Abstract
Genetic forms of polycystic kidney diseases (PKDs), including nephronophthisis, are characterized by formation of fluid-filled cysts in the kidneys and progression to end-stage renal disease. No therapies are currently available to treat cystic diseases, making it imperative to dissect molecular mechanisms in search of therapeutic targets. Accumulating evidence suggests a pathogenic role for glucosylceramide (GlcCer) in multiple forms of PKD. It is not known, however, whether other structural glycosphingolipids (GSLs) or bioactive signaling sphingolipids (SLs) modulate cystogenesis. Therefore, we set out to address the role of a specific GSL (ganglioside GM3) and signaling SL (sphingosine-1-phosphate, S1P) in PKD progression, using the jck mouse model of nephronopthisis. To define the role of GM3 accumulation in cystogenesis, we crossed jck mice with mice carrying a targeted mutation in the GM3 synthase (St3gal5) gene. GM3-deficient jck mice displayed milder PKD, revealing a pivotal role for ganglioside GM3. Mechanistic changes in regulation of the cell-cycle machinery and Akt-mTOR signaling were consistent with reduced cystogenesis. Dramatic overexpression of sphingosine kinase 1 (Sphk1) mRNA in jck kidneys suggested a pathogenic role for S1P. Surprisingly, genetic loss of Sphk1 exacerbated cystogenesis and was associated with increased levels of GlcCer and GM3. On the other hand, increasing S1P accumulation through pharmacologic inhibition of S1P lyase had no effect on the progression of cystogenesis or kidney GSL levels. Together, these data suggest that genes involved in the SL metabolism may be modifiers of cystogenesis, and suggest GM3 synthase as a new anti-cystic therapeutic target.
INTRODUCTION
Nephronophthisis is an autosomal recessive cystic kidney disease that is a common cause of end-stage renal disease in the first three decades of life (1,2). Nephronophthisis can be caused by mutations in at least 12 distinct genes; however, all of the proteins encoded by these genes localize to the primary cilia or centrosome (1,3). Ciliary dysfunction has been recently discovered as a common abnormality leading to multiple forms of polycystic kidney disease (PKD) (1,4–6). Cystogenesis is accompanied by characteristic changes in tubular epithelial cells, including increased proliferation, apoptosis, acquisition of a secretory phenotype, dysregulation of the cell cycle, intracellular calcium and cAMP signaling and activation of several molecular pathways, including the Ras/Raf/MEK/ERK, Akt/mTOR and Wnt, among others (7–18) (also reviewed in 4,19,20). Recent advances in mechanistic understanding of pathogenic changes underlying cystogenesis have yielded potential therapeutic approaches to slow cyst growth in preclinical models (17,18,21–25).
Both glycosphingolipids (GSLs) and bioactive signaling sphingolipids (SLs) are known as important regulators of proliferation, differentiation, apoptosis and receptor signaling, all processes which are disrupted in multiple forms of PKD, including nephronopthisis (26–30). Recent findings demonstrate that increased biosynthesis of GSLs promotes cystogenesis and therefore can be targeted for therapeutic intervention (27,31,32). GSL synthesis begins with the glucosylation of ceramide to form glucosylceramide (GlcCer), which is a precursor to the formation of many other structural GSLs, including globosides and gangliosides (26,30). We have shown previously that specific inhibition of GlcCer synthase effectively decreases cystogenesis in mouse models orthologous to human autosomal dominant PKD (ADPKD) and nephronophthisis (32). Notably, the reduction of cystogenesis was accompanied by the reduction of not only GlcCer, but also the ganglioside GM3 (32). Although GSL imbalances clearly play a role in cystogenesis, the specific GSLs responsible for this role, and the mechanisms by which this occurs, remain to be identified.
Although there is a role for GSLs in the modulation of PKD, it is not known whether bioactive lipids contribute to the pathology of PKD. Importantly, the synthesis/degradation pathways of GSL and SL metabolism comprise a complex network, with ceramide (itself a signaling SL) playing a central role. As described above, ceramide forms the basis for the GSLs. Additionally, ceramide can be formed from sphingomyelin and act directly on cellular functions, or give rise to sphingosine (SPH), another bioactive signaling SLs (33). SPH is then phosphorylated by SPH kinase to form sphingosine-1-phosphate (S1P). Collectively, bioactive SLs, including ceramide and S1P, play important roles regulating cell survival, apoptosis and differentiation (26,28,34,35). S1P can be secreted from the cell, allowing it to interact with G-protein-coupled receptors; alternatively, S1P or its derivatives can function intracellularly to mediate signaling activity (reviewed in 35,36). S1P can be degraded by the S1P lyase (SPL) in an irreversible reaction that generates hexadecenal and phosphoethanolamine (35). It has been shown that many enzymes in the GSL and SL pathways are regulated by growth factors and cytokines, leading to changes in SL levels in a cell- and tissue-specific manner (37,38). Thus, the regulation of lipid flux through the network in response to cellular stimuli or pharmacologic intervention cannot be predicted, and must be experimentally determined in order to understand disease mechanisms and the impact of therapeutic lipid modulation.
We set out to assess the unique contribution of GSLs and signaling SLs to PKD progression in the jck mouse model of human nephronophthisis. Here we demonstrate for the first time that the ganglioside GM3 plays a pivotal role in cystogenesis by crossing jck mice with mice carrying a targeted mutation in the GM3 synthase gene (St3gal5). Genetic loss of GM3 synthase (GM3S) prevented GM3 synthesis and inhibited PKD progression in jck mice. This was accompanied by alterations to cell-cycle regulatory protein expression and Akt-mTOR signaling, consistent with reduced cystogenesis. We also show an elevation of SPH and S1P levels in cystic kidneys, with a strong overexpression of Sphk1 mRNA. We hypothesized that the reduction in S1P levels may inhibit cystogenesis in jck mice. We crossed jck mice with mice carrying a targeted mutation in the Sphk1 gene. Unexpectedly, the loss of Sphk1 accelerated PKD and was associated with increased levels of GlcCer and GM3. Therefore, we tested the hypothesis that S1P is protective by pharmacologic inhibition of SPL activity. Although SPL inhibition significantly increased kidney S1P levels, there was no effect on cystogenesis or kidney GSL levels. Together, these data demonstrate that GSLs and signaling SLs play important roles in cystogenesis, and suggest GM3S as a novel target for therapeutic intervention.
RESULTS
Structural and bioactive SL metabolism is dysregulated in cystic kidneys of jck mice
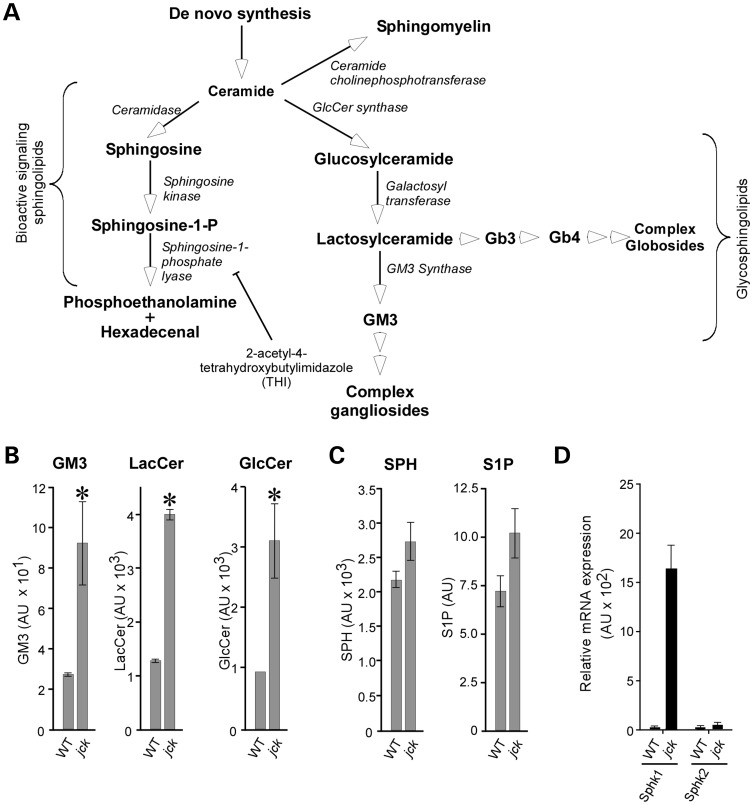
Because aberrant expression of SLs and GSLs is known to influence cellular processes such as growth, differentiation and survival, we considered the possibility that GSLs as well as bioactive signaling lipids may modify cystic disease progression in the jck mouse model (orthologous to human nephronophthisis with a mutation in the NPHP9 gene) (1,39). We compared lipid levels in cystic kidneys from jck mice with the levels present in age-matched wild-type controls, using LC-MS analysis, as previously described (Fig. 1) (32). The levels of all GSLs analyzed were elevated in cystic kidneys compared with normal controls, which is in line with previous reports (Fig. 1B) (27,31). We have previously reported no significant change in ceramide levels in this model (32). Interestingly, the levels of bioactive SLs, including SPH, and S1P were also elevated, although to a lesser extent than GSLs (Fig. 1C). S1P is generated through the actions of two distinct genes, Sphk 1 or 2. Therefore, we assessed Sphk1 and Sphk2 mRNA levels in wild-type and jck mouse kidney samples, using quantitative RT-PCR. Significant overexpression of Sphk1 mRNA was observed in cystic kidneys compared with wild-type controls (Fig. 1D). Analysis of human ADPKD SAGE data also revealed increased expression of SPHK1 mRNA levels (40). These results suggest that structural GSLs, as well as bioactive lipids, may play a role in the progression of cystic growth.
Figure 1.
Altered SL and GSL metabolism in PKD. (A) Schematic of normal GSL and SL metabolism. Bioactive SLs, including ceramide and S1P, play important roles in apoptosis and cell survival. GSLs, including GlcCer, LacCer, the ganglioside GM3 and the globosides Gb3 and Gb4, are components of lipid rafts and play a role in modulating cell-surface receptor signaling. Enzymes mediating the production of these lipids are shown in italics. THI inhibitor of SPL is indicated. (B) LC-MS analysis of GSL levels in the 64-day-old male wild-type (WT) and jck mouse kidney. Note the statistically significant increases in GlcCer, LacCer and GM3 accumulation in jck kidneys. (C) LC-MS analysis of bioactive SL levels in the 64-day-old wild-type (WT) and jck mouse kidney. Increased levels of SPH and S1P are seen in jck kidneys compared with wild-type controls (P = 0.14 and P = 0.11, respectively). (D) Sphk1 and Sphk2 mRNA levels. Whole-kidney mRNA from 64-day-old wild-type (wt) or jck mice was assayed for gene expression, using TaqMan analysis. Data shown are the mean ± SEM of three independent samples. *P < 0.05 compared with wild-type.
Loss of GM3S slows PKD progression in jck mice
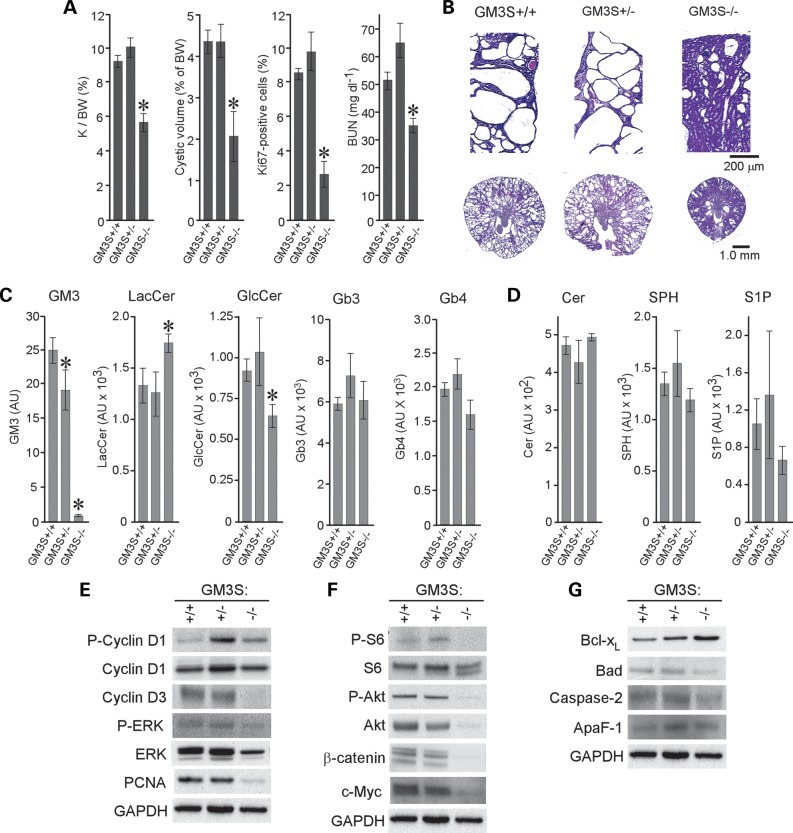
We have shown previously that elevated levels of GlcCer promote cystogenesis and that pharmacologic blockade of GlcCer synthase attenuates PKD in jck mice (32). Importantly, the reduction of cystogenesis was accompanied not only by decreased levels of GlcCer, but GM3 as well (32). To determine whether elevated kidney GM3 levels play a direct pathogenic role in PKD progression, we crossed jck animals with GM3S knockout animals to generate cystic animals that specifically lack GM3 (Fig. 2) (41). PKD progression was highly inhibited in jck mice lacking GM3S activity (GM3S−/−), as indicated by a significantly lower kidney/body weight ratio, cyst volume, blood urea nitrogen (BUN) (Fig. 2A; Supplementary Material, Table S1) and preservation of normal tissue (Fig. 2B).
Figure 2.
Loss of GM3S protects from cystogenesis in jck mice. (A) PKD progression in 64-day-old GM3S:jck males. Shown are quantitative analyses of kidney/body weight ratio (K/BW), cyst volume, percentage of Ki67-positive cells and BUN. Data are shown as mean ± SEM (9–23 animals per group for all but percentage of Ki67-positive cells, which is 3 representative animals per group). (B) Representative H&E-stained sections from 64-day-old wild-type (GM3S+/+), heterozygous mutant (GM3S+/−) and homozygous mutant (GM3S−/−) jck mice. (C) LC-MS analysis of GSL levels in kidneys of 64-day-old jck male mice carrying GM3S mutations. (D) LC-MS analysis of bioactive SL levels in kidneys of 64-day-old jck male mice carrying GM3S mutations. *P < 0.05 compared with GM3S+/+. Data shown are the mean ± SEM of three representative animals. (E) Immunoblot analysis of cell-cycle regulatory protein expression in GM3S mutant jck mice. P-cyclin D1, phosphorylated cyclin D1; cyclin D1, total cyclin D1; cyclin D3, total cyclin D3; P-ERK, phosphorylated ERK1/2; ERK, total ERK1/2; GAPDH, glyceraldehyde-3-phosphate dehydrogenase. (F) Immunoblot analysis of the Akt-mTOR pathway in GM3S mutant jck mice. P-S6, phosphorylated ribosomal protein S6; S6, total ribosomal protein S6; P-Akt, phosphorylated Akt; Akt, total Akt; β-catenin, total β-catenin; c-Myc, total c-Myc. (G) Immunoblot analysis of proteins regulating apoptosis in GM3S mutant jck mice. GAPDH staining was used to control for loading differences.
Next, we analyzed GSL levels in the kidneys of GM3S−/− jck animals (Fig. 2C). As expected, GM3 levels in the GM3S−/− mice were below the limit of detection for the LC-MS assay employed. Also, GM3S−/− mice showed reduced GlcCer levels and elevated lactosylceramide (LacCer) levels (Fig. 2C). The accumulation of LacCer is expected if the LacCer that would normally form gangliosides is not shunted into the globoside pathway in GM3S-null animals; indeed, we did not observe an increase in globosides Gb3 or Gb4 in these animals (Fig. 2C). It is unclear whether the reduction in GlcCer results from a feedback mechanism regulating glycosphinglipid accumulation, possibly due to increased LacCer, or as a secondary consequence of reduced cystogenesis. A slight but not significant reduction in GlcCer levels was observed in non-cystic GM3S mice (Supplementary Material, Fig. S1), with no change in LacCer, suggesting that the change in GlcCer may be secondary to reduced cystogenesis, but further work will be necessary to determine how the GSL metabolism is regulated in normal and cystic animals. These data suggest that ganglioside GM3 plays a pivotal role in modifying cystogenesis in jck mice. Heterozygous GM3S+/− animals demonstrated slightly decreased GM3 levels with unchanged GlcCer and LacCer abundance, which was not apparently sufficient to modify PKD progression (Fig. 2A and B; Supplementary Material, Table S1). Analysis of bioactive SLs showed a slight decrease in S1P in GM3S−/− cystic kidneys, with no changes in ceramide and SPH levels (Fig. 2D). A decrease of S1P was also observed in non-cystic GM3S−/− mice, suggesting that S1P levels are influenced by GM3 levels (Supplementary Material, Fig. S1). These data show that ganglioside GM3 is an important modulator of cystic growth.
Molecular pathways modulated by the loss of GM3S in cystic kidneys
To determine whether dysregulated cell cycle, apoptosis and signaling pathways are affected by the loss of GM3S activity, we performed western blot analysis on kidney extracts from GM3S+/+, +/− and −/− jck mice. Analysis of cell-cycle regulatory proteins showed decreased expression of cyclin D3 and increased phosphorylation of cyclin D1 in GM3S−/− jck mice, suggesting potent inhibition of the cell cycle (Fig. 2E; Supplementary Material, Fig. S2). The loss of GM3S also affected MEK-ERK signaling, known to regulate cyclin D1 (Fig. 2E). Proliferation was greatly reduced in GM3S−/− jck kidneys, evident by a decrease in the percentage of Ki67-positive cells and decreased proliferating cell nuclear antigen (PCNA) levels (Fig. 2A and E; Supplementary Material, Fig. S2). Decreased total and phosphorylated (Ser-473) Akt, as well as decreased ribosomal protein S6 phosphorylation, was observed in GM3S−/− animals, demonstrating reduced Akt/mTOR signaling (Fig. 2F; Supplementary Material, Fig. S2). Decreased Wnt/β-catenin signaling, known to be influenced by the cilium (42), was observed in cystic epithelial cells lacking GM3, as evidenced by reduced β-catenin and c-Myc expression (Fig. 2F; Supplementary Material, Figs S2 and S3). Apoptosis was not markedly affected in GM3S−/− jck mice, with only a slight increase in anti-apoptotic Bcl-xL protein and a slight decrease in Bad (Fig. 2G; Supplementary Material, Fig. S2). These data demonstrate that GM3 loss is likely to modulate several pathways of cystogenesis, including cell cycle, growth-factor-regulated signaling pathways and, possibly, ciliary signaling. It is important to note, however, that this data set does not distinguish primary effects of GM3 loss from effects that are secondary to reduced disease burden, as only terminal timepoints were analyzed.
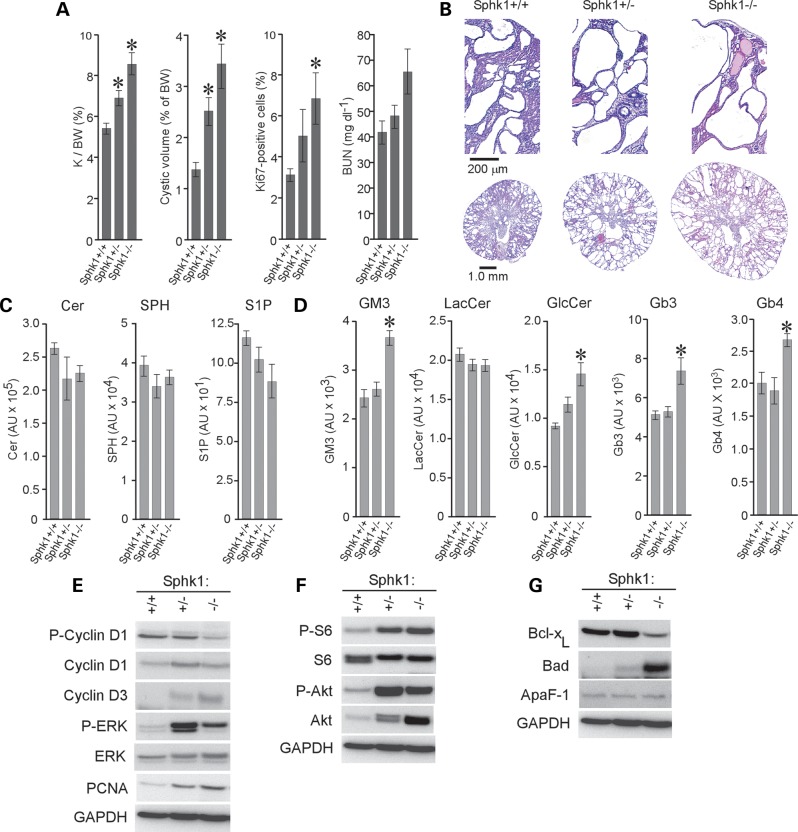
Loss of Sphk1 aggravates PKD in jck mice
Overexpression of Sphk1 mRNA, but not Sphk2 mRNA, and elevated levels of S1P in jck kidneys (Fig. 1C and D) suggested that the loss of Sphk1 may inhibit cystic disease progression. To test this hypothesis, we crossed jck mice with Sphk1 knockout mice to generate Sphk1+/+, +/− or −/− jck mice (Fig. 3) (43). Surprisingly, mutations in the Sphk1 gene significantly accelerated PKD in jck mice, and the level of disease correlated with the Sphk1 mutant gene dosage (Fig. 3A and B; Supplementary Material, Table S2). Comparative analysis of signaling SLs showed a reduction in kidney S1P levels in both the Sphk1+/− and Sphk1−/− jck kidneys; Sphk2 activity prevents the complete loss of S1P in kidneys from the Sphk1−/− animals (Fig. 3C). Although the reduction in S1P levels did not reach statistical significance, the data are suggestive of a gene dose–response relationship (Fig. 3C). Kidney ceramide and SPH levels were largely unaffected in Sphk1+/− and Sphk1−/− animals. A significant reduction of S1P was observed in non-cystic animals lacking Sphk1, with no significant changes in other SLs (Supplementary Material, Fig. S4). Interestingly, analysis of structural GSLs showed significantly increased levels of GM3 and GlcCer, but not LacCer, in Sphk1−/− jck kidneys (Fig. 3D). The lack of change in LacCer levels can be explained by increased synthesis of globosides; therefore, we assessed the level of globosides Gb3 and Gb4 in these mice (Fig. 3D). Gb3 and Gb4 levels are elevated in these animals, suggesting that LacCer levels are maintained in Sphk1−/− animals by increased production of gangliosides and globosides. As we have shown that increased GlcCer and GM3 levels are associated with increased cystogenesis, it is possible that the loss of Sphk1 results in the aggravation of PKD because it leads to the accumulation of the GSLs GlcCer and GM3.
Figure 3.
The loss of Sphk1 activity exacerbates cystogenesis in jck mice. (A) PKD progression in 50-day-old Sphk1:jck males. Kidney/body weight ratio (K/BW), cyst volume, percentage of Ki67-positive cells and BUN are shown. Data shown are the mean ± SEM of 13–25 animals per group for all but percentage of Ki67-positive cells, which is 3 representative animals per group. (B) Representative H&E-stained sections from (Sphk1+/+), heterozygous mutant (Sphk1+/−) and homozygous mutant (Sphk1−/−) jck mice. (C) LC-MS analysis of bioactive lipids in kidneys of 50-day-old jck males carrying Sphk1 mutations. A gene-dosage dependent reduction in S1P levels is noted (P = 0.084 for Sphk1−/− compared with Sphk1+/+). Data shown are the mean ± SEM of three representative animals. (D) LC-MS analysis of GSL levels in kidneys of 50-day-old jck males carrying Sphk1 mutations. *P < 0.05 compared with Sphk1+/+ control. Data shown are the mean ± SEM of three representative animals. (E) Immunoblot analysis of cell-cycle regulatory protein expression in Sphk1 mutant jck mice. P-cyclin D1, phosphorylated cyclin D1; cyclin D1, total cyclin D1; cyclin D3, total cyclin D3; P-ERK, phosphorylated ERK1/2; ERK, total ERK1/2; GAPDH, glyceraldehyde-3-phosphate dehydrogenase. (F) Immunoblot analysis of the Akt-mTOR pathway in Sphk1 mutant jck mice. P-S6, phosphorylated ribosomal protein S6; S6, total ribosomal protein S6; P-Akt, phosphorylated Akt; Akt, total Akt. (G) Immunoblot analysis of proteins regulating apoptosis in Sphk1 mutant jck mice. GAPDH staining was used to control for loading differences.
Molecular pathways modulated by Sphk1 loss in cystic kidneys
To test whether the aggravation of PKD associated with Sphk1 loss is mechanistically linked to the accumulation of GlcCer and GM3, which in turn leads to cell-cycle dysregulation and Akt-mTOR signaling, we performed western blot analysis on kidney extracts from Sphk1+/+, +/− and −/− jck mice (Fig. 3E–G; Supplementary Material, Fig. S5). Loss of Sphk1 function increased the expression of the cell-cycle regulatory protein cyclin D3, reduced phosphorylated cyclin D1 and increased ERK phosphorylation (Fig. 3E). Elevated Akt-mTOR signaling was evident by increased phosphorylation of ribosomal protein S6 and Akt (Fig. 3F). Increased apoptosis was detected in Sphk1−/− jck kidneys by decreased expression of the anti-apoptotic protein Bcl-xL and increased expression of the pro-apoptotic protein Bad (Fig. 3G).
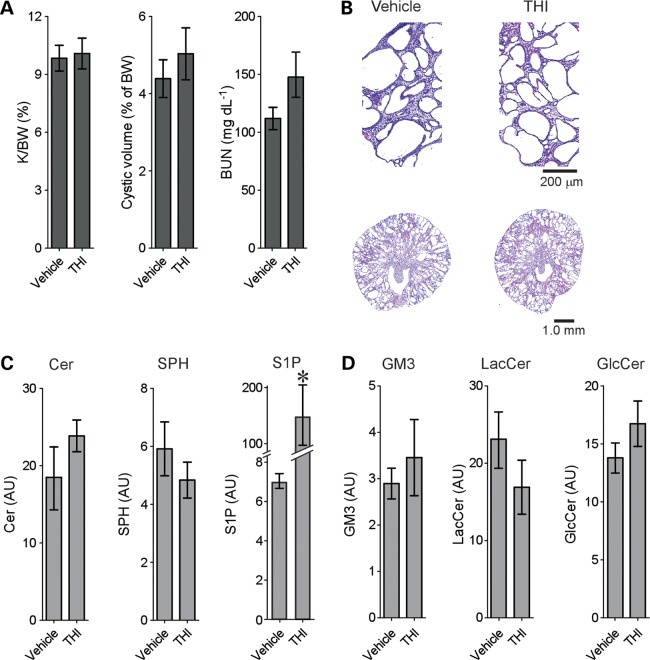
SPL inhibition increases S1P levels without affecting cystogenesis
Since genetic deletion of Sphk1 exacerbates PKD and reduces S1P levels, we next tested the hypothesis that increasing S1P levels might inhibit cystogenesis by treating jck mice with the SPL inhibitor 2-acetyl-4-tetrahydroxybutylimidazole (THI) (44). Interestingly, THI treatment had no effect on cystogenesis (Fig. 4A and B) despite a significant increase in kidney S1P levels (Fig. 4C). Therefore, increasing S1P levels does not alter cystogenesis. No significant change was observed in kidney GlcCer or GM3 levels (Fig. 4D). These data suggest that changes in GSL levels play major roles in modulating cystogenesis.
Figure 4.
Inhibition of SPL activity increases S1P but does not inhibit cystogenesis in jck mice. Male jck mice were treated from 26–64 days of age with 50 mg/l THI in the drinking water. (A) Quantitative analysis of kidney/body weight ratio (K/BW), cyst volume and BUN from jck mice treated with THI or vehicle controls. Data shown are the mean ± SEM, n = 10 vehicle and 8 THI-treated animals. (B) Representative H&E-stained sections from THI-treated jck mice or vehicle controls. (C) LC-MS analysis of bioactive SL levels in kidneys of THI- or vehicle-treated jck mice. *P < 0.05 compared with vehicle. (D) LC-MS analysis of structural GSL levels in kidneys of THI- or vehicle-treated jck mice. Data shown are the mean ± SEM of three representative animals.
DISCUSSION
GSLs and signaling SLs play important roles in regulating proliferation, signal transduction and apoptosis, processes that are known to be dysregulated during cystogenesis (26–29). Here we show that metabolic pathways for structural GSLs and signaling SLs are significantly altered in the jck mouse model, a murine model of nephronophthisis (1,45). Although previous studies identified similar GSL imbalances in preclinical models of nephronophthisis and ADPKD (32), future analysis will be necessary to directly extend our findings to other forms of PKD, including autosomal recessive PKD and ADPKD. We demonstrate that genetic loss of GM3S protects against PKD progression in jck mice, mediated through the inhibition of the cell cycle and Akt-mTOR signaling. In contrast, reducing the level of the bioactive SL S1P through genetic loss of Sphk1 increases kidney GlcCer and GM3, and therefore aggravates cystogenesis. The inhibition of SPL activity increases kidney S1P levels but not GSL levels, and has no effect on cystogenesis. We have recently shown that pharmacologic inhibition of GlcCer synthase reduces cystogenesis in two mouse models orthologous to nephronophthisis, as well as a mouse model orthologous to human ADPKD (32). GlcCer is a precursor to GM3 biosynthesis, and therefore the inhibition of GlcCer synthesis could reduce GM3 production by limiting the available substrate for GM3S. Indeed, reduced GM3 levels were observed in animals treated with a GlcCer synthase inhibitor, suggesting that the effects of GlcCer synthase inhibition could be mediated, in whole or in part, by reduction of GM3 biosynthesis (32). Interestingly, reduced levels of GlcCer were found in the GM3S−/− jck animals. It is possible that the loss of GM3 activates a feedback mechanism that inhibits GlcCer accumulation, possibly due to the accumulation of LacCer in these animals.
As a component of lipid rafts, GM3 can interact with cell signaling receptors for growth factors such as the epidermal growth factor (EGF) or insulin (46,47). The EGF and insulin-like growth factor 1 have been shown to increase cystogenesis in PKD (48,49). It is reasonable to speculate that the accumulation of GM3 may alter its interactions with cell-surface receptors, resulting in aberrant cell signaling associated with cystogenesis. In support of this hypothesis, we find that mice lacking GM3 have reduced Mek-Erk signaling and Akt-mTOR activity, both of which are modulated by cell-surface receptors and are important drivers of cystogenesis (20). Since the data provided here are from terminal timepoints, some of the changes we observe may be secondary to changes in cumulative kidney damage. For example, cumulative kidney damage can activate mTOR signaling to induce compensatory hypertrophy (50). We have shown previously, however, that brief treatment of cystic kidneys with a GlcCer synthase inhibitor reduces both GM3 levels and mTOR signaling prior to an effect on cyst burden, arguing that GM3 may directly regulate this pathway (32). Of note, the gangliosides GM1 and GM3 were found to be localized along the entire length of the primary cilia, an organelle that plays an important role in regulating cystogenesis (51). Here we demonstrate altered canonical Wnt signaling in cystic GM3S−/− mice, suggesting that GM3 can modulate ciliary signaling, providing an additional link between the GSL metabolism and processes that drive cystogenesis. Importantly, GM3S−/− animals are viable and fertile (41,52), suggesting that compounds that inhibit GM3S activity might be effective and safe therapies to treat PKDs.
Although recent data strongly suggest a role of structural GSLs in the pathogenesis of PKD, the role of signaling SLs has not been addressed previously (27,31,32). Ceramide, a central molecule in SL metabolism, was found to be associated with the base of the primary cilium (53). The inhibition of ceramide synthesis prevents the formation of the primary cilia, whereas exogenous addition of a ceramide analog rescues primary cilia formation (53). In vitro manipulation of ceramide levels in NIH 3T3 cells suggests that increased ceramide levels can inhibit cell-cycle progression (54). Ceramide is a central molecule in both the GSL and bioactive SL metabolic pathways and the relative levels of ceramide and S1P have been proposed to regulate apoptosis, which plays an important role in cystogenesis (25,28). Interestingly, the SPH kinase 1 and 2 proteins, encoded by the Sphk1 and Sphk2 genes, respectively, have been localized to the centrosome, suggesting a role in regulating cell division (29). Furthermore, Sphk1 can promote cellular proliferation, another potential mechanism by which altered S1P levels could influence PKD (55,56).
SPH kinase 1 expression was shown to be increased in human colon carcinomas and in mouse models of adenomas, and genetic loss of Sphk1, but not Sphk2, reduces polyp growth in these mouse models (55,56). This was accompanied by a reduction of cyclin-dependent kinase 4 and c-Myc protein expression (56). Dysregulation of the cell cycle and overexpression of c-Myc are hallmarks of PKD, and therapies targeting both have proven to be effective in preclinical models of PKD (21,57). Although our data show a significant upregulation of Sphk1 mRNA in jck mice, genetic loss of Sphk1 aggravates PKD in jck mice, suggesting that S1P produced by Sphk1 is protective. This could work through S1P-mediated activation of G-protein-coupled receptors, or through the reduction of the cellular SL pool necessary for ceramide production. Although we do not see an increase in Sphk2 expression, we cannot rule out a role for Sphk2 in PKD. Recent studies have demonstrated increased Sphk1 mRNA expression and kinase activity after renal ischemia–reperfusion injury (IRI), without changes in Sphk2 mRNA or kinase activity (58,59). Jo et al. (58) reported that Sphk2 loss was associated with an increased level of kidney damage following IRI and increased expression of the S1P3 receptor, suggesting that Sphk2 is required by Sphk1 to limit renal injury by limiting the activation of the S1P3 receptor. Our observation that S1P levels are only partially reduced in Sphk1−/− jck mouse kidneys demonstrates that Sphk2 is present and functional in these animals. It would therefore be interesting to explore the potential role of Sphk2 in PKD.
The ratio of S1P to ceramide has been proposed to function as a ‘rheostat’ to allow growth and survival of cells when S1P predominates, or apoptosis when ceramide accumulates (28). Apoptosis is associated with human and murine PKD (reviewed in 60). Inhibition of apoptosis was shown to reduce cystogenesis in mouse and rat models of PKD (25,61). Conversely, the disruption of the anti-apoptotic gene Bcl-2 causes PKD (62,63). Here we show that the deletion of Sphk1 reduces S1P levels, which would be expected to disrupt the S1P:ceramide balance to favor apoptosis. In support of this, western blot analysis demonstrates reduced expression of the anti-apoptotic protein Bcl-xL and increased expression of the pro-apoptotic Bad. This could be a mechanism by which Sphk1 loss exacerbates cystogenesis. However, if this were the case, SPL inhibition would be expected to drive the S1P:ceramide ratio to inhibit apoptosis, thereby inhibiting cystogenesis. This is not what we observe. Therefore, apoptosis may not be the primary mechanism increasing cystogenesis in the Sphk1−/− jck mice. Similarly, if increased cystogenesis in Sphk1-knockout mice was the result of decreased S1P-mediated activation of S1P receptors, we would have expected that increasing S1P levels through SPL inhibition would decrease cystogenesis. Again, our data are not consistent with this hypothesis. A third hypothesis is that Sphk1 inhibits cystogenesis by acting as a sink to remove ceramide from the pool available for GSL production. The observation that jck mouse kidney GlcCer and GM3 levels increase upon Sphk1 loss, coupled with the fact that SPL inhibition has no effect on GSL levels or cystogenesis, is consistent with this model. We note that the elevation of GlcCer and GM3 in Sphk1+/− animals is small, despite a significant increase in cystogenesis, which could argue against this model and requires further investigation. It would be interesting to test the progression of cystogenesis in jck mice lacking both GM3S and Sphk1 in future studies.
These data demonstrate that altered SL metabolism can impact cyst growth, suggesting that genes involved in SL metabolism may act as modifier genes to influence disease severity. A number of studies have reported loci that modify cystogenesis in murine models of PKD (64–67). Although the regions identified in these studies include potentially hundreds of distinct genes, it is intriguing that a region of mouse chromosome 4 containing a modifier of cystogenesis in the jck and pcy mouse models of nephronopthisis (66,67) includes the mouse GlcCer synthase gene, Ugcg. There is strong evidence that time to end-stage renal disease is controlled by modifier loci in human ADPKD (68–70), making the identification of these genes especially important.
Understanding the mechanisms that promote cystogenesis is necessary to develop therapeutics to slow cyst growth. Here we demonstrate that elevated GM3 levels promote cyst growth in a mouse model of nephronophthisis, and that genetic deletion of GM3S protects from cystogenesis. We further demonstrate that overexpression of Sphk1, one of two enzymes that produces the signaling SL S1P, is not pathogenic in jck kidneys. These data suggest that a greater understanding of the role of GSL and SL imbalances in nephronophthisis and other forms of PKD may lead to the identification of viable therapeutic targets.
MATERIALS AND METHODS
Animal handling and treatment
Mice were handled in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. The protocol was approved by the Genzyme Institutional Animal Care and Use Committee. Jck mice were genotyped as described previously (71). GM3S knockout mice (St3gal5tm1Rlp/tm1Rlp, a kind gift of Dr Richard Proia, NIDDK) were genotyped using the primers 5′-TTACTCCTTCTATTCTGTGAG-3′, 5′-AAGACTTCCTGAGCATAGCT-3′ and 5′-CAATCCATCTTGTTCAATCA-3′, in PCR reactions containing 1.5 mm MgCl2 to generate reaction products of 506 (wild-type GM3S allele) or ∼450 bp (GM3S-null allele). Cycling parameters were: 94°C × 5 min; 35 × (94°C × 30 s, 65°C × 30 s, 72°C × 2 min); 72°C × 10 min. Sphk1 knockout mice (Sphk1tm1Rlp/tm1Rlp, kindly provided by Dr Richard Proia, NIDDK) were genotyped by Transnetyx, Inc., using a proprietary assay. The SPL inhibitor THI was ordered from Albany Molecular Research, Inc., and was administered ad libitum at 50 mg/l in drinking water containing 1% glucose (pH 4.0) (44). Histologic and BUN analyses were performed as described previously (71).
GSL analysis
To assess the relative levels of GSLs and SLs, we performed LC-MS analysis; all GSLs except S1P were analyzed as described previously (32). For Gb3 and Gb4 analysis, kidney homogenates were extracted with a mixture of 50% (1% acetic acid 5 mm ammonium acetate in 97:2:1 acetonitrile:methanol:water) and 50% (1% acetic acid 5 mm ammonium acetate in 99:1 methanol/water). Extracts were separated by HPLC using an Acquity UPLC system with a BEH HILIC column (Waters Corporation) and analyzed using an API 4000 mass spectrometer (Applied Biosystems). The low abundance of S1P in kidney tissues necessitated the following modifications: separation was achieved with an Xbridge Phenyl 2.1 × 100 mm column (Waters Corporation), and eluates were analyzed with an API-5000 mass spectrometer (Applied Biosystems). Data shown are the mean ± SEM of three representative animals.
Quantitative RT-PCR analysis
RNA was extracted and processed as previously described (72). Quantitative RT-PCR for Sphk1 and Sphk2 was performed using Applied Biosystems-predesigned TaqMan Gene Expression Assays, and normalized with rodent 18S RNA.
Western blot analysis
Kidney extracts were prepared, run and transferred for western blot analysis as described previously (72). Three kidneys of each genotype were analyzed, and representative examples are shown. The following primary antibodies were used: Bcl-xL, Bad, Akt, ApaF-1, cyclin D3, β-catenin (all from BD Biosciences, San Jose, CA, USA), phospho-Akt (Ser473), S6 ribosomal protein, phospho-S6 ribosomal protein (Ser235/236), cyclin D1, phospho-cyclin D1 (Thr286), total ERK, phospho-ERK (Thr202/Tyr204), c-Myc (all from Cell Signaling Technologies, Danvers, MA, USA), caspase-2 (Millipore, Billerica, MA, USA), PCNA and GAPDH (US Biological, Swampscott, MA, USA).
Immunohistochemistry and immunofluorescence
Paraffin-embedded tissue sections were deparaffinized through a xylene/graded ethanol series, and antigen retrieval was performed by heat-treating in a citrate buffer (Dako Corporation, Carpinteria, CA, USA) for 20–30 min, using a pressure cooker. Slides for immunohistochemistry (IHC) were blocked with a peroxidase-blocking solution (Dako Corporation). All slides were pre-incubated with a serum-free protein-blocking agent (Dako Corporation) prior to incubation with primary antibodies diluted in an antibody dilution reagent (Dako Corporation). Primary antibody binding was revealed using either an Envision+ HRP system followed by 3,3′-diaminobenzidine tetrahydrochloride (DAB) (Dako Corporation) as recommended by the manufacturer for IHC, or by Alexa 488-conjugated secondary antibodies (Life Technologies, Grand Island, NY, USA) for immunofluorescence (IF). Slides for IHC were counterstained with hematoxylin prior to mounting. Slides for IF were mounted with Vectashield plus DAPI (Vector Laboratories, Burlingame, CA, USA). An anti-Ki67 antibody (Abcam, Cambridge, MA, USA) was used for IHC; anti-c-Myc (Abcam) and anti-β-catenin (BD Biosciences) antibodies were used for IF. For quantification of Ki67 staining, 15–20 representative 20× fields were photographed from each of three representative animals per genotype. The percentage of Ki67-positive nuclei was measured using the Metamorph Imaging Series® software (Molecular Devices Corporation, Dowington, PA, USA).
Statistical analysis
Data are expressed as mean ± SEM. Data were analyzed with a D'Agostino and Pearson omnibus normality test. Comparisons were made by a two-tailed t-test (if normally distributed) or Mann–Whitney test (if not normally distributed) using the GraphPad Prism software (GraphPad Software, Inc., LaJolla, CA, USA); significance was accepted at the 0.05 level of probability (P < 0.05).
SUPPLEMENTARY MATERIAL
Supplementary Material is available at HMG online.
ACKNOWLEDGEMENTS
We thank R. Proia for providing the GM3S and Sphk1 knockout mice, M. Troutt and M. Phipps for breeding and genotyping of mice; the staff of the Departments of Comparative Medicine and Histology, Genzyme Corporation, for help with the in vivo studies and sample preparation; S. Komarnitsky and R. Finnegan for help with project management; A. Smith, J. Burns and R. Gregory for helpful discussions; W.R. Dackowski, S. Moreno, R.J. Russo and H. Ling for expert technical assistance.
Conflict of Interest statement. The authors are all employees of Genzyme Corporation, a Sanofi company.
FUNDING
This work was supported by Genzyme Corporation, a Sanofi company. Funding to pay the Open Access publication charges for this article was provided by Genzyme Corporation, a Sanofi Company.
REFERENCES
- 1.Hurd T.W., Hildebrandt F. Mechanisms of nephronophthisis and related ciliopathies. Nephron Exp. Nephrol. 2011;118:e9–e14. doi: 10.1159/000320888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hildebrandt F., Attanasio M., Otto E. Nephronophthisis: disease mechanisms of a ciliopathy. J. Am. Soc. Nephrol. 2009;20:23–35. doi: 10.1681/ASN.2008050456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Otto E.A., Hurd T.W., Airik R., Chaki M., Zhou W., Stoetzel C., Patil S.B., Levy S., Ghosh A.K., Murga-Zamalloa C.A., et al. Candidate exome capture identifies mutation of SDCCAG8 as the cause of a retinal-renal ciliopathy. Nat. Genet. 2010;42:840–850. doi: 10.1038/ng.662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Patel V., Chowdhury R., Igarashi P. Advances in the pathogenesis and treatment of polycystic kidney disease. Curr. Opin. Nephrol. Hypertens. 2009;18:99–106. doi: 10.1097/MNH.0b013e3283262ab0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Joly D., Hummel A., Ruello A., Knebelmann B. Ciliary function of polycystins: a new model for cystogenesis. Nephrol. Dial. Transplant. 2003;18:1689–1692. doi: 10.1093/ndt/gfg256. [DOI] [PubMed] [Google Scholar]
- 6.Yoder B.K. Role of primary cilia in the pathogenesis of polycystic kidney disease. J. Am. Soc. Nephrol. 2007;18:1381–1388. doi: 10.1681/ASN.2006111215. [DOI] [PubMed] [Google Scholar]
- 7.Nadasdy T., Laszik Z., Lajoie G., Blick K.E., Wheeler D.E., Silva F.G. Proliferative activity of cyst epithelium in human renal cystic diseases. J. Am. Soc. Nephrol. 1995;5:1462–1468. doi: 10.1681/ASN.V571462. [DOI] [PubMed] [Google Scholar]
- 8.Bhunia A.K., Piontek K., Boletta A., Liu L., Qian F., Xu P.N., Germino F.J., Germino G.G. PKD1 induces p21(waf1) and regulation of the cell cycle via direct activation of the JAK-STAT signaling pathway in a process requiring PKD2. Cell. 2002;109:157–168. doi: 10.1016/s0092-8674(02)00716-x. [DOI] [PubMed] [Google Scholar]
- 9.Woo D. Apoptosis and loss of renal tissue in polycystic kidney diseases. N. Engl. J. Med. 1995;333:18–25. doi: 10.1056/NEJM199507063330104. [DOI] [PubMed] [Google Scholar]
- 10.Li X., Luo Y., Starremans P.G., McNamara C.A., Pei Y., Zhou J. Polycystin-1 and polycystin-2 regulate the cell cycle through the helix-loop-helix inhibitor Id2. Nat. Cell Biol. 2005;7:1202–1212. doi: 10.1038/ncb1326. [DOI] [PubMed] [Google Scholar]
- 11.Grantham J.J., Ye M., Gattone V.H., II, Sullivan L.P. In vitro fluid secretion by epithelium from polycystic kidneys. J. Clin. Invest. 1995;95:195–202. doi: 10.1172/JCI117638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Babich V., Zeng W.Z., Yeh B.I., Ibraghimov-Beskrovnaya O., Cai Y., Somlo S., Huang C.L. The N-terminal extracellular domain is required for polycystin-1-dependent channel activity. J. Biol. Chem. 2004;279:25582–25589. doi: 10.1074/jbc.M402829200. [DOI] [PubMed] [Google Scholar]
- 13.Hanaoka K., Qian F., Boletta A., Bhunia A.K., Piontek K., Tsiokas L., Sukhatme V.P., Guggino W.B., Germino G.G. Co-assembly of polycystin-1 and -2 produces unique cation-permeable currents. Nature. 2000;408:990–994. doi: 10.1038/35050128. [DOI] [PubMed] [Google Scholar]
- 14.Koulen P., Cai Y., Geng L., Maeda Y., Nishimura S., Witzgall R., Ehrlich B.E., Somlo S. Polycystin-2 is an intracellular calcium release channel. Nat. Cell Biol. 2002;4:191–197. doi: 10.1038/ncb754. [DOI] [PubMed] [Google Scholar]
- 15.Calvet J.P., Grantham J.J. The genetics and physiology of polycystic kidney disease. Semin. Nephrol. 2001;21:107–123. doi: 10.1053/snep.2001.20929. [DOI] [PubMed] [Google Scholar]
- 16.Yamaguchi T., Wallace D.P., Magenheimer B.S., Hempson S.J., Grantham J.J., Calvet J.P. Calcium restriction allows cAMP activation of the B-Raf/ERK pathway, switching cells to a cAMP-dependent growth-stimulated phenotype. J. Biol. Chem. 2004;279:40419–40430. doi: 10.1074/jbc.M405079200. [DOI] [PubMed] [Google Scholar]
- 17.Shillingford J.M., Murcia N.S., Larson C.H., Low S.H., Hedgepeth R., Brown N., Flask C.A., Novick A.C., Goldfarb D.A., Kramer-Zucker A., et al. The mTOR pathway is regulated by polycystin-1, and its inhibition reverses renal cystogenesis in polycystic kidney disease. Proc. Natl Acad. Sci. USA. 2006;103:5466–5471. doi: 10.1073/pnas.0509694103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wahl P.R., Serra A.L., Le Hir M., Molle K.D., Hall M.N., Wuthrich R.P. Inhibition of mTOR with sirolimus slows disease progression in Han:SPRD rats with autosomal dominant polycystic kidney disease (ADPKD) Nephrol. Dial. Transplant. 2006;21:598–604. doi: 10.1093/ndt/gfi181. [DOI] [PubMed] [Google Scholar]
- 19.Song X., Di Giovanni V., He N., Wang K., Ingram A., Rosenblum N.D., Pei Y. Systems biology of autosomal dominant polycystic kidney disease (ADPKD): computational identification of gene expression pathways and integrated regulatory networks. Hum. Mol. Genet. 2009;18:2328–2343. doi: 10.1093/hmg/ddp165. [DOI] [PubMed] [Google Scholar]
- 20.Torres V.E., Harris P.C. Autosomal dominant polycystic kidney disease: the last 3 years. Kidney Int. 2009;76:149–168. doi: 10.1038/ki.2009.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bukanov N.O., Smith L.A., Klinger K.W., Ledbetter S.R., Ibraghimov-Beskrovnaya O. Long-lasting arrest of murine polycystic kidney disease with CDK inhibitor roscovitine. Nature. 2006;444:949–952. doi: 10.1038/nature05348. [DOI] [PubMed] [Google Scholar]
- 22.Chen N.X., Moe S.M., Eggleston-Gulyas T., Chen X., Hoffmeyer W.D., Bacallao R.L., Herbert B.S., Gattone V.H., II Calcimimetics inhibit renal pathology in rodent nephronophthisis. Kidney Int. 2011;80:612–619. doi: 10.1038/ki.2011.139. [DOI] [PubMed] [Google Scholar]
- 23.Gattone V.H., II, Sinders R.M., Hornberger T.A., Robling A.G. Late progression of renal pathology and cyst enlargement is reduced by rapamycin in a mouse model of nephronophthisis. Kidney Int. 2009;76:178–182. doi: 10.1038/ki.2009.147. [DOI] [PubMed] [Google Scholar]
- 24.Gattone V.H., II, Wang X., Harris P.C., Torres V.E. Inhibition of renal cystic disease development and progression by a vasopressin V2 receptor antagonist. Nat. Med. 2003;9:1323–1326. doi: 10.1038/nm935. [DOI] [PubMed] [Google Scholar]
- 25.Tao Y., Kim J., Faubel S., Wu J.C., Falk S.A., Schrier R.W., Edelstein C.L. Caspase inhibition reduces tubular apoptosis and proliferation and slows disease progression in polycystic kidney disease. Proc. Natl Acad. Sci. USA. 2005;102:6954–6959. doi: 10.1073/pnas.0408518102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shayman J.A. Sphingolipids. Kidney Int. 2000;58:11–26. doi: 10.1046/j.1523-1755.2000.00136.x. [DOI] [PubMed] [Google Scholar]
- 27.Chatterjee S., Shi W.Y., Wilson P., Mazumdar A. Role of lactosylceramide and MAP kinase in the proliferation of proximal tubular cells in human polycystic kidney disease. J. Lipid Res. 1996;37:1334–1344. [PubMed] [Google Scholar]
- 28.Cuvillier O., Pirianov G., Kleuser B., Vanek P.G., Coso O.A., Gutkind S., Spiegel S. Suppression of ceramide-mediated programmed cell death by sphingosine-1-phosphate. Nature. 1996;381:800–803. doi: 10.1038/381800a0. [DOI] [PubMed] [Google Scholar]
- 29.Gillies L., Lee S.C., Long J.S., Ktistakis N., Pyne N.J., Pyne S. The sphingosine 1-phosphate receptor 5 and sphingosine kinases 1 and 2 are localised in centrosomes: possible role in regulating cell division. Cell Signal. 2009;21:675–684. doi: 10.1016/j.cellsig.2009.01.023. [DOI] [PubMed] [Google Scholar]
- 30.Lahiri S., Futerman A.H. The metabolism and function of sphingolipids and glycosphingolipids. Cell. Mol. Life Sci. 2007;64:2270–2284. doi: 10.1007/s00018-007-7076-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Deshmukh G.D., Radin N.S., Gattone V.H., II, Shayman J.A. Abnormalities of glycosphingolipid, sulfatide, and ceramide in the polycystic (cpk/cpk) mouse. J. Lipid Res. 1994;35:1611–1618. [PubMed] [Google Scholar]
- 32.Natoli T.A., Smith L.A., Rogers K.A., Wang B., Komarnitsky S., Budman Y., Belenky A., Bukanov N.O., Dackowski W.R., Husson H., et al. Inhibition of glucosylceramide accumulation results in effective blockade of polycystic kidney disease in mouse models. Nat. Med. 2010;16:788–792. doi: 10.1038/nm.2171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Reynolds C.P., Maurer B.J., Kolesnick R.N. Ceramide synthesis and metabolism as a target for cancer therapy. Cancer Lett. 2004;206:169–180. doi: 10.1016/j.canlet.2003.08.034. [DOI] [PubMed] [Google Scholar]
- 34.Hannun Y.A., Obeid L.M. Principles of bioactive lipid signalling: lessons from sphingolipids. Nat. Rev. Mol. Cell Biol. 2008;9:139–150. doi: 10.1038/nrm2329. [DOI] [PubMed] [Google Scholar]
- 35.Fyrst H., Saba J.D. An update on sphingosine-1-phosphate and other sphingolipid mediators. Nat. Chem. Biol. 2010;6:489–497. doi: 10.1038/nchembio.392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Strub G.M., Maceyka M., Hait N.C., Milstien S., Spiegel S. Extracellular and intracellular actions of sphingosine-1-phosphate. Adv. Exp. Med. Biol. 2010;688:141–155. doi: 10.1007/978-1-4419-6741-1_10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bourteele S., Hausser A., Doppler H., Horn-Muller J., Ropke C., Schwarzmann G., Pfizenmaier K., Muller G. Tumor necrosis factor induces ceramide oscillations and negatively controls sphingolipid synthases by caspases in apoptotic Kym-1 cells. J. Biol. Chem. 1998;273:31245–31251. doi: 10.1074/jbc.273.47.31245. [DOI] [PubMed] [Google Scholar]
- 38.Hannun Y.A., Luberto C., Argraves K.M. Enzymes of sphingolipid metabolism: from modular to integrative signaling. Biochemistry. 2001;40:4893–4903. doi: 10.1021/bi002836k. [DOI] [PubMed] [Google Scholar]
- 39.Otto E.A., Trapp M.L., Schultheiss U.T., Helou J., Quarmby L.M., Hildebrandt F. NEK8 mutations affect ciliary and centrosomal localization and may cause nephronophthisis. J. Am. Soc. Nephrol. 2008;19:587–592. doi: 10.1681/ASN.2007040490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Husson H., Manavalan P., Akmaev V.R., Russo R.J., Cook B., Richards B., Barberio D., Liu D., Cao X., Landes G.M., et al. New insights into ADPKD molecular pathways using combination of SAGE and microarray technologies. Genomics. 2004;84:497–510. doi: 10.1016/j.ygeno.2004.03.009. [DOI] [PubMed] [Google Scholar]
- 41.Yamashita T., Hashiramoto A., Haluzik M., Mizukami H., Beck S., Norton A., Kono M., Tsuji S., Daniotti J.L., Werth N., et al. Enhanced insulin sensitivity in mice lacking ganglioside GM3. Proc. Natl Acad. Sci. USA. 2003;100:3445–3449. doi: 10.1073/pnas.0635898100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lancaster M.A., Schroth J., Gleeson J.G. Subcellular spatial regulation of canonical Wnt signalling at the primary cilium. Nat. Cell Biol. 2011;13:700–707. doi: 10.1038/ncb2259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Allende M.L., Sasaki T., Kawai H., Olivera A., Mi Y., van Echten-Deckert G., Hajdu R., Rosenbach M., Keohane C.A., Mandala S., et al. Mice deficient in sphingosine kinase 1 are rendered lymphopenic by FTY720. J. Biol. Chem. 2004;279:52487–52492. doi: 10.1074/jbc.M406512200. [DOI] [PubMed] [Google Scholar]
- 44.Schwab S.R., Pereira J.P., Matloubian M., Xu Y., Huang Y., Cyster J.G. Lymphocyte sequestration through S1P lyase inhibition and disruption of S1P gradients. Science. 2005;309:1735–1739. doi: 10.1126/science.1113640. [DOI] [PubMed] [Google Scholar]
- 45.Atala A., Freeman M.R., Mandell J., Beier D.R. Juvenile cystic kidneys (jck): a new mouse mutation which causes polycystic kidneys. Kidney Int. 1993;43:1081–1085. doi: 10.1038/ki.1993.151. [DOI] [PubMed] [Google Scholar]
- 46.Kabayama K., Sato T., Saito K., Loberto N., Prinetti A., Sonnino S., Kinjo M., Igarashi Y., Inokuchi J. Dissociation of the insulin receptor and caveolin-1 complex by ganglioside GM3 in the state of insulin resistance. Proc. Natl Acad. Sci. USA. 2007;104:13678–13683. doi: 10.1073/pnas.0703650104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang X.Q., Sun P., Paller A.S. Ganglioside GM3 blocks the activation of epidermal growth factor receptor induced by integrin at specific tyrosine sites. J. Biol. Chem. 2003;278:48770–48778. doi: 10.1074/jbc.M308818200. [DOI] [PubMed] [Google Scholar]
- 48.Parker E., Newby L.J., Sharpe C.C., Rossetti S., Streets A.J., Harris P.C., O'Hare M.J., Ong A.C. Hyperproliferation of PKD1 cystic cells is induced by insulin-like growth factor-1 activation of the Ras/Raf signalling system. Kidney Int. 2007;72:157–165. doi: 10.1038/sj.ki.5002229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Richards W.G., Sweeney W.E., Yoder B.K., Wilkinson J.E., Woychik R.P., Avner E.D. Epidermal growth factor receptor activity mediates renal cyst formation in polycystic kidney disease. J. Clin. Invest. 1998;101:935–939. doi: 10.1172/JCI2071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bell P.D., Fitzgibbon W., Sas K., Stenbit A.E., Amria M., Houston A., Reichert R., Gilley S., Siegal G.P., Bissler J., et al. Loss of primary cilia upregulates renal hypertrophic signaling and promotes cystogenesis. J. Am. Soc. Nephrol. 2011;22:839–848. doi: 10.1681/ASN.2010050526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Janich P., Corbeil D. GM1 and GM3 gangliosides highlight distinct lipid microdomains within the apical domain of epithelial cells. FEBS Lett. 2007;581:1783–1787. doi: 10.1016/j.febslet.2007.03.065. [DOI] [PubMed] [Google Scholar]
- 52.Sandhoff R., Geyer R., Jennemann R., Paret C., Kiss E., Yamashita T., Gorgas K., Sijmonsma T.P., Iwamori M., Finaz C., et al. Novel class of glycosphingolipids involved in male fertility. J. Biol. Chem. 2005;280:27310–27318. doi: 10.1074/jbc.M502775200. [DOI] [PubMed] [Google Scholar]
- 53.Wang G., Krishnamurthy K., Bieberich E. Regulation of primary cilia formation by ceramide. J. Lipid Res. 2009;50:2103–2110. doi: 10.1194/jlr.M900097-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rani C.S., Abe A., Chang Y., Rosenzweig N., Saltiel A.R., Radin N.S., Shayman J.A. Cell cycle arrest induced by an inhibitor of glucosylceramide synthase. Correlation with cyclin-dependent kinases. J. Biol. Chem. 1995;270:2859–2867. doi: 10.1074/jbc.270.6.2859. [DOI] [PubMed] [Google Scholar]
- 55.Kawamori T., Kaneshiro T., Okumura M., Maalouf S., Uflacker A., Bielawski J., Hannun Y.A., Obeid L.M. Role for sphingosine kinase 1 in colon carcinogenesis. FASEB J. 2009;23:405–414. doi: 10.1096/fj.08-117572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kohno M., Momoi M., Oo M.L., Paik J.H., Lee Y.M., Venkataraman K., Ai Y., Ristimaki A.P., Fyrst H., Sano H., et al. Intracellular role for sphingosine kinase 1 in intestinal adenoma cell proliferation. Mol. Cell. Biol. 2006;26:7211–7223. doi: 10.1128/MCB.02341-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ricker J.L., Mata J.E., Iversen P.L., Gattone V.H. c-myc antisense oligonucleotide treatment ameliorates murine ARPKD. Kidney Int. 2002;61:S125–131. doi: 10.1046/j.1523-1755.2002.0610s1125.x. [DOI] [PubMed] [Google Scholar]
- 58.Jo S.K., Bajwa A., Ye H., Vergis A.L., Awad A.S., Kharel Y., Lynch K.R., Okusa M.D. Divergent roles of sphingosine kinases in kidney ischemia-reperfusion injury. Kidney Int. 2009;75:167–175. doi: 10.1038/ki.2008.400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Park S.W., Kim M., D'Agati V.D., Lee H.T. Sphingosine kinase 1 protects against renal ischemia-reperfusion injury in mice by sphingosine-1-phosphate1 receptor activation. Kidney Int. 2011;80:1315–1327. doi: 10.1038/ki.2011.281. [DOI] [PubMed] [Google Scholar]
- 60.Edelstein C.L. What is the role of tubular epithelial cell apoptosis in polycystic kidney disease (PKD)? Cell Cycle. 2005;4:1550–1554. doi: 10.4161/cc.4.11.2185. [DOI] [PubMed] [Google Scholar]
- 61.Tao Y., Zafar I., Kim J., Schrier R.W., Edelstein C.L. Caspase-3 gene deletion prolongs survival in polycystic kidney disease. J. Am. Soc. Nephrol. 2008;19:749–755. doi: 10.1681/ASN.2006121378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kamada S., Shimono A., Shinto Y., Tsujimura T., Takahashi T., Noda T., Kitamura Y., Kondoh H., Tsujimoto Y. bcl-2 deficiency in mice leads to pleiotropic abnormalities: accelerated lymphoid cell death in thymus and spleen, polycystic kidney, hair hypopigmentation, and distorted small intestine. Cancer Res. 1995;55:354–359. [PubMed] [Google Scholar]
- 63.Nakayama K., Negishi I., Kuida K., Sawa H., Loh D.Y. Targeted disruption of Bcl-2 alpha beta in mice: occurrence of gray hair, polycystic kidney disease, and lymphocytopenia. Proc. Natl Acad. Sci. USA. 1994;91:3700–3704. doi: 10.1073/pnas.91.9.3700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Iakoubova O.A., Dushkin H., Beier D.R. Genetic analysis of a quantitative trait in a mouse model of polycystic kidney disease. Am. J. Respir. Crit. Care Med. 1997;156:S72–S77. doi: 10.1164/ajrccm.156.4.12-tac-0. [DOI] [PubMed] [Google Scholar]
- 65.Guay-Woodford L.M., Wright C.J., Walz G., Churchill G.A. Quantitative trait loci modulate renal cystic disease severity in the mouse bpk model. J. Am. Soc. Nephrol. 2000;11:1253–1260. doi: 10.1681/ASN.V1171253. [DOI] [PubMed] [Google Scholar]
- 66.Kuida S., Beier D.R. Genetic localization of interacting modifiers affecting severity in a murine model of polycystic kidney disease. Genome Res. 2000;10:49–54. [PMC free article] [PubMed] [Google Scholar]
- 67.Woo D.D., Nguyen D.K., Khatibi N., Olsen P. Genetic identification of two major modifier loci of polycystic kidney disease progression in pcy mice. J. Clin. Invest. 1997;100:1934–1940. doi: 10.1172/JCI119724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Fain P.R., McFann K.K., Taylor M.R., Tison M., Johnson A.M., Reed B., Schrier R.W. Modifier genes play a significant role in the phenotypic expression of PKD1. Kidney Int. 2005;67:1256–1267. doi: 10.1111/j.1523-1755.2005.00203.x. [DOI] [PubMed] [Google Scholar]
- 69.Paterson A.D., Magistroni R., He N., Wang K., Johnson A., Fain P.R., Dicks E., Parfrey P., St George-Hyslop P., Pei Y. Progressive loss of renal function is an age-dependent heritable trait in type 1 autosomal dominant polycystic kidney disease. J. Am. Soc. Nephrol. 2005;16:755–762. doi: 10.1681/ASN.2004090758. [DOI] [PubMed] [Google Scholar]
- 70.Persu A., Duyme M., Pirson Y., Lens X.M., Messiaen T., Breuning M.H., Chauveau D., Levy M., Grunfeld J.P., Devuyst O. Comparison between siblings and twins supports a role for modifier genes in ADPKD. Kidney Int. 2004;66:2132–2136. doi: 10.1111/j.1523-1755.2004.66003.x. [DOI] [PubMed] [Google Scholar]
- 71.Smith L.A., Bukanov N.O., Husson H., Russo R.J., Barry T.C., Taylor A.L., Beier D.R., Ibraghimov-Beskrovnaya O. Development of polycystic kidney disease in juvenile cystic kidney mice: insights into pathogenesis, ciliary abnormalities, and common features with human disease. J. Am. Soc. Nephrol. 2006;17:2821–2831. doi: 10.1681/ASN.2006020136. [DOI] [PubMed] [Google Scholar]
- 72.Natoli T.A., Gareski T.C., Dackowski W.R., Smith L., Bukanov N.O., Russo R.J., Husson H., Matthews D., Piepenhagen P., Ibraghimov-Beskrovnaya O. Pkd1 and Nek8 mutations affect cell-cell adhesion and cilia in cysts formed in kidney organ cultures. Am. J. Physiol. Renal Physiol. 2008;294:F73–F83. doi: 10.1152/ajprenal.00362.2007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.