Abstract
Adrenalectomy in the mature rat leads to death of granule cells in the dentate gyrus of the hippocampal formation. The mechanisms underlying this cell death have not been fully clarified: It has been considered that the granule cells require adrenal steroids for their survival, since corticosterone replacement prevents their death. However, adrenalectomy-induced loss of negative feedback also increases levels of corticotropin releasing hormone (CRH) in several limbic brain regions. CRH is known to induce neuronal death in hippocampal regions rich in CRH receptors. This study tested the hypothesis that adrenalectomy-induced granule cell death is mediated via the enhanced activation of CRH receptors. The extent of granule cell degeneration was compared among 4 groups of young adult male rats: Sham-adrenalectomy controls, adrenalectomized rats, adrenalectomized rats infused with a CRH antagonist from the onset of steroid deprivation to the time of sacrifice, and adrenalectomized rats infused with vehicle only. (9–41)-alpha-helical CRH was administered using an osmotic pump into the cerebral ventricles. Adrenalectomy led to robust granule cell degeneration, which was maximal in the suprapyramidal blade of the dentate gyrus. Infusion of the CRH antagonist in doses shown to block CRH actions on limbic neurons did not decrease the number of degenerating granule cells compared with the untreated or vehicle-infused adrenalectomized groups. Therefore, blocking the actions of CRH does not prevent adrenalectomy-induced granule cell death, consistent with a direct effect of corticoids on the survival of these neurons.
Keywords: CRF, cell death, glucocorticoid, mineralocorticoid, hippocampus, receptor, granule cells
INTRODUCTION
Adrenalectomy (ADX) of the adult rat leads to degeneration of a significant proportion of granule cells in the dentate gyrus of the hippocampal formation (Sloviter et al., 1989; Sloviter et al., 1993a,b). The structural features of this selective cell death are consistent with apoptosis (Sloviter et al., 1993b), but the mechanisms activating this cell death are not fully understood. Cell death commences within days of steroid withdrawal, and is clearly evident by 5–7 days (Gould et al., 1990; Hornsby et al., 1996). As adrenalectomy results in depletion of steroids and selective steroid replacement prevents granule cell death (Joels and deKloet, 1994; Hornsby, et al., 1996), it has been assumed that adrenal steroids are required for survival of granule cells. However, adrenalectomy, via loss of negative feedback, also increases the synthesis and levels of corticotropin releasing hormone (CRH; Vale et al., 1981) in a number of limbic brain regions (Young et al., 1986; Makino et al., 1994). CRH may play a pivotal role in ADX-induced death of dentate gyrus granule cells: First, previous studies have shown that CRH administration to the immature rat can lead to cell death in the hippocampal CA3 region, which is rich in CRH receptors during that developmental age (Baram and Ribak, 1995); in the adult rat, the dentate gyrus contains higher levels of CRH receptors (Avishai-Eliner et al., 1996). Next, CRH antagonists have been shown to protect from ischemic and excitotoxic neuronal death (Lyons et al., 1991; Maecker et al., 1997). Finally. CRH receptors and their messenger-RNA are abundant in the dentate gyrus of the adult rat (De Souza et al., 1985; Potter et al., 1994; Avishai-Eliner et al., 1996), which may provide a basis for the apparent selective loss of these cells after ADX.
The goal of this study was to test the hypothesis that ADX results in granule cell death via excess activation of CRH receptors (Fig. 1). The hypothesis predicts that ADX-induced granule cell loss is prevented by inhibiting activation of CRH receptors. Therefore, the ability of a chronic infusion of a competitive CRH receptor antagonist to prevent ADX-induced granule cell death was investigated.
FIGURE 1.
A cartoon illustrating several potential mechanisms for the death of granule cells after adrenalectomy. The top illustration demonstrates the situation in an intact animal. Granule cells are shown to possess receptors for corticotropin releasing hormone (CRH), as well as the two types of steroid receptors: mineralocorticoid (MR) and glucocorticoid (GR). The occupancy of these receptors under these “normal” circumstances is depicted. The two bottom illustrations demonstrate two alternative scenarios for granule cell death. On the left, a marked excess of CRH. due to elimination of the negative feedback action of adrenal steroids, leads to activation of CRH receptors (CRH-R) and to excitotoxicity. Alternatively, as shown on the right, absence of adrenal steroid ligands for the MR receptors interferes with cell survival. B = corticosterone.
MATERIALS AND METHODS
Animals
Male Sprague-Dawley derived rats (125–150 gm) were purchased from Zivic-Miller (Zelienople, PA). Rats were maintained (two per cage) in a NIH-approved animal facility, kept on a 12-h light, 12-h dark cycle, and given access to unlimited lab chow and water. ADX rats were offered saline, and all post-surgical rats received oral antibiotics in their drinking solutions. Experiments were approved by the institutional committee for animal care.
Experimental Design
Four experimental groups (n = 6 / group) were compared for the extent of granule cell death: 1) Sham-operated controls; 2) ADX rats 3) ADX rats who received CRH-antagonist via infusion into the lateral cerebral ventricle from the onset of steroid deprivation to the time of sacrifice (ADX-Antagonist), and 4) ADX rats infused with the vehicle for the antagonist only (ADX-Vehicle). All ADX groups were permitted to recuperate for three days and were provided with corticosterone (20 mg/liter) in their drinking solution. The ADX-Antagonist and the ADX-Vehicle groups were then subjected to cannula- and micropump-implantation. Corticosterone was withdrawn from the drinking solutions of all ADX groups on the morning after the onset of CRH-antagonist or vehicle infusion, and all groups were sacrificed 5–7 days later.
Surgical Procedures
ADX was performed in the morning, to prevent potential circadian variation in CRH levels (Watts and Swanson, 1989), under halothane anesthesia, using a dorsal approach. The completeness of the ADX was verified in each animal using two methods: first, a postmortem inspection excluded visible adrenal tissue; second, plasma corticosterone was measured to exclude ectopic steroid-secreting adrenal tissue. Animals with evidence of incomplete ADX by either method were excluded from further analysis.
Implantation of osmotic micro-pumps: Following ADX, the ADX-Antagonist and ADX-Vehicle groups received (9–41)-alpha-helical CRH antagonist and vehicle, respectively, via an Alzet micro-pump (0.5 × 0.25″; ALZA Co., Palo Alto, CA). Animals were anesthetized with halothane and secured in the stereotaxic apparatus while a cannula was inserted into the lateral cerebral ventricle as previously described (Baram and Schultz, 1991; Baram et al., 1992). A length of plastic tubing connected to the cannula was threaded subcutaneously to the micro-pump which was implanted dorsally at the shoulder area. The accurate placement of the cannula was histologically verified for each animal.
CRH-antagonist and vehicle treatment
The inhibitory peptide analog (9–41)-alpha-helical CRH (Rivier et al., 1984) was utilized. The peptide is a competitive antagonist at both members of the CRH receptor family (CRF1 and CRF2). The peptide was dissolved in saline at a concentration of 5 mg/ml and the osmotic pump was filled according to the manufacturer’s instructions. The pumps were set to deliver 0.5 microliter per hour or 60 μg per rat per day. The vehicle-infused control group received the same volume of saline.
Hormonal and physiological monitoring
Plasma corticosterone was determined by radioimmunoassay using a commercial assay (ICN, Irvine, CA) as described previously (Baram and Schultz, 1992). Assay sensitivity was 0.005 μg/dl. All animals were weighed at the onset of the experiment and on the day of sacrifice.
Tissue Processing
Under pentobarbital anesthesia, animals were perfused intracardially with saline for 5 minutes, followed by a fresh, buffered 4% paraformaldehyde solution for 12 minutes. Brains were kept in situ for 24 hours, then removed, cryoprotected in 25% sucrose, and stored at −80°C. Brains were cut into 20 μm coronal sections in a cryostat, mounted on gelatin-coated slides and stored at 4°C.
Nissl Staining for visualization of apoptotic neurons
Coronal sections were chosen to represent the septal, middle, and temporal regions of the hippocampus as described below. Staining procedures employed 0.1% cresyl violet for 5–8 minutes. The sections were immersed in distilled water containing 0.15% acetic acid (15 seconds) followed by graded alcohols (70%, 80%, 95% containing 0.15% acetic acid, and 100% twice) and finally cleared in Histoclear (Fisher Scientific) and coverslipped.
Quantitation of Apoptotic Granule Cells
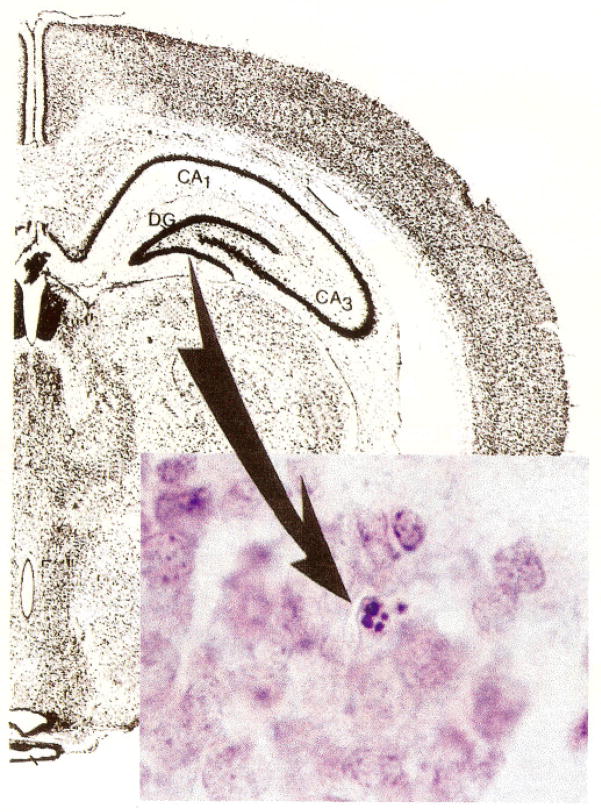
Granule cells were examined for features of apoptosis in both the ipsilateral and contralateral dentate gyrus to the side of cannula placement and at all levels of the hippocampal formation as detailed below. Granule cells were considered to be undergoing apoptosis based on morphological characteristics, including a pyknotic, shrunken nucleus and an eosinophilic cytoplasm. For purposes of quantitation, only cells that exhibited dense chromatin, forming crescent or ringlike structures or discrete clumps, were counted (Fig. 2). Pyknotic single nuclear clumps were excluded. Cells were not counted if their cytoplasm lay outside the boundary of the granule cell layer, as defined by a line connecting the outside-most boundary of adjacent cells.
FIGURE 2.
A composite showing the dentate gyrus and a photomicrograph of a granule cell in the granule cell layer of a male rat adrenalectomized 5 days prior to sacrifice. The cell displays the morphological features of apoptosis. i.e., clumping of the chromatin. Nissl stain, 1000x prior to reduction.
Analysis and statistical considerations
Only animals considered to have a complete ADX were analyzed. Each animal was inspected for residual adrenal tissue after sacrifice, and its plasma was subjected to corticosterone assay as described above. Animals with visible residual adrenals and/or plasma corticosterone levels of >0.2 μg/dl were excluded from analysis. Therefore, after exclusion of incompletely ADX rats, the hippocampi from three each ADX, ADX-Antagonist and ADX-vehicle rats were analyzed. To avoid major differences in variance, only three, randomly-selected sham-ADX controls were analyzed.
The number of apoptotic cells was determined in distinct subregions of the dentate gyrus. These included the infra-pyramidal and supra-pyramidal blades, from the septal (anterior), middle, and temporal (caudal) regions of the hippocampal formation. The septal region of the dentate gyrus corresponded to (−1.8 mm to −3 3 mm) posterior to Bregma (Plates 18–21, Paxinos and Watson, 1986). The middle region encompassed (−3.8 to −4.8) posterior to Bregma (Plates 22–24), while the temporal (posterior) region included plates 25–27 (−5.3 to −6.3 mm) posterior to Bregma. A complete series of consecutive sections encompassing the antero-posterior extent of the hippocampus was Nissl stained, and sections were screened to determine the boundaries of the septal, middle, and temporal regions. The septal region. 1500 μm in length, averaged 75 (20 μm) sections, while the middle and temporal regions averaged 50 sections each. For each rat, every tenth section in each region was analyzed, leading to a mean of 10.92 ± 0.97 sections per rat (median 12 sections). Also, the left dentate gyrus, contralateral to the cannula implantation was evaluated separately from the right, ipsilateral side.
The significance of differences among the experimental groups was determined using the appropriate statistical tests: For comparing Gaussian-distribution parameters (weight, plasma corticosterone) of each experimental group to the control, student’s t-test (with Welch’s correction, if appropriate) was used. For multiple group analysis, non-parametric (Kruskal-Wallis) ANOVA with post-hoc Dunn’s test were utilized (e.g., Table III) using the PRISM software package, (San Diego, CA).
TABLE III.
Effect of CRH antagonist infusion on ADX-induced granule cell death
| Treatment: | Dentate Gyrus Region
|
||
|---|---|---|---|
| Septal | Middle | Temporal | |
| Sham-ADX | 0.67 ± 0.2 | 0.38 ± 0.7 | 0.66 ± 0.33 |
| ADX | 10.7 ± 1.6* | 17.0 ± 3.9* | 15.0 ± 3.2* |
| ADX-Antagonist | 9.0 ± 1.4* | 22.4 ± 7.0* | 28.8 ± 18.1* |
| ADX-Vehicle | 16.3 ± 1.8* | 18.1 ± 2.1* | 22.4 ± 3.9* |
The number of apoptotic granule cells was determined in both the left and the right side of the dentate gyrus. The number of sections and the anatomic boundaries of the septal, middle, and temporal regions of the hippocampal formation have been defined in the Methods’ section.
Treatment group medians are significantly different from the control group’s (p < 0.05; Kruskal-Wallis non-parametric ANOVA test). ADX-Vehicle is not significantly different from ADX-Antagonist (post-hoc Dunn’s test).
RESULTS
ADX led to drastic reduction of plasma corticosterone levels (a mean of 0.08 μg/dl in the three ADX groups, compared with 2.8 μg/dl in controls), and to a decreased body weight gain (Table 1). Animals carrying the Alzet-pump (ADX-Antagonist and ADX-Vehicle groups) gained less weight than those subjected to ADX alone, potentially because of the added surgical stress.
ADX resulted in significant numbers of apoptotic neurons in the granule cell layer of the dentate gyrus (Fig. 2). The distribution of these cells was not random and demonstrated a distinct pattern. A higher number of degenerating cells were found in the suprapyramidal blade, as compared with the infrapyramidal blade of the dentate gyrus (171 versus 79 in a sampling of nine sections from a typical rat), as is shown in Table II (see also Fig. 3).
TABLE II.
Distribution of apoptotic cells in sub-regions of the dentate gyrus of a typical Adrenalectomized (ADX) rat
| Region | Sections(N) | Right Dentate Gyrus
|
Left Dentate Gyrus
|
Total | ||
|---|---|---|---|---|---|---|
| Infrapyramidal | Suprapyramidal | Infrapyramidal | Suprapyramidal | |||
| Septal | 4 | 11 | 24 | 8 | 21 | 64 |
| Middle | 3 | 12 | 33 | 6 | 19 | 70 |
| Temporal | 7 | 16 | 38 | 26 | 36 | 116 |
| Total | 14 | 39 | 95 | 40 | 76 | |
| Average | N/A | 13 | 32 | 13 | 25 | |
Actual quantitation of apoptotic cells in the various regions of the dentate gyrus from an adrenalectomized rat (III-5). A sample of fourteen sections, selected as described in the Methods section, were counted without knowledge of treatment. The criteria for designation of a neuron as “apoptotic” are found in the Methods section.
N/A = not applicable.
FIGURE 3.
A graph showing the numbers of apoptotic granule cells in the septal (anterior) region of the dentate gyrus of four experimental groups. The three most anterior. sections from each animal were subjected to counting of apoptotic cells as described in the Methods section. The septal region is the closest to the site of cannula placement and thus to the maximal concentration of the CRH-antagonist infused via the cannula. Values are means ± S.E.M. of 9 sections per group. For each of the four regions, experimental groups are significantly different (p=0.0028 – 0.014; Kruskal-Wallis test). The apoptotic cell numbers of the three ADX groups (ADX alone, or with infusion of vehicle or CRH antagonist) are not significantly different from each other (p > 0.05; Dunn’s multiple comparisons post-hoc test). Abbreviations: INF = infra-pyramidal; SUP = suprapyramidal: IPSI = ipsilateral to the cannula (right); CONTRA = contralateral to the cannula (left).
Administration of 60 μg per day of (9–41)-alpha-helical CRH did not alter the number of apoptotic cells (Tables III and IV). Specifically, when compared to either ADX alone, or to ADX and vehicle infusion, administration of CRH antagonist did not reduce the number of apoptotic granule cells in any region of the dentate gyrus. To avoid missing a local protective effect of the antagonist in regions close to the site of infusion, the number of apoptotic cells was analyzed in several ways. First, both ipsilateral and contralateral sides of the dentate gyrus, (septal, middle, and temporal) were compared among the experimental groups (Table III). Next, since the side ipsilateral to the cannula may have been exposed to higher antagonist concentrations, the left and right sides of the dentate gyrus were analyzed separately, and the three most anterior sections, closest to the cannula site, were compared among groups (Fig. 3). Also, since the infra-pyramidal blade may be closer to the CRH antagonist infused into the lateral ventricle, these two blades were analyzed separately (Fig. 3; Table IV). Finally, whether the region closest to the site of infusion—the infra-pyramidal dentate gyrus blade in the anterior, septal region—preferentially manifested fewer apoptotic granule cells in the antagonist treated group in comparison to the control groups was evaluated as well (Table IV). No trend for decreased numbers of apoptotic granule cells in areas closer to the cannula was established (Fig. 3; Table IV).
TABLE IV.
Comparison of apoptotic cell number in the infra-pyramidal and supra-pyramidal blades of the septal dentate gyrus in adrenalectomized (ADX) rats infused with CRH antagonist compared with sham-ADX, ADX alone and ADX rats infused with vehicle
| Treatment: | Infra-pyramidal | Supra-pyramidal |
|---|---|---|
| Sham ADX | 0.7±0.26 | 0.6±0.30 |
| ADX | 2.1±0.34 | 3.2±0.53 |
| ADX-Antagonist | 2.7±0.39 | 7.2±3.10 |
| ADX-Vehicle | 2.8±0.44 | 4.9±0.79 |
The values are means ± S.E.M. of apoptotic cells counted in both the left and right dentate gyrus, in the three most anterior sections of each of three rats per group within the septal region of the hippocampal formation. The anterior septal region was chosen since it is the closest to the cannula site. The infra-pyramidal blade, which is closer to the cerebral lateral ventricle, is compared to the suprapyramidal blade, to look for a local protective effect of the CRH antagonist against ADX-induced granule cell death. For both the inferior and the superior blades, experimental group medians arc significantly different (p < 0.0004; Kruskal-Wallis nonparametic ANOVA test). Post-hoc Dunn’s multiple comparison test reveals a significant difference between the control and each of the ADX groups, but no significant difference among the three ADX groups.
DISCUSSION
This study demonstrates that ADX for 5–7 days leads to the death of significant numbers of dentate gyrus granule cells, which is not prevented by a competitive CRH antagonist, suggesting that this death is not mediated by corticotropin releasing hormone (Fig. 1).
The model of ADX-induced cell death has been described in detail previously (Sloviter et al., 1993a; Sloviter et al., 1993b). The original authors commented on the significant variability, both qualitative and quantitative, in the number and distribution of apoptotic neurons. In this study, we attempted to control as fully as possible for inter-animal variability and the extent of steroid deprivation. First, young males were used (Joels, M, personal communication), which minimized age and sex variability. Second, ADX was performed in our lab and each animal was inspected postmortem. Third, animals were monitored for weight gain (Table 1), since ADX typically leads to retarded growth. Finally, corticosterone assays were performed and rats with residual levels above 0.2 μg/dl were excluded from the study. This low level of residual corticosterone is similar to that reported by McNeill et al., 1991, and is far lower than levels which “protect” from granule cell loss (one μg/dl, Hornsby et al., 1996). The residual corticosterone levels permitted in the current study are also lower than those which suppress adrenalectomy-induced upregulation of CRH levels in the hypothalamus (Swanson and Simmons, 1989; Baram and Schultz, 1992).
The pattern of distribution of apoptotic granule cells found in young male Sprague-Dawley rats in this study is in overall agreement with previous reports using Long-Evans (Sloviter et al., 1993a) and Wistar rats (Hornsby et al., 1996). The supra-pyramidal blade of the dentate gyrus contained more apoptotic neurons than the infra-pyramidal blade (Table II). As did Sloviter et al., and Hornsby et al., we observed a tendency towards congregation of apoptotic cells closer to the molecular layer. This is consistent with the preferential ADX-induced death of “older” granule cells (Cameron and Gould, 1996). As far as the rostrocaudal distribution of the apoptotic granule cells, our short-term ADX study suggested a slight predominance of affected neurons posteriorly (Table II). Sloviter et al., using a longer ADX period, found more dying granule cells in the septal region, while Jaarsma et al., (1992) suggested that the middle third of the hippocampus was predominantly involved.
Although the ADX-induced granule cell death model has been amply described, the precise mechanisms leading to the almost exclusive loss of granule cells are not clear. For example, ADX results in loss of a number of different steroids, which exert their effects through two distinct receptors (Reul and de Kloet, 1985; Joels and de Kloet. 1994). ADX also leads to elimination of a major source of cate-cholamines. Because administration of corticosterone prevents ADX-induced cell loss (Sloviter et al., 1989; Gould et al., 1990), it has been assumed that lass of steroids constitutes the major feature of ADX which promotes granule cell death. However, since the adrenal steroids have almost ubiquitous actions, drastic reduction of their plasma levels is expected to initiate a complex cascade of “disinhibitory” events. One of the more obvious effects of elimination of the negative feedback of glucocorticoids within the central nervous system is up-regulation of the synthesis and levels of the major stress-neurohormone, CRH. As early as 1985, ADX has been shown to increase steady-state CRH-mRNA levels in the hypothalamus (Jingami et al., 1985; Young et al., 1986). Increased levels of the CRH peptide itself were documented using both radioimmunoassay (Moldow and Fischman, 1982) and immunocytochemistry (Kovacs et al., 1986; Sawchenko, 1987) The regulatory effect of glucocorticoids on CRH synthesis has been found in both the hypothalamus and the amygdala (Kovacs et al., 1986; Makino et al., 1994). That this increase in CRH synthesis is due to blocking of the effects of adrenal glucocorticoids on their receptors has also been established (Kovacs et al., 1986; Sawchenko, 1987; Yi et al., 1993).
CRH has been shown to lead to excitation of hippocampal neurons (Aldenhoff et al., 1983; Baram et al., 1992; Smith and Dudek, 1994; Hollrigel et al., 1997). Administration of picomole doses of CRH may lead to neuronal death in the hippocampus of the immature rat (Baram and Ribak, 1995; Ribak and Baram. 1996). On ultrastructural analysis, the CRH-induced cell death is characterized by the morphological features of apoptosis (Ribak and Baram, 1996). Although (CRH seems to increase glutamate release in the hippocampus (Hollrigel et al., 1997). the excitotoxic effect of CRH does not depend on glutamate receptor activation (Baram et al., 1995) and is most likely mediated by CRF1, (Baram et al., 1997). In the immature rat (second postnatal week) the neurons affected by CRH have consisted of pyramidal cells of the CA3 region. This may well be due to the exceptionally high abundance of CRH receptors, specifically the CRF1, type, in this region during development (Avishai-Eliner et al., 1996). In the adult rat, the pattern of CRH receptor distribution in the hippocampus differs, with elevated CRF1, concentration in the dentate gyrus (Wong et al., 1994; Chalmers et al., 1995; Avishai-Eliner et al., 1996).
Competitive blockers of CRH receptors have been shown to decrease ischemic neuronal loss (Lyons et al., 1991) and excitotoxic hippocampal cell death (Maecker et al., 1997), implicating CRH in these processes of neuronal death. A potential mechanism by which CRH could lead to neuronal death may involve increased intracellular calcium (Kuryshev et al., 1996; Weiss et al., 1996). An ADX-induced cell death which is mediated via “excess” activation of CRH receptors at sites of their high concentration would thus provide an explanation for the unique specificity of this phenomenon to the granule cells of the dentate gyrus.
The study described demonstrated that infusion of (9–41)-alpha-helical CRH, a competitive analog of CRH, did not block ADX-induced cell death. The antagonist was infused into the lateral cerebral ventricles (icv). and it could be argued that due to an insufficient dose or limited diffusion the antagonist did not reach CRH receptors on the granule cells in adequate concentrations. This possibility is considered remote for several reasons:
First, although distinct effects of CRH require different molar ratios of antagonist for their attenuation (Fisher et al., 1991 ). the doses of (9–41 )-alpha-helical CRH needed for complete abolishment of the excitatory effects of CRH on limbic neurons have been established (Baram and Schultz, 1991; Baram et al., 1996). The molar ratio of (9–41)-alpha-helical CRH: CRH leading to elimination of CRH-induced seizures was 12:1 (Baram and Schultz, 1991). An additional study demonstrated that a ten-fold excess of the antagonist given icv abolished CRH-induced neuronal excitation in 33% of rats and markedly attenuated excitation in the remainder (Baram et al., 1996). Although the precise amounts of native CRH in the hippocampus are unknown, total forebrain content of CRH is in the low nanogram range (Palkovits et al., 1985). A daily delivery of 60 μg of CRH antagonist thus provides at least one thousand-fold excess. Furthermore, protection of hippocampal neurons from transient forebrain ischemia-induced death was observed even with (9–41)-alpha-helical CRH doses of 1 and 10 μg given icv (Lyons et al., 1991)
Second, the extent of diffusion of the antagonist to sites of CRH action may be a concern. However, earlier studies (Baram and Schultz, 1991; Baram et al., 1992) have documented that CRH-induced seizures originated in the amygdala, and that amygdala-origin behavioral seizures and epileptic electroencephalographic discharges were eliminated by the icv administered CRH antagonist. This provides strong evidence that the alpha-helical CRH reaches the amygdala, which is significantly further from the cerebral ventricles than the dentate gyrus. Similar effects of (9–41)-alpha-helical CRH on amygdala-mediated parameters of anxiety and stress-behaviors have been published by others (Heinrichs et al., 1992).
Additionally, if issues of antagonist concentration. due to insufficient quantity or penetration problems. were the cause for the observed lack of protection against ADX-induced granule cell death, a local protective effect around the cannula site or an alteration of the distribution pattern of the granule cell loss would be expected. As is evident from Tables III and IV and from Figure 3, no decrease in the number of apoptotic granule cells was found anteriorly (septally, closest to the site of cannula implantation) in rats treated with the CRH antagonist. Similarly, no reduction of the number of apoptotic cells ipsilateral to the cannula was observed.
These data lead to the conclusion that the tested hypothesis–that ADX-induced apoptosis of dentate gyrus granule cells is mediated by CRH receptor activation—is not correct. Refuting this hypothesis leaves open the ongoing quest for the molecular mechanisms leading from elimination of the adrenal gland to the specific death of a unique population of limbic neurons (Hornsby et al., 1996). Issues under consideration include the “permissive” role of low steroid levels for neuronal survival as opposed to the well established degeneration induced by high steroid levels (McEwen et al., 1992). Other unresolved issues awaiting both theoretical and experimental approaches include the basis for the specificity of the observed neuronal death to the granule cells which are not particularly rich in either mineralocorticoid or glucocorticoid receptors, and the relative importance of other factors such as glutamate-mediated neurotransmission (Magarinos and McEwen, 1995).
TABLE I.
Hormonal and physiological parameters of Sham-adrenalectomized (ADX), ADX and ADX-rats treated with the CRH antagonist, (9–41)-alpha-helical CRH or with the saline vehicle
| Treatment group | Weight gain (gm) | plasma corticosterone
|
|---|---|---|
| (μg/dl) | ||
| Control | 46.5 ± 0.5 | 2.8 ± 0.23 |
| ADX | 25.0 ± 5.0+ | 0.09 ± 0.02* |
| ADX+ Antagonist | 15.3 ± 2.9+ | 0.06 ± 0.02* |
| ADX+ Vehicle | 14.7 ± 7.8+ | 0.09 ± 0.04* |
Control rats were sham-ADX. All the ADX rats were given corticosterone for three days. On the fourth day the ADX-antagonist and ADX-vehicle groups underwent cannula implantation in the cerebral lateral ventricle, which was connected to a subcutaneously implanted osmotic micropump (Alza). Animals were sacrificed 5–7 days later. Weight gain was defined as the difference between the animals’ weight on the day of sacrifice and on the day of elimination of corticosterone from the drinking water.
Significantly different from the sham-adrenalectomy control group (p < 0.01).
Significantly different from the control group (p < 0.0001, Student’s t-test with Welch’s correction).
Acknowledgments
Supported by an award from the Markey Foundation (Principal investigators: Drs. I. Lipkin & Carl Cotman). and by NIH RO-1, NS 28912. The technical assistance of L. Schultz is appreciated.
References
- Aldenhoff JB, Gruol DL, Rivier J, Vale W, Siggins GR. Corticotropin-releasing factor decreases postburst hyper-polarization and excites hippocampal neurons. Science. 1983;221:875–877. doi: 10.1126/science.6603658. [DOI] [PubMed] [Google Scholar]
- Avishai-Eliner S, Yi SJ, Baram TZ. Developmental profile of CRH-receptor messenger RNA in the rat limbic system. Dev Brain Res. 1996;91:159–163. doi: 10.1016/0165-3806(95)00158-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baram TZ, Schultz L. CRH is a rapid and potent convulsant in the infant rat. Dev Brain Res. 1991;61:97–101. doi: 10.1016/0165-3806(91)90118-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baram TZ, Hirsch E, Snead OCIII, Schultz L. CRH induced seizures in the infant brain originate in the amygdala. Ann Neurol. 1992;31:488–494. doi: 10.1002/ana.410310505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baram TZ, Schultz L. CRH gene expression in the fetal rat is not increased after pharmacological adrenalectomy. Neurosci Lett. 1992;142:215–218. doi: 10.1016/0304-3940(92)90376-i. [DOI] [PubMed] [Google Scholar]
- Baram TZ, Ribak CE. Peptide-induced infant status epilepticus causes neuronal death and synaptic reorganization. NeuroReport. 1995;6:277–280. doi: 10.1097/00001756-199501000-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baram TZ, Avishai-Eliner S, Schultz L. Seizure threshold to kainic acid in infant rats is markedly decreased by corticotropin releasing hormone. Epilepsia. 1995;36(supp):abs B-05. [Google Scholar]
- Baram TZ, Kotsoukos Y, Schultz L, Rivier J. The effect of “Astressin”a novel antagonist of corticotropin releasing hormone (CRH) on CRH-induced seizures in the infant rat: Comparison with two other antagonists. Molec Psychiatr. 1996;1:223–226. [PMC free article] [PubMed] [Google Scholar]
- Baram TZ, Chalmers DT, Chen C, Kotsoukos Y, De Sauza EB. The CRF1 receptor mediates the excitatory actions of corticotropin releasing factor in the developing rat brain. Brain Res. 1997;770:89–95. doi: 10.1016/s0006-8993(97)00759-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron HA, Gould E. Distinct populations of cells in the adult dentate gyrus undergo mitosis or apoptosis in response to adrenalectomy. J Camp Neurol. 1996;369:56–63. doi: 10.1002/(SICI)1096-9861(19960520)369:1<56::AID-CNE4>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- Chalmers DT, Lovenberg TW, De Souza EB. Localization of novel corticotropin releasing factor receptor (CRF2) mRNA expression to specific subcortical nuclei in rat brain: Comparison with CRF1 mRNA expression. J Neurosci. 1995;15:6340–6350. doi: 10.1523/JNEUROSCI.15-10-06340.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Souza EB, Insel TR, Perrin MH, Rivier J, Vale WW, Kuhar MJ. Corticotropin-releasing factor receptors are widely distributed within the rat CNS: an autoradiographic study. J Neurosci. 1985;5:3189–3203. doi: 10.1523/JNEUROSCI.05-12-03189.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher L, Rivier C, Rivier J, Brown M. Differential antagonist activity of helical CRF9–4, in 3 bioassay systems. Endocrinology. 1991;129:1312–1316. doi: 10.1210/endo-129-3-1312. [DOI] [PubMed] [Google Scholar]
- Gould E, Woolley CS, McEwen BS. Short term glucocorticoid manipulation affect neuronal morphology and survival in the adult dentate gyrus. Neuroscience. 1990;37:367–375. doi: 10.1016/0306-4522(90)90407-u. [DOI] [PubMed] [Google Scholar]
- Heinrichs SC, Merlo-Pich E, Miczek KA, Britton KT, Koob GF. Corticotropin releasing factor antagonist reduces emotionality in socially defeated rats via direct neurotropic action. Brain Res. 1992;581:190–197. doi: 10.1016/0006-8993(92)90708-h. [DOI] [PubMed] [Google Scholar]
- Hollrigel G, Baram TZ, Soltesz I. Corticotropin releasing hormone increases excitatory synaptic transmission in the hippocampus of infant rats. Neuroscience. 1997 doi: 10.1016/s0306-4522(97)00499-5. (In press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hornsby CD, Grootendorst J, de Kloet ER. Dexamethasone does not prevent seven-day ADX-induced apoptosis in the dentate gyrus of the rat hippocampus. Stress. 1996;1:51–65. doi: 10.3109/10253899609001095. [DOI] [PubMed] [Google Scholar]
- Jaarsma D, Postema F, Korf J. Time course and distribution of neuronal degeneration in the dentate gyrus of the rat after adrenalectomy: a silver impregnation study. Hippocampus. 1992;2:143–150. doi: 10.1002/hipo.450020206. [DOI] [PubMed] [Google Scholar]
- Joels M, de Kloet ER. Mineralocorticoid and glucocorticoid receptors in the brain. Implications for ion permeability and transmitter systems. Prog Neurobiol. 1994;43:1–36. doi: 10.1016/0301-0082(94)90014-0. [DOI] [PubMed] [Google Scholar]
- Jingami H, Matsukura S, Numa S, Imura H. Effects of adrenalectomy and dexamethasone administration on the levels of pre-pro-CRF-mRNA in the hypothalamus and ACTH/β-lipotropin precursor mRNA in the pituitary in rats. Endocrinology. 1985;117:1314–1420. doi: 10.1210/endo-117-4-1314. [DOI] [PubMed] [Google Scholar]
- Kovacs KJ, Kiss JZ, Makara GB. Glucocorticoid implants around the hypothalamic PVN prevent the increase of CRF and AVP immunostaining induced by adrenalectomy. Neuroendocrinology. 1986;44:229–234. doi: 10.1159/000124650. [DOI] [PubMed] [Google Scholar]
- Kuryshev YA, Child GV, Ritchie AK. CRH stimulates calcium entry through L-type and P-type Ca++ channels in rat corticotrophs. Endocrinology. 1996;137:2269–2277. doi: 10.1210/endo.137.6.8641175. [DOI] [PubMed] [Google Scholar]
- Lyons MK, Anderson RE, Meyer F. CRF antagonist reduces ischemic hippocampal neuronal injury. Brain Res. 1991;545:339–342. doi: 10.1016/0006-8993(91)91310-w. [DOI] [PubMed] [Google Scholar]
- Maecker H, Desai A, Dash R, Rivier J, Vale W, Sapolsky R. Astressin, a novel and potent CRF antagonist, is neuroprotective in the hippocampus when administered after a seizure. Brain Res. 1997;744:166–170. doi: 10.1016/s0006-8993(96)01207-3. [DOI] [PubMed] [Google Scholar]
- Magarinos AM, McEwen BS. Stress-induced atrophy of apical dendrites of hippocampal CA3c neurons: Involvement of glucocorticoid secretion and excitatory amino acid receptors. Neuroscience. 1995;68:89–98. doi: 10.1016/0306-4522(95)00259-l. [DOI] [PubMed] [Google Scholar]
- Makino S, Gold PW, Schulkin J. Corticosterone effects on CRH-mRNA in the central nucleus of the amygdala and the parvocellular region of the paraventricular nucleus of the hypothalamus. Brain Res. 1994;640:105–112. doi: 10.1016/0006-8993(94)91862-7. [DOI] [PubMed] [Google Scholar]
- McEwen BS, Angulo J, Cameron H, Chao HM, Daniels D, Gannon MN, Gould E, Mendelson S, Sakai R, Spencer R, Woolley C. Paradoxical effects of adrenal steroids on the brain: protection versus degeneration. Biol Psychiatry. 1992;31:177–199. doi: 10.1016/0006-3223(92)90204-d. [DOI] [PubMed] [Google Scholar]
- McNeill TH, Masters JN, Finch CE. Effect of chronic adrenalectomy on neuron loss and distribution of sulfated glycoprotein-2 in the dentate gyrus of prepubertal rats. Exp-Neurol. 1991;111:140–144. doi: 10.1016/0014-4886(91)90062-h. [DOI] [PubMed] [Google Scholar]
- Moldow RL, Fischman AJ. Hypothalamic CRF-like immunoreactivity in the rat after hypophysectomy or adrenalectomy. Peptides. 1982;3:143–147. doi: 10.1016/0196-9781(82)90043-2. [DOI] [PubMed] [Google Scholar]
- Palkovits M, Brownstein MJ, Vale W. Distribution of CRF in the rat brain. Fed, Proceed. 1985;44:215–219. [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 2. Academic Press; Sydney: 1986. [DOI] [PubMed] [Google Scholar]
- Potter E, Sutton S, Donaldson C, Chen R, Perrin MH, Lewis K, Sawchenko PE, Vale WW. Distribution of Corticotropin-releasing factor receptor mRNA expression in the rat brain and pituitary. Proc, Natl, Acad, Sci, USA. 1994;91:8777–8781. doi: 10.1073/pnas.91.19.8777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reul JMHM, de Kloet ER. Two receptor systems for corticosterone in rat brain: Microdistribution and differential occupation. Endocrinology. 1985;117:2505–2511. doi: 10.1210/endo-117-6-2505. [DOI] [PubMed] [Google Scholar]
- Ribak CE, Baram TZ. Selective death of hippocampal CA3 pyramidal cells with mossy fiber afferents after CRH-induced status epilepticus in infant rats. Dev Brain Res. 1996;91:245–251. doi: 10.1016/0165-3806(95)00183-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivier J, Rivier C, Vale W. Synthetic competitive antagonists of corticotropin releasing factor: Effect on ACTH secretion in the rat. Science. 1984;224:889–891. doi: 10.1126/science.6326264. [DOI] [PubMed] [Google Scholar]
- Sawchenko PE. Evidence for a local site of action for glucocorticoids in inhibiting CRF and vasopressin expression in the paraventricular nucleus. Brain Res. 1987;403:213–224. doi: 10.1016/0006-8993(87)90058-8. [DOI] [PubMed] [Google Scholar]
- Sloviter RS, Valiquette G, Abrams GM, Ronk EC, Sollas AL, Paul LA, Neubort S. Selective loss of hippocampal granule cells in the mature rat brain after adrenalectomy. Science. 1989;243:535–538. doi: 10.1126/science.2911756. [DOI] [PubMed] [Google Scholar]
- Sloviter RS, Sollas AL, Dean E, Neubort S. Adrenalectomy-induced granule cell degeneration in the rat hippocampal dentate gyrus: Characterization of an in vivo model of controlled neuronal death. J Comp Neurol. 1993a;330:324–336. doi: 10.1002/cne.903300304. [DOI] [PubMed] [Google Scholar]
- Sloviter RS, Dean E, Neubort S. Electron microscopic analysis of adrenalectomy induced hippocampal granule cell degeneration in the rat: Apoptosis in the adult central nervous system. J Comp Neurol. 1993b;330:337–351. doi: 10.1002/cne.903300305. [DOI] [PubMed] [Google Scholar]
- Smith BN, Dudek FE. Age-related epileptogenic effects of corticotropin releasing hormone in the isolated CA1 region of rat hippocampal slices. J Neurophysiol. 1994;72:2328–2333. doi: 10.1152/jn.1994.72.5.2328. [DOI] [PubMed] [Google Scholar]
- Swanson LW, Simmons DM. Differential steroid hormone and neural influences on peptide mRNA levels in CRH cells of the paraventricular nucleus: A hybridization histochemistry Study. J Comp Neurol. 1989;285:413–435. doi: 10.1002/cne.902850402. [DOI] [PubMed] [Google Scholar]
- Vale W, Spiess J, Rivier C, Rivier J. Characterization of a 41-residue ovine hypothalamic peptide that stimulates secretion of corticotropin and beta-endorphin. Science. 1981;213:1394–1397. doi: 10.1126/science.6267699. [DOI] [PubMed] [Google Scholar]
- Watts AG, Swanson LW. Diurnal variation in the content of CRH-mRNA in the hypothalamic PVN of rats of both sexes as measured by in situ hybridization. Endocrinology. 1989;125:1734–1738. doi: 10.1210/endo-125-3-1734. [DOI] [PubMed] [Google Scholar]
- Wong ML, Licinio J, Gold PW. Localization of CRH receptor mRNA in adult rat brain by ISH. Endocrinology. 1994;135:2275–2278. doi: 10.1210/endo.135.5.7956950. [DOI] [PubMed] [Google Scholar]
- Weiss J, Yin HZ, Baram TZ. Corticotropin releasing hormone (CRH) increases intracellular calcium in a subset of large hippocampal neurons. Soc, Neurosci Abst. 1996;26:822.18. [Google Scholar]
- Yi SJ, Masters JN, Baram TZ. The effect of a specific glucocorticoid receptor antagonist on CRH gene expression in the neonatal rat hypothalamus. Dev, Brain Res. 1993;73:253–259. doi: 10.1016/0165-3806(93)90145-z. [DOI] [PubMed] [Google Scholar]
- Young WS, III, Mezey E, Siegel RE. Quantitative in situ hybridization histochemistry reveals increased levels of corticotropin releasing factor mRNA after adrenalectomy. Neuroscience Lett. 1986;70:198–203. doi: 10.1016/0304-3940(86)90463-5. [DOI] [PubMed] [Google Scholar]