction. (c) Same as (b), but at different contour level, showing the interactions (black arrows) between the bottom of the fiber shaft and the penton base. (d) Density map of the fiber extracted from (b), showing the ‘stay-cables’ (protrusions) and sites (black arrows) of interaction with the penton base. (e) Ribbon models (same region as the red box in Fig. 2c) showing a hydrophobic ring at the rim of the central pore on the top surface of penton base. Hydrophobic side chains are colored blue. (f) Side view of the penton complex and the enlargement region (inset) showing the fiber tail interacts with the domain of penton base containing an RGD motif.
In addition to the connections made by the ‘stay-cables’, the fiber also interacts with the penton base at the bottom of the fiber shaft around the rim of the central pore on the top surface of the penton base (black arrows in Fig. 5c). These interactions are about 10Å distance from the connection sites (protrusion) between the ‘stay-cables’ and the fiber shaft (Fig. 5d). This rim is formed entirely by hydrophobic amino acids (hydrophobic side chains colored blue in Fig. 5e, including Phe475, tyr476 and Phr489, Tyr482 and Leu485 from every penton-base monomer), suggesting that the interactions between the bottom of the fiber shaft and the penton base are likely to be hydrophobic.
A third type of interaction is between the N terminus of the fiber tail and the domain of the penton base containing an Arginine-Glycine-Aspartic acid (RGD) motif, a site of integrin binding.27, 28 This interaction is consistent with that suggested from an 18 Å resolution cryoEM structure of the T=1 dodecahedral penton particle assembled by the penton-base protein and fiber protein.17 The distal end of this domain is disordered in the crystal structure (Fig. 5f, inset). As discussed above, each fiber tail interacts with two penton base monomers (Fig. 2c). Fitting the atomic models of penton-base monomers and the fiber tail to the 10Å cryoEM density map shows that the N-terminus (black arrow in Fig. 5f) of the fiber tail interacts with the domain containing the RGD motif of the penton-base protein.
Discussion
In this study, we show the in situ high-resolution structure of Ad5 fiber and how this trimeric fiber attaches to the pentameric penton base. The bottom of the fiber shaft binds to a hydrophobic ring (blue ring in Fig. 6a) around the central pore on the top surface of the penton base. This binding is further secured by three ‘stay-cables’ (fiber tails) located above the hydrophobic ring (schematic Fig. 6a,b), thus overcoming a symmetry mismatch and forming a meta-stable cable-stayed trimeric fiber on top of the penton base.
Fig. 6.
Schematic illustrations of the attachment of the trimeric fiber (magenta) to the pentameric penton base (black). (a) Top view showing the trimeric fiber attaches to the pentameric penton base at a hydrophobic ring (blue) and three fiber tails (stay-cables). The three sides (a–c) of the magenta triangle denote the three subunits of each fiber. (b) Side view of (a), showing the locations of the hydrophobic interaction (blue), ‘stay-cable’/tail, and the flexible fiber shaft.
Fitting the cryoEM density with the model of the fiber (Fig. 4) suggests a possible arrangement for the trimeric fiber and the pentameric penton base as depicted in Fig. 6a, where the three sides (a, b and c) of the magenta triangle denote the three subunits of a trimeric fiber. The first subunit (a) crosses two penton-base monomers symmetrically, and the other two subunits (b and c) cross three penton-base monomers, respectively (schematic Fig. 6a). The hydrophobic interactions site between the bottom of the fiber shaft and the penton base would allow relatively free rotation of the shaft around the hydrophobic ring on the top surface of the penton base, thus permitting symmetry mismatch between the trimeric fiber and the pentameric penton base. The extended N termini of the ‘stay-cables’ then bind to the grooves between two adjacent penton-base monomers and extend to the RGD motif of penton-base protein to provide the necessary restraints to secure the attachment of the fiber (schematic Fig. 6b). Such attachment mechanism for the long fibers means that the fibers can be quite flexible and can readily dissociate from the virion, a likely requirement for intra-cellular virion transport across the microtubules to reach nuclear pore during infection. Therefore, alterations to these interactions sites can be targeted to change adenovirus uptake and intra cellular transport for vaccine and gene therapy applications.
Also depicted in Fig. 6 is the interaction between the N-terminus of the fiber tail and the domain of penton base containing an RGD motif. This interaction can explain a previous observation where the conformation of the RGD motif changed remarkably upon fiber binding.29 Since the RGD motif is related to binding of integrin on host cell surface, a link between the fiber tail and the RGD motif may provide a means to signal the dissociation of the fiber upon sensing binding at and entry into a host cell.
Materials and Methods
Sample preparation, CryoEM Imaging and data processing
The details of Ad5 sample preparation, cryoEM imaging and data processing were described previously.11 The 3.6Å resolution map of Ad5 was obtained from images recorded by an FEI Titan Krios cryo-electron microscope and processed with the IMIRS package, 30 which is enhanced by icosahedral symmetry-adapted functions for 3D reconstruction31 and by the incorporation of astigmatism in the CTF correction in both orientation/center refinement and 3D reconstruction steps.
Due to the large particle radius and the symmetry mismatch between trimeric fiber and the pentameric penton base, the fiber’s density becomes discontinuous in our 3.6Å structure. In order to reveal the fiber feature and interactions, we filtered the 3.6Å 3D map to 4Å (Fig. 1a) and 10Å (Fig. 1b) resolutions using the proc3d routine in EMAN.32 Visualization of the 3D reconstructions and segmented densities of individual molecular components and fitting of the atomic models to cryoEM density maps were performed with Chimera.33
Atomic model building for the fiber tail from cryoEM density
The fiber tail density was segmented out from the cryoEM density map of Ad5 virion and used to build an atomic model of the 13 amino acids (aa7–19) of the tail domain. We first used the Baton_build utility in the crystallographic programs O (ref.34) to build Cα model. Amino-acid sequence registration was accomplished based on the three clear densities of bulky side chains, which serve as ‘landmarks’ (Phe11, Tyr15 and Tyr17). Then we used Coot 35 to produce the full-atom model and improve the fit of the atomic model to the density map and geometry refinement with iterative cycles of model rebuilding until no obvious improvement could be obtained. Our final model matches the cryoEM density well and has good geometry.
Full pseudo-atomic modeling for the Ad5 fiber
By careful examining the amino acid sequence of the shaft domain of Ad5, we identified and aligned the 21 repeats each with 15 amino acid residues (Fig. 3b). This pattern of repeats is identical to that of Ad2 (ref.19), suggesting that the Ad5 fiber shaft has a structure similar to that of Ad2. Therefore, using the crystal structure of four repeats of the Ad2 fiber shaft19 as a template, we built a model of the entire Ad5 shaft with the following procedure. First, we copied the atomic model of one 15-residue repeat (three copies for aa361–376) of the fiber shaft of Ad2. Second, we transformed this repeat to the next one by shifting 13.2Å and rotating 51.4° along the shaft axis. The full 21 repeats of the fiber shaft were formed in this way. Third, the amino acids were changed according to the Ad5 fiber to form the pseudo-atomic model of the fiber shaft of Ad5. Fourth, this pseudo-atomic model of fiber shaft was merged with the atomic model of the Ad5 fiber knob from the X-ray crystal structure26 and the atomic model of the Ad5 fiber tail from our cryoEM density to form the full pseudo-atomic model of Ad5 fiber. The density model (Fig. 4a) was generated from its pseudo-atomic model using the pdb2mrc program in EMAN32.
Data deposition and accession numbers
The electron density maps and associated atomic models have been deposited with the Electron Microscopy Data Bank at the European Bioinformatics Institute under accession code EMD-5172 and with the Protein Data Bank under accession code 3IZO.
Note added in proof
Upon submission of this paper, we noted the publication of a crystal structure of the human adenovirus with a shorter fiber (~90Å), which was engineered for the purpose of crystallization.36 The crystal structure reveals two major differences from the structure presented here. First, the crystal structure shows a hole (~50 Å) at the center of the penton base that is much larger than the hole (~15 Å) in the wild type adenovirus structure described here. Second, the fiber shaft density in the crystal structure is inserted into the hole of the penton base; in contrast, there is no inserted density in our wild-type adenovirus structure. The authors of the crystal structure suggested a possible relationship of these different structures to early events in cell entry.36
Supplementary Material
Acknowledgments
We thank Ivo Atanasov and Wong H. Hui for imaging; Lei Jin, Stan Schein and Peng Ge for discussion; Dr. Sok Boon S. Koh, Chae-Ok Yun and Oh-Joon Kwon for providing the Ad-ΔE1B19/55 virus sample. This project is supported in part by grants from the National Institutes of Health (GM071940 and AI069015 to ZHZ) and (CA101904 to LW). We acknowledge the use of cryoEM facilities at the Electron Imaging Center for NanoMachines supported in part by NIH (1S10RR23057). HL thanks the National Natural Scientific Foundations of China for support (31070663 and 10874144).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Roberts DM, Nanda A, Havenga MJE, Abbink P, Lynch DM, Ewald BA, Liu J, Thorner AR, Swanson PE, Gorgone DA, Lifton MA, Lemckert AA, Holterman L, Chen B, Dilraj A, Carville A, Mansfield KG, Goudsmit J, Barouch DH. Hexon-chimaeric adenovirus serotype 5 vectors circumvent pre-existing anti-vector immunity. Nature. 2006;441:239–243. doi: 10.1038/nature04721. [DOI] [PubMed] [Google Scholar]
- 2.Everts M, Curiel DT. Transductional targeting of adenoviral cancer gene therapy. Curr. Gene Ther. 2004;4:337–346. doi: 10.2174/1566523043346372. [DOI] [PubMed] [Google Scholar]
- 3.Tatsis N, Ertl HCJ. Adenoviruses as vaccine vectors. Mol. Ther. 2004;10:616–629. doi: 10.1016/j.ymthe.2004.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kalyuzhniy O, Di Paulo NC, Silvestry M, Hofherr SE, Barry MA, Stewart PL, Shayakhmetov DM. Adenovirus serotype 5 hexon is critical for virus infection of hepatocytes in vivo. Proc. Natl Acad. Sci. U S A. 2008;105:5483–5488. doi: 10.1073/pnas.0711757105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Waddington SN, McVey JH, Bhella D, Parker AL, Barker K, Atoda H, Pink R, Buckley SMK, Greig JA, Denby L, Custers J, Morita T, Francischetti IMB, Monteiro RQ, Barouch DH, Rooijen Nv, Napoli C, Havenga MJE, Nicklin SA, Baker AH. Adenovirus serotype 5 hexon mediates liver gene transfer. Cell. 2008;132:397–409. doi: 10.1016/j.cell.2008.01.016. [DOI] [PubMed] [Google Scholar]
- 6.Burnett RM. The structure of adenovirus capsid II. The packing symmetry of hexon and its implications for viral architecture. J. Mol. Biol. 1985;185:125–143. doi: 10.1016/0022-2836(85)90187-1. [DOI] [PubMed] [Google Scholar]
- 7.Berk AJ. In: Adenoviridae: The Viruses and Their Replication. in Fields Virology. Knipe DM, Howley PM, editors. Vol.II. Philadelphia, Pennsylvania: Lippincott Williams & Wilkins; 2007. [Google Scholar]
- 8.Stewart PL, Fuller SD, Burnett RM. Difference imaging of adenovirus: bridging the resolution gap between X-ray crystallography and electron microscopy. EMBO J. 1993;12:2589–2599. doi: 10.1002/j.1460-2075.1993.tb05919.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stewart PL, Burnett RM, Cyrklaff M, Fuller SD. Image reconstruction reveals the complex molecular organization of adenovirus. Cell. 1991;67:145–154. doi: 10.1016/0092-8674(91)90578-m. [DOI] [PubMed] [Google Scholar]
- 10.Saban SD, Silvestry M, Nemerow GR, Stewart PL. Visualization of α-helices in a 6 Angstrom resolution cryoelectron microscopy structure of adenovirus allows refinement of capsid protein assignments. J. Virol. 2006;80:12049–12059. doi: 10.1128/JVI.01652-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu H, Jin L, Koh SBS, Atanasov I, Schein S, Wu L, Zhou ZH. Atomic structure of human adenovirus by cryo-EM reveals interactions among protein networks. Science. 2010;329:1038–1043. doi: 10.1126/science.1187433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bergelson JM, Cunningham JA, Droguett G, Kurt-Jones EA, Krithivas A, Hong JS, Horwitz MS, Crowell RL, Finberg RW. Isolation of a common receptor for coxsackie B viruses and adenoviruses 2 and 5. Science. 1997;275:1320–1323. doi: 10.1126/science.275.5304.1320. [DOI] [PubMed] [Google Scholar]
- 13.Gaggar A, Shayakhmetov DM, Lieber A. CD46 is a cellular receptor for group B adenoviruses. Nat. Med. 2003;9:1408–1412. doi: 10.1038/nm952. [DOI] [PubMed] [Google Scholar]
- 14.Nemerow GR, Pache L, Reddy V, Stewart PL. Insights into adenovirus host cell interactions from structural studies. Virology. 2009;384:380–388. doi: 10.1016/j.virol.2008.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Saban SD, Nepomuceno RR, Gritton LD, Nemerow GR, Stewart PL. CryoEM structure at 9 Å resolution of an adenovirus vector targeted to hematopoietic cells. J Mol. Biol. 2005;349:526–537. doi: 10.1016/j.jmb.2005.04.034. [DOI] [PubMed] [Google Scholar]
- 16.Chiu CY, Wu E, Brown SL, Seggern DJV, Nemrow GR, Stewart PL. Structural analysis of a fiber-pseudotyped adenovirus with ocular tropism suggests differential modes of cell receptor interactions. J Virol. 2001;75:5375–5380. doi: 10.1128/JVI.75.11.5375-5380.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fuschiotti P, Schoehn G, Fender P, Fabry CMS, Hewat EA, Chroboczek J, Ruigrok RWH, Conway JF. Structure of the dodecahedral penton particle from human adenovirus type 3. J Mol. Biol. 2006;356:510–520. doi: 10.1016/j.jmb.2005.11.048. [DOI] [PubMed] [Google Scholar]
- 18.Zubieta C, Schoehn G, Chroboczek J, Cusack S. The structure of the human adenovirus 2 penton. Mol. Cell. 2005;17:121–135. doi: 10.1016/j.molcel.2004.11.041. [DOI] [PubMed] [Google Scholar]
- 19.Van Raaij MJ, Mitraki A, Lavigne G, Cusack S. A triple β-spiral in the adenovirus fibre shaft reveals a new structural motif for a fibrous protein. Nature. 1999;401:935–938. doi: 10.1038/44880. [DOI] [PubMed] [Google Scholar]
- 20.Green NM, Wrigley NG, Russell WC, Martin SR, McLachlan AD. Evidence for a repeating cross-β sheet structure in the adenovirus fibre. EMBO J. 1983;2:1357–1365. doi: 10.1002/j.1460-2075.1983.tb01592.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ruigrok RWH, Barge A, Albiges-Rizo C, Dayan S. Structure of adenovirus fibre II. morphology of single fibres. J. Mol. Biol. 1990;215:589–596. doi: 10.1016/S0022-2836(05)80170-6. [DOI] [PubMed] [Google Scholar]
- 22.Wu E, Pache L, Seggern DJV, Mullen T-M, Mikyas Y, Stewart PL, Nemerow GR. Flexibility of the adenovirus fiber is required for efficient receptor interaction. J Virol. 2003;77:7225–7235. doi: 10.1128/JVI.77.13.7225-7235.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Seki T, Dmitriev I, Kashentseva E, Takayama K, Rots M, Suzuki K, Curiel DT. Artificial extension of the adenovirus fiber shaft inhibits infectivity in coxsackievirus and adenovirus receptor-positive cell lines. J Virol. 2002;76:1100–1108. doi: 10.1128/JVI.76.3.1100-1108.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fabry CM, Rosa-Calatrava M, Conway JF, Zubieta C, Cusach S, Ruigrok RW, Schoehn G. A quasi-atomic model of human adenovirus type 5 capsid. EMBO J. 2005;24:1645–1654. doi: 10.1038/sj.emboj.7600653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Van Raaij MJ, Louis N, Chroboczek J, Cusack S. Structure of the human adenovirus serotype 2 fiber head domain at 1.5Å resolution. Virology. 1999;262:333–343. doi: 10.1006/viro.1999.9849. [DOI] [PubMed] [Google Scholar]
- 26.Xia D, Henry LJ, Gerard RD, Deisenhofer J. Crystal structure of the receptor-binding domain of adenovirus type 5 fiber protein at 1.7Å resolution. Structure. 1994;2:1259–1270. doi: 10.1016/s0969-2126(94)00126-x. [DOI] [PubMed] [Google Scholar]
- 27.Wickham TJ, Mathias P, Cheresh DA, Nemerow GR. Integrins αvβ3 and αvβ5 promote adenovirus internalization but not virus attachment. Cell. 1993;73:309–319. doi: 10.1016/0092-8674(93)90231-e. [DOI] [PubMed] [Google Scholar]
- 28.Mathias P, Wickham T, Moore M, Nemerow G. Multiple adenovirus serotypes use αv integrins for infection. J Virol. 1994;68:6811–6814. doi: 10.1128/jvi.68.10.6811-6814.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schoehn G, Fender P, Chroboczek J, Hewat EA. Adenovirus 3 penton dodecahedron exhibits structural changes of the base on fibre binding. EMBO J. 1996;15:6841–6846. [PMC free article] [PubMed] [Google Scholar]
- 30.Liang Y, Ke EY, Zhou ZH. IMIRS: a high-resolution 3D reconstruction package integrated with a relational image database. J. Struct. Biol. 2002;137:292–304. doi: 10.1016/s1047-8477(02)00014-x. [DOI] [PubMed] [Google Scholar]
- 31.Liu H, Cheng L, Zeng S, Cai C, Zhou ZH, Yang Q. Symmetry-adapted spherical harmonics method for high-resolution 3D single-particle reconstructions. J. Struct. Biol. 2008;161:64–73. doi: 10.1016/j.jsb.2007.09.016. [DOI] [PubMed] [Google Scholar]
- 32.Ludtke SJ, Baldwin PR, Chiu W. EMAN: Semiautomated software for high-resolution single particle-reconstructions. J. Struct. Biol. 1999;128:82–97. doi: 10.1006/jsbi.1999.4174. [DOI] [PubMed] [Google Scholar]
- 33.Pettersen EF, Goddard TD, Huang CC, Couch GS, Greenblatt DM, Meng EC, Ferrin TE. UCSF Chimera--a visualization system for exploratory research and analysis. J. Comput. Chem. 2004;25:1605–1612. doi: 10.1002/jcc.20084. [DOI] [PubMed] [Google Scholar]
- 34.Jones TA, Zou JY, Cowan SW, Kjeldgaard M. Improved methods for building protein models in electron density maps and the location of errors in these models. Acta Cryst. 1991;A47:110–119. doi: 10.1107/s0108767390010224. [DOI] [PubMed] [Google Scholar]
- 35.Emsley P, Cowtan K. Coot: model-building tools for molecular graphics. Acta Cryst. 2004;D60:2126–2132. doi: 10.1107/S0907444904019158. [DOI] [PubMed] [Google Scholar]
- 36.Reddy VS, Natchiar SK, Stewart PL, Nemerow1 GR. Crystal structure of human adenovirus at 3.5 Å resolution. Science. 2010;329:1071–1075. doi: 10.1126/science.1187292. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.