Abstract
Lymphangioleiomyomatosis (LAM) is a rare, multisystem disease affecting primarily premenopausal women. The disease is characterized by cystic lung disease, at times leading to respiratory compromise, abdominal tumors (in particular, renal angiomyolipomas), and involvement of the axial lymphatics (e.g., adenopathy, lymphangioleiomyomas). Disease results from the proliferation of neoplastic cells (LAM cells), which, in many cases, have a smooth muscle cell phenotype, express melanoma antigens, and have mutations in one of the tuberous sclerosis complex genes (TSC1 or TSC2). In the lung, LAM cells found in the vicinity of cysts are, at times, localized in nodules and may be responsible for cyst formation through the production of proteases. Lymphatic channels, expressing characteristic lymphatic endothelial cell markers, are found within the LAM lung nodules. LAM cells may also be localized within the walls of the axial lymphatics, and, in some cases, penetrate the wall and proliferate in the surrounding adipose tissue. Consistent with extensive lymphatic involvement in LAM, the serum concentration of VEGF-D, a lymphangiogenic factor, is higher in LAM patients than in healthy volunteers.
Keywords: lymphangiogenesis, metastasis, lymphangioleiomyomatosis, VEGF-D, VEGF-C
Introduction
Lymphangioleiomyomatosis (LAM), a rare multisystem disorder primarily affecting premenopausal women, is characterized by cystic lung destruction, abdominal tumors, in particular, renal angiomyolipomas (AMLs), and involvement of the thoracic and abdominal axial lymphatics (e.g., adenopathy, lymphangioleiomyomas).1-7 Initial clinical presentation consists of symptoms resulting from lung, kidney, or lymphatic involvement. Disease progression is variable. The course may be insidious: 5 or 6 years may elapse between appearance of symptoms and diagnosis.5,8 Delayed diagnosis of this rare disease may account for the poor prognosis seen in earlier studies; recent reports indicate a 78% survival in 8.5 years or 90% in 10 years.5,9
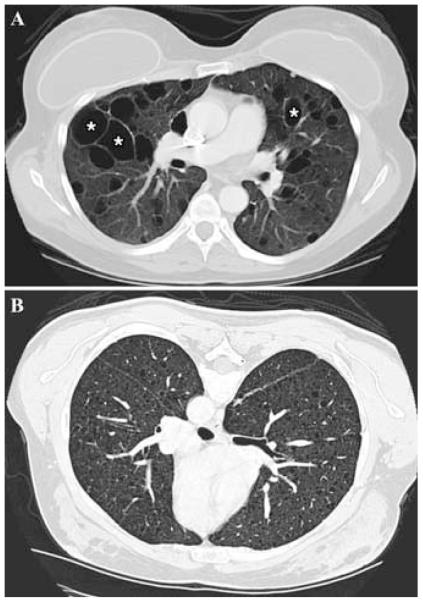
Cystic lung destruction appears to be responsible for the progressive decline in pulmonary function, which can lead to respiratory failure, dependence on supplemental oxygen, lung transplantation, or death.8 Pulmonary signs and symptoms include dyspnea, reactive airways disease, and recurrent pneumothoraces. Pulmonary function abnormalities include airflow obstruction (decreased FEV1) and reduced diffusion capacity (DLco).1,4,10 High-resolution computed tomography (HRCT) scans reveal thin-walled cysts homogeneously distributed in both lungs and interspersed with normal parenchyma (Figs. 1A and B).1,4,5 Ventilation/perfusion scans are consistent with air-trapping in the cystic areas.4,11
FIGURE 1.
Lung CT scan of a patient with LAM (A) showing numerous thin-walled cysts (*) distributed through-out otherwise normal-appearing lungs. (B) CT scan of a patient with severe LAM; the lung parenchyma is almost completely replaced by very small cysts.
The hallmark histologic characteristic of LAM is the presence of abnormal-appearing, smooth muscle-like LAM cells.12 LAM lesions in both pulmonary and extrapulmonary sites consist of LAM cells, in some cases localized in nodules, with slit-like lymphatic channels.12,13 In the lung, LAM cells are seen in nodular clusters near the vasculature, lymphatics, and bronchioles, and appear to be present in the walls of the cystic lesions; LAM nodules may be responsible for the thin-walled cystic lesions seen on HRCT scans.1,4,12 LAM cells in lung nodules are morphologically heterogeneous, with both spindle-shaped and epithelioid-like cells observed within the nodule.12 The LAM nodulesappear to be covered by hyperplastic type II cells.14 Spindle-shaped cells are immunoreactive for proliferating cell nuclear antigen, an indicator of mitotic activity and proliferation; the large epithelioid cells are immunoreactive with HMB45, a monoclonal antibody that recognizes gp100, a premelanosomal protein.12,15 LAM cells, in particular, those with an epithelioid appearance, express progesterone and estrogen receptors, consistent with evidence that hormonal factors may play an important role in disease pathogenesis.16,17 The LAM nodules may also be regulated by the renalangiotensin and insulin-like growth factor systems.18,19
Extracellular matrix is a structural and functional part of the pulmonary parenchyma.20 Matrix metalloproteinases (MMPs), a group of Zn2+-dependent endopeptidases, and their regulators, the tissue inhibitors of metalloproteinases (TIMPs), are primary effectors of extracellular matrix turnover and remodeling.21 LAM lung lesions are immunoreactive for MMPs-1, 2, 9, and 14, and show decreased expression of TIMP-3, an inhibitor of some MMPs.12,22-25 An imbalance in the MMP/TIMP ratio caused by the release of MMPs from LAM cells may, in part, result in degradation of the extracellular matrix and thus result in the cystic changes in the LAM lung parenchyma.12,22,23
One-third of women with tuberous sclerosis complex (TSC), an autosomal dominant disorder with variable penetrance, characterized by neurologic (tubers, astrocytomas), renal (AMLs), and dermatologic (facial angiofibroma) manifestations and mutations in the TSC1 or TSC2 tumor suppressor genes, have cystic lung lesions seen on HR CT scans.26-28 LAM cells from the kidney (AMLs), lung, and lymph nodes of patients with sporadic LAM (no evidence of germline TSC mutations) may possess identical TSC2 mutations and/or loss of heterozygosity for the allele.29-31 TSC2 mutations result in activation of the guanine nucleotide-binding protein Rheb, and its downstream target, mammalian target of rapamycin (mTOR), leading to increases in cell size and cell number.32-34 These studies are consistent with the conclusion that the TSC genes are involved in the susceptibility to LAM.
Extrapulmonary manifestations, which occur in more than 70% of patients with LAM, include AMLs, lymphadenopathy, lymphangioleiomyomas, chylous ascites, and pleural effusions.6,8,12,13 AMLs are benign hamartomatous lesions, with abnormal, heterogenous smooth muscle-like cells similar to those in pulmonary and other extrapulmonary LAM lesions. Unlike these other LAM lesions, AMLs may be associated with adipose tissue and underdeveloped thick-walled blood vessels12,13,35-38 (Fig. 2). The adipose tissues and blood vessels may possess TSC gene mutations.39
FIGURE 2.
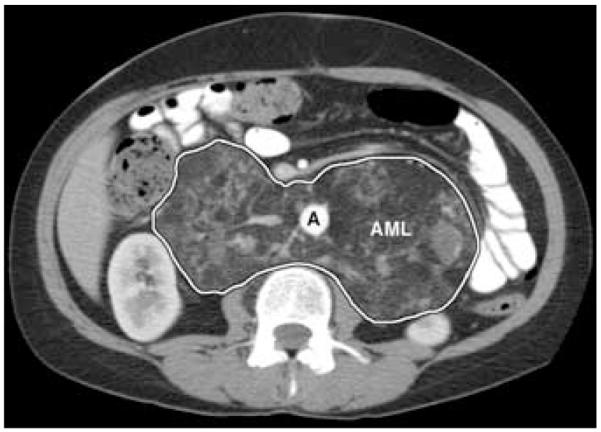
Abdominal CT scan of a patient with LAM showing a very large angiomyolipoma (AML, outlined area) completely involving the retroperitoneal area, resulting in anterior displacement of the aorta (A).
Patients with LAM frequently present with pulmonary symptoms, but initial symptoms may originate from extrapulmonary LAM lesions.12,13,40,41 Roentgenographic appearance of diffusely distributed pulmonary cysts, coupled with CT evidence of extrathoracic disease (e.g., AMLs, lymphatic involvement, chylous effusions), and/or manifestations of are sufficient for a diagnosis of pulmonary LAM. If these extrapulmonary manifestations of disease are not evident, a lung biopsy may be necessary to confirm the diagnosis.4,8,37,42
Improved understanding of prognostic indicators would facilitate selection of subjects for clinical trials and, perhaps, provide new insight into the etiology of LAM. To this end, Matsui et al. correlated histopathologic features and predictors of survival by developing a grading system, the LAM histologic score (LHS), based on a review of surgical biopsy specimens of lung from 105 LAM patients.43 Scores based on the percentage of lung tissue involved with both cystic lesions and areas of proliferation in the lung tissue sample, were: LHS-1 <25% lung involvement, LHS-2=25-50% involvement, and LHS-3 >50% involvement. LHS-3 was correlated with poor prognosis and more rapid progression to lung transplantation or death. In an analysis of pulmonary function tests data from 143 LAM patients, Taveira-DaSilva et al. concluded that DLco correlated with the LHS and could also, potentially, be a predictor of survival or time to lung transplantation.10
Avila et al. proposed a grading system using HRCT lung scans of LAM patients.11 Lungs were divided into three equal zones for assessment of the extent of cystic involvement of parenchyma: grade 0 = none, grade 1 <30%, grade 2=30%-60%, grade 3 >60% judged abnormal. In 39 LAM patients, comparison of these grades with pulmonary function tests data revealed an inverse correlation between CT grade and both FEV1 and DLco.11 In another study of 80 patients with LAM, a correlation was observed between severity of lung disease, assessed by HRCT scans (CT grade), and the presence of enlarged abdominal lymph nodes.44
There is no effective treatment for preventing the progression of pulmonary LAM. Hormonal manipulations were, in the past, a frequent choice of treatment, but no study has confirmed their efficacy.5,8,45 One serious complication of extrapulmonary LAM is AML hemorrhage.46 The initial symptom is abdominal pain, with hematuria, and shock caused by blood loss. Treatment may involve embolization of the artery responsible for the bleeding or surgical resection of the tumor.46 There is no effective treatment for lymphangioleiomyomas. Chylothorax, an accumulation of lymph or chyle in the pleural space, is a complication, which occurs in fewer than 20% of LAM patients.1,4,5,47 Depending on the size and clinical effects of the effusion, thoracentesis, pleurodesis, parietal pleurectomy, and thoracic duct ligation are possible options.47 Pneumothorax can be treated by thoracostomy, but because of its frequent recurrence, pleurodesis may be necessary, even though the procedure may complicate subsequent lung transplantation.48 Evidence of pleural repair may be seen on HRCT after pleurodesis.49 The presence of pleurodesis has variable influence on the correlation of quantitative thin-section CT with pulmonary function tests.50 The occurrence of pneumothoraces may have genetic and morphologic determinants.51
There is genetic evidence that cells in the recurrent LAM lesions in transplanted lungs originate in the recipient.52,53 These observations are consistent with a model of metastatic disease in which cells move from AML, lymphatics, or a presently unknown site of origin, into the lung. Isolation of LAM cells from blood and other body fluids (e.g., urine, chylous pleural effusions, ascites) is additional support for the metastatic model.54
Lymphatic Studies in LAM
Extrapulmonary LAM
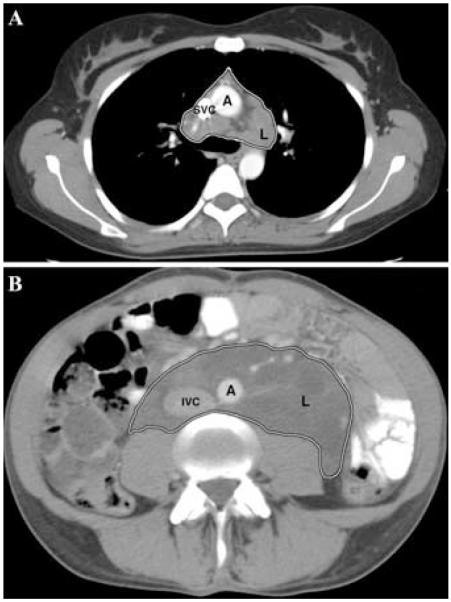
Lymphatic involvement in LAM, which may include the posterior mediastinum, and upper retroperitoneal, or pelvic regions, appears to be related to the distribution of lymphatic vessels.4,13,40,41 Lymphan-gioleiomyomas on CT scans are well-circumscribed lesions of variable dimensions that are seen as thick- or thin-walled lobulated masses with a central fluid-rich region (Figs. 3A and B). These lesions are most frequently found in the retroperitoneal and mediastinal regions.13,44,55 Abdominal lymphangioleiomyomas, formed from a collection of chyle in lymphatic vessels, when overdistended, can rupture or leak, thus leading to chylous ascites.13,44 Enlarged lymph nodes appear round or oval and are most often found in the retroperitoneal area.4,40,44 They have also been observed in the mediastinum and pelvic regions, and are commonly associated with other extrapulmonary lesions.44,56,57 Unlike pulmonary LAM cells, most extrapulmonary lymphatic LAM cells are found in lymph nodes along the lymphatic vessels of the mediastinum and retroperitoneum, where they form fascicles and papillary patterns, rather than nodules, and have been reported to extend beyond the connective tissue capsule of the lymphatics.4,12,13,40,41 Mutations in the TSC genes have been observed in lymphatic LAM cells and in the constituent tissues of the AMLs.29,39
FIGURE 3.
Chest CT scan of a patient with LAM (A) showing a large lymphangioleiomyoma (L, outlined area) involving the pre-tracheal and left lung hilar area, and an abdominal CT scan (B) of a patient with LAM showing a very large lymphangioleiomyoma (L, outlined area) located in the retroperitoneal area surrounding the aorta and inferior vena cava. SVC, superior vena cava; IVC, inferior vena cava; A, aorta.
With the exception of AMLs, the different manifestations of extrapulmonary LAM appear to arise from a common pathology. Proliferating LAM cellsappear to compress or obstruct lymphatic vessels, leading to obstruction of the flow of chyle, and the observed lymphangioleiomyomas or dilatation of lymphatic vessels seen on abdominal CT scans of patients with LAM.13,44,58 Biopsies of involved lymph nodes, with characteristic adenopathy, reveal replacement of normal components by LAM cells.45
In two separate studies, lymphangioleiomyomas in retroperitoneal, pelvic, thoracic, and/or cervical regions were found to have a diurnal variation in size in patients with LAM. Patients underwent CT or sonogram examinations in the morning and afternoon of the same day. The studies showed that the lesions in 12/13 patients and 12/21 patients, respectively, exhibited an increase between morning and afternoon.59,60 The median percent changes in volume were 140% and 38%, respectively.59,60 Diurnal variation may be due to the greater lymph flow resulting from food intake and exercise, and/or the effect of gravity on lymph flow. Lymphangioleiomyomas, viewed by CT or sonography, are sometimes difficult to distinguish from neoplasms such as lymphoma or sarcoma. Documentation of diurnal variation may aid in diagnosis and obviate the need for biopsy.
Lymphatic Involvement in TSC
Hamartomas in patients with TSC are most frequently found in the skin, brain (tubers, subependymal lesions), kidneys (AMLs), and heart (rhabdomyomas).61 Angiogenesis in subependymal giant cell astrocytoma and AML lesions has been demonstrated by immunoreactivity with anti-CD31 antibody.62 In agreement, AMLs have been shown to produce VEGF in vitro.62
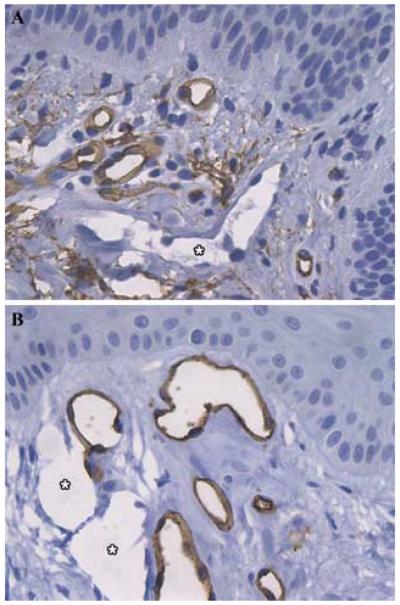
Lymphatic involvement (thoracic duct dilation, chylous pleural effusion, ascites, and lymphangioleiomyomas) is less common in patients with TSC/LAM than in those with sporadic LAM.63 Patients with TSC develop multiple different types of skin lesions, including facial angiofibromas, forehead plaques, and ungual fibromas. The papules and plaques are typically pink to red, and contain accumulated collagen plus increased numbers of ectatic vessels in the dermis.64 Sections of TSC skin tumors have been studied using immunohistochemical markers for angiogenesis. Skin tumor lesions (angiofibromas) of TSC patients express VEGF.65 Many dilated tubular structures are blood vessels that contain immunoreactive CD31 or CD34.62,66 Not all of the endothelial-lined spaces in angiofibromas and periungual fibromas react with anti-CD34 antibodies (Figs. 4A and B). The vessels in TSC skin tumors have not been closely examined, and although blood vessels are often assumed to be present, some reports mentioned increased numbers of lymphatic as well as blood vessels.67,68 These data are consistent with the presence of dilated lymphatic vessels, since anti-CD34 antibodies do not react with lymphatic endothelial cells in skin.69
FIGURE 4.
Lymphangiogenesis in TSC skin lesions. Sections of angiofibromas (A) and periungual fibromas (B) were stained for CD34, a marker for blood vessel endothelial cells. Vessels negative for CD34 (*), presumed to be lymphatic vessels, are interspersed with blood vessels positive for CD34 (darkly stained endothelial cells). Original magnification ×400.
Histopathologic Studies of LAM Cells and Lymphatics in LAM
Genetic and pathologic evidence suggests a metastatic potential for LAM cells. Angiogenesis and lymphangiogenesis have been postulated as mediators of metastatic spread and tumor growth by providing new vessels for the invading tumor cells.70,71 The role of angiogenesis in tumor spread is well documented, but research regarding lymphangiogenesis in tumor progression presents a new avenue of approach and has been facilitated by the identification of specific lymphatic endothelial cell markers, such as LYVE-1 (transports hyaluronan by lymphatic endothelial cells to lymph), podoplanin (marker for lymphatic endothelial cells), VEGF-R3 (growth factor receptor for ligands VEGF-C and VEGF-D), and PROX-1 (transcription factor required for lymphatic vessel growth).70
Lymphatic studies in LAM focus on defining the role of lymphangiogenesis and angiogenesis in the dissemination of LAM cells. Among three recent LAM studies that focus on lymphatic involvement, the first used immunohistochemistry to analyze 21 samples of LAM tissue (lung, lymph nodes, uterus, ovary) obtained from autopsy or lung explants.72 Angiogenesis and lymphangiogenesis were evaluated, respectively, with antibodies against CD31, a vascular endothelial cell marker and VEGFR-3 (Flt-4), a specific lymphatic endothelial cell marker. LAM lesions exhibited minimal cellular reactive CD31, but were reactive with anti-VEGFR-3 (Flt-4) antibodies in both pulmonary and extrapulmonary tissue samples of the lung, lymph nodes (cervical, axillary, supraclavicular, mediastinal, retroperitoneal, and mesenteric regions), uterus and ovary. The slit-like spaces surrounding and infiltrating LAM cell foci and nodules were identified in this way as lymphatic tracts. Lymphatic endothelial cells, immunoreactive with Flt-4 antibodies, were observed in retroperitoneal and mediastinal lymph nodes to surround LAM cell foci, which were formed in bundle-like structures. LAM cell clusters (LCCs), or LAM cell nests covered by lymphatic endothelial clusters appeared to be fragments of LAM foci and were observed in lymphatic vessels, some near lymph nodes, indicating a region of potential interaction of LAM cells and lymphatic endothelial clusters. Lymphangiogenesis was given a semiquantitative assessment based on the extent of each lesion occupied by lymphatic endothelial cells.72
In the same study, LAM tissue was analyzed immunohistochemically for VEGF-C as a potential mediator of lymphangiogenesis. Diffuse immunoreactivity with antibodies against VEGF-C and with monoclonal antibody HMB45 was observed in the cytoplasm of pulmonary and extrapulmonary LAM tissue, including lung samples from biopsy and explanted lung, as well as lymph nodes, uterus, and lung cells in primary cultures. Relationships between the intensity of VEGF-C immunostaining in lung tissue, the extent of lymphangiogenesis in lung lesions (as reflected by estimates of relative amounts of Flt-4 immunostaining), and the LHS score developed by Matsui et al.43 were evaluated. A statistically significant correlation between greater lymphatic involvement and higher VEGF-C content was demonstrated, and both were associated with worse prognosis and time to lung transplantation as expressed by LHS.72 The results of this study would support a lymphangiogenic model for the progression of LAM, in which VEGF-C produced by LAM cells enhances lymphangiogenesis via the VEGFR-3 receptor.
Immunologic examination of chylous fluid and retroperitoneal lymphangioleiomyoma samples from six patients with LAM, and examination of tissue from five autopsy cases that included samples of the thoracic duct, and left jugular subclavian angle, and cervical, axillary, supraclavicular, mediastinal, retroperitoneal, and mesenteric region lymph nodes were employed to assess the presence of LAM cells in lymphatic structures containing chyle and to evaluate a potential route for LAM cell entry into the lymphatic system.73 LAM cells, immunoreactive with both HMB45 and anti-α-SMA (smooth muscle actin) antibodies, were observed, in all cases, as clusters covered by lymphatic endothelial clusters in chylous effusions and within lymphatic vessels, and were also detected floating in the extralymphatic spaces. The clusters showed an outer layer of lymphatic endothelial cells containing immunoreactive VEGFR-3. In addition, in the lymphangioleiomyoma sample, LAM cell lesions appeared as LAM cell clusters surrounded by lymphatic endothelial clusters. Retrospective examination of the autopsy samples confirmed the involvement of LAM cells extending along the axial lymphatics, including the region of thoracic duct drainage into the venous circulation (left jugular and subclavian junction). LAM cell-positive lymph nodes were most frequently identified in the retroperitoneal region. After LCCs were incubated in vitro on collagen-coated slides for several days, clusters were no longer visible, but two types of cells were seen, spindle-shaped LAM cells (containing immunoreactive α-SMA) and lymphatic endothelial cells (immunopositive for VEGFR-3).73
Based on the results of these two studies, a model for the dissemination of AM cells was formulated in which lymphatic endothelial cells lining lymphatic channels in LAM cell nodules, cystic lesions, and lymph nodes eventually envelop LAM cells, and the resulting clusters may be shed into the lymphatic circulation or extrapulmonary space. In the lymphatic channels, interaction of the lymphatic endothelial cells on the cluster and the lymphatic endothelial cells lining lymphatic vessels, initiate fragmentation, separating LCCs into LAM cells and lymphatic endothelial cells. LAM cells are able then to invade the extracellular matrix, which is degraded by LAM cell-derived MMPs with facilitation by downregulation of TIMPs. LAM cell invasion and proliferation results in the formation of a new LAM lesion.73
In the most recent report of LAM-associated lymphangiogenesis, it was proposed that since the pathogenesis and progression of LAM (evaluated by LHS) is associated with extent of lymphatic involvement, serum levels of lymphatic growth factors might be elevated, and correlated with clinical characteristics of LAM.74 Such a growth factor could be considered a biological marker for lymphangiogenesis and disease severity. Serum levels of VEGF-A, an angiogenic factor, and VEGF-C and VEGF-D, described lymphangiogenic factors, in 44 patients with LAM were quantified by serum enzyme-linked immunosorbent assay. Serum concentrations of VEGF-A in patients with LAM were similar to those of the control group, whereas the levels of VEGF-C were significantly lower in LAM than in control groups. Serum VEGF-D was, however, significantly higher in patients with LAM than in controls. There was a significant negative correlation between serum levels of VEGF-D and pulmonary function as measured by FEV1/FVC (forced expiratory volume in 1 second/forced vital capacity) or percent predicted DLco/VA (diffusing capacity for carbon monoxide/alveolar volume).
A subset of LAM patients was divided into those who had been given hormonal treatment and those who had not.74 The group of patients who had undergone hormone therapy had more restricted air flow (lower FEV1 percent predicted, FEV1/FVC), lower diffusing capacity (percent predicted lower DLco/VA), and higher serum VEGF-D concentration than those patients with no history of recent hormone therapy. This study established an association between serum levels of VEGF-D, a lymphangiogenic growth factor, and clinical characteristics of patients with LAM, suggesting that VEGF-D could be a marker for disease severity.
Conclusion and Future Directions
Extensive association of lymphangiogenesis was found with pulmonary and extrapulmonary lesions in LAM. The production of VEGF-C and VEGF-D by pulmonary and extrapulmonary LAM lesions might reflect LAM cell-induction of lymphangiogenesis through VEGFR-3 signaling. The interesting observation of LCCs covered with lymphatic endothelial cells located in lymphatic vessels suggests a potential mechanism for a lymphangiogenesis-driven metastatic process. In vitro evidence of LCC fragmentation suggests a mechanism whereby LAM cells have the potential to interact with the extracellular matrix to form new lesions and to reach the vascular circulation via the thoracic duct. Future studies may reveal histopathologic evidence of anatomic connections between blood and lymphatic vessels in LAM lesions.
Findings from the first two reports, based on immunohistochemical evidence of protein expression, implicated the growth factor VEGF-C as a driving force for LAM-directed lymphangiogenesis.72,73 In contrast, on the basis of serum levels of VEGF growth factors in the latest study, VEGF-D appears much more likely to be a biomarker that correlates with lymphangiogenesis and disease progression.74 Protein expression studies and studies correlating clinical phenotypes to serum levels of VEGF-D and VEGF-C would help to define more clearly the differential roles of VEGF-C and VEGF-D in LAM. The study of lymphangiogenesis and LAM presents a promising avenue to elucidate a model for dissemination of LAM cells.
Acknowledgments
This study was supported by the Intramural Research Program of the National Institutes of Health, NHLBI. We thank Dr. Martha Vaughan for helpful discussions and critical review of the manuscript. We thank the LAM Foundation and the Tuberous Sclerosis Alliance for patient referrals, and we thank the patients with LAM for their commitment and inspiration.
Footnotes
Conflicts of Interest
The authors declare no conflicts of interest.
References
- 1.Kitaichi M, Nishimura K, et al. Pulmonary lymphangioleiomyomatosis: a report of 46 patients including a clinicopathologic study of prognostic factors. Am. J. Respir. Crit. Care Med. 1995;151:527–533. doi: 10.1164/ajrccm.151.2.7842216. [DOI] [PubMed] [Google Scholar]
- 2.Urban T, Lazor R, et al. Pulmonary lymphangioleiomyomatosis: a study of 69 patients. Medicine. 1999;78:321–337. doi: 10.1097/00005792-199909000-00004. [DOI] [PubMed] [Google Scholar]
- 3.Ryu JH, Moss J, et al. The NHLBI lymphangioleiomyomatosis registry: characteristics of 230 patients at enrollment. Am. J. Respir. Crit. Care Med. 2006;173:105–111. doi: 10.1164/rccm.200409-1298OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chu SC, Horiba K, et al. Comprehensive evaluation of 35 patients with lymphangioleiomyomatosis. Chest. 1999;115:1041–1052. doi: 10.1378/chest.115.4.1041. [DOI] [PubMed] [Google Scholar]
- 5.Taylor JR, Ryu J, et al. Lymphangioleiomyomatosis: clinical course in 32 patients. N. Engl. J. Med. 1990;323:1254–1260. doi: 10.1056/NEJM199011013231807. [DOI] [PubMed] [Google Scholar]
- 6.Johnson SR, Tattersfield AE. Lymphangioleiomyomatosis. Semin. Respir. Crit. Care Med. 2002;23:85–92. doi: 10.1055/s-2002-25298. [DOI] [PubMed] [Google Scholar]
- 7.Hayashida M, Seyama K, et al. The epidemiology of lymphangioleiomyomatosis in Japan: a nationwide cross-sectional study of presenting features and prognostic factors. Respirology. 2007;12:523–530. doi: 10.1111/j.1440-1843.2007.01101.x. [DOI] [PubMed] [Google Scholar]
- 8.Taveira-DaSilva AM, Steagall WK, et al. Lymphangioleiomyomatosis. Cancer Control. 2006;13:276–285. doi: 10.1177/107327480601300405. [DOI] [PubMed] [Google Scholar]
- 9.Johnson SR, Whale CI, et al. Survival and disease progression in UK patients with lymphangioleiomyomatosis. Thorax. 2004;59:800–803. doi: 10.1136/thx.2004.023283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Taveira-DaSilva AM, Hedin C, et al. Reversible airflow obstruction: proliferation of abnormal smooth muscle cells, and impairment of gas exchange as predictors of outcome in lymphangioleiomyomatosis. Am. J. Respir. Crit. Care Med. 2001;164:1072–1076. doi: 10.1164/ajrccm.164.6.2102125. [DOI] [PubMed] [Google Scholar]
- 11.Avila NA, Chen CC, et al. Pulmonary lymphangioleiomyomatosis: correlation of ventilation-perfusion scintigraphy, chest radiography, and CT with pulmonary function tests. Radiology. 2000;214:441–446. doi: 10.1148/radiology.214.2.r00fe41441. [DOI] [PubMed] [Google Scholar]
- 12.Ferrans VJ, Yu Z-X, et al. Lymphangioleiomyomatosis (LAM): a review of clinical and morphological features. J. Nippon Med. Sch. 2000;67:311–329. doi: 10.1272/jnms.67.311. [DOI] [PubMed] [Google Scholar]
- 13.Matsui K, Tatsuguchi A, et al. Extrapulmonary lymphangioleiomyomatosis (LAM): clinicopathologic features in 22 cases. Hum. Pathol. 2000;31:1242–1248. doi: 10.1053/hupa.2000.18500. [DOI] [PubMed] [Google Scholar]
- 14.Matsui K, Riemenscneider WK, et al. Hyperplasia of type II pneumocytes in pulmonary lymphangioleiomyomatosis: immunohistochemical and electron microscopic study. Arch. Pathol. Lab. Med. 2000;124:1642–1648. doi: 10.5858/2000-124-1642-HOTIPI. [DOI] [PubMed] [Google Scholar]
- 15.Matsumoto Y, Horiba K, et al. Markers of cell proliferation and expression of melanosomal antigen in lymphangioleiomyomatosis. Am. J. Respir. Cell Mol. Biol. 1999;21:327–336. doi: 10.1165/ajrcmb.21.3.3693. [DOI] [PubMed] [Google Scholar]
- 16.Ohori NP, Yousem SA, et al. Estrogen and progesterone receptors in lymphangioleiomyomatosis, epithelioid hemangioendothelioma, and sclerosing hemangioma of the lung. Am. J. Clin. Pathol. 1991;96:529–535. doi: 10.1093/ajcp/96.4.529. [DOI] [PubMed] [Google Scholar]
- 17.Matsui K, Takeda K, et al. Downregulation of estrogen and progesterone receptors in the abnormal smooth muscle cells in pulmonary lymphangioleiomyomatosis following therapy: an immunohistochemical study. Am. J. Respir. Crit. Care Med. 2000;161:1002–1009. doi: 10.1164/ajrccm.161.3.9904009. [DOI] [PubMed] [Google Scholar]
- 18.Valencia JC, Pacheco-Rodriguez G, et al. Tissue-specific renin-angiotensin system in pulmonary lymphangioleiomyomatosis. Am. J. Respir. Cell Mol. Biol. 2006;35:40–47. doi: 10.1165/rcmb.2005-0387OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Valencia JC, Matsui K, et al. Distribution and mRNA expression of insulin-like growth factor system in pulmonary lymphangioleiomyomatosis. J. Invest. Med. 2001;49:421–433. doi: 10.2310/6650.2001.33787. [DOI] [PubMed] [Google Scholar]
- 20.Shapiro SD. Chairman’s summary. Proc. Am. Thorac. Soc. 2006;3:397–400. [Google Scholar]
- 21.Parks WC, Shapiro S. Review: matrix metalloproteinases in lung biology. Respir. Res. 2001;2:10–19. doi: 10.1186/rr33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Matsui K, Takeda K, et al. Role for activation of matrix metalloproteinases in the pathogenesis of pulmonary lymphangioleiomyomatosis. Arch. Pathol. Lab. Med. 2000;124:267–275. doi: 10.5858/2000-124-0267-RFAOMM. [DOI] [PubMed] [Google Scholar]
- 23.Hayashi T, Fleming MV, et al. Immunohistochemical study of matrix metalloproteinases (MMPs) and their tissue inhibitors (TIMPs) in pulmonary lymphangioleiomyomatosis (LAM) Hum. Pathol. 1997;28:1071–1078. doi: 10.1016/s0046-8177(97)90061-7. [DOI] [PubMed] [Google Scholar]
- 24.Zhe X, Yang Y, et al. Tissue inhibitor of metalloproteinase-3 downregulation in lymphangioleiomyomatosis: potential consequence of abnormal serum response factor expression. Am. J. Respir. Cell Mol. Biol. 2003;28:504–511. doi: 10.1165/rcmb.2002-0124OC. [DOI] [PubMed] [Google Scholar]
- 25.Krymskaya VP, Shipley JM. Lymphangioleiomyomatosis: a complex tale of serum response factor-mediated tissue inhibitor of metalloproteinase-3 regulation. Am. J. Respir. Cell Mol. Biol. 2003;28:546–550. doi: 10.1165/rcmb.F267. [DOI] [PubMed] [Google Scholar]
- 26.Moss J, Avila NA, et al. Prevalence and clinical characteristics of lymphangioleiomyomatosis (LAM) in patients with tuberous sclerosis complex. Am. J. Respir. Crit. Care Med. 2001;163:669–671. doi: 10.1164/ajrccm.164.4.2101154. [DOI] [PubMed] [Google Scholar]
- 27.Costello LC, Hartman TE, et al. High frequency of pulmonary lymphangioleiomyomatosis in women with tuberous sclerosis complex. Mayo Clin. Proc. 2000;75:591–594. doi: 10.4065/75.6.591. [DOI] [PubMed] [Google Scholar]
- 28.Franz DN, Brody A, et al. Mutational and radiographic analysis of pulmonary disease consistent with lymphangioleiomyomatosis and micronodular pneumocyte hyperplasia in women with tuberous sclerosis. Am. J. Respir. Crit. Care Med. 2001;164:661–668. doi: 10.1164/ajrccm.164.4.2011025. [DOI] [PubMed] [Google Scholar]
- 29.Smolarek TA, Wessner LL, et al. Evidence that lymphangioleiomyomatosis is caused by TSC2 mutations: chromosome 16p13 loss of heterozygosity inangiomyolipomas and lymph nodes from women with lymphangiomyomatosis. Am. J. Hum. Genet. 1998;62:810–815. doi: 10.1086/301804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Carsillo T, Astrinidis A, et al. Mutations in the tuberous sclerosis complex gene TSC2 are a cause of sporadic pulmonary lymphangioleiomyomatosis. Proc. Natl. Acad. Sci. USA. 2000;97:6085–6090. doi: 10.1073/pnas.97.11.6085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sato T, Seyama K, et al. Mutation analysis of the TSC1 and TSC2 genes in Japanese patients with pulmonary lymphangioleiomyomatosis. J. Hum. Genet. 2002;47:20–28. doi: 10.1007/s10038-002-8651-8. [DOI] [PubMed] [Google Scholar]
- 32.Goncharova EA, Goncharov DA, et al. Tuberin regulates p70 S6 kinase activation and ribosomal protein S6 phosphorylation: a role for the TSC2 tumor suppressor gene in pulmonary lymphangioleiomyomatosis (LAM) J. Biol. Chem. 2002;277:30958–30967. doi: 10.1074/jbc.M202678200. [DOI] [PubMed] [Google Scholar]
- 33.Tee AR, Fingar DC, et al. Tuberous sclerosis complex-1 and -2 gene products function together to inhibit mammalian target of rapamycin (mTOR)-mediated downstream signaling. Proc. Natl. Acad. Sci. USA. 2002;99:13571–13576. doi: 10.1073/pnas.202476899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tee AR, Manning BD, et al. Tuberous sclerosis complex gene products, Tuberin and Hamartin, control mTOR signaling by acting as a GTPase-activating protein complex toward Rheb. Curr. Biol. 2003;13:1259–1268. doi: 10.1016/s0960-9822(03)00506-2. [DOI] [PubMed] [Google Scholar]
- 35.Maziak DE, Keston S, et al. Extrathoracic angiomyolipomas in lymphangioleiomyomatosis. Eur. Respir. J. 1996;9:402–405. doi: 10.1183/09031936.96.09030402. [DOI] [PubMed] [Google Scholar]
- 36.Kerr LA, Blute ML, et al. Renal angiomyolipoma in association with pulmonary lymphangioleiomyomatosis: forme fruste of tuberous sclerosis? Urology. 1993;41:440–444. doi: 10.1016/0090-4295(93)90504-4. [DOI] [PubMed] [Google Scholar]
- 37.Kelly J, Moss J. Lymphangioleiomyomatosis. Am. J. Med. Sci. 2001;321:17–25. doi: 10.1097/00000441-200101000-00004. [DOI] [PubMed] [Google Scholar]
- 38.McIntosh GS, Dutoit SH, et al. Multiple unilateral renal angiomyolipomas with regional lymphangioleiomyomatosis. J. Urol. 1989;142:1305–1307. doi: 10.1016/s0022-5347(17)39067-5. [DOI] [PubMed] [Google Scholar]
- 39.Henske EP, Neumann HP, et al. Loss of heterozygosity in the tuberous sclerosis (TSC2) region of chromosome band 16p13 occurs in sporadic as well as TSC-associated renal angiomyolipomas. Genes Chromosomes Cancer. 1995;13:295–298. doi: 10.1002/gcc.2870130411. [DOI] [PubMed] [Google Scholar]
- 40.Kebria M, Black D, et al. Primary retroperitoneal lymphangioleiomyomatosis in a postmenopausal woman: a case report and review of the literature. Int. J. Gynecol. Cancer. 2007;17:528–532. doi: 10.1111/j.1525-1438.2007.00785.x. [DOI] [PubMed] [Google Scholar]
- 41.Lam B, Ooi GC, et al. Extrapulmonary presentation of asymptomatic pulmonary lymphangioleiomyomatosis. Respirology. 2003;8:544–547. doi: 10.1046/j.1440-1843.2003.00498.x. [DOI] [PubMed] [Google Scholar]
- 42.Johnson SR. Lymphangioleiomyomatosis. Eur. Respir. J. 2006;27:1056–1065. doi: 10.1183/09031936.06.00113303. [DOI] [PubMed] [Google Scholar]
- 43.Matsui K, Beasley MB, et al. Prognostic significance of pulmonary lymphangioleiomyomatosis histologic score. Am. J. Surg. Pathol. 2001;29:1356–1366. doi: 10.1097/00000478-200104000-00007. [DOI] [PubMed] [Google Scholar]
- 44.Avila NA, Kelly JA, et al. Lymphangioleiomyomatosis: abdominopelvic CT and US findings. Radiology. 2000;216:147–153. doi: 10.1148/radiology.216.1.r00jl42147. [DOI] [PubMed] [Google Scholar]
- 45.Taveira-DaSilva AM, Stylianou MP, et al. Decline in lung function in patients with lymphangioleiomyomatosis treated with or without progesterone. Chest. 2004;126:1867–1874. doi: 10.1378/chest.126.6.1867. [DOI] [PubMed] [Google Scholar]
- 46.Bissler JJ, Kingswood JC. Renal angiomyolipomata. Kidney Int. 2004;66:924–934. doi: 10.1111/j.1523-1755.2004.00838.x. [DOI] [PubMed] [Google Scholar]
- 47.Ryu JH, Doerr CH, et al. Chylothorax in lymphangioleiomyomatosis. Chest. 2003;123:623–627. doi: 10.1378/chest.123.2.623. [DOI] [PubMed] [Google Scholar]
- 48.Almoosa KF, Ryu JH, et al. Management of pneumothorax in lymphangioleiomyomatosis: effects on recurrence and lung transplantation complications. Chest. 2006;129:1274–1281. doi: 10.1378/chest.129.5.1274. [DOI] [PubMed] [Google Scholar]
- 49.Avila NA, Dwyer AJ, et al. CT of pleural abnormalities in lymphangioleiomyomatosis and comparison of pleural findings after different types of pleurodesis. AJR Am. J. Roentgenol. 2006;186:1007–1012. doi: 10.2214/AJR.04.1912. [DOI] [PubMed] [Google Scholar]
- 50.Avila NA, Kelly JA, et al. Lymphangioleiomyomatosis: correlation of qualitative and quantitative thinsection CT with pulmonary function tests and assessment of dependence on pleurodesis. Radiology. 2002;223:189–197. doi: 10.1148/radiol.2231010315. [DOI] [PubMed] [Google Scholar]
- 51.Steagall WK, Glasgow CG, et al. Genetic and morphologic determinants of pneumothorax in lymphangioleiomyomatosis. Am. J. Physiol. Lung Cell. Mol. Physiol. 2007;293:L800–L808. doi: 10.1152/ajplung.00176.2007. [DOI] [PubMed] [Google Scholar]
- 52.Bittman I, Rolf B, et al. Recurrence of lymphangioleiomyomatosis after single lung transplantation: new insights into pathogenesis. Hum. Pathol. 2003;34:95–98. doi: 10.1053/hupa.2003.50. [DOI] [PubMed] [Google Scholar]
- 53.Karbowniczek M, Astrinidis A, et al. Recurrent lymphangiomyomatosis after transplantation: genetic analyses reveal a metastatic mechanism. Am. J. Respir. Crit. Care Med. 2003;167:976–982. doi: 10.1164/rccm.200208-969OC. [DOI] [PubMed] [Google Scholar]
- 54.Crooks DM, Pacheco-Rodriguez G, et al. Molecular and genetic analysis of disseminated neoplastic cells in lymphangioleiomyomatosis. Proc. Natl. Acad. Sci. USA. 2004;101:17462–17467. doi: 10.1073/pnas.0407971101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tai H-C, Lin J-W, et al. Pulmonary lymphangioleiomyomatosis followed by a localized retroperitoneal lymphangioleiomyoma. A.P.M.I.S. 2006;114:821–824. doi: 10.1111/j.1600-0463.2006.apm_489.x. [DOI] [PubMed] [Google Scholar]
- 56.Cornog JL, Jr., Enterline HT. Lymphangiomyoma, a benign lesion of chyliferous lymphatics synonymous with lymphangiopericytoma. Cancer. 1966;19:1909–1930. doi: 10.1002/1097-0142(196612)19:12<1909::aid-cncr2820191219>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 57.Woodring JH, Howard RS, II, et al. Massive low-attenuation mediastinal, retroperitoneal, and pelvic lymphadenopathy on CT from lymphangioleiomyomatosis case report. Clinical Imaging. 1994;18:7–11. doi: 10.1016/0899-7071(94)90138-4. [DOI] [PubMed] [Google Scholar]
- 58.Pallisa E, Sanz P, et al. Lymphangioleiomyomatosis: pulmonary and abdominal findings with pathologic correlation. RadioGraphics. 2002;22:S185–S198. doi: 10.1148/radiographics.22.suppl_1.g02oc13s185. [DOI] [PubMed] [Google Scholar]
- 59.Avila NA, Bechtle J, et al. Lymphangioleiomyomatosis: CT of diurnal variation of lymphangioleiomyomas. Radiology. 2001;221:415–421. doi: 10.1148/radiol.2212001448. [DOI] [PubMed] [Google Scholar]
- 60.Avila NA, Dwyer AJ, et al. Sonography of lymphangioleiomyoma in lymphangioleiomyomatosis: demonstration of diurnal variation in lesion size. AJR Am. J. Roentgenol. 2005;184:459–464. doi: 10.2214/ajr.184.2.01840459. [DOI] [PubMed] [Google Scholar]
- 61.Leung AKC, Robson WLM. Tuberous sclerosis complex: a review. J. Pediatr Health Care. 2007;21:108–114. doi: 10.1016/j.pedhc.2006.05.004. [DOI] [PubMed] [Google Scholar]
- 62.Arbiser JL, Brat D, et al. Tuberous sclerosis-associated lesions of the kidney, brain, and skin are angiogenic neoplasms. J. Am. Acad. Dermatol. 2002;46:376–380. doi: 10.1067/mjd.2002.120530. [DOI] [PubMed] [Google Scholar]
- 63.Avila NA, Dwyer AJ, et al. Sporadic lymphangioleiomyomatosis and tuberous sclerosis complex with lymphangioleiomyomatosis: comparison of CT features. Radiology. 2007;242:277–285. doi: 10.1148/radiol.2421051767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Webb DW, Clarke A, et al. The cutaneous features of tuberous sclerosis: a population study. Br. J. Dermatol. 1996;135:1–5. [PubMed] [Google Scholar]
- 65.Nguyen-Vu P-A, Facker I, et al. Loss of tuberin, the tuberous-sclerosis-complex-2 gene product is associated with angiogenesis. J. Cutan. Pathol. 2001;28:470–475. doi: 10.1034/j.1600-0560.2001.028009470.x. [DOI] [PubMed] [Google Scholar]
- 66.Li S, Takeuchi F, et al. MCP-1 overexpressed in tuberous sclerosis lesions acts as a paracrine factor for tumor development. J. Exp. Med. 2005;202:617–624. doi: 10.1084/jem.20042469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Nickel WR, Reed WB. Tuberous sclerosis: special reference to the microscopic alterations in the cutaneous hamartomas. Arch. Dermatol. 1962;85:89–106. doi: 10.1001/archderm.1962.01590020049006. [DOI] [PubMed] [Google Scholar]
- 68.Sanchez NP, Wick MR, et al. Adenoma sebaceum of Pringle: a clinicopathologic review, with a discussion of related pathologic entities. J. Cutan. Pathol. 1981;8:395–403. doi: 10.1111/j.1600-0560.1981.tb01028.x. [DOI] [PubMed] [Google Scholar]
- 69.Hirakawa S, Hong Y-K, et al. Identification of vascular lineage-specific genes by transcriptional profiling of isolated blood vascular and lymphatic endothelial cells. Am. J. Pathol. 2003;162:575–586. doi: 10.1016/S0002-9440(10)63851-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Stacker SA, Baldwin ME, et al. The role of tumor lymphangiogenesis in metastatic spread. FASEB J. 2002;16:922–934. doi: 10.1096/fj.01-0945rev. [DOI] [PubMed] [Google Scholar]
- 71.Sleeman JP. The lymph node as a bridgehead in the metastatic dissemination of tumors. Rec. Results Cancer Res. 2000;157:55–81. doi: 10.1007/978-3-642-57151-0_6. [DOI] [PubMed] [Google Scholar]
- 72.Kumasaka T, Seyama K, et al. Lymphangiogenesis in lymphangioleiomyomatosis: its implication in the progression of lymphangioleiomyomatosis. Am. J. Surg Pathol. 2004;8:1007–1015. doi: 10.1097/01.pas.0000126859.70814.6d. [DOI] [PubMed] [Google Scholar]
- 73.Kumasaka T, Seyama K, et al. Lymphangiogenesis-mediated shedding of LAM cell clusters as a mechanism for dissemination in lymphangioleiomyomatosis. Am. J. Surg. Pathol. 2005;29:1356–1366. doi: 10.1097/01.pas.0000172192.25295.45. [DOI] [PubMed] [Google Scholar]
- 74.Seyama K, Kumasaka T, et al. Vascular endothelial growth factor-D is increased in serum of patients with lymphangioleiomyomatosis. Lymph. Res. Biol. 2006;3:143–152. doi: 10.1089/lrb.2006.4.143. [DOI] [PubMed] [Google Scholar]