Abstract
Introduction
Exercise is protective against ventricular arrhythmias induced by ischemia (I), the condition of inadequate blood flow, and reperfusion (R), the reestablishment of blood flow. This protection is observed clinically and scientifically by decreased incidence in ECG abnormalities. Numerous scoring systems exist for the quantification of ventricular arrhythmia severity. On the basis of preventricular contractions, ventricular tachycardia, and ventricular fibrillation frequency, these scoring systems are intended to provide more robust ECG outcome indicators than individual arrhythmia variables. Scoring systems vary primarily on continuous versus discontinuous treatment of the data, which should be considered when matching these arrhythmia metrics to scientific applications.
Purpose
The aim of this investigation was to evaluate seven ECG scoring systems in the assessment of ventricular arrhythmia severity after IR in male Sprague–Dawley rats.
Methods
Animals remained sedentary or exercised (3 d of treadmill exercise for 60 min) before surgically induced IR. A subset of sedentary animals served as sham, undergoing surgical procedure without IR. ECGs were evaluated under blinded conditions by three trained individuals. Single arrhythmia data and the parametric score were analyzed by one-way ANOVA, whereas the Kruskal–Wallis was used to compare group means for all nonparametric scoring systems between groups.
Results
IR produced a significant arrhythmic response in exercised and sedentary rats as determined by all arrhythmia scoring systems. Four arrhythmia metrics resulted in significant differences between exercised and sedentary treatments (P < 0.001), whereas three metrics did not.
Conclusions
Continuous versus discontinuous treatment of the data may account for variation in scoring system outcomes. These data confirm that exercise protects against IR-induced arrhythmias, and care must be taken when selecting an arrhythmia scoring system for ECG evaluation.
Keywords: ELECTROCARDIOGRAM, HEART ATTACK, PREVENTRICULAR CONTRACTION, VENTRICULAR
Exercise-induced cardioprotection against ischemia–reperfusion (IR) has been well established during the last 10–15 yr (2,12,19,20,30,32,33). Robust protection is elicited by extended-duration exercise regimens (months) as well as short-duration (days) exercise exposure (1,3–5,19,20,25,30,33). Cardioprotective adaptation to short-duration exercise exposure is similarly responsive to both moderate-intensity and high-intensity exercises, suggesting that the threshold for stimulus is relatively low (17). IR injury resistance is established against arrhythmia generation in addition to myocardial stunning and tissue death; although the underlying mechanisms are unique to each level of IR injury (1,3,18,23,26,32). Recent efforts in our laboratory use short-duration exercise and surgically induced IR in a rodent model to elucidate the mechanisms responsible for exercise-induced cardioprotection against IR-mediated ventricular arrhythmias (15,29). The scopes of these laboratory-based studies reveal a mechanistic understanding of the exercised heart in a clinically relevant manner.
The clinical relevance of these in vivo animal studies is emphasized when ECG-related outcome measures are used as key dependent study variables (6,9,10,14,15,21,27). In this regard, application of clinically based ECG scoring systems for ventricular arrhythmias has revealed several important considerations for use in exercise-based cardioprotection studies. First, hearts exposed to even 3 d of moderate-intensity treadmill running and the associated habituation period are more resistant to IR-generated arrhythmias compared with sedentary counterparts (11,15,21,27). Second, the magnitude of exercise-mediated protection is most apparent when the major ventricular arrhythmias, including premature ventricular contractions (PVCs), ventricular tachycardia (VT), and ventricular fibrillation (VF), are analyzed collectively rather than individually (15,27). Third, we proposed that the use of continuous parametric scoring equations provides a more robust evaluation of group differences for arrhythmic responses to IR than discontinuous nonparametric scoring systems (9). This notion is because parametric systems score on a continuum and prevent score clustering. Given the established model of exercise as a cardioprotective intervention against IR-induced arrhythmias, the experiments outlined in this article address these latter two points in novel detail.
For these experiments, we examined a single data set across seven clinically based ECG scoring systems previously applied to exercise cardioprotection research (Table 1) adapted from Curtis and Walker (9,10,15,27). Each of the seven scoring systems (A–G) assigns a numeric value based upon the severity of arrhythmia, with larger values indicating greater severity (9). Scoring systems A, B, C, D, E, and F are nonparametric, discontinuous systems, and score G is an equation-based parametric system. Quantification of arrhythmia severity is based on the occurrence of PVCs, VT, and VF, listed in order of individual severity (9). Variations between scoring methods include accounting for VT and VF by duration versus number of episodes, the inclusion of spontaneously and/or nonspontaneously converting VT and VF, as well as the numerical scoring scale (i.e., 0–6 vs 0–7) (9). The sensitivity of these ECG scoring systems in the identification of significant group differences in ECG outcomes is established (9). Yet to be determined, however, is the applicability of these various scoring systems to a physiologically meaningful paradigm of exercise-induced cardioprotection. Therefore, the purpose of this study was to evaluate the sensitivity of seven established ECG ventricular arrhythmia scoring systems in a model of exercise-induced cardioprotection.
TABLE 1.
ECG scoring systems (A–G) criteria.
| Scoring system A | |
| 0 | 0–49 PVCs |
| 1 | 50–499 PVCs |
| 2 | >499 PVCs and/or 1 episode of SVT or VF |
| 3 | >1 episode of VT or VF or both (<60-s combined duration) |
| 4 | VT or VF or both (60–119-s total combined duration) |
| 5 | VT or VF or both (>119-s total combined duration) |
| 6 | Fatal VF starting at >15 min after occlusion |
| 7 | Fatal VF starting between 4 and 14 min 59 s after occlusion |
| 8 | Fatal VF starting between 1 min and 3 min 59 s after occlusion |
| 9 | Fatal VF starting < 1 min after occlusion |
| Scoring system B | |
| 0 | <10 PVCs |
| 1 | >10 PVCs |
| 2 | 1–5 episodes of VT |
| 3 | >5 episodes VT and/or any number episodes SVF and/or 1 episode of NVF (provided VT and VF total duration is <40 s) |
| 4 | 2–5 episodes NVF provided VT and VF total duration <80 s |
| 5 | >5 episodes of NVF provided VT and VF total combined duration <160 s |
| 6 | VT or VF or both (total combined duration <300 s) |
| 7 | VT or VF or both (total combined duration >300 s) |
| Scoring system C | |
| 0 | No PVCs, VT, or VF |
| 1 | PVCs |
| 2 | 1–5 episodes of VT |
| 3 | >5 episodes of VT or 1 episode of VF or both |
| 4 | 2–5 episodes of VF |
| 5 | >5 episodes of NVF |
| Scoring system D | |
| 0 | <10 PVCs |
| 1 | 10–50 PVCs or 1 episode of VT or both |
| 2 | >50 PVCs or >1 episode of VT or both |
| 3 | SVF or 1 episode of NVF |
| 4 | 2–4 episodes of NVF |
| 5 | >4 episodes of NVF |
| Scoring system E | |
| 0 | <20 PVCs |
| 1 | 21–100 PVCs |
| 2 | >100 PVCs or 1–3 episodes of VT or both |
| 3 | >3 episodes of VT |
| 4 | SVF |
| 5 | 1 or 2 episodes of NVF |
| 6 | 3–5 episodes of NVF |
| 7 | >5 episodes of NVF |
| Scoring system F | |
| 0 | <50 PVCs |
| 1 | Q50 PVCs |
| 2 | 1–5 episodes of VT |
| 3 | Q6 episodes of VT |
| 4 | SVF or 1 episode of NVF or both |
| 5 | 2–5 episodes of NVF |
| 6 | >5 episodes of NVF |
| Scoring system G | |
| Score | (log10 PVCs) + (log10 episodes VT) + 2[(log10 episodes of VF) + (log10 total duration of VF)] |
Scoring system criterion modified from Curtis and Walker (9).
NVF, nonspontaneous converting VF.
METHODS
Animals and experimental design
The experimental protocol was approved by the Appalachian State University Animal Care and Use Committee and followed guidelines established by the American Physiological Society for the ethical use of animals in research and the animal care standards of the American College of Sports Medicine. Twenty-two male Sprague–Dawley rats were used in this study (n = 6–9/treatment group). Rats were randomly assigned to one of three experimental groups: 1) control–sedentary, 2) exercise training, or 3) sham–sedentary (no IR, surgical controls). During the experimental period, all animals were housed on a 12:12-h light–dark cycle and provided food (AIN93 diet) and water ad libitum.
Exercise training protocol
Animals assigned to the exercise group were habituated to the treadmill for five consecutive days. This habituation period involved a gradual increase in running time beginning with 10 min on day 1 and increased an additional 10 min·d−1 to 50 min of exercise on habituation day 5. After 2 d of rest, the exercise-habituated animals performed three consecutive days of treadmill exercise for 60 min·d−1 at 30 m·min−1, 0% grade (estimated work rate of ~70% maximum oxygen consumption). Exercise intensities are based on previous understanding of rodent metabolic responses to treadmill running (7,16). Rats were exposed to a surgical in vivo IR challenge 24 h after the conclusion of the 3-d exercise protocol.
Surgical procedure
Surgical procedure was followed as previously described (15,28,29). Briefly, rats were anesthetized with 60 mg·kg−1 sodium pentobarbital and ventilated with room air through a tracheotomy tube. Continuous direct measurement of blood pressure was recorded by computer software connected with a pressure transducer attached to a saline-filled catheter inserted into the right carotid artery (Biopac Student Lab Pro 2005, Goleta, CA). A catheter was also placed in the right jugular vein for administration of sodium pentobarbital as needed throughout the experimental procedure to maintain a surgical plane anesthesia. Left thoracotomy was performed to expose the left side of the heart. Rats were then exposed to an in vivo IR challenge consisting of 20 min of ischemia followed by 30 min of reperfusion or sham surgery (50 min of no ischemia). Ischemia was induced by placing a ligature around the left descending coronary artery; sham animals received ligature placement without tightening. Reperfusion was initiated by loosening of the ligature in sedentary and exercised animals. After 30 min of reperfusion, ligatures were replaced on all animals, and 1% Evans Blue dye was infused through the coronary artery catheter for visualization of the area at risk (AAR), defined as the area receiving no blood flow due to coronary artery occlusion. Hearts were then immediately excised and sliced into 1.5-mm-thick cross sections and imaged digitally for analysis (Kodak image analysis device, Rochester, NY) under blinded conditions for at risk (non-perfused) and perfused areas.
ECG analysis
Digital ECG tracings were recorded for all animals using Biopac Student Lab Pro 2005 software and analyzed by three trained blinded individuals for PVCs, VT, and VF in accordance with the recommendations of the Lambeth Conventions (31). PVCs were identified by the presence of a premature QRS complex; VT was classified as three or more consecutive PVCs. Seven ECG scoring methods (A–G) of Curtis and Walker (9) were used (Table 1). Score G is an equation-based parametric system, whereas scores A–F are nonparametric ECG assessments.
Data analysis
A one-way ANOVA was performed to assess group differences for all group-dependent measures and the parametric ECG scoring method (score G) with a Tukey post hoc when appropriate. Significance was established a priori at P ≤ 0.05. Nonparametric ECG scoring methods (scores A–F) were analyzed with Kruskal–Wallis nonparametric test and a Mann–Whitney post hoc test. Because of the lenient nature of this nonparametric post hoc, significance was established a priori at P ≤ 0.016 (P ≤ 0.05 per three groups) to minimize likelihood of committing type I errors (8). Data are presented as mean ± SEM unless otherwise stated.
RESULTS
Animal characteristics
Physical characteristics of the animals are presented in Table 2. No significant differences existed in body weight (P = 0.053), total heart weight (P = 0.369), ischemic AAR weight (P = 0.915), or perfused weight (P = 0.465). Percent of the total heart AAR (%AAR) was also not significantly different between groups (P = 0.905), indicating that all animals received a similar magnitude coronary occlusion.
TABLE 2.
Animal characteristic data.
| Group | Body Weight (g) | Heart Weight (g) | AAR Weight (g) | Perfused Weight (g) | % AAR (g) |
|---|---|---|---|---|---|
| Sham | 345.8 ± 16.9 | 1.24 ± 0.03 | 0.28 ± 0.01 | 0.97 ± 0.03 | 0.225 ± 0.009 |
| Sedentary | 345.0 ± 14.3 | 1.25 ± 0.05 | 0.30 ± 0.05 | 0.94 ± 0.06 | 0.243 ± 0.098 |
| Exercised | 305.4 ± 9.8 | 1.32 ± 0.04 | 0.30 ± 0.03 | 1.02 ± 0.04 | 0.229 ± 0.021 |
Values are group means ± SE.
No significant differences were found between groups for any variable, P > 0.05.
Ventricular arrhythmia analysis
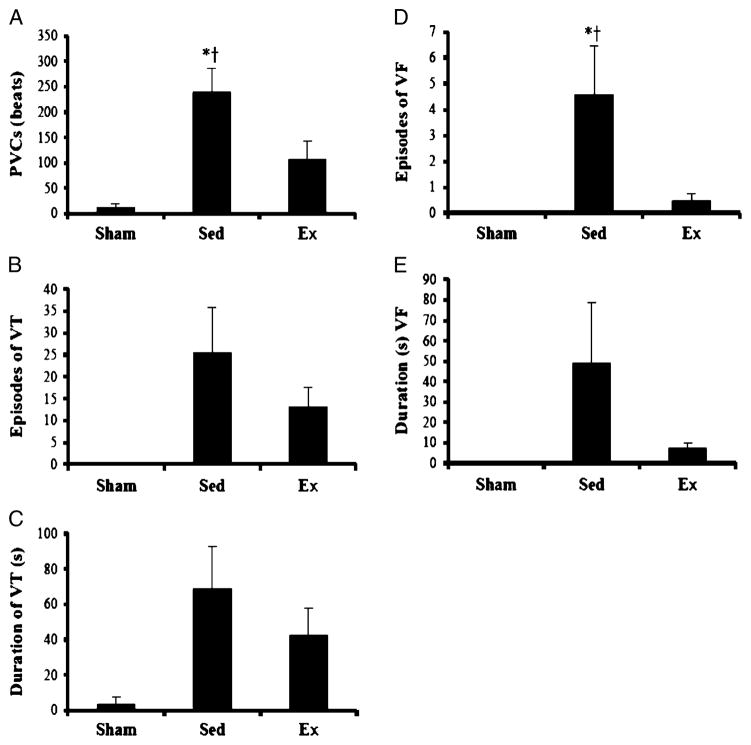
Group means for ventricular arrhythmia occurrence and duration are presented in Figure 1. Incidences of PVCs were significantly different between groups (P = 0.002). The PVC incidence of the sedentary group was significantly greater compared with sham and exercise groups; however, a significant difference was not found between sham and exercise groups. The episodes and duration of VT approached significance between groups (P = 0.052 and P = 0.060). Episodes of VF was significantly different between groups (P = 0.010). The sedentary group experienced significantly more episodes of VF compared with both sham and exercise groups, whereas no significant difference was found between sham and exercise groups. Statistical differences were not observed between groups for duration of VF (P = 0.095).
FIGURE 1.
Average arrhythmia for each group; values are means ± SEM. Significantly different from *sham and †exercise, P < 0.05. A. PVCs. B. Episodes of VT. C. Duration of VT (s). D. Episodes of VF. E. Duration of VF (s).
Parametric ECG scoring systems
Group means are reported in Table 3. The parametric scoring system (score G) indicated significant differences between groups (P ≤ 0.001). The sedentary group score was significantly elevated from both sham and exercise groups. A significant difference was also indicated between the sham and exercise groups.
TABLE 3.
Nonparametric and parametric scoring systems.
| n | Nonparametric
|
Parametric
|
||||||
|---|---|---|---|---|---|---|---|---|
| A | B | C | D | E | F | G | ||
| Sham | 6 | 0.00 ± 0.00 | 0.10 ± 0.22 | 0.40 ± 0.54 | 0.20 ± 0.45 | 0.10 ± 0.22 | 0.10 ± 0.22 | 0.29 ± 0.41† |
| Sedentary | 7 | 3.50 ± 0.84* | 4.83 ± 0.93*† | 5.00 ± 0.00* | 3.33 ± 0.82* | 5.40 ± 0.99*† | 4.78 ± 0.51*† | 8.76 ± 2.49*† |
| Exercised | 9 | 2.63 ± 1.90 | 2.13 ± 1.48 | 3.00 ± 2.29 | 2.00 ± 1.35 | 2.29 ± 1.67 | 2.29 ± 1.67 | 3.59 ± 2.64* |
Values are group means ± SEM.
Nonparametric scores significantly different from * sham and † exercise, P < 0.016.
Parametric scores significantly different from * sham and † exercise, P < 0.05.
Nonparametric ECG scoring systems
Group means are reported in Table 3. Three of the nonparametric scoring systems (A, C, and D) indicated a significantly elevated score for the sedentary group compared with sham (P < 0.016), without significant differences between exercise and sham or sedentary groups. Three of the six nonparametric scoring systems (B, E, and F) indicated a significant difference between sedentary and sham groups, as well as between sedentary and exercise groups (P < 0.016). No significant differences between exercise and sham groups were indicated by the six nonparametric scoring systems.
DISCUSSION
Principal findings
Using an established animal model of exercise-induced cardioprotection against IR-induced ventricular arrhythmias (15,29), our aim was to compare the sensitivity of seven (one parametric and six nonparametric) ECG scoring systems to ECG tracings from hearts exposed to IR in vivo. Examination of a single data set of ventricular arrhythmias across the seven scoring methods demonstrated sensitivity differences exist in regard the detection of statistically meaningful differences between sham, sedentary, and exercise treatments. Only the parametric scoring system (score G) indicated significant differences between each group and, therefore, may be advantageous in that it provides a greater level of sensitivity compared with other nonparametric scoring systems. Three of the six nonparametric scoring systems (B, E, and F) indicated significant differences between the sham and sedentary groups, as well as between sedentary and exercise groups, whereas the other three (A, C, and D) only indicated significant differences between sham and sedentary groups. None of the nonparametric scoring systems indicated a significant difference between sham and exercise groups. Given these sensitivity differences between individual systems in the context of exercise-induced cardioprotection, care should be used when applying a scoring system to a particular experimental design. Further discussion of the application of these findings proceeds in the following sections.
Exercise antiarrhythmic protection and ECG scoring systems
We evaluated seven clinically relevant ECG scoring systems in the context of exercise-derived protection against IR-induced ventricular arrhythmias. This approach is founded on the fact that exercise is a potent stimulus of protection against IR-induced arrhythmias (14,15,21,29). Functional and clinically relevant outcome measures of exercise-induced cardioprotection research are needed in the search to discover protective mechanisms involved in this observed protection. The ECG is a clinically relevant outcome measure that has been repeatedly used by our laboratory, as well as others (6,14,15,21,29). ECG analysis is commonly used as a prognostic indicator in human medicine (22,24) and is also used in research models as an indicator of IR damage severity (15,27).
Although arrhythmias may be analyzed singly (PVCs, VT, and VF), this quantitative approach does not lend itself well to clinical and research scenarios when arrhythmic frequency and severity may be vast. Examination of single arrhythmias in the current data set revealed significant increases in PVC and VF incidences in sedentary animals compared with sham and exercise groups, whereas VT incidence was not significantly different between groups (Fig. 1). Furthermore, exercised animals did not differ from sham animals in PVC, VT, or VF incidences. Thus, there is inconsistency between the physiologic benefit of exercise and the statistically meaningful outcomes of individual arrhythmia analysis.
Based decades of medical science practice, we approached the current investigation with the working assumption that an ECG scoring metric is more sensitive than quantification of individual ventricular events (e.g., PVCs alone) for understanding the clinical relevance of IR-induced arrhythmias. To illustrate this point, examination of individual ECG arrhythmias, without the ECG scoring metrics, does not reveal statistically meaningful group differences. On the basis of the mean differences and respective SDs in the current study, many more experimental observations are required to find significant group differences for PVCs (n = 12–18 per group), VT episodes and duration (n = 18–27 per group), and VF episodes (n = 48–64 per group). Collectively, these data illustrate the need for arrhythmia scoring systems where analysis of the collective arrhythmic response provides a more robust evaluation of the ventricular arrhythmias experienced.
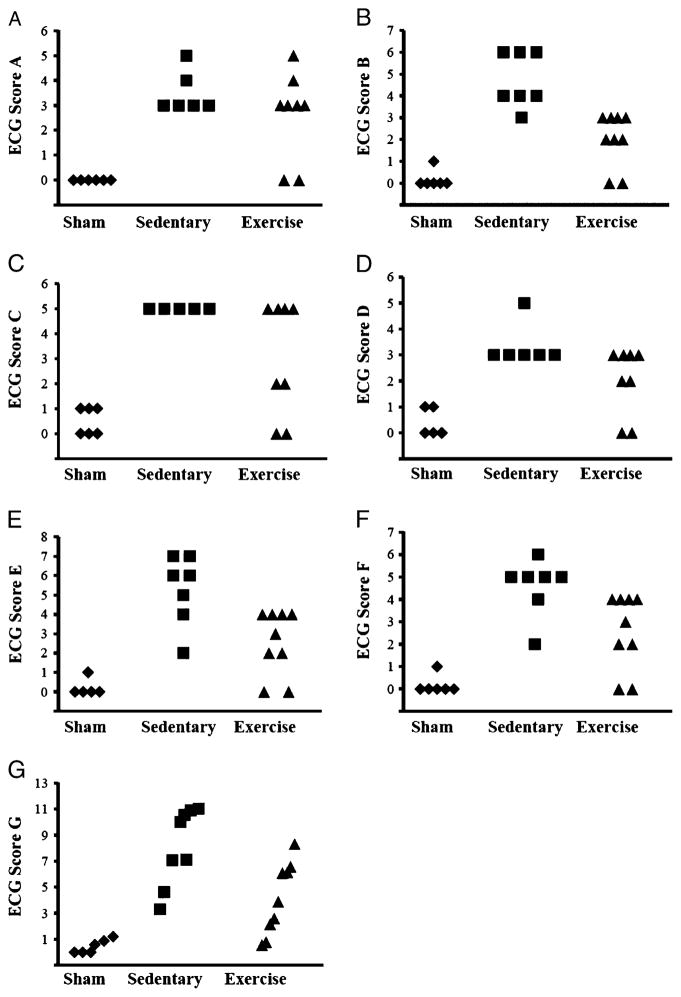
Existing scoring systems generally differ according to parametric and nonparametric treatment of data analysis. This approach is generally based on which arrhythmic events are included and the severity/frequency of these episodes. We found the parametric scoring system (score G) was sensitive to group differences with a relatively low group number (n = 6–9). Nonparametric systems are generally advantageous in that they enable rapid categorical analysis of arrhythmia load, which can include considerations of mortality (9). However, our results suggest that nonparametric derived scores can suffer from a lack of sensitivity when scores are tiered on a discontinuous scoring system. The categorical score clustering (scores A–F) versus score continuum (score G) for ventricular arrhythmias experienced in the current study are illustrated in Figure 2. The prevention of score clustering by the parametric scoring system, as visualized in Figure 2, may increase the sensitivity to group differences. Our data illustrate that three nonparametric scoring systems (A, C, and D) indicate significance between only sedentary and sham groups but fail to find exercise–sedentary differences because of score clustering. While nonparametric scoring systems B, E, and F did observe sham–sedentary and sedentary–exercise group differences for this particular data set, it is worth noting that alternative outcomes may be observed in other clinical or scientific applications. Because outcome variations across these nonparametric scoring systems is dependent on the types and frequency of arrhythmias (up to and including death) of a given experimental paradigm, one could conceivably avoid the confusion described in this study by careful selection of the metric to match an experimental design. It is conceivable, however, that many researchers may not be able to identify which of the similar nonparametric scoring systems is the most valid fit to their experimental model. Only the parametric scoring system indicated a significant difference between each group at the current sample size.
FIGURE 2.
Representation of tiered discontinuous nonparametric scoring systems and linear equation-based parametric scoring system. ◆, sham; ■, sedentary; ▲, exercise.
As with the analysis of individual ventricular arrhythmias described earlier, the number of sampling observations will affect study outcomes from a given scoring system. We determined that doubling the sample size (n = 12–18) would have resulted in significant differences between each group for five of the nonparametric scoring systems (B, C, D, E, and F), whereas score A would have required eight times the observation number (n = 48–72). Given that this study model uses a nonsurvival IR insult in an animal model, scientists are ethically obliged to minimize the number of animals needed to complete their research (13). This mandate of reduction was best observed using the continuous scoring system G, where fewer animals were required to observed exercise-derived treatment effects in comparison to sedentary counterparts.
CONCLUSIONS
The use of ECG scoring systems in an in vivo model of exercise-derived protection against IR-induced arrhythmia may serve as a powerful tool to elucidate group differences in arrhythmic load. Data from the current study suggest that minor variations between the systems may make interstudy comparisons difficult because of differences in sensitivity. Our data demonstrate that the parametric scoring system provides greater sensitivity by using equation-based outcomes compared with nonparametric data treatment by preventing tiered data clustering. These findings indicate that subtle but important variation in scoring system sensitivity exists between individual metrics, and care should be used when selecting a scoring system in exercise-induced cardioprotection research. Our data support the notion that an equation-based parametric scoring system is the most sensitive to intergroup differences. Thus, we propose the use of parametric scoring systems in research applications of the exercise-induced cardioprotection model similar to the current investigation. Moreover, application of the current findings may benefit future investigations of exercise and antiarrhythmic protection. Examination of ECG outcome differences across age, sex, animal strain, experimental treatment, or exercise protocol differences would be best served by application of the most sensitive analytical metrics.
Acknowledgments
This work was supported by grants from the National Institutes of Health (HL087256) awarded to Dr. Quindry, Appalachian State University Research Council.
Footnotes
The authors declare no conflict of interest.
The results of this study do not constitute endorsement by the American College of Sports Medicine.
References
- 1.Bowles DK, Farrar RP, Starnes JW. Exercise training improves cardiac function after ischemia in the isolated, working rat heart. Am J Physiol. 1992;263(3 pt 2):H804–9. doi: 10.1152/ajpheart.1992.263.3.H804. [DOI] [PubMed] [Google Scholar]
- 2.Bowles DK, Starnes JW. Exercise training improves metabolic response after ischemia in isolated working rat heart. J Appl Physiol. 1994;76(4):1608–14. doi: 10.1152/jappl.1994.76.4.1608. [DOI] [PubMed] [Google Scholar]
- 3.Brown DA, Chicco AJ, Jew KN, et al. Cardioprotection afforded by chronic exercise is mediated by the sarcolemmal, and not the mitochondrial, isoform of the KATP channel in the rat. J Physiol. 2005;569(pt 3):913–24. doi: 10.1113/jphysiol.2005.095729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brown DA, Jew KN, Sparagna GC, Musch TI, Moore RL. Exercise training preserves coronary flow and reduces infarct size after ischemia–reperfusion in rat heart. J Appl Physiol. 2003;95(6):2510–8. doi: 10.1152/japplphysiol.00487.2003. [DOI] [PubMed] [Google Scholar]
- 5.Chicco AJ, Johnson MS, Armstrong CJ, et al. Sex-specific and exercise-acquired cardioprotection is abolished by sarcolemmal KATP channel blockade in the rat heart. Am J Physiol Heart Circ Physiol. 2007;292(5):H2432–7. doi: 10.1152/ajpheart.01301.2006. [DOI] [PubMed] [Google Scholar]
- 6.Collins HL, Loka AM, Dicarlo SE. Daily exercise-induced cardioprotection is associated with changes in calcium regulatory proteins in hypertensive rats. Am J Physiol Heart Circ Physiol. 2005;288(2):H532–40. doi: 10.1152/ajpheart.00873.2004. [DOI] [PubMed] [Google Scholar]
- 7.Criswell D, Powers S, Dodd S, et al. High intensity training–induced changes in skeletal muscle antioxidant enzyme activity. Med Sci Sports Exerc. 1993;25(10):1135–40. [PubMed] [Google Scholar]
- 8.Cronk BC. How to Use SPSS: A Step-by-step Guide to Analysis and Interpretation. 5. Glendale (CA): Pyrczak Publishing; 2008. pp. 90–3. [Google Scholar]
- 9.Curtis MJ, Walker MJ. Quantification of arrhythmias using scoring systems: an examination of seven scores in an in vivo model of regional myocardial ischaemia. Cardiovasc Res. 1988;22(9):656–65. doi: 10.1093/cvr/22.9.656. [DOI] [PubMed] [Google Scholar]
- 10.Das B, Sarkar C. Mitochondrial K ATP channel activation is important in the antiarrhythmic and cardioprotective effects of non-hypotensive doses of nicorandil and cromakalim during ischemia/reperfusion: a study in an intact anesthetized rabbit model. Pharmacol Res. 2003;47(6):447–61. doi: 10.1016/s1043-6618(02)00335-3. [DOI] [PubMed] [Google Scholar]
- 11.Demirel HA, Powers SK, Zergeroglu MA, et al. Short-term exercise improves myocardial tolerance to in vivo ischemia–reperfusion in the rat. J Appl Physiol. 2001;91(5):2205–12. doi: 10.1152/jappl.2001.91.5.2205. [DOI] [PubMed] [Google Scholar]
- 12.French JP, Quindry JC, Falk DJ, et al. Ischemia–reperfusion-induced calpain activation and SERCA2a degradation are attenuated by exercise training and calpain inhibition. Am J Physiol Heart Circ Physiol. 2006;290(1):H128–36. doi: 10.1152/ajpheart.00739.2005. [DOI] [PubMed] [Google Scholar]
- 13.Grossblatt N, editor. Guide for the Care and Use of Laboratory Animals. 8. Washington (DC): National Academy Press; 2010. pp. 1–20. [Google Scholar]
- 14.Hajnal A, Nagy O, Litvai A, Papp J, Parratt JR, Vegh A. Nitric oxide involvement in the delayed antiarrhythmic effect of treadmill exercise in dogs. Life Sci. 2005;77(16):1960–71. doi: 10.1016/j.lfs.2005.02.015. [DOI] [PubMed] [Google Scholar]
- 15.Hamilton KL, Quindry JC, French JP, et al. MnSOD antisense treatment and exercise-induced protection against arrhythmias. Free Radic Biol Med. 2004;37(9):1360–8. doi: 10.1016/j.freeradbiomed.2004.07.025. [DOI] [PubMed] [Google Scholar]
- 16.Lawler JM, Powers SK, Hammeren J, Martin AD. Oxygen cost of treadmill running in 24-month-old Fischer-344 rats. Med Sci Sports Exerc. 1993;25(11):1259–64. [PubMed] [Google Scholar]
- 17.Lennon SL, Quindry JC, French JP, Kim S, Mehta JL, Powers SK. Exercise and myocardial tolerance to ischaemia–reperfusion. Acta Physiol Scand. 2004;182(2):161–9. doi: 10.1111/j.1365-201X.2004.01346.x. [DOI] [PubMed] [Google Scholar]
- 18.Lennon SL, Quindry JC, Hamilton KL, et al. Elevated MnSOD is not required for exercise-induced cardioprotection against myocardial stunning. Am J Physiol Heart Circ Physiol. 2004;287(2):H975–80. doi: 10.1152/ajpheart.01208.2003. [DOI] [PubMed] [Google Scholar]
- 19.Locke M, Tanguay RM, Klabunde RE, Ianuzzo CD. Enhanced postischemic myocardial recovery following exercise induction of HSP 72. Am J Physiol. 1995;269(1 pt 2):H320–5. doi: 10.1152/ajpheart.1995.269.1.H320. [DOI] [PubMed] [Google Scholar]
- 20.McElroy CL, Gissen SA, Fishbein MC. Exercise-induced reduction in myocardial infarct size after coronary artery occlusion in the rat. Circulation. 1978;57(5):958–62. doi: 10.1161/01.cir.57.5.958. [DOI] [PubMed] [Google Scholar]
- 21.Nagy O, Hajnal A, Parratt JR, Vegh A. Delayed exercise-induced protection against arrhythmias in dogs—effect of celecoxib. Eur J Pharmacol. 2004;499(1–2):197–9. doi: 10.1016/j.ejphar.2004.07.068. [DOI] [PubMed] [Google Scholar]
- 22.Nikus KC, Birnbaum Y. Symposium on electrocardiogram in myocardial ischemia and infarction. J Electrocardiol. 2009;42(1):1–5. doi: 10.1016/j.jelectrocard.2008.10.003. [DOI] [PubMed] [Google Scholar]
- 23.Noakes TD, Higginson L, Opie LH. Physical training increases ventricular fibrillation thresholds of isolated rat hearts during normoxia, hypoxia and regional ischemia. Circulation. 1983;67(1):24–30. doi: 10.1161/01.cir.67.1.24. [DOI] [PubMed] [Google Scholar]
- 24.Pahlm US, Chaitman BR, Rautaharju PM, Selvester RH, Wagner GS. Comparison of the various electrocardiographic scoring codes for estimating anatomically documented sizes of single and multiple infarcts of the left ventricle. Am J Cardiol. 1998;81(7):809–15. doi: 10.1016/s0002-9149(98)00016-2. [DOI] [PubMed] [Google Scholar]
- 25.Powers SK, Demirel HA, Vincent HK, et al. Exercise training improves myocardial tolerance to in vivo ischemia–reperfusion in the rat. Am J Physiol. 1998;275(5 pt 2):R1468–77. doi: 10.1152/ajpregu.1998.275.5.R1468. [DOI] [PubMed] [Google Scholar]
- 26.Quindry J, French J, Hamilton K, Lee Y, Mehta JL, Powers S. Exercise training provides cardioprotection against ischemia–reperfusion induced apoptosis in young and old animals. Exp Gerontol. 2005;40(5):416–25. doi: 10.1016/j.exger.2005.03.010. [DOI] [PubMed] [Google Scholar]
- 27.Quindry JC, French J, Hamilton KL, Lee Y, Selsby J, Powers S. Exercise does not increase cyclooxygenase-2 myocardial levels in young or senescent hearts. J Physiol Sci. 2010;60(3):181–6. doi: 10.1007/s12576-009-0082-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Quindry JC, Hamilton KL, French JP, et al. Exercise-induced HSP-72 elevation and cardioprotection against infarct and apoptosis. J Appl Physiol. 2007;103(3):1056–62. doi: 10.1152/japplphysiol.00263.2007. [DOI] [PubMed] [Google Scholar]
- 29.Quindry JC, Schreiber L, Hosick P, Wrieden J, Irwin JM, Hoyt E. Mitochondrial KATP channel inhibition blunts arrhythmia protection in ischemic exercised hearts. Am J Physiol Heart Circ Physiol. 2010;299(1):H175–83. doi: 10.1152/ajpheart.01211.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Starnes JW, Taylor RP, Park Y. Exercise improves postischemic function in aging hearts. Am J Physiol Heart Circ Physiol. 2003;285(1):H347–51. doi: 10.1152/ajpheart.00952.2002. [DOI] [PubMed] [Google Scholar]
- 31.Walker MJ, Curtis MJ, Hearse DJ, et al. The Lambeth Conventions: guidelines for the study of arrhythmias in ischaemia infarction, and reperfusion. Cardiovasc Res. 1988;22(7):447–55. doi: 10.1093/cvr/22.7.447. [DOI] [PubMed] [Google Scholar]
- 32.Yamashita N, Hoshida S, Otsu K, Asahi M, Kuzuya T, Hori M. Exercise provides direct biphasic cardioprotection via manganese superoxide dismutase activation. J Exp Med. 1999;189(11):1699–706. doi: 10.1084/jem.189.11.1699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang KR, Liu HT, Zhang HF, et al. Long-term aerobic exercise protects the heart against ischemia/reperfusion injury via PI3 kinase–dependent and Akt-mediated mechanism. Apoptosis. 2007;12(9):1579–88. doi: 10.1007/s10495-007-0090-8. [DOI] [PubMed] [Google Scholar]