Abstract
Slip flow occurs in colloidal crystals made of 470 nm silica spheres that are chemically modified with hydrocarbon, giving enhanced volume flow rates and a narrower distribution of fluid velocities. Bovine serum albumin separates by pressure-driven flow with a zone that is 15-fold narrower than the theoretical limit for Hagen-Poiseuille flow. The zone variance, normalized for separation length, is 15 nm, which is 500-fold smaller than previous reports for pressure-driven protein chromatography. A colloidal crystal is shown to separate a monoclonal antibody from its aggregates in only 40 s, representing a ten-fold increase in speed. Slip flow thus has profound implications for protein chromatography.
Slip flow in nanofluidics arises from weak intermolecular interactions between a fluid and the surrounding walls, giving significantly enhanced volume flow rates of water through hydrophobic nanoscale channels, such as carbon nanotubes,1–4 nanopipes,5–6 and nanoscale carbon sheets.7–9 The nature of slip flow is illustrated in Figure 1. Enhanced flow rates arise from a nonzero fluid velocity at the walls. The enhancement decreases with larger channel width, giving only slight flow enhancements as the channel width reaches the microscale.10
FIGURE 1.
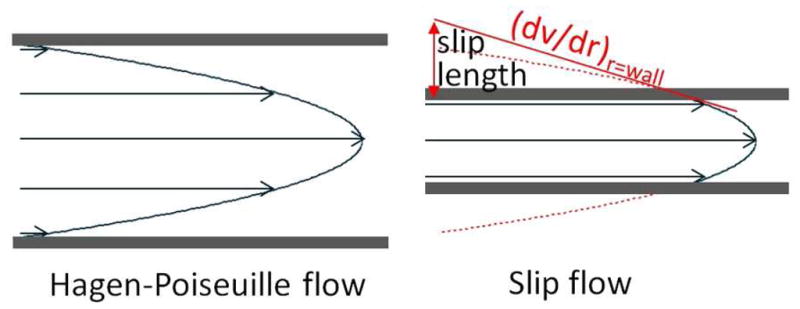
Contrast between slip flow and Hagen-Poiseuille (no-slip) flow. Slip flow has only a segment of the parabolic flow profile inside the channel, reducing the velocity distribution.
An exciting property of slip flow is the narrowed velocity distribution that is depicted in Figure 1. In chromatography, the velocity distribution translates to a distribution of arrival times at the end of the column, therefore, resolution in chromatography is presently limited by the wide distribution of velocities in the Hagen-Poiseuille flow profile.11–12 This problem is only partially offset by molecular diffusion across the channel. To reduce diffusion distances, commercial columns use spheres as small as 1.5 μm in diameter,13 and spheres as small as 1 μm have been explored.11 Nonetheless, the advances are modest: zone broadening in chromatography has been reduced by less than a factor of three in the last 20 years. The problem is worse for proteins because these have ten-fold lower diffusion coefficients. Improved protein chromatography is needed for many applications: proteomics,14–15 pharmaceutical biotechnology16 and biomarker discovery.17 This is because a typical protein has many post-translational modifications, glycoforms, substitutions, oxidations and other changes that make it a complex mixture of very similar species.18 The phenomenon of slip flow needs to be explored as a potential new means of improving resolution in chromatography.
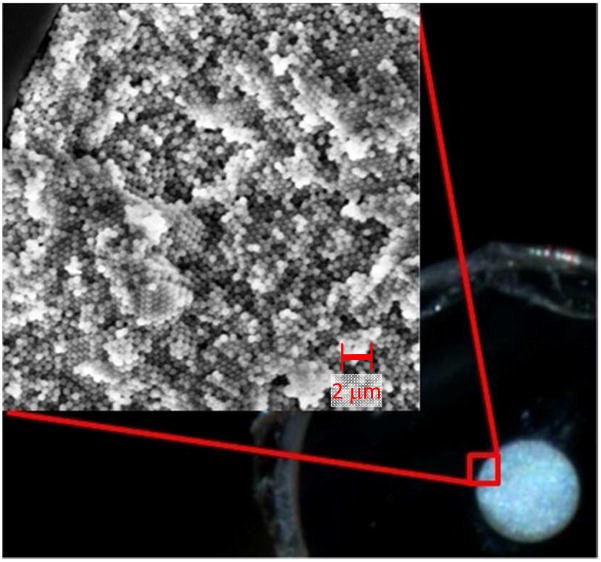
Slip flow effects require that the channel radius approaches the slip length. As detailed in the Supporting Information, this requirement can be met by using silica colloidal crystals because these have sufficiently narrow interstices, i.e., on the order of 30 to 40 nm. Packing these in capillaries enables facile connection to high pressure pumps.19–20 Figure 2 shows a photograph of a cross-section of a cleaved capillary packed with 470 nm particles. The opalescence indicates crystalline domains. The SEM image confirms face-centered cubic (fcc) domains, which is the expected for colloidal crystals,12,20–22 and the domain sizes are consistent with the photograph.
FIGURE 2.
Photograph of cross-section of a packed 75 μm i.d. capillary. The SEM image shows the individual 470 nm particles.
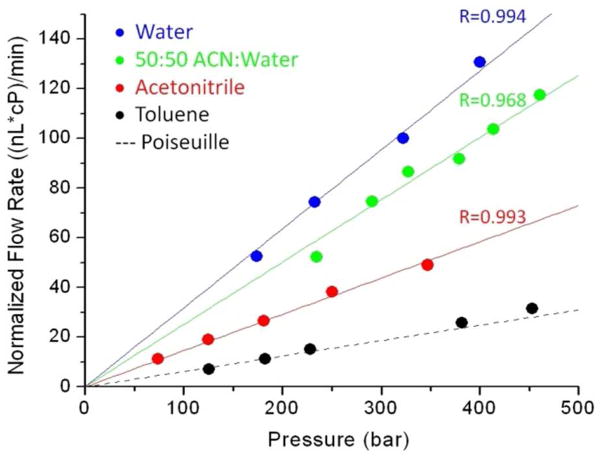
The flow behavior of a 21 mm long colloidal crystal in a capillary was characterized. The colloidal crystal was first chemically modified, after packing, by self-assembly of mixed hydrophobic trichloroalkylsilanes12,20, using C4 and C1 chain lengths in this case. The trichlorosilane polymerization also served to stabilize the colloidal crystal inside the capillary. The porosity (interstitial volume fraction), ε, was determined from the volume of hexadecane that wicked inside a known length of colloidal crystal, revealing ε=0.277±0.008. The porosity was independently checked by fluorescence recovery after photobleaching: the ratio of the diffusion coefficients of fluorescein inside the colloidal crystal to that in an open capillary is 0.28, in agreement. The raw data are in the Supporting Information. The porosity thus approaches the limiting value of 0.26 for fcc geometry. The volume flow rates measured for various liquids are presented in Figure 3 as a function of pressure. The flow rates are all scaled by viscosity, expressed in centipoise (cP) to facilitate comparisons. The flow rate for toluene is shown to approach the case of no slip, as expected, based on its strong agreement with the flow rate predicted by the Kozeny-Carman equation (dashed line), which is detailed in the Supporting Information. For the hydrocarbon-immiscible liquids, the flow enhancements relative to Hagen-Poiseuille flow are 5.3(±0.2), 4.1(±0.3) and 2.4(±0.1) for water, 50:50 acetonitrile:water and acetonitrile, respectively. These flow rate enhancements progressively increase with greater immiscibility, as expected for slip flow. The results demonstrate that there is significant slip flow.
FIGURE 3.
Flow rate data. Volume flow rates are shown for four liquids relative to that of Hagen-Poiseuille flow (dashed line), which was calculated by the Kozeny-Carman equation.
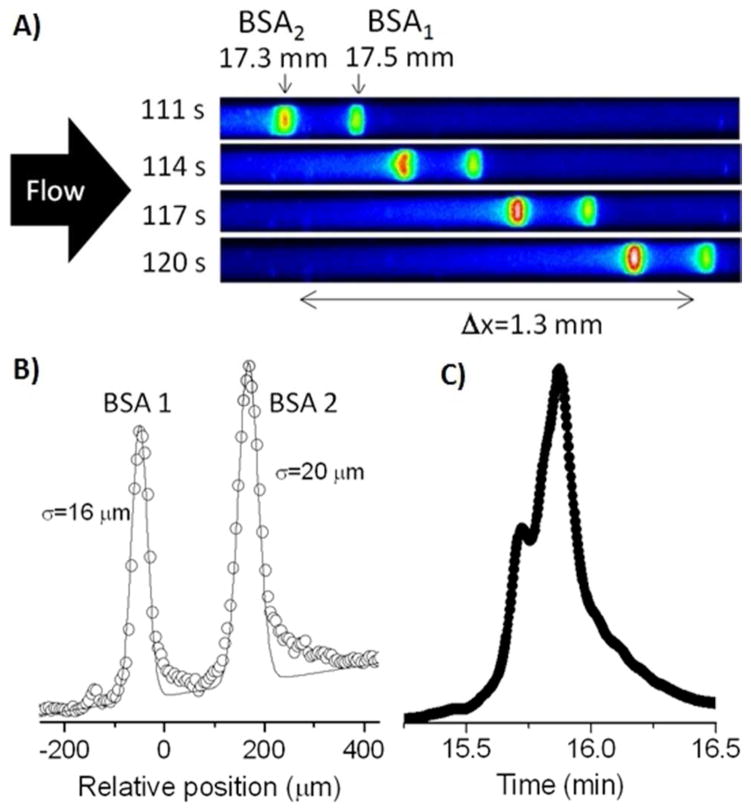
The benefit of slip flow is especially attractive for chromatography of proteins because they have smaller diffusion coefficients than do small molecules. The zone widths of proteins were measured after pressure-driven flow through the colloidal crystal. Bovine serum albumin with a fluorescent label was injected from a strongly aqueous solution to form a narrow, immobilized zone at the entrance to the capillary, as described previously.12 Then a high pressure pump was used to flow a mixture of 25:75 acetonitrile:water (V:V) with 0.1% trifluoroacetic acid at a pressure of 330 bar. Figure 4A shows a series of images in 3-s increments as two narrow zones emerge after almost 2 min from the first 17 mm of the 21 mm long silica colloidal crystal. The two sharp peaks are well separated, with at least one low, broad peak near the baseline, likely due to known aggregation of this protein.23 The image data from the last frame are plotted in Figure 4B to present a spatial chromatogram. For comparison, Figure 4C shows a temporal chromatogram for the same protein using a leading commercial column for protein separations, which was 50-mm long, and made of porous 1.7 μm particles chemically modified with butyl groups. The chromatogram from the commercial column agrees with the presence of two peaks, which are presumably from over-labeling, but it does not separate them. The data in Figure 4B allow calculation of the plate height for the two peaks in the case of the colloidal crystal: 15 nm and 23 nm. The plate number, a figure of merit which is (L/σ)2, exceeds 106, a milestone that had never been reached for a pressure-driven liquid chromatographic separation of any species. The plate height is 500x lower and the plate number is 200x higher than present materials for protein chromatography.13
FIGURE 4.
Separation of labeled BSA. A) Time series of fluorescence micrographs over the field of view. B) Plot of the image data from the last frame of part A: data points (○) and Gaussian fit (—). C) Gradient elution chromatogram of the same protein sample using a 50-mm long commercial UHPLC column.
To interpret how much of the improvement is from slip flow, as opposed to smaller particle size24 and more uniform packing11, these results are compared with simulations of no-slip flow in fcc crystals.25 The simulations showed that the plate height normalized for particle diameter, which is called the reduced plate height, has a minimum possible value of 0.5 for fcc geometry. The lower limit has been approached only once, with a reduced plate height of 1.0 for a small-molecule separation.26 The data of BSA1 in Figure 4 show a reduced plate height of 0.032, which is a factor of 15 lower than the simulated value for no-slip flow. It is concluded that it is slip flow that allows the zones to remain so narrow.
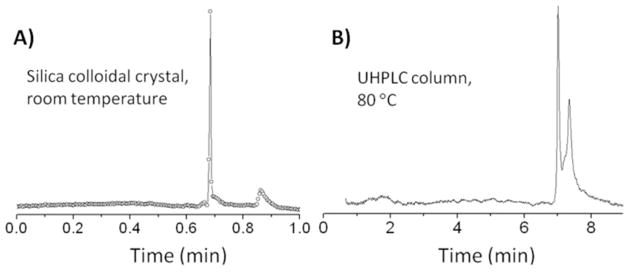
Hydrophobic silica is often used as to characterize protein drugs, such as therapeutic monoclonal antibodies, because aggregates cause side effects from immunogenicity.16 The separation of a monoclonal antibody from its aggregates was studied for the hydrophobic colloidal crystal, in this case using labeled monoclonal anti-prostate specific antigen. The experiment was identical to the one for bovine serum albumin except that more acetonitrile was used, 45%, which only slightly retains the protein and its aggregates. Also, a higher pump pressure was used: 460 bar. Figure 5A shows the chromatogram obtained by recording intensity at a fixed position of 14.5 mm from the capillary entrance. The chromatogram shows a zone so sharp that it is under-sampled by the 5 Hz sampling rate. The aggregates eluted later, as expected. A chromatogram for the same sample, using the same commercial column used earlier, is shown in Figure 5B. The general appearance is the same but the resolution is much lower and the time scale is ten-fold longer. The colloidal crystal separation was at room temperature, whereas the commercial column required 80 °C and gradient elution. The higher speed and the ability to operate at room temperature are consequences of the very low adsorptivity. It is the ultra-sharp zones that enable resolution with such low adsorptivity. Image data in the Supporting Information show that when one accounts for the 0.2 s acquisition time, which introduces a predictable contribution of 30 nm to the plate height,27 the net plate height is 70 nm for the sharp zone, giving a reduced plate height of 0.15. This again is more than three-fold lower than the minimum predicted value of 0.5 for no-slip flow.
FIGURE 5.
Chromatogram of a monoclonal antibody for A) silica colloidal crystal and B) commercial column
The results demonstrate that slip flow drastically improves chromatography, particularly for proteins.
Supplementary Material
Acknowledgments
We thank Professor Fred Regnier for helpful suggestions on the manuscript. This work was supported by grants NIH R01 GM65980 and R01 GM101464.
ABBREVIATIONS
- fcc
face-centered cubic
- C1
trichloromethylsilane
- C4
trichlorobutylsilane
- BSA
bovine serum albumin
- UHPLC
ultra-high performance liquid chromatography
Footnotes
ASSOCIATED CONTENT
Supporting Information. Materials and Methods, additional introduction, raw data flow rate and diffusion coefficient, additional discussion and references.
References
- 1.Holt JK, Park HG, Wang YM, Stadermann M, Artyukhin AB, Grigoropoulos CP, Noy A, Bakajin O. Science. 2006;312:1034. doi: 10.1126/science.1126298. [DOI] [PubMed] [Google Scholar]
- 2.Majumder M, Chopra N, Andrews R, Hinds BJ. Nature. 2005;438:44. doi: 10.1038/43844a. [DOI] [PubMed] [Google Scholar]
- 3.Thomas JA, McGaughey AJH. Nano Lett. 2008;8:2788. doi: 10.1021/nl8013617. [DOI] [PubMed] [Google Scholar]
- 4.Qin XC, Yuan QZ, Zhao YP, Xie SB, Liu ZF. Nano Lett. 2011;11:2173. doi: 10.1021/nl200843g. [DOI] [PubMed] [Google Scholar]
- 5.Sinha S, Rossi MP, Mattia D, Gogotsi Y, Bau HH. Phys Fluids. 2007:19. [Google Scholar]
- 6.Whitby M, Cagnon L, Thanou M, Quirke N. Nano Lett. 2008;8:2632. doi: 10.1021/nl080705f. [DOI] [PubMed] [Google Scholar]
- 7.Karan S, Samitsu S, Peng XS, Kurashima K, Ichinose I. Science. 2012;335:444. doi: 10.1126/science.1212101. [DOI] [PubMed] [Google Scholar]
- 8.Nair RR, Wu HA, Jayaram PN, Grigorieva IV, Geim AK. Science. 2012;335:442. doi: 10.1126/science.1211694. [DOI] [PubMed] [Google Scholar]
- 9.Paul DR. Science. 2012;335:413. doi: 10.1126/science.1216923. [DOI] [PubMed] [Google Scholar]
- 10.Choi CH, Westin KJA, Breuer KS. ASME Conference Proceedings. 2002;2002:557. [Google Scholar]
- 11.Patel KD, Jerkovich AD, Link JC, Jorgenson JW. Anal Chem. 2004;76:5777. doi: 10.1021/ac049756x. [DOI] [PubMed] [Google Scholar]
- 12.Wei B, Malkin DS, Wirth MJ. Anal Chem. 2010;82:10216. doi: 10.1021/ac102438w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wu NJ, Liu YS, Lee ML. J Chromatogr A. 2006;1131:142. doi: 10.1016/j.chroma.2006.07.042. [DOI] [PubMed] [Google Scholar]
- 14.Tran JC, Zamdborg L, Ahlf DR, Lee JE, Catherman AD, Durbin KR, Tipton JD, Vellaichamy A, Kellie JF, Li MX, Wu C, Sweet SMM, Early BP, Siuti N, LeDuc RD, Compton PD, Thomas PM, Kelleher NL. Nature. 2011;480:254. doi: 10.1038/nature10575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McLafferty FW. Proc Natl Acad Sci U S A. 2008;105:18088. doi: 10.1073/pnas.0800784105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vazquez-Rey M, Lang DA. Biotechnol Bioeng. 2011;108:1494. doi: 10.1002/bit.23155. [DOI] [PubMed] [Google Scholar]
- 17.Rafalko A, Dai SJ, Hancock WS, Karger BL, Hincapie M. J Proteome Res. 2012;11:808. doi: 10.1021/pr2006704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhiqiang A. Therapeutic Monoclonal Antibodies: From Bench to Clinic. J. Wiley & Sons; Hoboken, NJ: 2009. [Google Scholar]
- 19.MacNair JE, Lewis KC, Jorgenson JW. Anal Chem. 1997;69:983. doi: 10.1021/ac961094r. [DOI] [PubMed] [Google Scholar]
- 20.Malkin DS, Wei B, Fogiel AJ, Staats SL, Wirth MJ. Anal Chem. 2010;82:2175. doi: 10.1021/ac100062t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kamp U, Kitaev V, von Freymann G, Ozin GA, Mabury SA. Adv Mater. 2005;17:438. [Google Scholar]
- 22.Moon JH, Kim S, Yi GR, Lee YH, Yang SM. Langmuir. 2004;20:2033. [Google Scholar]
- 23.de Frutos M, Cifuentes A, Diez-Masa JC, Camafeita E, Mendez E. HRC-J High Resolut Chromatogr. 1998;21:18. [Google Scholar]
- 24.MacNair JE, Patel KD, Jorgenson JW. Anal Chem. 1999;71:700. doi: 10.1021/ac9807013. [DOI] [PubMed] [Google Scholar]
- 25.Schure MR, Maier RS, Kroll DM, Davis HT. J Chromatogr A. 2004;1031:79. doi: 10.1016/j.chroma.2003.12.030. [DOI] [PubMed] [Google Scholar]
- 26.Witowski SR, Kennedy RT. J Microcolumn Sep. 1999;11:723. [Google Scholar]
- 27.Moore AW, Jorgenson JW. Anal Chem. 1993;65:3550. doi: 10.1021/ac00072a004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.