Inflammatory bowel disease likely derives from a loss of homeostasis between the host and the commensal microbiota in a genetically susceptible host 1. Our insight into the three major components that underlie this model has vastly expanded: namely host genetic susceptibility, the commensal microbiota; and the immunologic basis of this disorder. The latter in particular has generated new therapeutic ideas that have been stimulated by preclinical studies in a wide variety of mouse models and to a limited extent studies in human tissues, with several of them having undergone testing in human clinical studies
One aspect of the immunobiology of these disorders, both Crohn’s disease (CD) and ulcerative colitis (UC), is the important role played by chronically activated T lymphocytes, which has become a central part of the paradigm of the immunologic basis of IBD. CD4+ and CD8+ T cells are increased within the lamina propria in IBD with T cell receptors (TCR) demonstrating common elements suggestive of an antigen driven T cell response 2. Additionally, the MHC region is associated with genetic risk for IBD (more so for UC compared to CD) as are several cytokine and –receptor genes that are linked to T cell function. Animal studies in models of IBD have further indicated that elimination of T cells, inhibition of secreted cytokines, or blockade of their activation results in disruption of different types of intestinal inflammation. This has led to a deep interest in understanding the mechanisms of T cell activation and resulted in specific approaches aimed at disrupting this activation. One such concept is based on blocking the co-stimulatory pathways involved in T cell activation. Blockade of co-stimulation has worked in other non-intestinal antigen specific inflammatory diseases where the antigen is not known. As reported by Sandborn and colleagues, phase III trials in moderate to severe CD and UC revealed no demonstrable evidence for a therapeutic benefit of Abatacept (CTLA4-Ig) 3, despite Abatacept’s efficacy in diseases such as psoriasis and rheumatoid arthritis (RA), which exhibit substantial immunogenetic overlap with IBD. How can this be interpreted?
What is co-stimulation?
T cells are educated in the thymus to avoid responses to self-antigens through complex pathways of positive and negative selection. T cells that then enter the peripheral circulation are considered to be naive, antigen inexperienced cells which migrate extensively through secondary lymphoid structures such as the spleen and lymph nodes. Activation of a naive T cell in a secondary lymphoid organ requires three signals. Signal 1 is provided by a major histocompatibility complex class II (HLA-DR, DP or DQ) or MHC class I (HLA-A, B or C) on an antigen presenting cell (APC) to a CD4+ or CD8+ T cell, respectively. An externally positioned groove of the MHC presents a small 10–20 amino acid peptide to a T cell, with the peptide deriving from the proteolytic intracellular digestion of a larger soluble protein within the APC. Dendritic cells (DCs) represent the most potent type of APC that are capable of activating a naive T cell. In the absence of further stimuli, signal 1 alone is a tolerogenic signal leading to deletion or anergy of that cell. To become an active, effector T cell, the naive T cell must simultaneously receive signal 2; the co-called co-stimulatory signal. The best studied co-stimulatory molecule on naïve T cells is CD28. Binding of CD80 and/or CD86 on an APC to CD28 on a T cell stimulates additional signals critical to activation of a naïve T cell. These include up-regulation of other co-stimulatory molecules on the T cell such as CD40-ligand (CD40L) which binds to CD40 on the APC and promotes the production of interleukin-2 (IL-2) and expression of the high affinity IL-2 receptor (CD25) on the T cell. The latter represents signal 3, which drives the T cell into proliferation and further differentiation. The subsequent short-term fate is determined by the local microenvironment that triggers different effector functions such as T helper (Th) 1 (secreting interferon gamma and tumor necrosis factor), Th2 (secreting IL-4 and IL-13), Th17 (secreting IL-17A, IL-17F, IL-21 and IL-22) or T regulatory (Treg; secreting transforming growth factor β, IL-10 and IL-35).
Conclusion of the immune response includes elimination of effector T cells via activation-induced programmed cell death, but also differentiation into memory cells 4. The latter allow the host to rapidly respond to subsequent exposure to the same antigen. Two major types of memory cells, effector-memory (EM) and central memory (CM) cells accumulate in tertiary (e.g. intestinal lamina propria) or secondary lymphoid organs (e.g. lymph nodes), respectively. In fact, the intestinal lamina propria is comprised almost exclusively of effector-memory T cells with very few naïve T cells 5. Different cell surface markers on TEM and TCM control their migration to these various destinations.
In addition, powerful forces from regulatory pathways restrain the functional activity of effector T cells. Cell intrinsic T cell regulation involves upregulation of co-inhibitory molecules on the cell surface of the T cells, such as CTLA4. CTLA4, expressed 4–7 days after T cell activation, has 100-fold increased avidity for CD80 and CD86 compared to CD28. CTLA4 thereby out-competes CD28 for CD80/CD86 binding and at the same time delivers an inhibitory signal to the T cell.
Cell extrinsic T cell regulation is imposed by other cell types such as regulatory T cells (Treg). Treg are also educated in the thymus or induced in peripheral tissues such as the gut, and their differentiation is controlled by a transcription factor, FOXP3. Humans with a genetic defect in FOXP3 do not develop Treg cells and suffer from IPEX, an inflammatory condition in various organ systems including the intestine, which resembles a similar condition called scurfy in mice lacking Foxp3. Treg cells control effector T cells by contact-independent or contact-dependent mechanisms, the latter involving secretion of inhibitory cytokines such as IL-10, TGF-β and IL-35. The contact-dependent mechanisms include the expression of CTLA4 on the Treg which binds to CD80 or CD86 on activated T cells and delivers an inhibitory signal 6, including the activation of indoleamine-dioxygenase (IDO) IDO catabolizes tryptophan (required for proliferation of activated T cells) and generates inhibitory metabolites such as indoleamine. CTLA4 on Treg can also remove CD80 and CD86 from the cell surface of the APC 7.
CTLA4-Ig and blockade of co-stimulation as an immunotherapeutic mechanism
Co-stimulatory blockade has emerged as an appealing methodologic approach in the treatment of a variety of inflammatory disorders considered to be T cell driven. Most experience has been gathered with blockade of the CD28–CD80 and CD86 system via a genetically engineered fusion protein that chimerizes CTLA4 to the Fc portion of IgG (CTLA4-Ig; Abatacept™). Mechanistically, due to its much higher affinity for CD80 and CD86, CTLA4-Ig is thought to outcompete CD28 binding. CTLA4-Ig has been well-known to block T cell activation for 20 years based upon beneficial CTLA4-Ig treatment in a model of pancreatic islet cell transplantation 8. Since that time, CTLA4-Ig (Abatacept™) has been tested in human RA 9, psoriatic arthritis 10, type 1 diabetes (T1D) 11, multiple sclerosis 12, alopecia totalis 13, systemic lupus erythematosus 14 and as now published in Gastroenterology, IBD 3.
CTLA4-Ig has exhibited efficacy in RA in phase III trials, and exhibits promise in phase II trials in psoriatic arthritis and T1D. In the current report, Sandborn and colleagues 3 describe the results of four placebo-controlled trials that evaluated Abatacept™ for induction and maintenance of remission in patients with CD and UC at doses of 30, 10 or 3 mg/kg with dosing at weeks 0, 2, 4 and 8. In the maintenance protocol, responders to Abatacept from the CD and UC induction trials were randomized to 10 mg/kg Abatacept™ or placebo. In all four trials, even though Abatacept™ was generally well tolerated, there was no evidence of efficacy. In the UC trials, patients underwent sigmoidoscopy at weeks 0, 8, 12, 36 and 52 which allowed for an assessment of histology and immunohistochemical expression of CD68 (a marker for macrophages), CD20 (a marker for B cells), CD86 (a marker for activated T cells and APC), CD4 (a marker for Th cells), FOXP3 (a marker for regulatory cells and activated T cells), caspase 3 (a marker of programmed cell death) and tenascin (a measure of the extracellular matrix). None of these markers exhibited differences over time. These observations add CTLA4-Ig to an increasingly large list of biologic therapies that have failed in IBD trials.
What can be learned from the Abatacept™ experience in IBD?
At a mechanistic level, there may be many potential, non mutually-exclusive, explanations for this clinical result. Perhaps the most cogent reason is the relative lack of dependence on co-stimulation that TEM cells, the predominant type of T cell in the gut, exhibit. Co-stimulation is generally much more critical during the activation of naive T cells and less so for memory and effector memory T cells 15. This may also underlie the preferential efficacy of CTLA4-Ig in the therapy of early compared to late T1D 11. Thus CTLA4-Ig would be more likely to be efficacious in circumstances wherein naive T cells are actively recruited into inflamed locales; something which may not likely be a major feature of IBD where almost exclusively TEM cells and virtually no naive T cells are present within the intestines. Another possibility is that CD28 related pathways are not important in the pathogenesis of IBD. Many CD8+ and CD4+ T cells especially within the epithelium, do not express CD28 in the gut 16 and models of IBD such as IL-2-deficient mice develop inflammation in the complete absence of CD28 17. The current trials may have revealed the important insight into the CD28 independence of co-stimulation in the intestines. Indeed, intestinal lamina propria and intraepithelial T cells have been long-known to respond better to TCR independent signals such as CD2. Moreover, studies of model systems suggest other co-stimulatory molecules such as OX40/OX40L not targeted by CTLA4-Ig might be important in intestinal inflammation 18.
A third possibility is that Abatacept might have impeded Treg function in addition to preventing effector T cell activation. CD28 and CTLA4 are critical for Treg cell development and function, respectively. CTLA4-Ig could deprive Tregs of an important CD28-dependent signal. In fact, blockade of CTLA4 function through Ipilimumab™, which is effective in metastatic melanoma via augmenting an anti-melanoma immune response, induces colitis as a characteristic adverse event 19. FOXP3+ cells are functional and present in normal or increased numbers in IBD. Although no changes in FOXP3 immunohistochemistry were noted in the studies by Sandborn and colleagues 3, in the absence of a functional analysis of the T cells in these studies, it cannot be determined whether CTLA4-Ig had an adverse effect on Treg cell populations since FOXP3 can also be expressed by activated T cells. Moreover, CTLA4 binding to CD80 and CD86 may induce tryptophan catabolism and the generation of regulatory metabolites such as indoleamine as discussed above. Perhaps CTLA4-Ig deprives the gut T cells from this important natural regulatory pathway that is normally provided by the interactions between endogenous CTLA4 and CD80 and CD86.
A fourth and final possibility relates to the important role played by CD28 and CTLA4 in T cell differentiation into Th1, Th2 and Th17 20. Each of these effectors can drive inflammation when present at pathologic levels, and they extensively cross-regulate each other. Blockade of CD28 pathways may diminish Th2 cytokines which would be predicted to result in increased Th1 cytokines. This might be problematic in CD wherein a Th1 cytokine excess is consistent with the IFNγ-dependent formation of granulomas in this disorder. In the absence of tissue profiling of cytokine expression in the reported studies3, such possibilities can only be speculated upon.
The path forward?
Do these studies represent the death-knell for blocking co-stimulation as a means to affect IBD? The answer is likely no. Next-generation forms of Abatacept™ such as CTLA4-Ig with higher affinity for CD80 and CD86 such as Belatacept™ (L104E and A29Y mutations in CTLA4) result in significantly improved clinical efficacy e.g. in transplant medicine 21. Another aspect worth considering is the confounding variable introduced by azathioprine or 6-mercaptopurine that target CD28 signaling and which 30–40% of the patients in the trials by Sandborn and colleagues were receiving 22. Treatment with azathrioprine may already neutralize CD28-mediated signals 22. Post-hoc analysis of the data by Sandborn and colleagues may possibly elucidate the importance or lack thereof of this potential factor. For future trials, it could be imagined that CTLA4-Ig would be effective in azathioprine naïve individuals through a top-down type approach prior to removal of active CD28 signaling through azathioprine treatment 22. Another possibility is that due to the unique TEM compartment in the intestines, blockade of other co-stimulatory pathways may be more important for treating IBD, such as OX40/OX40L or CD2–CD58 interactions 23. CD58 is particularly interesting because of the extremely important role that CD2 activation has in the gut and the relatively high expression of CD58 on epithelial cells; an atypical APC that is unique to the intestines and does not typically express CD80 or CD86.
Does the failure of Abatacept and other strategies that targeted T cells indicate that IBD is a non-T cell disease associated with abnormalities in innate immunity alone? The answer is likely no again. Interpretation of the negative results of the study of the anti-CD3 antibody Visilizumab™ in steroid-refractory UC24 is severely hampered by a 47% placebo response rate and specific aspects of initial CD3 activation upon binding of this antibody to T cells. Results of trials with the anti-CD25/IL-2RA antibodies Basiliximab™ and Daclizumab™ which also failed in steroid-refractory UC could easily be explained by potential depletion or functional impairment of Treg cells, which typically express CD25 and represent the vast majority of CD4+CD25+ cells 25; as discussed above for Abatacept™. The failure of the aforementioned antibodies is in stark contrast to the success of strategies that target T cell immigration into the intestine such as those reported for the anti-α4 integrin antibody Natalizumab™ in CD and, in particular, the anti-α4β7 antibody Vedolizumab in UC 26. These strikingly different results of various T cell-targeted strategies, which partly contrast with their effects in e.g. rheumatoid arthritis, may highlight the particular complexities and specifics of the intestinal immune compartment, rather than indicate the importance (or lack thereof) of T cells in IBD in general.
In the end, the studies reported by Sandborn and colleagues points towards the conclusion that not all T cell mediated inflammatory diseases are alike. What works in one may not work in another and highlights the unique rules that govern T cell activation in specific tissue sites such as the apparent CD28-independence of lamina propria lymphocytes with the caveats discussed. These and other possible considerations must be part of the ongoing dialogue going forward in developing future studies in this area. Whatever the target, it cannot be emphasized more emphatically the importance of carefully designed clinical studies together with basic experimental investigations. Such additional experimentation can hopefully enhance the information provided by the clinical studies and allow for the interrogation of biologic events associated with the therapeutic intervention in order to inform future clinical studies. In their absence, we risk not having a future road-map such that the therapeutic agent as applied becomes a “dead-end” study. Carefully performed clinical studies such as those reported by Sandborn and colleagues provide perhaps the best opportunity for understanding disease mechanisms in humans.
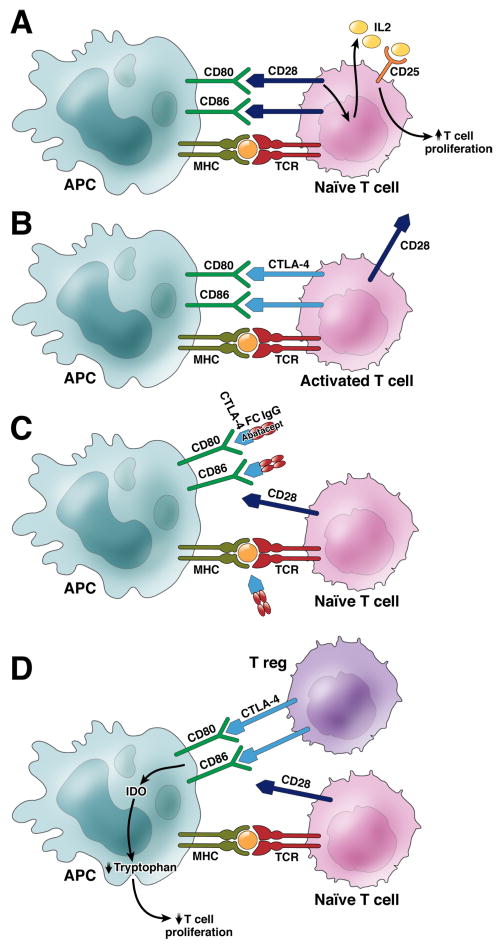
Figure 1.
A. Activation of naive T cells requires three signals. Antigen presented on MHC molecules on professional antigen presenting cells (APC) engage the T cell receptor (TCR) on a naïve T cell (Signal 1). Co-stimulation (Signal 2) is provided through B7 (CD80 and CD86) on APCs interacting with CD28 on T cells, which induces IL-2 secretion. IL-2 in turn engages the high affinity IL-2 receptor CD25 (Signal 3), which results in T cell proliferation. B. Activated T cells upregulate CTLA-4 4–7 days after TCR activation. CTLA-4 has higher affinity to CD80 and CD86 and thereby out-competes CD28. C. CTLA4-Ig (AbataceptTm) targets CD80/CD86 on APCs therefore depriving naïve T cells from its co-stimulatory signal (Signal 2). D. Regulatory T cells (T reg) constitutively express CTLA-4, which upon engagement with CD80/86 on APCs are downregulated and express indoleamine 2,3-dioxygenase (IDO), the rate limiting enzyme in tryptophan catabolism, which inhibits T cell proliferation via tryptophan deprivation. In addition, Treg secrete inhibitory cytokines such as IL-10, TGF-β and IL-35.
Acknowledgments
We thank Drs Josh Korzenik for helpful discussions and Lukas Niederreiter for help with the graphical illustration. RSB was supported by NIH DK051362, DK044319, DK053056, DK088199, Harvard Digestive Diseases Center (NIH DK034854); AK by the European Research Council Grant agreement n° 26 0961 and the National Institute for Health Research Cambridge Biomedical Research Centre. LM was supported by NIH AI044236, AI084952, DK072201 and DK086605.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kaser A, Zeissig S, Blumberg RS. Inflammatory Bowel Disease. Annu Rev Immunol. 2010;28:573–621. doi: 10.1146/annurev-immunol-030409-101225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Probert CS, Saubermann LJ, Balk S, et al. Repertoire of the alpha beta T-cell receptor in the intestine. Immunol Rev. 2007;215:215–25. doi: 10.1111/j.1600-065X.2006.00480.x. [DOI] [PubMed] [Google Scholar]
- 3.Sandborn WJ, Colombel JF, Sands BE, et al. Abatacept for Crohn’s disease and ulcerative colitis. Gastroenterology. 2012 doi: 10.1053/j.gastro.2012.04.010. in press. [DOI] [PubMed] [Google Scholar]
- 4.Krammer PH, Arnold R, Lavrik IN. Life and death in peripheral T cells. Nat Rev Immunol. 2007;7:532–42. doi: 10.1038/nri2115. [DOI] [PubMed] [Google Scholar]
- 5.Sheridan BS, Lefrancois L. Regional and mucosal memory T cells. Nat Immunol. 2011;12:485–91. doi: 10.1038/ni.2029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wing K, Yamaguchi T, Sakaguchi S. Cell-autonomous and -non-autonomous roles of CTLA-4 in immune regulation. Trends Immunol. 2011;32:428–33. doi: 10.1016/j.it.2011.06.002. [DOI] [PubMed] [Google Scholar]
- 7.Qureshi OS, Zheng Y, Nakamura K, et al. Trans-endocytosis of CD80 and CD86: a molecular basis for the cell-extrinsic function of CTLA-4. Science. 2011;332:600–3. doi: 10.1126/science.1202947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lenschow DJ, Zeng Y, Thistlethwaite JR, et al. Long-term survival of xenogeneic pancreatic islet grafts induced by CTLA4lg. Science. 1992;257:789–92. doi: 10.1126/science.1323143. [DOI] [PubMed] [Google Scholar]
- 9.Kremer JM, Westhovens R, Leon M, et al. Treatment of rheumatoid arthritis by selective inhibition of T-cell activation with fusion protein CTLA4Ig. N Engl J Med. 2003;349:1907–15. doi: 10.1056/NEJMoa035075. [DOI] [PubMed] [Google Scholar]
- 10.Mease P, Genovese MC, Gladstein G, et al. Abatacept in the treatment of patients with psoriatic arthritis: results of a six-month, multicenter, randomized, double-blind, placebo-controlled, phase II trial. Arthritis Rheum. 2011;63:939–48. doi: 10.1002/art.30176. [DOI] [PubMed] [Google Scholar]
- 11.Orban T, Bundy B, Becker DJ, et al. Co-stimulation modulation with abatacept in patients with recent-onset type 1 diabetes: a randomised, double-blind, placebo-controlled trial. Lancet. 2011;378:412–9. doi: 10.1016/S0140-6736(11)60886-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Viglietta V, Bourcier K, Buckle GJ, et al. CTLA4Ig treatment in patients with multiple sclerosis: an open-label, phase 1 clinical trial. Neurology. 2008;71:917–24. doi: 10.1212/01.wnl.0000325915.00112.61. [DOI] [PubMed] [Google Scholar]
- 13.Rosenblum MD, Gratz IK, Paw JS, et al. Treating human autoimmunity: current practice and future prospects. Science translational medicine. 2012;4:125sr1. doi: 10.1126/scitranslmed.3003504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Merrill JT, Burgos-Vargas R, Westhovens R, et al. The efficacy and safety of abatacept in patients with non-life-threatening manifestations of systemic lupus erythematosus: results of a twelve-month, multicenter, exploratory, phase IIb, randomized, double-blind, placebo-controlled trial. Arthritis Rheum. 2010;62:3077–87. doi: 10.1002/art.27601. [DOI] [PubMed] [Google Scholar]
- 15.Smith-Garvin JE, Koretzky GA, Jordan MS. T cell activation. Annu Rev Immunol. 2009;27:591–619. doi: 10.1146/annurev.immunol.021908.132706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ebert EC, Roberts AI. Costimulation of the CD3 pathway by CD28 ligation in human intestinal lymphocytes. Cell Immunol. 1996;171:211–6. doi: 10.1006/cimm.1996.0195. [DOI] [PubMed] [Google Scholar]
- 17.Boone DL, Dassopoulos T, Lodolce JP, et al. Interleukin-2-deficient mice develop colitis in the absence of CD28 costimulation. Inflamm Bowel Dis. 2002;8:35–42. doi: 10.1097/00054725-200201000-00005. [DOI] [PubMed] [Google Scholar]
- 18.Griseri T, Asquith M, Thompson C, et al. OX40 is required for regulatory T cell-mediated control of colitis. J Exp Med. 2010;207:699–709. doi: 10.1084/jem.20091618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hodi FS, O’Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711–23. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Littman DR, Rudensky AY. Th17 and regulatory T cells in mediating and restraining inflammation. Cell. 2010;140:845–58. doi: 10.1016/j.cell.2010.02.021. [DOI] [PubMed] [Google Scholar]
- 21.Snanoudj R, Frangie C, Deroure B, et al. The blockade of T-cell co-stimulation as a therapeutic stratagem for immunosuppression: Focus on belatacept. Biologics. 2007;1:203–13. [PMC free article] [PubMed] [Google Scholar]
- 22.Tiede I, Fritz G, Strand S, et al. CD28-dependent Rac1 activation is the molecular target of azathioprine in primary human CD4+ T lymphocytes. J Clin Invest. 2003;111:1133–45. doi: 10.1172/JCI16432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ebert EC, Panja A, Praveen R. Human intestinal intraepithelial lymphocytes and epithelial cells coinduce interleukin-8 production through the CD2–CD58 interaction. Am J Physiol Gastrointest Liver Physiol. 2009;296:G671–7. doi: 10.1152/ajpgi.90497.2008. [DOI] [PubMed] [Google Scholar]
- 24.Sandborn WJ, Colombel JF, Frankel M, et al. Anti-CD3 antibody visilizumab is not effective in patients with intravenous corticosteroid-refractory ulcerative colitis. Gut. 2010;59:1485–92. doi: 10.1136/gut.2009.205443. [DOI] [PubMed] [Google Scholar]
- 25.Sands BE, Sandborn WJ, Creed TJ, et al. Basiliximab Does Not Increase Efficacy of Corticosteroids in Patients with Steroid-Refractory Ulcerative Colitis. Gastroenterology. 2012 doi: 10.1053/j.gastro.2012.04.043. [DOI] [PubMed] [Google Scholar]
- 26.Feagan BG, Greenberg GR, Wild G, et al. Treatment of ulcerative colitis with a humanized antibody to the alpha4beta7 integrin. N Engl J Med. 2005;352:2499–507. doi: 10.1056/NEJMoa042982. [DOI] [PubMed] [Google Scholar]