Abstract
Membrane nanotubes are a recently discovered form of cellular protrusion between two or more cells whose functions include cell communication, environmental sampling, and protein transfer. Although clearly demonstrated in vitro, evidence of the existence of membrane nanotubes in mammalian tissues in vivo has until now been lacking. Confocal microscopy of whole-mount corneas from wild-type, enhanced GFP chimeric mice, and Cx3cr1gfp transgenic mice revealed long (>300 μm) and fine (<0.8 μm diameter) membrane nanotube-like structures on bone marrow-derived MHC class II+ cells in the corneal stroma, some of which formed distinct intercellular bridges between these putative dendritic cells. The frequency of these nanotubes was significantly increased in corneas subjected to trauma and LPS, which suggests that nanotubes have an important role in vivo in cell-cell communication between widely spaced dendritic cells during inflammation. Identification of these novel cellular processes in the mammalian cornea provides the first evidence of membrane nanotubes in vivo.
Long membrane nanotubular structures that connect mammalian cells have been identified in vitro in a number of cell types including rat pheochromocytoma cell lines, normal rat kidney cells (1), and primary cultures of dendritic cells (DCs),3 macrophages, human peripheral blood NK cells, and B cells (2, 3). Membrane nanotubes, sometimes referred to as “tunneling nanotubes,” range in diameter from between 50 and 200 nm in cultured neural cells to 700 nm in macrophage cell lines (1–4). In primary cultures of human NK cells the average length of nanotubes is ~30 μm; however, some intercellular connections between NK cells and macrophages spanning up to 140 μm have been described (2). Speculative functions for membrane nanotubes in vitro include a novel means of intercellular communication via calcium fluxes, the transfer of cytoplasmic vesicles and cell surface proteins including MHC class I molecules (2), and BCR-Ag complexes (3). Until now, evaluating the relevance of membrane nanotubes during immune responses has been limited by the lack of evidence that these structures exist in vivo in complex tissues (5–7), which has raised the question that nanotubes may in fact be purely an in vitro phenomenon.
The mammalian cornea was considered to be virtually devoid of resident tissue macrophages and DCs, and this absence of potential APCs was thought to represent one element underlying the so-called “immune privileged” status of the cornea (8). However, recent studies of bone marrow (BM)-derived cells in the mouse cornea have revealed a network of cells, including CD11c+MHC class II+ DCs and CD11b+ macrophages (8–10). These studies have been made possible by the transparency of the cellular and extra-cellular matrix of this critical refractive layer of the eye and its ready accessibility for both intravital and ex vivo microscopic investigations (11, 12). During studies of these cells in corneal whole mounts from wild-type, transgenic, and enhanced GFP (eGFP) chimeric mice we noted the presence of extremely long, fine cellular processes arising from MHC class II+ putative DCs in the corneal stroma. These structures, some of which form distinct intercellular bridges between MHC class II+ cells, may represent the first evidence of membrane nanotubes in vivo in the mammalian immune system. We hypothesize that membrane nanotube-bearing DCs in the “APC-poor” avascular environment of the dense corneal stroma may act as a potential means of transfer of Ag/receptor complexes between widely separated DCs. Although present in only low numbers in naive corneas, the incidence of membrane nanotubes increased markedly, particularly in the central cornea, following inflammatory stimuli. These data suggest a potential novel means of amplification of immune surveillance by corneal DCs.
Materials and Methods
Animals
C57BL/6 mice, aged 6–12 wk, were used for immunostaining of corneal whole mounts as described previously (12, 13). In BM chimeric studies, 6- to 12-wk-old eGFP C57BL/6TgN (ACTbEGFP)10sb mice were used as donors, with C57BL/6 and TLR4−/− mice as recipients (The Jackson Laboratory). TLR4−/− mice (Shizuo Akira) were fully backcrossed to C57BL/6 mice, and age-matched littermates were used as controls. Transgenic heterozygote Cx3cr1+/gfp mice and homozygote Cx3cr1gfp/gfp mice were also used in the present study (14). No difference was noted between heterozygote and homozygote mice; therefore, the data have been pooled. Mice were housed at the Animal Resources Centre, Murdoch, Western Australia and were treated in accordance with the animal welfare guidelines at the University of Western Australia, Crawley (Perth), Australia. A total of 48 mice were used in the present investigation.
Generation of eGFP chimeric mice
Recipient mice received two 600-Gy doses of whole-body irradiation 3 h apart. Mice were injected i.v. with 5 × 106 BM cells from C57BL/6TgN eGFP mice in 200 μl of DMEM (15).
Murine model of keratitis using TLR ligands
Mice were anesthetized by i.p. injection of either 2,2,2-tribomoethanol (TBE; 1.2%) (Sigma-Aldrich) or ketamine (40 mg/ml) and xylazine (20 mg/ml). A 1-mm diameter epithelial debridement wound in the central cornea was created using an Algerbrush II corneal rust ring remover (16, 17) and either 20 μg of Ultra Pure Escherichia coli LPS (strain K12; Invivogen) or sterile HBSS was applied to the surface of each eye. Mice were sacrificed 24 h later.
Corneal whole mount immunostaining
Corneas were dissected from eyes that had been fixed in 4% paraformaldehyde. Each cornea was examined either whole for quantitative and qualitative analysis (n = 23) or cut into pie-shaped wedges by radial incisions (n = 25) and processed as previously described for immunostaining (12). Corneas from eGFP chimeric mice and Cx3cr1gfp mice were double stained with anti-GFP Ab (Chemicon) and a range of anti-leukocyte mAbs including MHC class II (clone M5/114; BD Pharmingen), CD45 (Serotec), CD11b, CD68, CD169 (Sero-tec), and CD11c (BD Pharmingen) were visualized by the appropriate conjugated secondary Abs (12). Corneas from nonchimeric mice were double stained with one of the primary anti-leukocyte mAbs and either rhodamine- or streptavidin-Alexa Fluor 488-conjugated phalloidin (1/40) for 60 min at room temperature. To visualize nuclei, tissues were incubated in 4′,6′-diamidino-2-phenylindole (DAPI; Roche Molecular Biochemicals) for 10 min at room temperature. As negative control, isotype rat IgG2b was substituted for primary Abs.
Quantitative examination of complete corneal whole mounts
Corneal whole mounts were examined using both conventional epifluorescence microscopy (Olympus; DMRBE, Leica) and confocal microscopy (Leica TCS SP2). Nanotubes on immune cells were quantified in three separate regions of the cornea: central (within 0.5 mm from the center of the cornea), paracentral (between 0.5 and 1.0 mm from the center), and peripheral (between 1.0 mm and the limbal border) (18). Cells with nanotubes were quantified in intact healthy (n = 5), epithelial debridement- and saline-treated (n = 9), and epithelial debridement- and LPS-treated (n = 9) corneas. Corneal wedges were examined by confocal microscopy and Z-stacks series were generated through the corneal stroma in 0.5 μm increments, with final compilation of images performed using Leica confocal software. The diameter and length of nanotubes were determined using MetaMorph 4.5.1 software.
Results and Discussion
Intercellular or bridging membrane nanotubes
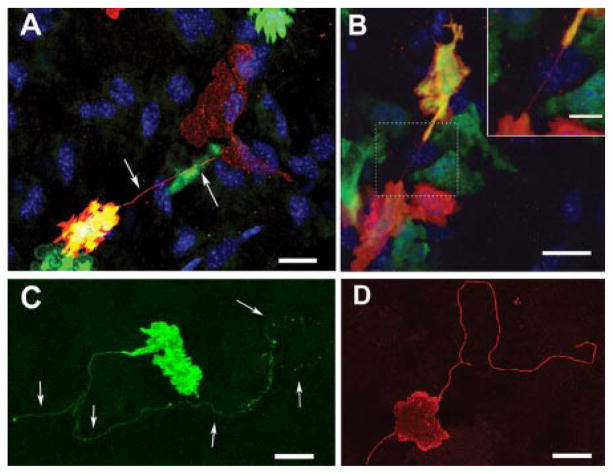
In inflamed corneas, short membrane bridges (<60 μm) were occasionally observed interconnecting two or more MHC class II+ cells (Fig. 1, A and B). Corneas of BM chimeric mice revealed rare physical membrane bridges or nanotubes connecting eGFP donor cells to either resident host MHC class II+ TLR4−/− cells (Fig. 1A) or other donor GFP+ MHC class II+ cells (Fig. 1B). These highly distinctive intercellular membrane nanotubes or bridges between two or more cells were generally short (22.13 ± 10 μm) and straight and accorded closely with previous descriptions of membrane nanotubes in vitro (1, 2, 4–7).
FIGURE 1.
Membrane nanotubes in the corneal stroma. A, Chimeric mouse corneal whole mount reveals a donor-derived (GFP+/green) MHC class II+ (red) and double positive (yellow) cell connecting via a fine membrane nanotube (arrows) to a resident MHC class II+ GFP+ cell (red only). B, Two donor-derived MHC class II+ cells expressing varying amounts of GFP joined by a fine, straight, membrane nanotube (inset shows the area within the dotted square at a higher magnification). C and D, Long, nonbridged membrane nanotubes on MHC class II+ cells in the naive (C) and inflamed (D) mouse corneal stroma. Connected neighboring cells were not identifiable. Scale bars =20 μm; inset scale bar = 10 μm.
Identification of long, possibly nonbridging membrane nanotubes in vivo
Besides the bridging nanotubular structures described above, qualitative analysis of BM-derived cells in the corneal stroma, particularly of inflamed corneas, revealed the presence of extremely fine, elongated cell processes on MHC class II+ cells (Fig. 1, C and D) with features identical to those of previously described membrane nanotubes in vitro (1, 2), with the exception that in many cases a second interconnected cell was not always detectable. Compared with conventional dendritic processes or filopodia, these nanotubes were usually single, of smaller diameter, less ramified, and up to several cell diameters in length. This category of nanotube-bearing MHC class II+ cell was more abundant in the central and paracentral regions of the cornea (Fig. 1, C and D) and were up to 333 μm in length in the inflamed central cornea (Table I). For reference, the radius of the mouse cornea is ~1500 μm, making it feasible for five or six cells to span its entire width. In the peripheral cornea, nanotubes were generally shorter but of a similar diameter. The cross-sectional diameter of a sample of membrane nanotubes in the present study (0.64 ±0.04 μm SEM, n =10) accords with descriptions of “thick” membrane nanotubes (2). The presence of focal bulges or lipid vesicles traveling along the lengths of nanotubes between HEK-293T cells (6) and macrophage cell lines in vitro (2) are one proposed means of cell-cell exchange. Similar bulges along the lengths and at the termination of some nanotubes were observed in the present study (Fig. 1C). Membrane nanotubes were not visualized on BM-derived cells reactive with the macrophage-specific mAbs anti-CD11b, anti-CD68, or anti-CD169 (12), indicating that membrane nanotubes are a feature of MHC class II-bearing putative DCs in the corneal stroma.
Table I.
Average nanotube length (μm) in various regions of the cornea
| Corneal Region
|
|||
|---|---|---|---|
| Central | Paracentral | Peripheral | |
| Naive corneas | 0 (0) | 188 ± 55 (4) | 58 ± 6 (7) |
| Inflamed corneasa | 151 ± 16 (50) | 107 ± 10 (31) | 73 ± 7 (17) |
Pooled data from LPS- and saline-treated corneas; mean length per cell ± SE (n = number of nanotubes).
The frequency of membrane nanotube-bearing MHC class II+ cells increases in the inflamed cornea
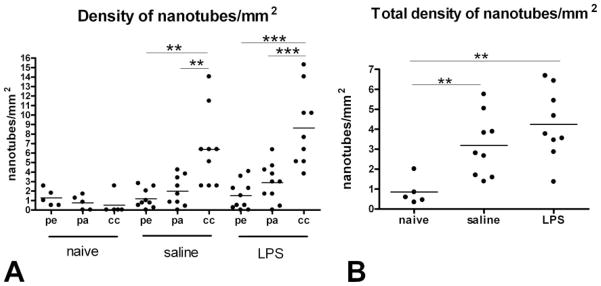
Quantitation of the distinctive membrane nanotubes during corneal inflammation revealed a significant increase in the density of cells possessing nanotubes, particularly in the central cornea (Fig. 2) where extremely long nanotubes up to 330 μm were observed (Table I). The increase in the density of nanotube-bearing cells in the entire cornea was not statistically significant between LPS- and saline-treated corneas; however the central corneal region in these mice contained significantly more nanotubes than the paracentral and peripheral regions (Fig. 2A). In summary, in conditions of inflammation (injury plus saline or LPS) the numbers of nanotubes in the cornea were significantly greater than in the normal cornea (Fig. 2B).
FIGURE 2.
Frequency of membrane nanotubes in naive and inflamed corneas. A, Significantly higher densities of nanotubes were identified in the central cornea (cc) compared with the peripheral (pe) and paracentral (pa) zones of the cornea in both saline- and LPS-treated animals. B, Pooled data from all regions demonstrate a significantly higher density of nanotubes in inflamed corneas (both saline- and LPS-treated eyes) compared with naive corneas (**, p < 0.005; ***, p < 0.001).
Visualization of BM-derived cells in the cornea of Cx3cr1gfp transgenic mice did not reveal membrane nanotubes
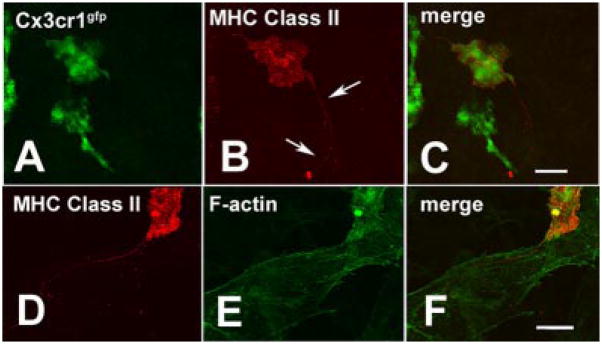
Because membrane nanotubes were not identifiable in corneas stained with anti-macrophage markers, we next examined corneal whole mounts from Cx3cr1gfp transgenic mice in which GFP expression occurs in the cytoplasm of myeloid-derived cells expressing the chemokine receptor Cx3cr1 (i.e., macrophages and DCs) (12). In these corneas, membrane nanotubes on MHC class II+ cells (Fig. 3, A–C) did not contain any detectable quantities of GFP, suggesting that the membrane nanotubes contain sparse cytoplasmic contents, which corresponds with previous descriptions (19, 20).
FIGURE 3.
A–C, GFP expression on BM-derived cells in the corneal stroma of Cx3cr1gfp transgenic mice did not extend along the MHC class II+ membrane nanotube (A, GFP only; B, red channel denotes MHC class II; C; merged image). D–F, Dual MHC class II (D) and F-actin (E) staining revealed the lack of F-actin in the membrane nanotubes (F, merged image). Strong F-actin reactivity was evident in both the BM-derived cell and the surrounding keratocytes. Scale bars = 20 μm.
F-actin staining of membrane nanotubes
Two theories on the formation of membrane nanotubes in vitro have been proposed (19, 20). The first, leading to the term “tunneling nanotubes,” implicates an F-actin-driven protrusive mechanism. The second mechanism suggests that membrane nanotubes may be derived from cell membrane bridges between closely apposed cells that elongate as these cells diverge or separate. Double staining (phalloidin and MHC class II staining) revealed the presence of rich intracytoplasmic F-actin in the abundant keratocytes or fibrocytes of the corneal stroma and in the cytoplasm of BM-derived cells (Fig. 3, D and F). Nonbridging membrane nanotubes were consistently F-actin negative; however, detection of small quantities of F-actin in such thin nanotubes in corneal whole mounts (~100 μm in thickness) may be a technical limitation of this study. Alternative explanations for these F-actin-negative in vivo nanotubes could include their formation from diverging cells (5) or an F-actin-independent protrusive mechanism involving curvature-driven self-assembly of interacting anisotropic raft elements as recently described in primary astrocytes grown on artificial decorated substrata (21).
The recent discovery that nanotube-mediated continuity between cells may act as a vehicle for cellular communication, particularly in the immunological setting (5, 7, 20), aroused our interest in the unusual morphology of a subpopulation of MHC class II+ putative DCs in the mouse cornea. Due to its transparent, rigid, and avascular nature, the cornea permits “en face” visualization of entire immune cell populations in situ without the need for extensive tissue processing or sectioning that could potentially damage the integrity of delicate nanotube structures that have been reported in vitro to be easily damaged by mechanical stress, fixation, and even exposure to light (19).
The identification of intercellular connections between putative DCs closely conformed in dimensions and morphology to previous descriptions of membrane nanotubes in vitro (1, 2, 6, 19, 20). However, membrane nanotubes discovered in naive and inflamed corneas differed from previous descriptions in several respects. First, they were often extremely long, (>300 μm) and had a tortuous or curved course. Interestingly, the latter feature has subsequently been confirmed in a study of T cells in vitro that was published after the submission of the present manuscript (22). Second, membrane nanotubes on corneal MHC class II+ cells apparently did not always form an obvious bridge to a neighboring cell. There are a number of possible explanations for the presence of these long nonbridged nanotubes. They may indeed be forming a connection to a neighboring but undetected cell or their processes could be seeking contact with distantly located cells in a manner akin to the recently described “dendrite surveillance extension and recycling habitude” (dSEARCH) behavior of DCs (23, 24). De novo nanotubes, yet to establish a connection to neighboring cells, have been described in culture conditions (1, 19).
In the central cornea the presence of extremely long membrane nanotubes may enable more isolated cells to communicate with widely spaced DCs, thereby forming a potential immunological “syncytium.” It is logical therefore that long nanotubes are less common in the peripheral cornea, where there is a greater density of MHC class II+ DCs (18), a conclusion borne out by our quantitative results. Previous studies have shown that membrane nanotubes allow calcium fluxes between cultured DCs and mediate phenotypic changes reminiscent of Ag stimulation in vitro (6). We speculate that corneal stromal DCs use membrane nanotubes as a means of transferring Ag-specific signals from cells that are distantly located from one another, especially during inflammatory conditions. This may represent a means of effectively increasing the functional capacity of the APC pool in a tissue that is “APC poor” similar to that recently proposed in the case of B cell Ag receptor (BCR) transfer from activated Ag-specific B cells to bystander B cells via short membrane nanotubes (3). These authors noted an increase in the number of nanotube-like structures following LPS activation, a feature consistent with our own in vivo studies where membrane nanotube-bearing MHC class II+ cells were more common in inflamed corneas (injury alone or injury plus LPS).
Examination of inflamed corneal whole mounts from GFP chimeric mice provided a unique opportunity to visualize the physical contact mediated by membrane nanotubes between newly arrived donor GFP+ cells and resident MHC class II+ GFP− host cells. Indeed, nanotubes from donor GFP+TLR4−/− MHC class II+ cells were observed extending to and making contact with resident GFP−TLR4−/− MHC class II+ host cells in inflamed corneas. Such cellular bridging may represent a route for the transfer of MHC class II-Ag complexes or TLR from donor GFP+ cells to host GFP− cells, similar to the MHC class I protein transfer via nanotubes (2) and the BCR transfer between B cells (3) discussed above.
The lack of detectable membrane nanotubes in tissues stained with macrophage phenotypic markers and their occurrence solely on MHC class II-bearing cells indicates that resident tissue macrophages in the cornea do not participate in this mode of cell-cell communication. Although we recognize that a sub-population of corneal macrophages express MHC class II, the majority of MHC class II-bearing cells in the mouse corneal stroma are likely DCs (8, 9, 12). Inconsistent immunostaining with anti-CD11c mAb in corneal whole mounts, a technical problem experienced by other groups (9, 25), prevented a more definitive determination of whether these MHC class II+ cells were in fact conventional CD11c+ tissue DCs.
To our knowledge, the current data provide the first evidence for the presence of membrane nanotubes in mammalian tissues in vivo and define a potential means of contact and integration between distant immune cells (5). We postulate that membrane nanotubes between DCs in vivo could represent a significant means of transmitting Ag, thus amplifying local immune surveillance in the “APC poor” environment of the mammalian cornea. Our quantitative analyses of the response to injury and LPS-mediated inflammation support this hypothesis. Determining the immunological function and dynamic behavior of these membrane nanotubes in the living eye using two-photon confocal microscopy represents an intriguing future research direction.
Acknowledgments
We thank Prof. John Forrester for advice in the early stages of interpretations of these observations and Dr. Eric Carlson for his help in generating the BM chimeras. All confocal microscopy was conducted using facilities at the Centre for Microscopy, Characterization and Analysis, University of Western Australia, which is supported by university, state and federal government funding.
Footnotes
This research was funded by an Ada Bartholemew Research Grant, National Institutes of Health Grants RO1EY14362 and P30EY11373, the Research to Prevent Blindness Foundation, and the Ohio Lions Eye Research Foundation.
Abbreviations used in this paper: DC, dendritic cell; BM, bone marrow; eGFP, enhanced GFP.
Disclosures
The authors have no financial conflict of interest.
References
- 1.Rustom A, Saffrich R, Markovic I, Walther P, Gerdes HH. Nanotubular highways for intercellular organelle transport. Science. 2004;303:1007–1010. doi: 10.1126/science.1093133. [DOI] [PubMed] [Google Scholar]
- 2.Onfelt B, Nedvetzki S, Yanagi K, Davis DM. Cutting edge: Membrane nanotubes connect immune cells. J Immunol. 2004;173:1511–1513. doi: 10.4049/jimmunol.173.3.1511. [DOI] [PubMed] [Google Scholar]
- 3.Quah BJC, Barlow VP, McPhun V, Matthaei KI, Hulett MD, Parish CR. Bystander B cells rapidly acquire antigen receptors from activated B cells by membrane transfer: a novel mechanism for enhancing specific antigen presentation. Nature Precedings. 2007 doi: 10.1038/npre.2007.1207.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Onfelt B, Nedvetzki S, Benninger RK, Purbhoo MA, Sowinski S, Hume AN, Seabra MC, Neil MA, French PM, Davis DM. Structurally distinct membrane nanotubes between human macrophages support long-distance vesicular traffic or surfing of bacteria. J Immunol. 2006;177:8476–8483. doi: 10.4049/jimmunol.177.12.8476. [DOI] [PubMed] [Google Scholar]
- 5.Davis DM. Intercellular transfer of cell-surface proteins is common and can affect many stages of an immune response. Nat Rev Immunol. 2007;7:238–243. doi: 10.1038/nri2020. [DOI] [PubMed] [Google Scholar]
- 6.Onfelt B, Davis DM. Can membrane nanotubes facilitate communication between immune cells? Biochem Soc Trans. 2004;32:676–678. doi: 10.1042/BST0320676. [DOI] [PubMed] [Google Scholar]
- 7.Watkins SC, Salter RD. Functional connectivity between immune cells mediated by tunneling nanotubules. Immunity. 2005;23:309–318. doi: 10.1016/j.immuni.2005.08.009. [DOI] [PubMed] [Google Scholar]
- 8.Dana MR. Corneal antigen-presenting cells: diversity, plasticity, and disguise; the Cogan lecture. Invest Ophthalmol Vis Sci. 2004;45:722–727. doi: 10.1167/iovs.03-0803. [DOI] [PubMed] [Google Scholar]
- 9.Brissette-Storkus CS, Reynolds SM, Lepisto AJ, Hendricks RL. Identification of a novel macrophage population in the normal mouse corneal stroma. Invest Ophthalmol Vis Sci. 2002;43:2264–2271. [PMC free article] [PubMed] [Google Scholar]
- 10.Hamrah P, Huq SO, Liu Y, Zhang Q, Dana MR. Corneal immunity is mediated by heterogeneous population of antigen-presenting cells. J Leukocyte Biol. 2003;74:172–178. doi: 10.1189/jlb.1102544. [DOI] [PubMed] [Google Scholar]
- 11.Camelo S, Shanley A, Voon AS, McMenamin PG. The distribution of antigen in lymphoid tissues following its injection into the anterior chamber of the rat eye. J Immunol. 2004;172:5388–5395. doi: 10.4049/jimmunol.172.9.5388. [DOI] [PubMed] [Google Scholar]
- 12.Chinnery HR, Ruitenberg MJ, Plant GW, Pearlman E, Jung S, McMenamin PG. The chemokine receptor CX3CR1 mediates homing of MHC class II-positive cells to the normal mouse corneal epithelium. Invest Ophthalmol Vis Sci. 2007;48:1568–1574. doi: 10.1167/iovs.06-0746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McMenamin PG. Optimal methods for preparation and immunostaining of iris, ciliary body, and choroidal wholemounts. Invest Ophthalmol Vis Sci. 2000;41:3043–3048. [PubMed] [Google Scholar]
- 14.Jung S, Aliberti J, Graemmel P, Sunshine MJ, Kreutzberg GW, Sher A, Littman DR. Analysis of fractalkine receptor CX3CR1 function by targeted deletion and green fluorescent protein reporter gene insertion. Mol Cell Biol. 2000;20:4106–4114. doi: 10.1128/mcb.20.11.4106-4114.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Carlson EC, Drazba J, Yang X, Perez VL. Visualization and characterization of inflammatory cell recruitment and migration through the corneal stroma in endotoxin-induced keratitis. Invest Ophthalmol Vis Sci. 2006;47:241–248. doi: 10.1167/iovs.04-0741. [DOI] [PubMed] [Google Scholar]
- 16.Johnson AC, Heinzel FP, Diaconu E, Sun Y, Hise AG, Golenbock D, Lass JH, Pearlman E. Activation of Toll-like receptor (TLR)2, TLR4, and TLR9 in the mammalian cornea induces MyD88-dependent corneal inflammation. Invest Ophthalmol Vis Sci. 2005;46:589–595. doi: 10.1167/iovs.04-1077. [DOI] [PubMed] [Google Scholar]
- 17.Sun Y, Hise AG, Kalsow CM, Pearlman E. Staphylococcus aureus-induced corneal inflammation is dependent on Toll-like receptor. 2 and myeloid differentiation factor 88. Infect Immun. 2006;74:5325–5332. doi: 10.1128/IAI.00645-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hamrah P, Zhang Q, Liu Y, Dana MR. Novel characterization of MHC class II-negative population of resident corneal Langerhans cell-type dendritic cells. Invest Ophthalmol Vis Sci. 2002;43:639–646. [PubMed] [Google Scholar]
- 19.Pontes B, Viana NB, Campanati L, Farina M, Neto VM, Nussenzveig HM. Structure and elastic properties of tunneling nanotubes. Eur Biophys J. 2007;37:121–129. doi: 10.1007/s00249-007-0184-9. [DOI] [PubMed] [Google Scholar]
- 20.Gerdes HH, Bukoreshtliev NV, Barroso JF. Tunneling nanotubes: a new route for the exchange of components between animal cells. FEBS Lett. 2007;581:2194–2201. doi: 10.1016/j.febslet.2007.03.071. [DOI] [PubMed] [Google Scholar]
- 21.Gimsa U, Iglic A, Fiedler S, Zwanzig M, Kralj-Iglic V, Jonas L, Gimsa J. Actin is not required for nanotubular protrusions of primary astrocytes grown on metal nano-lawn. Mol Membr Biol. 2007;24:243–255. doi: 10.1080/09687860601141730. [DOI] [PubMed] [Google Scholar]
- 22.Sowinski S, Jolly C, Berninghausen O, Purbhoo MA, Chauveau A, Kohler K, Oddos S, Eissmann P, Brodsky FM, Hopkins C, et al. Membrane nanotubes physically connect T cells over long distances presenting a novel route for HIV-1 transmission. Nat Cell Biol. 2008;10:211–219. doi: 10.1038/ncb1682. [DOI] [PubMed] [Google Scholar]
- 23.Nishibu A, Ward BR, Jester JV, Ploegh HL, Boes M, Takashima A. Behavioral responses of epidermal Langerhans cells in situ to local pathological stimuli. J Invest Dermatol. 2006;126:787–796. doi: 10.1038/sj.jid.5700107. [DOI] [PubMed] [Google Scholar]
- 24.Ward BR, Jester JV, Nishibu A, Vishwanath M, Shalhevet D, Kumamoto T, Petroll WM, Cavanagh HD, Takashima A. Local thermal injury elicits immediate dynamic behavioural responses by corneal Langerhans cells. Immunology. 2007;120:556–572. doi: 10.1111/j.1365-2567.2006.02533.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sosnova M, Bradl M, Forrester JV. CD34+ corneal stromal cells are bone marrow-derived and express hemopoietic stem cell markers. Stem Cells. 2005;23:507–15. doi: 10.1634/stemcells.2004-0291. [DOI] [PubMed] [Google Scholar]