Abstract
Human leukaemic HL-60 cells are widely used for studying interactions involving adhesion molecules [e.g. P-selectin and PSGL-1 (P-selectin glycoprotein ligand-1)] since their rolling behaviour has been shown to mimic the dynamics of leucocyte rolling in vitro. HL-60 cells are neutrophilic promyelocytes that can undergo granulocytic differentiation upon exposure to compounds such as DMSO (dimethylsulfoxide). Using a parallel plate flow chamber functionalized with recombinant P-selectin–Fc chimaera, undifferentiated and DMSO-induced (48, 72 and 96 h) HL-60 cells were assayed for rolling behaviour. We found that depending on P-selectin incubation concentration, undifferentiated cells incurred up to a 6-fold increase in rolling velocity while subjected to an approximately 10-fold increase in biologically relevant shear stress. HL-60 cells exposed to DMSO for up to 72 h incurred up to a 3-fold increase in rolling velocity over the same shear stress range. Significantly, cells exposed for up to 96 h incurred up to a 9-fold decrease in rolling velocity, compared with undifferentiated HL-60 cells. Although cell surface and nuclear morphological changes were evident upon exposure to DMSO, flow cytometric analysis revealed that PSGL-1 expression was unchanged, irrespective of treatment duration. The results suggest that DMSO-treated HL-60 cells may be problematic as a substitute for neutrophils for trafficking studies during advanced stages of the LAC (leucocyte adhesion cascade). We suggest that remodelling of the cell surface during differentiation may affect rolling behaviour and that DMSO-treated HL-60 cells would behave differently from the normal leucocytes during inflammatory response in vivo.
Keywords: DMSO, HL-60 cell rolling interaction, leucocyte adhesion cascade (LAC), parallel plate flow chamber
Abbreviations: BN, banded nuclei; CD11b, cluster of differentiation molecule 11b; DMF, dimethylformamide; FBS, fetal bovine serum; FOV, field of view; GN, cytoplasmic granules; ICAM, intercellular adhesion molecule; LAC, leucocyte adhesion cascade; LFA-1, lymphocyte function-associated antigen-1; Mac-1, macrophage-1 antigen; NT, no treatment; PBA, PBS containing 1% FBS and 0.02% sodium azide; PE, phycoethryin; PSGL-1, P-selectin glycoprotein ligand-1
INTRODUCTION
In the microcirculation, the innate immune response is often described as a cascade of events starting with the recruitment or trafficking of leucocytes from the circulatory pool to the area of injury. The immune response begins with the release of inflammatory cytokines and other signalling molecules by host cells, which activates vascular endothelial cells and induces up-regulation of P- and E-selectins [1] in order to attract freestream leucocytes. Initial adhesive contact also depends on PSGL-1 (P-selectin glycoprotein ligand-1) and L-selectin, which are found on the tips of leucocyte microvilli [2]. Eventually, the activated leucocyte transmigrates out of the blood vessel, facilitated by integrins, in order to carry out its immune function.
For leucocytes in the near-wall environment, cell rolling is enabled by the formation and rapid dissociation of receptor–ligand bonds between P-selectin and PSGL-1 [3,4]. Rolling is considered when the translational velocity is less than or equal to 50% of the associated stream velocity at that point [5]. It has been demonstrated in vitro for leucocytes on artificial lipid bilayers containing purified P-selectin [6], for leucocytes on P-selectin–Fc-coated surfaces [7], for cell-free rolling assays with ligand-coated microspheres [8,9], and in vivo in exposed rat mesentery [10]. Studies have shown that deficiency in P- and E-selectins can eliminate cellular inflammatory response even when integrins and their respective ligands are available [11], demonstrating that selectin-mediated rolling is critical for inflammatory response [12].
The HL-60 leukaemic cell line, derived from a female patient with acute promyelocytic leukaemia [13], is multipotent and can be induced to terminally differentiate towards cell types with distinct lineages. Treatment with DMSO (dimethylsulfoxide) and other polar compounds, and butyric acid have been shown to induce differentiation into myelocytes, metamyelocytes and banded and segmented neutrophils [13,14]. Several additional studies have shown that incubation with all-trans-retinoic acid in growth media will induce differentiation to functionally mature granulocytes [15,16]. One important surface marker that can be up-regulated is CD11b, the αM protein subunit for the β2-integrin αMβ2 or Mac-1 (macrophage-1 antigen) [16,17]. Although mature myeloid cells are positive for CD11b [18], undifferentiated HL-60 cells do not express or only weakly express CD11b surface antigen or mRNA [17]. β2-Integrins, including LFA-1 (lymphocyte function-associated antigen-1; integrin αLβ2) and p195,50 (integrin αXβ2), play key roles in the recruitment of leucocytes to sites of tissue injury [19] and therefore their expression and function are important in leucocyte trafficking. Hence, it is important whether or not differentiated HL-60s mimic leucocyte rolling behaviour by binding tightly to ligand-coated surfaces representing an inflamed endothelium for advanced stages of the LAC (leucocyte adhesion cascade).
The ultrastructure of the HL-60 cell is similar to that of the early promyelocyte in size and shape of nucleus, structure of chromatin and the presence of well-developed Golgi apparatus [20]. The constitutive expression of PSGL-1 [4,21] by HL-60 cells makes them an excellent candidate cell line for studies involving the initial stages of leucocyte recruitment and multiple researchers [4,7,22] have used these cells as surrogates for neutrophils in order to investigate the rolling of leucocytes over models of inflamed endothelium, or to investigate cell-sorting techniques via receptor-mediated adhesion [23,24]. In the present study, we investigated the rolling behaviour of DMSO-exposed HL-60 cells in order to assess whether the treated HL-60 cells would reproduce selectin-mediated rolling adhesion events. Using a parallel plate flow chamber, we found that depending on P-selectin incubation concentration untreated cells incurred up to a 6-fold increase in rolling velocity while subjected to an approximately 10-fold increase in biologically relevant shear stress. HL-60 cells exposed to DMSO for up to 72 h incurred up to a 3-fold increase in rolling velocity over the same shear stress range. Significantly, cells exposed for up to 96 h incurred up to a 9-fold decrease in rolling velocity, compared with undifferentiated HL-60 cells. Flow cytometry data indicated that PSGL-1 expression was unchanged, irrespective of treatment duration. The results suggest that DMSO-treated HL-60 cells may be problematic as a substitute for neutrophils for trafficking studies during advanced stages of the LAC.
MATERIALS AND METHODS
Cell lines
HL-60 cells were donated by Dr Michael King (Department of Biomedical Engineering, Cornell University, Ithaca, NY, U.S.A.) and maintained in suspension culture in RPMI 1640 medium supplemented with 10% heat-inactivated FBS (fetal bovine serum), 2 mM L-glutamine and antibiotics in a humidified 5% CO2 incubator. Viability was quantified by Trypan Blue dye exclusion. To induce differentiation, viable log-phase cells were resuspended in complete RPMI 1640 growth medium containing 1.25% DMSO and incubated for 24–96 h. To prepare cells for rolling, cultures were centrifuged and washed two times in PBS. Cells were resuspended at a density of 106 cells/ml in HBSS (Hanks' balanced salt solution; Invitrogen) supplemented with 2 mM CaCO3, 10 mM Hepes and 0.5% BSA (Sigma).
Cell rolling assay
rhP-selectin (recombinant human P-selectin)–Fc chimaera (R&D Systems) was dissolved in PBS to yield a final concentration of 100 μg/ml. Two different concentrations were used in these experiments and were prepared on the morning of an experiment from stock solution by diluting in PBS. To functionalize the flow chamber, a rectangular double-well flexiPERM gasket (Sigma) with the interior wall removed was placed on the lower surface of a 35-mm polystyrene culture dish (Corning). A portion (300 μl) of dilute P-selectin concentration was placed inside the well and the plate was gently rocked for 2 h (Orbit). The surface was washed three times with 300 μl of PBS and blocked for non-specific adhesion with 2% BSA in PBS for 1 h. The flow chamber (2.5 mm wide×0.127 mm thick, GlycoTech) was assembled on top of the dish and the device was mounted on an Olympus IX71 inverted microscope (Olympus America Inc.). A ×10 objective (UPlanFL ×10/0.30) (×100 total magnification) was used to observe cell rolling events. Cells were perfused through the flow chamber at a specific volume flow rate using a syringe pump (KD Systems). The flow rate was adjusted in order to reproduce wall shear stresses in the range 0.5–5 dyn/cm2, which have been measured in postcapillary venules [25]. In calculations, we assumed a fluid viscosity of 1 cP (centipoises).
Data acquisition and cell tracking
Cell rolling was monitored using a microscope-linked Hitachi CCD camera KP-M1AN (Hitachi) and recorded on a DVD recorder (Sony DVO-1000MD). Cell-rolling videos (recorded at 30 frames/s) were converted into AVI-formatted files (WinXDVD Ripper) and individual tracks were converted into a series of JPEG images using SC Video Decompiler. Tracking of cells was accomplished using ImageJ software (ImageJ 1.44) and individual cells were generally tracked for 30 s, although some were only tracked for 10–20 s. Rolling velocity was computed by converting pixel data into coordinate data and dividing by the corresponding time interval. Rolling flux was computed by counting the number of cells rolling across the FOV (field of view) for a 1-min period. Specifically, we simply averaged counts of rolling cells taken at the beginning and end of a 1-min period and divided by the FOV.
Flow cytometry
HL-60 cells (106 cells/ml; treated ±1.25% DMSO) were washed in freshly made PBA (PBS containing 1% FBS and 0.02% sodium azide) and harvested by centrifugation at 300 g for 5 min. Resuspended cells were blocked in 4 μl of 1:1 mixture of Fc-block (cat. no. 553142; BD Pharmingen) and PBA for 10 min on ice. Cells were stained with 1:50 dilution of PE (phycoerythrin)-conjugated anti-PSGL-1 antibody (cat. no. 556055; BD Pharmingen) for 60 min on ice (protected from light). Control cells were pre-incubated with 1:50 dilution of unconjugated anti-PSGL-1 (cat. no. 556053; BD Pharmingen) for 60 min (after blocking) before being stained with PE-conjugated anti-PSGL-1 antibody. After staining, volume was increased to 1 ml with PBA and cells were centrifuged at 300 g for 5 min and resuspended in 200 μl PBS. Cells were run on a BD Flow Cytometry 12-colour LSRII (BD Biosciences) with at least 10000 events routinely analysed for each sample. Data analysis was performed using FlowJo (Tree Star).
Cell staining
Suspensions of 4×105 HL-60 cells were cytocentrifuged on to glass slides for 10 min at 800 rev./min. Cells were fixed and stained according to the ‘Diff-Quik®’ staining protocol [26]. Cells were imaged using a Zeiss Axiovert 40 CFL inverted microscope and digital camera.
Data analysis
All results are presented as means±S.E.M., and represent the average of a minimum of 39 rolling interactions (n≥39, unless otherwise indicated) from two separate independent experiments. Differences among groups were statistically analysed using an unpaired two-tailed Student's t test; P<0.05 was considered statistically significant.
RESULTS
Undifferentiated HL-60 cells interact strongly with P-selectin–Fc surfaces
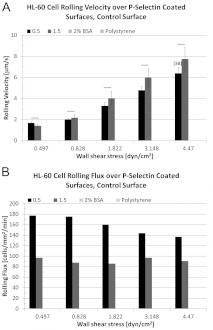
The acute promyelocytic leukaemia HL-60 cell line expresses the adhesion molecule PSGL-1 and HL-60 cells roll on P-selectin coated surfaces [27–29]. Using the parallel plate flow chamber, we examined the rolling interactions of HL-60 cells for two different incubation concentrations of P-selectin: 0.5 and 1.5 μg/ml. Cells were initially pulled into the flow chamber and the pump was turned off for ~1 min. Some cells settling on to the substrate were observed and those cells appeared buoyant. Once the flow rate was selected and flow initiated, cells were observed to form an immediate attachment to immobilized P-selectin cell adhesion molecules. Cell rolling followed immediately thereafter in response to hydrodynamic shear forces and short-lived tether bonds. In Figure 1, rolling velocity results are presented as means±S.E.M. and represent the average of a minimum of 39 rolling interactions (n≥39, unless otherwise indicated) from two separate independent experiments. For 0.5 μg/ml P-selectin concentration and over the course of an approximate 10-fold increase in wall shear stress, rolling velocity is observed to increase approximately 4-fold (Figure 1A). The same trend is evident at the higher concentration, producing a 5–6-fold increase. A control surface treated with blocking buffer (2% BSA) but no selectin did not support rolling. HL-60 cells are also negative for rolling on plain polystyrene surfaces (Figure 1).
Figure 1. Rolling interactions of undifferentiated HL-60 cells over P-selectin surfaces; incubation concentration in μg/ml.
Rolling velocity data are presented as means±S.E.M. and represent the average of a minimum of 39 rolling interactions (n≥39, unless otherwise indicated) from two separate independent experiments. (A) For 0.5 μg/ml and over an approximate 10-fold increase in wall shear stress, rolling velocity is seen to increase approximately 4-fold. This same trend is evident for 1.5 μg/ml P-selectin, producing a 5–6-fold increase. Differences in rolling velocity for the conditions tested were not significant (P>0.05). (B) Cell rolling flux is reasonably stable at the higher concentration even as the absolute numbers of rolling cells is decreased. Control surface consisting of dish treated with blocking buffer (2% BSA) but no selectin did not support rolling. HL-60 cells are also negative for rolling on plain polystyrene surfaces.
The cell rolling flux is reasonably stable at both incubation concentrations over the shear stress range tested, even as the absolute numbers of rolling cells are lower for the higher incubation concentration (Figure 1B). The flux data represent the simple average of two independent rolling experiments. While examining the video of rolling interactions, there was no visual evidence of a discernible increase in the number of rolling cells suddenly released from the surface at the higher incubation concentration.
We also examined the effect of DMSO (e.g. 48, 72 and 96 h exposure) in order to ascertain its effect, if any, on HL-60 rolling interactions. DMSO is one of several small molecule agents known to induce granulocytic differentiation of HL-60 cells [13,30,31] and up-regulate CD11b expression.
Morphological changes induced by exposure to DMSO
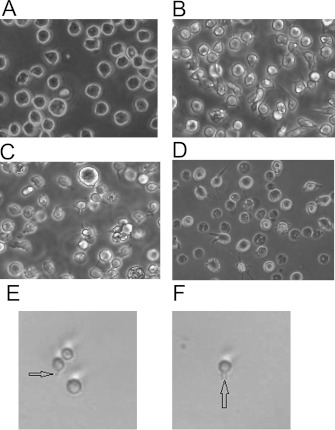
HL-60 cells were imaged after NT (no treatment) or treatment with 1.25% DMSO in growth media for 48, 72 and 96 h. The viability of undifferentiated HL-60 cells and cells exposed to DMSO for up to 96 h averaged 85–95% using Trypan Blue exclusion assays. Figure 2 shows that morphological changes can be observed in cells exposed to DMSO. Cells that were not treated were consistent in shape with a spherical appearance; Figure 2(A). Morphological changes, evident using phase-contrast imaging, are apparent upon DMSO exposure and include the formation of membrane extensions resulting in the loss of uniformity of shape (Figures 2B–2D). Thin membrane filaments extending from the surface are also clearly visible on many of the cells exposed to DMSO (Figure 2E, indicated with arrow). Figure 2(F) illustrates that multiple filaments can extend from the surface. It should be noted that we have observed these structures on untreated cells as well (results not shown). These surface features are similar in appearance to those found in HL-60 cells exposed to DMF (dimethylformamide) for 96 h [32].
Figure 2. Morphological changes to HL-60 cells after exposure to DMSO.
With NT, HL-60 cells are relatively circular and uniform in shape and size (A). After exposure to 1.25% DMSO in growth media for 48, 72 and 96 h, HL-60 cells exhibit membrane projections, changes in shape and loss of uniformity (B–D) respectively. In addition, HL-60 cells exposed to DMSO display thin-filament-membrane extensions (E, F). Cell viability averaged between 85 and 95% for all exposure periods (results not shown).
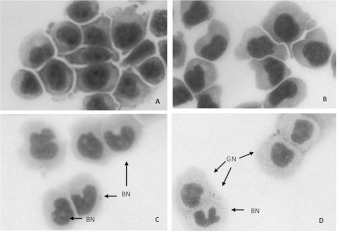
In order to image the cytosolic and nuclear compartments, HL-60 cells at various times of DMSO exposure were cytocentrifuged, fixed and stained with ‘Diff-Quik®’ stain. Although gross cellular morphology is not preserved after cytocentrifugation, DMSO-induced HL-60 cells display nuclear and cytoplasmic changes; Figure 3. NT HL-60 cells have large, dark-staining, uniform nuclear compartments; Figure 3(A). After 48 h, alteration in nuclear shape is evident; Figure 3(B). At 72 and 96 h time-points, there are cells with obvious lobular nuclei or BN (banded nuclei) and GN (cytoplasmic granules), both hallmarks of granulocyte differentiation [14,33]; Figures 3(C) and 3(D).
Figure 3. HL-60 cells exposed to DMSO display nuclear and cytoplasmic changes.
Undifferentiated HL-60 cells (A) have large, dark-staining, uniform nuclear compartments. After 48 h of 1.25% DMSO treatment (B) nuclear shape is altered with BN and GN becoming more apparent after 72 h (C) and 96 h (D) of treatment.
HL-60 exposure to DMSO does not affect PSGL-1 expression
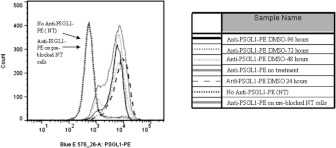
PE-conjugated anti-PSGL-1 antibody was used to stain PSGL-1 surface expression for HL-60 cells that were NT (not treated) or treated with 1.25% DMSO for 24, 48, 72 and 96 h. These samples were run on a BD Flow Cytometry 12-colour LSRII flow cytometer. All conditions showed very similar profiles for PSGL-1 surface expression (Figure 4). Populations of NT cells that were unstained or cells that were pre-incubated with unconjugated anti-PSGL-1 antibody were indistinguishable.
Figure 4. Using flow cytometric analysis, PSGL-1 expression on HL-60 cells does not significantly change during time-course treatment with 1.25% DMSO.
PE-conjugated anti-PSGL-1 antibody was used to stain PSGL-1 surface expression from HL-60 cells that were NT or treated with 1.25% DMSO for 24, 48, 72 and 96 h. All conditions showed very similar profiles for PSGL-1 surface expression. Populations of NT cells that were unstained or cells that were pre-incubated with unconjugated anti-PSGL-1 antibody were indistinguishable.
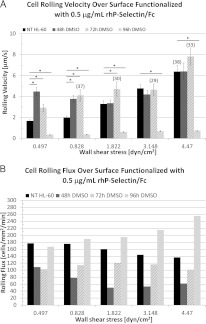
Exposure to DMSO can significantly affect rolling interactions
Figure 5(A) illustrates the effect of DMSO exposure on rolling velocity. Irrespective of the exposure period, induced cells generally increase their rolling velocity as a function of fluid shear stress, similar to NT HL-60 cells. However, cells exposed to DMSO for 48 and 72 h roll significantly faster (P<0.05) than NT cells at the lower shear stresses. This difference in rolling velocity as a function of exposure duration becomes less pronounced as shear stress increases, and rolling velocities are largely similar at the higher shear stresses, independent of exposure duration. By contrast, cells exposed to DMSO for 96 h roll significantly slower than NT cells, even as they increase their rolling velocity with shear stress.
Figure 5. Effect of DMSO exposure on rolling interactions.
Rolling velocity data are presented as means±S.E.M. and represent the average of a minimum of 39 rolling interactions (n≥39, unless otherwise indicated) from two separate independent experiments (*P<0.05). (A) For cells treated up to 72 h, the rolling velocity is significantly higher (compared with NT cells), but this effect is seen to gradually diminish with increasing shear stress. For cells treated up to 96 h, rolling velocity is significantly lower, compared with NT cells. (B) Cell rolling flux is reasonably robust as a function of the exposure period. For NT cells, flux is monotonic non-increasing as a function of shear stress. For cells treated up to 96 h, flux is monotonic non-decreasing over the tested shear stress range.
Cell rolling flux (i.e. number of cells rolling across the FOV over a 1-min period) under the same conditions is illustrated in Figure 5(B). For NT HL-60 cells and cells exposed to DMSO for up to 72 h, the rolling flux is observed to remain approximately constant or decrease moderately as a function of increasing shear stress. After 96 h of exposure, cell rolling flux is observed to increase as a function of shear stress. For NT HL-60 cells, rolling flux is monotonic non-increasing as a function of shear stress, whereas for 96 h cells rolling flux is monotonic non-decreasing as a function of shear stress.
DISCUSSION
HL-60 cells express LFA-1, a member of the β2 family of leucocyte integrins [34,35]. Although expression of LFA-1 remains consistent with DMSO treatment [17,34], HL-60 cells exposed to DMSO in culture up-regulate the αM (CD11b) and αX (CD11c) subunits for Mac-1 and p150/95 respectively, suggesting that DMSO-treated HL-60 cells might interact with a model of inflamed endothelium much like neutrophils do; rolling on selectin-coated surfaces initially and then adhering to ICAM (intercellular adhesion molecule)-1, ICAM-2 or VCAM-1 (vascular cell adhesion molecule-1) surfaces upon integrin activation. This might increase the value of the cell line as a surrogate for fresh leucocytes in trafficking studies as long as rolling interactions mediated by selectins are not adversely affected.
Using the parallel plate flow chamber, we examined rolling interactions of undifferentiated HL-60 cells for two different incubation concentrations of P-selectin: 0.5 and 1.5 μg/ml. In both cases, the rolling velocities were comparable and increased uniformly with increasing shear stress (Figure 1A). The cell rolling flux exhibited some sensitivities to selectin concentration, but was only mildly responsive to increase in fluid shear stress (Figure 1B). Next, we examined the effect of various treatment periods with DMSO (48, 72 and 96 h) on rolling velocity for 0.5 μg/ml P-selectin incubation concentration and found similar trends when compared with undifferentiated cells; namely that cell-rolling velocity exhibited mostly an upward trend as a function of fluid shear stress. However, and in particular, at the lower shear stresses, significant differences in absolute value of rolling velocity were calculated as a function of the exposure period. These differences tended to disappear with increasing shear stresses. Also of note, for cells exposed to DMSO for 96 h cell-rolling velocity was significantly lower than for any other condition tested, even though surface expression of PSGl-1 remained approximately constant (Figure 4).
Upon exposure to DMSO, HL-60 cells undergo differentiation towards metamyelocytes, band and segmented neutrophils. We observed characteristic transformation of the nuclear compartment from a rounded appearance (typical of immature granulocytes) to lobular appearance (typical of mature granulocytes) that was dependent on exposure time (Figure 3). Changes in cell morphology were also observed with formation of membrane extensions (Figure 2). Similar changes to surface morphology have been captured for HL-60 cells exposed to DMF, another known terminal differentiation inducing agent [32]. Some of these features appear as soon as 24 h after treatment (results not shown) and, in addition, many cells appear to lose their uniform spherical shape. Indeed, it has been shown that DMSO-induced differentiation of HL-60 cells into mature granulocytes takes approximately 7 days and that by this time only 2% of cells remain with S+G2M DNA content [30], characteristic of proliferating cells. It seems likely, then, that remodelling of the cell surface during differentiation plays a key role in modulating the observed cell rolling behaviour, perhaps through increased cell deformability, even as PSGL-1 expression remains consistent over the course of the exposure period studied here.
In summary, we examined the rolling interactions of undifferentiated HL-60 leukaemic cells and HL-60 cells exposed to DMSO, a known terminal differentiating agent, over surfaces containing immobilized P-selectin–Fc. Undifferentiated HL-60 cells incur a 4–6-fold increase (depending on the concentration) in rolling velocity over the approximately 10-fold increase in wall shear stress examined. The numbers of rolling cells are relatively high, regardless of incubation concentration. HL-60 cells that were induced towards terminal differentiation by exposure to 1.25% DMSO in growth media for 48 and 72 h continue to roll on these surfaces in numbers reasonably comparable with undifferentiated cells. However, we have observed some significant changes to cell rolling velocity over P-selectin surfaces for cells transiting through the various stages of differentiation. After 96 h of exposure, we detected a significant decrease in cell rolling velocity, compared with undifferentiated cells, across the shear stress range tested. Treated cells became less uniform in appearance and extended membrane projections. BN and GN became more apparent after 72 and 96 h of DMSO treatment. Although important integrin protein subunits appear in conjunction with granulocytic differentiation [17,34], the specific time-course treatment with DMSO significantly alters rolling interactions with P-selectin surfaces, as well as surface morphology. Thus, for the exposure periods tested, integrin-mediated interactions of differentiated HL-60 cells may not be comparable with normal leucocytes during immune response. Future work will investigate changes in the cytoskeleton in HL-60 cells during differentiation.
AUTHOR CONTRIBUTION
David Gee performed the flow chamber assays and carried out cell-rolling data analysis. Kate Wright performed the flow cytometry, cell staining and tissue culture. Jonathan Zimmerman, Kayla Cole, Karen Soule and Michelle Ubowski contributed to maintaining the tissue culture, flow experiments, flow cytometry and data analysis.
ACKNOWLEDGEMENTS
We thank Dr Jing Wang and Dr Samir Bhagwat (University of Rochester) for their assistance with the FACS analysis.
FUNDING
This study was supported by the Office of the Vice President for Research at the Rochester Institute of Technology [grant number 15651].
References
- 1.Kansas G. S. Selectins and their ligands: current concepts and controversies. Blood. 1996;88:3259–3287. [PubMed] [Google Scholar]
- 2.Springer T. A. Traffic signals on endothelium for lymphocyte recirculation and leukocyte emigration. Annu. Rev. Physiol. 1995;57:827–872. doi: 10.1146/annurev.ph.57.030195.004143. [DOI] [PubMed] [Google Scholar]
- 3.Moore K. L., Patel K. D., Bruehl R. E., Li F., Johnson D. A., Lichenstein H. S., Cummings R. D., Bainton D. F., McEver R. P. P-selectin glycoprotein ligand-1 mediates rolling of human neutrophils on P-selectin. J. Cell Biol. 1995;128:661–671. doi: 10.1083/jcb.128.4.661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Norman K. E., Moore K. L., McEver R. P., Ley K. Leukocyte rolling in vivo is mediated by P-selectin glycoprotein ligand-1. Blood. 1995;86:4417–4421. [PubMed] [Google Scholar]
- 5.Goldman A. J., Cox R. G., Brenner H. Slow viscous motion of a sphere parallel to a plane wall. II. Couette flow. Chem. Eng. Sci. 1967;22:653–660. [Google Scholar]
- 6.Lawrence M. B., Springer T. A. Leukocytes roll on a selectin at physiologic flow rates: distinction from and prerequisite for adhesion through integrins. Cell. 1991;65:859–873. doi: 10.1016/0092-8674(91)90393-d. [DOI] [PubMed] [Google Scholar]
- 7.Lawrence M. B., Kansas G. S., Kunkel E. J., Ley K. Threshold levels of fluid shear promote leukocyte adhesion through selectins (CD62L,P,E) J. Cell Biol. 1997;136:717–727. doi: 10.1083/jcb.136.3.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brunk D. K., Goetz D. J., Hammer D. A. Sialyl Lewisx/E-selectin mediated rolling in a cell-free system. Biophys. J. 1996;71:2902–2907. doi: 10.1016/S0006-3495(96)79487-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rodgers S. D., Camphausen R. T., Hammer D. A. Sialyl Lewisx-mediated, PSGL-1-independent rolling adhesion on P-selectin. Biophys. J. 2000;79:694–706. doi: 10.1016/S0006-3495(00)76328-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ley K., Gaehtgens P., Fennie C., Singer M. S., Lasky L. A., Rosen S. D. Lectin-like cell adhesion molecule 1 mediates leukocyte rolling in mesenteric venules in vivo. Blood. 1991;77:2553–2555. [PubMed] [Google Scholar]
- 11.Bullard D., Kunkel E., Kubo H., Hicks M., Lorenzo I., Doyle N., Doerschuk C., Ley K., Beaudet A. Infectious susceptibility and severe deficiency of leukocyte rolling and recruitment in E-selectin and P-selectin double mutant mice. J. Exp. Med. 1996;18:2329–2336. doi: 10.1084/jem.183.5.2329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Arfors K. E., Lundberg C., Lindborn L., Lundberg K., Beatty P. G., Harlan J. M. A monoclonal antibody to the membrane glycoprotein complex CD18 inhibits polymorphonuclear leukocyte accumulation and plasma leakage in vivo. Blood. 1987;69:338–340. [PubMed] [Google Scholar]
- 13.Collins S. J., Gallo R. C., Gallagher R. E. Continuous growth and differentiation of human myeloid leukemic cells in suspension culture. Nature. 1977;270:347–349. doi: 10.1038/270347a0. [DOI] [PubMed] [Google Scholar]
- 14.Collins S. J., Ruscetti F. W., Gallagher R. E., Gallo R. C. Terminal differentiation of human promyelocytic leukemia cells induced by dimethyl sulfoxide and other polar compounds. Proc. Natl. Acad. Sci. U.S.A. 1978;75:2458–2462. doi: 10.1073/pnas.75.5.2458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Breitman T. R., Selonick S. E., Collins S. J. Induction of differentiation of the human promyelocytic leukemia cell line (HL-60) by retinoic acid. Proc. Natl. Acad. Sci. U.S.A. 1980;77:2936–2940. doi: 10.1073/pnas.77.5.2936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huang A. C., Hu L., Kauffman S. A., Zhang W., Shmulevich I. Using cell fate attractors to uncover transcriptional regulation of HL60 neutrophil differentiation. BMC Sys. Biol. 2009;3:20. doi: 10.1186/1752-0509-3-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Carrigan S. O., Weppler A. L., Issekutz A. C., Stadnyk A. W. Neutrophil differentiated HL-60 cells model Mac-1 (CD11b/CD18)-independent neutrophil transepithelial migration. Immunology. 2005;115:108–117. doi: 10.1111/j.1365-2567.2005.02131.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hickstein D. D., Baker D. M., Gollahon K. A., Back A. L. Identification of the promoter of the myelomonocytic leukocyte integrin CD11b. Proc. Natl. Acad. Sci. U.S.A. 1992;89:2105–2109. doi: 10.1073/pnas.89.6.2105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Anderson D. C., Springer T. A. Leukocyte adhesion deficiency: an inherited defect in the Mac-1, LFA-1, and p150, 95 glycoproteins. Annu. Rev. Med. 1987;38:175–194. doi: 10.1146/annurev.me.38.020187.001135. [DOI] [PubMed] [Google Scholar]
- 20.Bainton D. F., Farquhar M. G. Differences in enzyme content of azurophil and specific granules of polymorphonuclear leukocytes. II Cytochemistry and electron microscopy of bone marrow cells. J. Cell Biol. 1968;39:299–317. doi: 10.1083/jcb.39.2.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Snapp K. R., Ding H., Atkins K., Warnke R., Luscinskas F. W., Kansas G. S. A novel P-Selectin glycoprotein ligand-1 monoclonal antibody recognizes an epitope within the tyrosine sulfate motif of human PSGL-1 and blocks recognition of both P- and L-selectin. Blood. 1998;91:154–164. [PubMed] [Google Scholar]
- 22.Dong C., Lei X. Biomechanics of cell rolling: shear flow, cell-surface adhesion, and cell deformability. J. Biomech. 2000;33:35–43. doi: 10.1016/s0021-9290(99)00174-8. [DOI] [PubMed] [Google Scholar]
- 23.Lee C. H., Bose S., Van Vliet K. J., Karp J. M., Karnik R. Examining the lateral displacement of HL60 cells rolling on asymmetric P-selectin patterns. Langmuir. 2011;27:240–249. doi: 10.1021/la102871m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Karnik R., Hong S., Zhang H., Anderson D. G., Karp J. M., Langer R. Nanomechanical control of cell rolling in two dimensions through surface patterning of receptors. Nano Lett. 2008;8:1153–1158. doi: 10.1021/nl073322a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Heisig N. Functional analysis of the microcirculation in the exocrine pancreas. Adv. Microcirc. 1968;1:89–94. [Google Scholar]
- 26.Skipper R., DeStephano D. B. A rapid stain for Campylobacter pylori in gastrointestinal tissue sections using Diff-Quik®. J. Histotechnol. 1989;12:303–304. [Google Scholar]
- 27.Moore K. L., Stults N. L., Diaz S., Smith D. F., Cummings R. D., Varki A., McEver R. P. Identification of a specific glycoprotein ligand for P-selectin (CD62) on myeloid cells. J. Cell Biol. 1992;118:445–456. doi: 10.1083/jcb.118.2.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sako D., Chang X. J., Barone K. M., Vachino G., White H. M., Shaw G., Veldman G. M., Bean K. M., Ahern T. J., Furie B., et al. Expression cloning of a functional glycoprotein ligand for P-selectin. Cell. 1993;75:1179–1186. doi: 10.1016/0092-8674(93)90327-m. [DOI] [PubMed] [Google Scholar]
- 29.Marathe D. D., Chandrasekaran E. V., Lau J. T., Matta K. L., Neelamegham S. Systems-level studies of glycosyltransferase gene expression and enzyme activity that are associated with the selectin binding function of human leukocytes. FASEB J. 2008;22:4154–4167. doi: 10.1096/fj.07-104257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Collins J. M., Foster K. A. Differentiation of promyelocytic (HL-60) cells into mature granulocytes: mitochondrial-specific rhodamine 123 fluorescence. J. Cell Biol. 1983;96:94–99. doi: 10.1083/jcb.96.1.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Watson R. W., Rotstein O. D., Parodo J., Bitar R., Hackam D., Marshall J. C. Granulocytic differentiation of HL-60 cells results in spontaneous apoptosis mediated by increased caspase expression. FEBS Lett. 1997;412:603–609. doi: 10.1016/s0014-5793(97)00779-5. [DOI] [PubMed] [Google Scholar]
- 32.Fleck R. A., Athwal H., Bygraves J. A., Hockley D. J., Feavers I. M., Stacey G. N. Optimization of NB-4 and HL-60 differentiation for use in opsonophagocytosis assays. In Vitro Cell. Dev. Biol.-Animal. 2003;39:235–242. doi: 10.1290/1543-706X(2003)039<0235:OONAHD>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 33.Janeway C. A., Jr, Travers P., Walport M., Shlomchik M. J. Immunobiology: The Immune System in Health and Disease. 5th edn. New York: Garland Science; 2001. [Google Scholar]
- 34.Burchard G. D., Moslein C., Brattig N. W. Adherence between Entamoeba histolytica trophozoites and undifferentiated or DMSO-induced HL-60 cells. Parasitol. Res. 1992;78:336–340. doi: 10.1007/BF00937093. [DOI] [PubMed] [Google Scholar]
- 35.Katagiri K., Kinashi T., Irie S., Katagiri T. Differential regulation of leukocyte function-associated antigen-1/intercellular adhesion molecules-1 -dependent adhesion and aggregation in HL-60 cells. Blood. 1996;87:4276–4285. [PubMed] [Google Scholar]