Introduction
Our most cherished sense, vision, begins with the process of phototransduction, a process performed by the highly specialized photoreceptor cells of the retina: the rods and cones (Rodieck, 1998). Rod photoreceptor cells are needed for night vision, as they are able to respond to very few photons, while cones are active under bright light conditions and are responsible for color vision. Many people are born with impaired vision, and many others experience loss of vision later in life. For example, age-related macular degeneration (AMD) occurs in ∼5% of people 80 years old in the U.S. (Rudnicka et al., 2012), while mutations in >200 genes can lead to loss of vision in younger individuals (RetNet; www.sph.uth.tmc.edu/Retnet). This review is focused on emerging strategies that employ gene therapy to combat vision loss, primarily due to mutations that affect photoreceptor cells. Exciting early results from the use of Adeno-associated virus (AAV) vectors in humans to combat Leber's congenital amaurosis (LCA) (Bainbridge et al., 2008; Maguire et al., 2008), a relatively rare form of congenital blindness, has inspired several groups to employ AAV in a number of ways to combat other genetic diseases. Here, the efforts aimed toward one particular disease, retinitis pigmentosa (RP) (Hartong et al., 2006), will be covered, as there are excellent animal models (Rivas and Vecino, 2009; Fletcher et al., 2011) and it offers some straightforward possibilities to save photoreceptor function and/or photoreceptor cells themselves. Ironically, one of the possibilities is to use optogenetics, rather than gene replacement or knock-down, for restoration of vision (Busskamp and Roska, 2011). It is hoped that we can apply the lessons learned from RP to other diseases that also result in loss of photoreceptor cells, including AMD.
Individuals with RP are typically born night-blind, due to rod dysfunction, but initially have full-field, high-acuity color vision. This is in keeping with the expression of many RP disease genes only in rods (www.sph.uth.tmc.edu/Retnet). For reasons that are still unclear (see Overview of therapeutic approaches to RP, below), loss of color vision then follows, and can occur as early as 5 years of age, or much later, in the fifth or sixth decade of life (Hartong et al., 2006; Berson, 2008). The progression of cone dysfunction and death eventually leads to total loss of vision, with the final loss in the center, due to loss of macular vision. The macula comprises only cones in its very center, and is the area of our highest acuity color vision. Invasion of retinal pigmented epithelial cells into the retina leads to the occurrence of black clumps within the retina, and hence the name of the disease. Attenuated retinal blood vessels and optic disc pallor are other hallmarks of the disease (Milam et al., 1998; Berson, 2008). There is no cure for RP, but there are several lines of therapeutic approaches that hold promise, including gene therapy.
Overview of therapeutic approaches to RP
Since many RP genes are expressed only in rods, one line of genetic therapy is to provide rods with a wild-type allele of a recessive disease gene, or to provide for an allele-specific loss of function (RNAi or ribozyme) in the case of a dominant disease gene. Vectors expressing such genes or knock-down cassettes have been developed and tested in animal models (LaVail et al., 2000; Schlichtenbrede et al., 2003; Pang et al., 2008; Chadderton et al., 2009; Raz-Prag et al., 2009; Palfi et al., 2010; Zou et al., 2011). While this approach may be successful for individual disease genes, and is a good one to establish the use of gene therapy vectors for ocular diseases, it likely will not be possible to extend such a targeted approach to all disease genes. The cost of clinical trials for gene therapy is quite high, and the number of individuals who can be treated for any individual disease gene is small. A more cost-effective strategy would be to develop therapies that can be used for people who have any of a larger number of disease genes. A generic way to prolong rod survival, even without rescuing their function, should prohibit the onset of cone death, which follows rod death. Similarly, gene therapy directly targeting cones to promote their function/survival should enable the treatment of individuals with many different disease genes.
As rods malfunction and die, there is a loss of cone function, followed by cone death. Several models for cone death in RP have been proposed (for review, see Punzo et al., 2011). Rods may supply a needed factor or factors for cone survival (Léveillard et al., 2004). This sets up a possible therapy, i.e., delivery of a growth factor, which is an approach currently in clinical trials (Sieving et al., 2006). Another class of models concerns toxicity due to rod death (Ripps, 2002). The release of a toxic factor by dying rods might kill the nearby cones. We believe that the kinetics of rod and cone death make this latter model unlikely (Punzo et al., 2009). If dying rods released a toxin, one would predict that there would be a close temporal and spatial association between rod and cone death. However, cone death often does not occur until many months after rod death (Carter-Dawson et al., 1978; Milam et al., 1998; Berson, 2008; Lin et al., 2009; Punzo et al., 2009). Another model holds that there is an increase in oxidative damage to cones once the rods have died (Yu et al., 2004; Shen et al., 2005; Komeima et al., 2006). This model also suggests a therapy, that is, delivery of antioxidants or genes encoding antioxidation enzymes. Finally, we recently proposed that the cones have a nutrient shortage and/or imbalance in metabolism due to a change in retinal architecture, brought on by the loss of the rods (Punzo et al., 2009, 2011).
Current strategies that might be more generic in their application, and use gene therapy, are considered in more detail in the following sections. Other approaches, such as engraftment of retinal neurons derived from stem cells (Comyn et al., 2010; Bermingham-McDonogh and Reh, 2011; Rowland et al., 2012) and implantation of prosthetic devices (Chader et al., 2009), are also being developed, but are beyond the scope of this paper. It is likely that some very effective therapies will be combinatorial, such as engineering engrafted photoreceptor cells to secrete growth factors. An example of a combinatorial strategy, one which combines sophisticated electronic devices with AAV-mediated delivery of a light-sensitive protein, will be described further in the last few sections.
Treatment with growth factors
An attractive model for cone death posits that there is loss of a trophic factor for cones that is made by rods (Mohand-Said et al., 1998, 2000; Léveillard and Sahel, 2010). Sahel and colleagues (Fintz et al., 2003; Léveillard et al., 2004) discovered a candidate for such a factor, RdCVF [rod-derived cone viability factor; also named nucleoredoxin 1 (Nxn1)]. Characterization of a recent mouse knock-out for this gene showed that cones in the mutant are more susceptible to oxidative damage (Cronin et al., 2010). Interestingly, this gene has a domain with homology to thioredoxin, which has a role in establishing the redox state of cells through a thiol-oxydoreductase activity. However, a short isoform of RdCVF, which does not have the thiol-oxydoreductase activity, is nonetheless able to promote cone survival (Léveillard et al., 2004). RdCVF protein has been injected into the eye of an RP rat model where it has shown survival-promoting activity for cones, along with preservation of cone activity (Yang et al., 2009). However, the protein is difficult to purify, and frequent delivery of any protein is problematic. Currently, an AAV vector is being developed to bypass the problem of protein purification and delivery (J. Sahel, personal communication).
Other growth factors have been tested by injection of the factors themselves into genetic models or into retinas where photoreceptor cells have been acutely damaged by light (LaVail et al., 1998). BDNF, CNTF, bFGF, and PEDF have activity when delivered to the subretinal space. CNTF is the factor that is furthest along (Wen et al., 2012), as it is now in clinical trials for RP (Sieving et al., 2006; Raz-Prag et al., 2009; Talcott et al., 2011) and AMD (K. Zhang et al., 2011). It also shows survival-promoting activity for retinal ganglion cells, the cell type affected in glaucoma (Hellström and Harvey, 2011). CNTF is being delivered by encapsulated cells that secrete the factor (Tao, 2006). The semipermeable membrane containing the cells is inserted into the ciliary margin, where it does not interfere with vision. This arrangement allows for the possibility of removal should any untoward effects develop.
Treatment with antioxidants
As mentioned above, oxidative stress has been suggested as one of the causes of cone dysfunction and death in RP (Yu et al., 2004; Shen et al., 2005; Komeima et al., 2006). As the rods die in RP, the flow of oxygen from the choroidal circulation does not abate, and thus each cone is exposed to increasing amounts of oxygen (Yu et al., 2000, 2004). Studies of the levels of oxidized proteins, nucleic acids, and lipids have shown that oxidative damage increases during the course of RP (Shen et al., 2005; Komeima et al., 2006). Oxidative stress is likely not only correlated with photoreceptor degeneration in RP, but contributes to it. Cone death was slowed in several mouse models of RP that were treated with exogenous antioxidants (Komeima et al., 2006, 2007). AAV delivery of endogenous antioxidant enzymes, including superoxide dismutase and glutathione peroxidase, in some RP mouse models decreased oxidative damage and prolonged cone survival (Lu et al., 2009; Usui et al., 2009). Also in keeping with the beneficial effects of antioxidant enzymes, a recent study showed that low-dose irradiation prolonged photoreceptor survival (Otani et al., 2012). The gene encoding the antioxidation enzyme, peroxiredoxin 2, was upregulated by this treatment, and its activity was required for the survival-promoting effect. Gene therapy directed to the photoreceptor cells will solve one problem posed by the delivery of chemical antioxidants through, e.g., the diet. The blood–retinal barrier, and the soluble nature of many of these compounds, does not enable a high, steady-state level of the antioxidants in the retina. Moreover, ROS are important signaling molecules and a wholesale decrease in ROS might not be without side effects, particularly with exposure of the entire body to these chemicals (Finkel, 2003). Viral gene delivery, ideally coupled with a cone-specific promoter, might provide a more effective approach, one that might be especially beneficial if the promoter was also regulated by the oxidation level of the tissue. Such vectors are being developed for use in other diseases and could be adapted for use in RP (Qi et al., 2007; Koilkonda et al., 2010).
Optogenetics
Seven transmembrane proteins that can capture the energy of light are ancient proteins that have evolved into many different forms. Some of these proteins are ion channels, some are ion pumps, and some are able to initiate a signal transduction cascade that can lead to changes in membrane polarization (Bamann et al., 2010; F. Zhang et al., 2011). Our own opsin proteins partner with a chromophore, 11-cis-retinal, to give our photoreceptor cells light sensitivity. Following a successful isomerization reaction, there is a series of signal transduction events that amplify this initial signal to the point where neurotransmitter release is altered. Rods and cones transduce the light signal so as to hyperpolarize, and thus reduce their rate of release of glutamate. An archaebacterium, Natronomonas pharaonis, uses its light-sensitive protein, halorhodopsin (NpHR), in a similar manner, as it too hyperpolarizes in response to light. In this case, the protein is a chloride pump, and thus does not need the elaborate signal transduction cascade used by photoreceptor cells. Another light-sensitive channel, channel rhodopsin2 (ChR2), from Chlamydomonas rheinhardtii, is a light-activated ion channel that triggers depolarization by the passage of sodium and other positively charged ions. When expressed in a neuron, this channel mimics neuronal activation. The utility of ChR2 for light activation of neurons has been exploited for many basic science applications (Kolstad et al., 2010; Fenno et al., 2011) and, more recently, is being considered for clinical applications.
The optogenetic approaches target several different cell types within the retina: the photoreceptor cells (Busskamp et al., 2010), the bipolar interneurons (Lagali et al., 2008; Doroudchi et al., 2011), and the ganglion cells (Bi et al., 2006; Lin et al., 2008; Ivanova and Pan, 2009; Zhang et al., 2009; Ivanova et al., 2010) (Table 1, Fig. 1). The retina has evolved a complex set of circuits (Gollisch and Meister, 2010) involving >60 cell types (Masland, 2001). It is not a camera, nor is it a simple filter that passes on fairly raw information to the brain. The information transformations performed by the local retinal circuits are still being discovered, as are the patterns of connections within these circuits. The type of information that is transmitted is highly processed to extract features of value to the organism. For example, there are multiple circuits that signal the direction of motion, or predict the path of an object in motion, or signal that an object is approaching. All of these circuits work with the initial signals emanating from photoreceptor cells. These signals are passed through bipolar interneurons, then onto the output cells, the ganglion cells, with inhibition along the way from horizontal cells and the many types of amacrine cells. Thus, when considering how to use optogenetics to preserve as much vision as possible, it is best to initiate the signal in photoreceptor cells, so as to use all of the downstream processing. In RP, histological characterizations suggested that the inner retinal cells survived for quite some time, even after photoreceptor death (Santos et al., 1997; Mazzoni et al., 2008; Sekirnjak et al., 2009). It was not known, however, if the circuits were still functional, particularly as changes in the processes of inner retinal cells had been noted (Marc et al., 2003). The studies using AAV to deliver optogenetic proteins to different retinal cell types suggest that at least some of the circuits are still functional, even after most photoreceptor cells have died (Table 1).
Table 1.
Optogenetic genes delivered to the retina
| Gene | Species (genotype) | Route | Transduced cells | Promoter | Assay | Reference |
|---|---|---|---|---|---|---|
| ChR2-GFP | Marmoset (WT)** | V | RGC and AII AC | CAG, CMV | Physiology | Ivanova, 2010 |
| ChR2-GFP | Mice (WT) | V | CAG | Physiology | Ivanova, 2009 | |
| ChR2-GFP | Mice (rd1) | V | RGCs, some ACs, HCs, few BP | CAG | Physiology, VEP | Bi, 2006 |
| Mice (WT) | ||||||
| Rats (WT) | ||||||
| ChR2-GFP | Mice (rd1) | V | RGCs, some ACs, HCs, few BP and PRs | CMV | Physiology | Zhang, 2009 |
| Mice (WT) | ||||||
| ChR2-GFP | Mice (rd1) | SR* | ON BP | GRM6 | Physiology, Optomotor | Lagali, 2008 |
| ChR2-GFP | Mice (rd1) | SR | ON BP | GRM6 | Physiology, Water maze | Doroudchi, 2011 |
| Mice (rd16) | ||||||
| Melanopsin | Mice (rd1) | V | RGCs | CMV | Light avoidance | Lin, 2009 |
| NpHR-YFP | Mice (Cnga3−/−; Rho−/−) | SR | Cones | Cone opsin or cone arrestin | Physiology, Optomotor | Busskamp, 2010 |
| NpHR-mCherry | Mice (rd1) | V | RGCs, some ACs, HCs, few BP and PRs | CMV | Physiology | Zhang, 2009 |
| Mice (WT) | ||||||
| Mice (rd1) |
ChR2, Channel rhodopsin 2; GFP, green fluorescent protein; NpHR, halorhodopsin; YFP, yellow fluorescent protein; WT, wild type; rd1, retinal degeneration 1, with a mutation in the phosphdiesterase beta gene; rd16, retinal degeneration 16, with a mutation in centrosomal protein 290; Cnga3, cyclic nucleotide gated channel alpha 3; rho, rhodopsin gene; V, vitreal injection; SR, subretinal injection; HC, horizontal cells; BP, bipolar cells; AC, amacrine cells; AII AC, AII amacrine cells; RGC, retinal ganglion cells; ON BP, bipolar cells that hyperpolarize in response to glutamate signaling when light is on; PRs, photoreceptor cells, CMV and CAG are broadly active promoters; VEP, visually evoked potentials measured from the visual cortex; GRM6, metabotropic glutamate receptor 6, expressed only in ON BP cells.
*Electroporation was used, in all other cases delivery was via AAV.
**Up to 18 months, the longest time assayed.
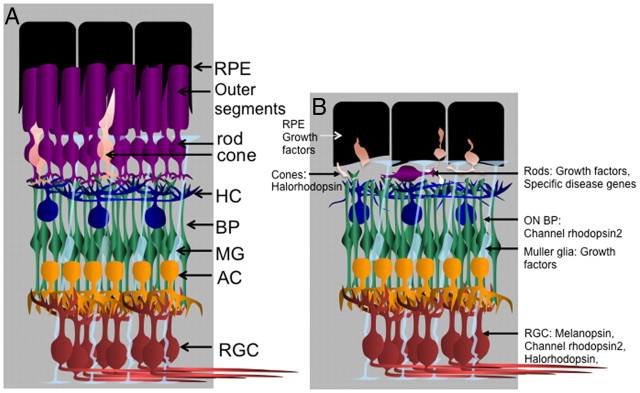
Figure 1.
Targeted cell types and types of genes to be delivered using gene therapy for RP. A, The cell types of the normal retina, with the associated support cells, the retinal pigmented epithelium (RPE) cells. Note that there are many more rods than cones in the photoreceptor layer, approximately 20:1, in both the mouse and the extramacular region of the human retina. In addition, note that the retinal pigmented epithelium processes surround the rod and cone outer segments, providing various types of support functions. The outer segments are membrane-rich and are the location of the phototransduction process. B, The rods typically die first in RP, with a concomitant collapse of the outer segment layer, and a loss of the normal association between the retinal pigmented epithelium processes and the remaining outer segments. Cones exhibit a dramatic change in morphology, modeled here after observations in mice (Lin et al., 2009). The cell types that are being targeted for gene therapy using AAV vectors are shown. For a description of the types of genes being delivered, see Table 1 and the text. HC, horizontal cell; BP, bipolar cells; AC, amacrine cells; MG, Mueller glia; RGC, retinal ganglion cells; ON BP, bipolar cells that hyperpolarize in response to glutamate when light is on. Drawing by Santiago Rompani and C. Cepko.
Delivery of NpHR to cone photoreceptor cells
As mentioned above, mammalian photoreceptor cells hyperpolarize in response to light after triggering an elaborate signal transduction cascade. NpHR also leads to hyperpolarization upon light stimulation by pumping chloride. Busskamp and colleagues (2010) used AAV to deliver NpHR to the cones in two RP mouse models. Virus was delivered after the point when the cones stopped carrying out their own light responses. The treated retinas were able to use the remaining inner retinal circuitry to give the mice several hallmarks of vision, including motion detection and dark–light discrimination. Moreover, the NpHR did not elicit an immune response nor lead to toxicity after >1 year in vivo. It has also been introduced into human retinal explants, where it was shown to permit light detection. However, for application to humans, this approach will need to be augmented to compensate for the lower sensitivity of NpHR. The sensitivity of the current alleles of NpHR is good enough to permit vision in bright outdoor light conditions, but it is still too low to permit useful vision in less bright situations, such as room light. To increase sensitivity, a specialized set of glasses can be worn. These glasses have an array of sensitive light detectors, much like the detectors in a conventional video camera. The detectors drive an array of micro-LEDs, which then emit the proper wavelength and intensity of light (Grossman et al., 2010) to activate the NpHR that has been transduced into the cones via AAV. This work is moving forward toward a Phase 1 clinical trial, and will be using AAV with NpHR driven by a human cone promoter (Roska et al., personal communication).
Delivery of ChR2 to ON bipolar cells
Bipolar cells and horizontal cells are the initial synaptic partners of photoreceptor cells. Bipolar cells can be classified as ON or OFF. The ON bipolar cells release glutamate when light is on, when their glutamate signal from photoreceptor cells is low, due to the action of a metabotropic glutamate receptor, Grm6. A promoter for Grm6 was used to express ChR2 either following electroporation (Lagali et al., 2008) or injection of an AAV vector (Doroudchi et al., 2011) into RP mouse models. Expression was restricted to ON bipolar cells, which constitute >50% of the bipolar cells in the retina. The downstream circuitry appeared to be functional in that the mice exhibited light-guided behavior, including motion detection and light–dark discrimination. This strategy too is being developed for human clinical trials, with encouraging signs in that toxicity studies performed in mice showed no toxicity (Doroudchi et al., 2011).
Delivery of ChR2 to ganglion cells
Retinal prosthetics that employ a light-sensitive chip have been implanted into diseased eyes to bypass the need for either photoreceptor cells or interneurons (Chader et al., 2009). One strategy is to stimulate the ganglion cells directly using the electronic signals initiated by light sensing by the chip. A biological approach to the same strategy is to use a light-sensitive protein, such as ChR2 or melanopsin, expressed in ganglion cells.
Melanopsin, a rhabdomeric type of opsin, was discovered to be expressed in ∼0.5% of retinal ganglion cells in the mouse (Do and Yau, 2010). These cells project to the suprachiasmatic nucleus and are important for the photoentrainment of the circadian rhythm. As many additional projections are seen, it is likely that they participate in other types of information processing as well. The majority of retinal ganglion cells involved in vision do not express melanopsin and they project to the lateral geniculate nucleus and superior colliculus. Lin et al. (2008) delivered melanopsin to the large number of retinal ganglion cells that do not normally express it. They delivered the gene using an AAV vector injected into the vitreous body, which borders the ganglion cell layer. The RP mice so treated were able to perform a visual discrimination task as well as avoid light. However, melanopsin has slow kinetics, too slow for such activities as motion detection. Still, the ability to discriminate light and dark will be welcomed by individuals who are otherwise unable to do so.
ChR2 exhibits faster kinetics than melanopsin. It too was delivered to retinal ganglion cells using intravitreal injections of AAV. Marmosets, rats, and mice have been infected with AAV transducing ChR2 (Bi et al., 2006; Ivanova and Pan, 2009; Zhang et al., 2009; Ivanova et al., 2010). All of the groups used physiological recordings to demonstrate responses to the appropriate wavelengths of light. In addition, Bi et al. (2006) could find evoked potentials in the visual cortex of infected wild-type and rd1 mice, demonstrating transmission of retinal ganglion cell signals. Zhang et al. (2009) also delivered NpHR, in some cases to the same retinal ganglion cells as were transduced by ChR2. Recording from such doubly expressing cells gave depolarization or hyperpolarization in response to the wavelengths that normally activate these two opsins.
An exciting possibility building upon this strategy is being developed by several groups (Nirenberg, 2010; Freeman et al., 2011). They are delivering ChR2 to retinal ganglion cells, also via AAV, but hope to make the signals from these transduced retinal ganglion cells more informative to the brain by using the normal coding mechanisms of retinal ganglion cells. The patterns of action potentials from different types of retinal ganglion cells receiving different types of input from retinal circuits is thought to encode much of the information distilled from the visual scene (Jacobs et al., 2009). They plan to use a camera that will transform the signals from a visual scene into light flashes that will, when flashed onto the transduced retinal ganglion cells, stimulate the ganglion cells to fire in a more meaningful way. If this can be correctly engineered using the growing appreciation of the rules that govern the coding of information by retinal ganglion cells, it could be a very exciting development for people who have lost all of their photoreceptor cells.
Footnotes
Editor's Note: Disease Focus articles provide brief overviews of a neural disease or syndrome, emphasizing potential links to basic neural mechanisms. They are presented in the hope of helping researchers identify clinical implications of their research. For more information, see http://www.jneurosci.org/misc/ifa_minireviews.dtl.
This work was supported by the Howard Hughes Medical Institute, The Foundation Fighting Blindness, The Retina Research Foundation, and the Edward N. and Della L. Thome Memorial Foundation. I thank Claudio Punzo and Wenjun Xiong for their suggestions regarding the manuscript, and Santiago Rompani for help in preparing Figure 1. I am also grateful for stimulating discussions with many colleagues who work in the area of retinal degenerations, particularly Botond Roska, Jean Bennett, Elliot Berson, Bo Chen, Claudio Punzo, Wenjun Xiong, and Yashodore Chinchore.
References
- Bainbridge JW, Smith AJ, Barker SS, Robbie S, Henderson R, Balaggan K, Viswanathan A, Holder GE, Stockman A, Tyler N, Petersen-Jones S, Bhattacharya SS, Thrasher AJ, Fitzke FW, Carter BJ, Rubin GS, Moore AT, Ali RR. Effect of gene therapy on visual function in Leber's congenital amaurosis. N Engl J Med. 2008;358:2231–2239. doi: 10.1056/NEJMoa0802268. [DOI] [PubMed] [Google Scholar]
- Bamann C, Nagel G, Bamberg E. Microbial rhodopsins in the spotlight. Curr Opin Neurobiol. 2010;20:610–616. doi: 10.1016/j.conb.2010.07.003. [DOI] [PubMed] [Google Scholar]
- Bermingham-McDonogh O, Reh TA. Regulated reprogramming in the regeneration of sensory receptor cells. Neuron. 2011;71:389–405. doi: 10.1016/j.neuron.2011.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berson E. Retinal degenerations: planning for the future. Adv Exp Med Biol. 2008;613:21–35. doi: 10.1007/978-0-387-74904-4_2. [DOI] [PubMed] [Google Scholar]
- Bi A, Cui J, Ma YP, Olshevskaya E, Pu M, Dizhoor AM, Pan ZH. Ectopic expression of a microbial-type rhodopsin restores visual responses in mice with photoreceptor degeneration. Neuron. 2006;50:23–33. doi: 10.1016/j.neuron.2006.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busskamp V, Roska B. Optogenetic approaches to restoring visual function in retinitis pigmentosa. Curr Opin Neurobiol. 2011;21:942–946. doi: 10.1016/j.conb.2011.06.001. [DOI] [PubMed] [Google Scholar]
- Busskamp V, Duebel J, Balya D, Fradot M, Viney TJ, Siegert S, Groner AC, Cabuy E, Forster V, Seeliger M, Biel M, Humphries P, Paques M, Mohand-Said S, Trono D, Deisseroth K, Sahel JA, Picaud S, Roska B. Genetic reactivation of cone photoreceptors restores visual responses in retinitis pigmentosa. Science. 2010;329:413–417. doi: 10.1126/science.1190897. [DOI] [PubMed] [Google Scholar]
- Carter-Dawson LD, LaVail MM, Sidman RL. Differential effect of the rd mutation on rods and cones in the mouse retina. Invest Ophthalmol Vis Sci. 1978;17:489–498. [PubMed] [Google Scholar]
- Chadderton N, Millington-Ward S, Palfi A, O'Reilly M, Tuohy G, Humphries MM, Li T, Humphries P, Kenna PF, Farrar GJ. Improved retinal function in a mouse model of dominant retinitis pigmentosa following AAV-delivered gene therapy. Mol Ther. 2009;17:593–599. doi: 10.1038/mt.2008.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chader GJ, Weiland J, Humayun MS. Artificial vision: needs, functioning, and testing of a retinal electronic prosthesis. Prog Brain Res. 2009;175:317–332. doi: 10.1016/S0079-6123(09)17522-2. [DOI] [PubMed] [Google Scholar]
- Comyn O, Lee E, MacLaren RE. Induced pluripotent stem cell therapies for retinal disease. Curr Opin Neurol. 2010;23:4–9. doi: 10.1097/WCO.0b013e3283352f96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cronin T, Raffelsberger W, Lee-Rivera I, Jaillard C, Niepon ML, Kinzel B, Clérin E, Petrosian A, Picaud S, Poch O, Sahel JA, Léveillard T. The disruption of the rod-derived cone viability gene leads to photoreceptor dysfunction and susceptibility to oxidative stress. Cell Death Differ. 2010;17:1199–1210. doi: 10.1038/cdd.2010.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Do MT, Yau KW. Intrinsically photosensitive retinal ganglion cells. Physiol Rev. 2010;90:1547–1581. doi: 10.1152/physrev.00013.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doroudchi MM, Greenberg KP, Liu J, Silka KA, Boyden ES, Lockridge JA, Arman AC, Janani R, Boye SE, Boye SL, Gordon GM, Matteo BC, Sampath AP, Hauswirth WW, Horsager A. Virally delivered channelrhodopsin-2 safely and effectively restores visual function in multiple mouse models of blindness. Mol Ther. 2011;19:1220–1229. doi: 10.1038/mt.2011.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenno L, Yizhar O, Deisseroth K. The development and application of optogenetics. Annu Rev Neurosci. 2011;34:389–412. doi: 10.1146/annurev-neuro-061010-113817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkel T. Oxidant signals and oxidative stress. Curr Opin Cell Biol. 2003;15:247–254. doi: 10.1016/s0955-0674(03)00002-4. [DOI] [PubMed] [Google Scholar]
- Fintz AC, Audo I, Hicks D, Mohand-Said S, Léveillard T, Sahel J. Partial characterization of retina-derived cone neuroprotection in two culture models of photoreceptor degeneration. Invest Ophthalmol Vis Sci. 2003;44:818–825. doi: 10.1167/iovs.01-1144. [DOI] [PubMed] [Google Scholar]
- Fletcher EL, Jobling AI, Vessey KA, Luu C, Guymer RH, Baird PN. Animal models of retinal disease. Prog Mol Biol Transl Sci. 2011;100:211–286. doi: 10.1016/B978-0-12-384878-9.00006-6. [DOI] [PubMed] [Google Scholar]
- Freeman DK, Rizzo JF, 3rd, Fried SI. Encoding visual information in retinal ganglion cells with prosthetic stimulation. J Neural Eng. 2011;8 doi: 10.1088/1741-2560/8/3/035005. 035005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gollisch T, Meister M. Eye smarter than scientists believed: neural computations in circuits of the retina. Neuron. 2010;65:150–164. doi: 10.1016/j.neuron.2009.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossman N, Poher V, Grubb MS, Kennedy GT, Nikolic K, McGovern B, Berlinguer Palmini R, Gong Z, Drakakis EM, Neil MA, Dawson MD, Burrone J, Degenaar P. Multi-site optical excitation using ChR2 and micro-LED array. J Neural Eng. 2010;7:16004. doi: 10.1088/1741-2560/7/1/016004. [DOI] [PubMed] [Google Scholar]
- Hartong DT, Berson EL, Dryja TP. Retinitis pigmentosa. Lancet. 2006;368:1795–1809. doi: 10.1016/S0140-6736(06)69740-7. [DOI] [PubMed] [Google Scholar]
- Hellström M, Harvey AR. Retinal ganglion cell gene therapy and visual system repair. Curr Gene Ther. 2011;11:116–131. doi: 10.2174/156652311794940746. [DOI] [PubMed] [Google Scholar]
- Ivanova E, Pan ZH. Evaluation of the adeno-associated virus mediated long-term expression of channelrhodopsin-2 in the mouse retina. Mol Vis. 2009;15:1680–1689. [PMC free article] [PubMed] [Google Scholar]
- Ivanova E, Hwang GS, Pan ZH, Troilo D. Evaluation of AAV-mediated expression of Chop2-GFP in the marmoset retina. Invest Ophthalmol Vis Sci. 2010;51:5288–5296. doi: 10.1167/iovs.10-5389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs AL, Fridman G, Douglas RM, Alam NM, Latham PE, Prusky GT, Nirenberg S. Ruling out and ruling in neural codes. Proc Natl Acad Sci U S A. 2009;106:5936–5941. doi: 10.1073/pnas.0900573106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koilkonda RD, Chou TH, Porciatti V, Hauswirth WW, Guy J. Induction of rapid and highly efficient expression of the human ND4 complex I subunit in the mouse visual system by self-complementary adeno-associated virus. Arch Ophthalmol. 2010;128:876–883. doi: 10.1001/archophthalmol.2010.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolstad KD, Dalkara D, Guerin K, Visel M, Hoffmann N, Schaffer DV, Flannery JG. Changes in adeno-associated virus-mediated gene delivery in retinal degeneration. Hum Gene Ther. 2010;21:571–578. doi: 10.1089/hum.2009.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komeima K, Rogers BS, Lu L, Campochiaro PA. Antioxidants reduce cone cell death in a model of retinitis pigmentosa. Proc Natl Acad Sci U S A. 2006;103:11300–11305. doi: 10.1073/pnas.0604056103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komeima K, Rogers BA, Campochiaro PA. Antioxidants slow photoreceptor cell death in mouse models of retinitis pigmentosa. J Cell Physiol. 2007;213:809–815. doi: 10.1002/jcp.21152. [DOI] [PubMed] [Google Scholar]
- Lagali PS, Balya D, Awatramani GB, Münch TA, Kim DS, Busskamp V, Cepko CL, Roska B. Light-activated channels targeted to ON bipolar cells restore visual function in retinal degeneration. Nat Neurosci. 2008;11:667–675. doi: 10.1038/nn.2117. [DOI] [PubMed] [Google Scholar]
- LaVail MM, Yasumura D, Matthes MT, Lau-Villacorta C, Unoki K, Sung CH, Steinberg RH. Protection of mouse photoreceptors by survival factors in retinal degenerations. Invest Ophthalmol Vis Sci. 1998;39:592–602. [PubMed] [Google Scholar]
- LaVail MM, Yasumura D, Matthes MT, Drenser KA, Flannery JG, Lewin AS, Hauswirth WW. Ribozyme rescue of photoreceptor cells in P23H transgenic rats: long-term survival and late-stage therapy. Proc Natl Acad Sci U S A. 2000;97:11488–11493. doi: 10.1073/pnas.210319397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Léveillard T, Sahel JA. Rod-derived cone viability factor for treating blinding diseases: from clinic to redox signaling. Sci Transl Med. 2010;2:26ps16. doi: 10.1126/scitranslmed.3000866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Léveillard T, Mohand-Saïd S, Lorentz O, Hicks D, Fintz AC, Clérin E, Simonutti M, Forster V, Cavusoglu N, Chalmel F, Dollé P, Poch O, Lambrou G, Sahel JA. Identification and characterization of rod-derived cone viability factor. Nat Genet. 2004;36:755–759. doi: 10.1038/ng1386. [DOI] [PubMed] [Google Scholar]
- Lin B, Koizumi A, Tanaka N, Panda S, Masland RH. Restoration of visual function in retinal degeneration mice by ectopic expression of melanopsin. Proc Natl Acad Sci U S A. 2008;105:16009–16014. doi: 10.1073/pnas.0806114105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin B, Masland RH, Strettoi E. Remodeling of cone photoreceptor cells after rod degeneration in rd mice. Exp Eye Res. 2009;88:589–599. doi: 10.1016/j.exer.2008.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu L, Oveson BC, Jo YJ, Lauer TW, Usui S, Komeima K, Xie B, Campochiaro PA. Increased expression of glutathione peroxidase 4 strongly protects retina from oxidative damage. Antioxidants Redox Signaling. 2009;11:715–724. doi: 10.1089/ars.2008.2171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire AM, Simonelli F, Pierce EA, Pugh EN, Jr, Mingozzi F, Bennicelli J, Banfi S, Marshall KA, Testa F, Surace EM, Rossi S, Lyubarsky A, Arruda VR, Konkle B, Stone E, Sun J, Jacobs J, Dell'Osso L, Hertle R, Ma JX, et al. Safety and efficacy of gene transfer for Leber's congenital amaurosis. N Engl J Med. 2008;358:2240–2248. doi: 10.1056/NEJMoa0802315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marc RE, Jones BW, Watt CB, Strettoi E. Neural remodeling in retinal degeneration. Prog Retin Eye Res. 2003;22:607–655. doi: 10.1016/s1350-9462(03)00039-9. [DOI] [PubMed] [Google Scholar]
- Masland RH. The fundamental plan of the retina. Nat Neurosci. 2001;4:877–886. doi: 10.1038/nn0901-877. [DOI] [PubMed] [Google Scholar]
- Mazzoni F, Novelli E, Strettoi E. Retinal ganglion cells survive and maintain normal dendritic morphology in a mouse model of inherited photoreceptor degeneration. J Neurosci. 2008;28:14282–14292. doi: 10.1523/JNEUROSCI.4968-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milam AH, Li ZY, Fariss RN. Histopathology of the human retina in retinitis pigmentosa. Prog Retin Eye Res. 1998;17:175–205. doi: 10.1016/s1350-9462(97)00012-8. [DOI] [PubMed] [Google Scholar]
- Mohand-Said S, Deudon-Combe A, Hicks D, Simonutti M, Forster V, Fintz AC, Léveillard T, Dreyfus H, Sahel JA. Normal retina releases a diffusible factor stimulating cone survival in the retinal degeneration mouse. Proc Natl Acad Sci U S A. 1998;95:8357–8362. doi: 10.1073/pnas.95.14.8357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohand-Said S, Hicks D, Dreyfus H, Sahel JA. Selective transplantation of rods delays cone loss in a retinitis pigmentosa model. Arch Ophthalmol. 2000;118:807–811. doi: 10.1001/archopht.118.6.807. [DOI] [PubMed] [Google Scholar]
- Nirenberg S, Pandarinath C. A retinal prosthetic strategy with the capacity to restore normal vision. Soc Neurosci Abstr. 2010;36:20.1. doi: 10.1073/pnas.1207035109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otani A, Kojima H, Guo C, Oishi A, Yoshimura N. Low-dose-rate, low-dose irradiation delays neurodegeneration in a model of retinitis pigmentosa. Am J Pathol. 2012;180:328–336. doi: 10.1016/j.ajpath.2011.09.025. [DOI] [PubMed] [Google Scholar]
- Palfi A, Millington-Ward S, Chadderton N, O'Reilly M, Goldmann T, Humphries MM, Li T, Wolfrum U, Humphries P, Kenna PF, Farrar GJ. Adeno-associated virus-mediated rhodopsin replacement provides therapeutic benefit in mice with a targeted disruption of the rhodopsin gene. Hum Gene Ther. 2010;21:311–323. doi: 10.1089/hum.2009.119. [DOI] [PubMed] [Google Scholar]
- Pang JJ, Boye SL, Kumar A, Dinculescu A, Deng W, Li J, Li Q, Rani A, Foster TC, Chang B, Hawes NL, Boatright JH, Hauswirth WW. AAV-mediated gene therapy for retinal degeneration in the rd10 mouse containing a recessive PDEbeta mutation. Invest Ophthalmol Vis Sci. 2008;49:4278–4283. doi: 10.1167/iovs.07-1622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Punzo C, Kornacker K, Cepko CL. Stimulation of the insulin/mTOR pathway delays cone death in a mouse model of retinitis pigmentosa. Nat Neurosci. 2009;12:44–52. doi: 10.1038/nn.2234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Punzo C, Xiong W, Cepko CL. Loss of daylight vision in retinal degeneration: are oxidative stress and metabolic dysregulation to blame? J Biol Chem. 2011;287:1642–1648. doi: 10.1074/jbc.R111.304428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi X, Sun L, Lewin AS, Hauswirth WW, Guy J. Long-term suppression of neurodegeneration in chronic experimental optic neuritis: antioxidant gene therapy. Invest Ophthalmol Vis Sci. 2007;48:5360–5370. doi: 10.1167/iovs.07-0254. [DOI] [PubMed] [Google Scholar]
- Raz-Prag D, Zeng Y, Sieving PA, Bush RA. Photoreceptor protection by adeno-associated virus-mediated LEDGF expression in the RCS rat model of retinal degeneration: probing the mechanism. Invest Ophthalmol Vis Sci. 2009;50:3897–3906. doi: 10.1167/iovs.08-3153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ripps H. Cell death in retinitis pigmentosa: gap junctions and the ‘bystander’ effect. Exp Eye Res. 2002;74:327–336. doi: 10.1006/exer.2002.1155. [DOI] [PubMed] [Google Scholar]
- Rivas MA, Vecino E. Animal models and different therapies for treatment of retinitis pigmentosa. Histol Histopathol. 2009;24:1295–1322. doi: 10.14670/HH-24.1295. [DOI] [PubMed] [Google Scholar]
- Rodieck R. The first steps in seeing. Sunderland, MA: Sinauer; 1998. [Google Scholar]
- Rowland TJ, Buchholz DE, Clegg DO. Pluripotent human stem cells for the treatment of retinal disease. J Cell Physiol. 2012;227:457–466. doi: 10.1002/jcp.22814. [DOI] [PubMed] [Google Scholar]
- Rudnicka AR, Jarrar Z, Wormald R, Cook DG, Fletcher A, Owen CG. Age and gender variations in age-related macular degeneration prevalence in populations of european ancestry: a meta-analysis. Ophthalmology. 2012;119:571–580. doi: 10.1016/j.ophtha.2011.09.027. [DOI] [PubMed] [Google Scholar]
- Santos A, Humayun MS, de Juan E, Jr, Greenburg RJ, Marsh MJ, Klock IB, Milam AH. Preservation of the inner retina in retinitis pigmentosa: a morphometric analysis. Arch Ophthalmol. 1997;115:511–515. doi: 10.1001/archopht.1997.01100150513011. [DOI] [PubMed] [Google Scholar]
- Schlichtenbrede FC, MacNeil A, Bainbridge JW, Tschernutter M, Thrasher AJ, Smith AJ, Ali RR. Intraocular gene delivery of ciliary neurotrophic factor results in significant loss of retinal function in normal mice and in the Prph2Rd2/Rd2 model of retinal degeneration. Gene Ther. 2003;10:523–527. doi: 10.1038/sj.gt.3301929. [DOI] [PubMed] [Google Scholar]
- Sekirnjak C, Hulse C, Jepson LH, Hottowy P, Sher A, Dabrowski W, Litke AM, Chichilnisky EJ. Loss of responses to visual but not electrical stimulation in ganglion cells of rats with severe photoreceptor degeneration. J Neurophysiol. 2009;102:3260–3269. doi: 10.1152/jn.00663.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen J, Yang X, Dong A, Petters RM, Peng YW, Wong F, Campochiaro PA. Oxidative damage is a potential cause of cone cell death in retinitis pigmentosa. J Cell Physiol. 2005;203:457–464. doi: 10.1002/jcp.20346. [DOI] [PubMed] [Google Scholar]
- Sieving PA, Caruso RC, Tao W, Coleman HR, Thompson DJ, Fullmer KR, Bush RA. Ciliary neurotrophic factor (CNTF) for human retinal degeneration: phase I trial of CNTF delivered by encapsulated cell intraocular implants. Proc Natl Acad Sci U S A. 2006;103:3896–3901. doi: 10.1073/pnas.0600236103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talcott KE, Ratnam K, Sundquist SM, Lucero AS, Lujan BJ, Tao W, Porco TC, Roorda A, Duncan JL. Longitudinal study of cone photoreceptors during retinal degeneration and in response to ciliary neurotrophic factor treatment. Invest Ophthalmol Vis Sci. 2011;52:2219–2226. doi: 10.1167/iovs.10-6479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao W. Application of encapsulated cell technology for retinal degenerative diseases. Expert Opin Biol Ther. 2006;6:717–726. doi: 10.1517/14712598.6.7.717. [DOI] [PubMed] [Google Scholar]
- Usui S, Komeima K, Lee SY, Jo YJ, Ueno S, Rogers BS, Wu Z, Shen J, Lu L, Oveson BC, Rabinovitch PS, Campochiaro PA. Increased expression of catalase and superoxide dismutase 2 reduces cone cell death in retinitis pigmentosa. Mol Ther. 2009;17:778–786. doi: 10.1038/mt.2009.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen R, Tao W, Li Y, Sieving PA. CNTF and retina. Prog Retin Eye Res. 2012;31:136–151. doi: 10.1016/j.preteyeres.2011.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Mohand-Said S, Danan A, Simonutti M, Fontaine V, Clerin E, Picaud S, Léveillard T, Sahel JA. Functional cone rescue by RdCVF protein in a dominant model of retinitis pigmentosa. Mol Ther. 2009;17:787–795. doi: 10.1038/mt.2009.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu DY, Cringle SJ, Su EN, Yu PK. Intraretinal oxygen levels before and after photoreceptor loss in the RCS rat. Invest Ophthalmol Vis Sci. 2000;41:3999–4006. [PubMed] [Google Scholar]
- Yu DY, Cringle S, Valter K, Walsh N, Lee D, Stone J. Photoreceptor death, trophic factor expression, retinal oxygen status, and photoreceptor function in the P23H rat. Invest Ophthalmol Vis Sci. 2004;45:2013–2019. doi: 10.1167/iovs.03-0845. [DOI] [PubMed] [Google Scholar]
- Zhang F, Vierock J, Yizhar O, Fenno LE, Tsunoda S, Kianianmomeni A, Prigge M, Berndt A, Cushman J, Polle J, Magnuson J, Hegemann P, Deisseroth K. The microbial opsin family of optogenetic tools. Cell. 2011;147:1446–1457. doi: 10.1016/j.cell.2011.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang K, Hopkins JJ, Heier JS, Birch DG, Halperin LS, Albini TA, Brown DM, Jaffe GJ, Tao W, Williams GA. Ciliary neurotrophic factor delivered by encapsulated cell intraocular implants for treatment of geographic atrophy in age-related macular degeneration. Proc Natl Acad Sci U S A. 2011;108:6241–6245. doi: 10.1073/pnas.1018987108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Ivanova E, Bi A, Pan ZH. Ectopic expression of multiple microbial rhodopsins restores ON and OFF light responses in retinas with photoreceptor degeneration. J Neurosci. 2009;29:9186–9196. doi: 10.1523/JNEUROSCI.0184-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou J, Luo L, Shen Z, Chiodo VA, Ambati BK, Hauswirth WW, Yang J. Whirlin replacement restores the formation of the USH2 protein complex in whirlin knockout photoreceptors. Invest Ophthalmol Vis Sci. 2011;52:2343–2351. doi: 10.1167/iovs.10-6141. [DOI] [PMC free article] [PubMed] [Google Scholar]