Abstract
Considerable attention has focused on regulation of central tryptophan hydroxylase (TPH) activity and protein expression. At the time of these earlier studies, it was thought that there was a single central TPH isoform. However, with the recent identification of TPH2, it becomes important to distinguish between regulatory effects on the protein expression and activity of the two isoforms. We have generated a TPH2-specific polyclonal antiserum (TPH2-6361) to study regulation of TPH2 at the protein level and to examine the distribution of TPH2 expression in rodent and human brain. TPH2 immunoreactivity (IR) was detected throughout the raphe nuclei, in lateral hypothalamic nuclei and in the pineal body of rodent and human brain. In addition, a prominent TPH2-IR fiber network was found in the human median eminence. We recently reported that glucocorticoid treatment of C57/Bl6 mice for 4 days markedly decreased TPH2 messenger RNA levels in the raphe nuclei, whereas TPH1 mRNA was unaffected. The glucocorticoid-elicited inhibition of TPH2 gene expression was blocked by co-administration of the glucocorticoid receptor antagonist mifepristone (RU-486). Using TPH2-6361, we have extended these findings to show a dose-dependent decrease in raphe TPH2 protein levels in response to 4 days of treatment with dexamethasone; this effect was blocked by co-administration of mifepristone. Moreover, the glucocorticoid-elicited inhibition of TPH2 was functionally significant: serotonin synthesis was significantly reduced in the frontal cortex of glucocorticoid-treated mice, an effect that was blocked by mifepristone co-administration. This study provides further evidence for the glucocorticoid regulation of serotonin biosynthesis via inhibition of TPH2 expression, and suggest that elevated glucocorticoid levels may be relevant to the etiology of psychiatric diseases, such as depression, where hypothalamic-pituitary-adrenal axis dysregulation has been documented.
Keywords: serotonin, 5-HTP, dexamethasone, corticosterone, mifepristone
Introduction
Tryptophan hydroxylase (TPH) catalyzes the first step in the biosynthesis of serotonin and melatonin and is the rate-l enzyme in the serotonin biosynthetic pathway.1–3 A second TPH isoform identified by Walther et al.,4 in 2003, is termed TPH2, while the original TPH isoform is now termed TPH1. TPH2 is the predominant central nervous system (CNS) isoform in all species examined thus far, with TPH2 messenger RNA expressed throughout the raphe nuclei.5,6 TPH1 mRNA is expressed in the CNS at extremely low levels,7 although TPH1 mRNA is robustly expressed in the pineal gland, where TPH mediates melatonin biosynthesis.5,8 Recent data have suggested a possible association between TPH2 polymorphisms and a variety of psychiatric disorders including major depression,9–14 suicide,13,14 attention-deficit/hyperactivity disorder,15–17 autism,18 bipolar disorder,19 and obsessive compulsive disorder.16 In addition, it has recently been shown that polymorphisms in the 3′-UTR of rhesus monkey TPH2 modulate hypothalamic-pituitary-adrenal (HPA) axis function presumably through changes in TPH2 expression.20 These data suggest that changes in the expression and/or function of TPH2 may be associated with psychiatric disease and emphasize the need to understand what makes TPH isoforms distinct with regard to function and regulation.
TPH mRNA, protein, and enzyme activity in the dorsal raphe nucleus (DRN) have all been shown to be regulated by ovarian and stress hormones.7,21–30 While these studies demonstrated hormonal regulation of serotonin synthesis, the existence of TPH isoforms was not yet appreciated and therefore not studied. With the identification of TPH2,4 it has become important to understand the effects of hormones on the individual isoforms. Because currently available TPH antibodies do not distinguish between the two isoforms,4 it has been impossible to study the distribution and regulation of the specific TPH protein isoforms in the CNS.
We recently showed that glucocorticoids significantly modulate TPH2, but not TPH1 mRNA expression in the murine DRN, while estradiol had no effect on TPH2 mRNA expression.31 We now describe the identification and characterization of TPH2-specific antiserum (TPH2-6361), which we have used to study TPH2 protein distribution in rodent and human brain. In addition, we used TPH2-6361 to show that glucocorticoid modulation of TPH2 mRNA is followed by changes in TPH2 protein expression in the murine raphe. Finally, we determined if glucocorticoid effects on expression of TPH2 message and protein result in changes in the in vivo synthesis of serotonin in the frontal cortex. Our data suggest that glucocorticoids regulate the serotonergic system at the level of serotonin biosynthesis by modulation of the TPH2 isoform.
Materials and methods
Animal treatment procedure
Adult ovariectomized (ovx) female C57/BL6 mice (13–16 weeks; Charles River, Wilmington, MA, USA) and adult intact female Sprague–Dawley rats (23 weeks; Taconic, Carlsbad, CA, USA) were housed in an AAALAC approved facility at Merck & Co., Rahway, New Jersey, with free access to soy-free rodent chow and water. Tissue from ovx mice and intact female rats were used for the immunocytochemical (ICC) and western blot analyses to characterize the TPH2 antisera. For ICC studies, rats or mice were anesthetized with ketamine/xylazine (100–200/5–10 mg/kg, i.p.), transcardially perfused with 4% paraformaldehyde and the brains were removed for sectioning.
Glucocorticoid treatment of ovx mice consisted of once daily subcutaneous (s.c.) dosing for 4 days with propylene glycol/DMA (vehicle) or glucocorticoids (5–7 per group; see individual experiments for doses used). Approximately 6 h following the final dose, mice were anesthetized with ketamine/xylazine, decapitated, brains removed and slices of the pons containing the raphe nuclei were dissected, frozen on dry ice and stored at −80°C for western blot analyses. For neurochemical studies, mice were dosed with the 3,4-dihydroxyphenylalanine (DOPA) decarboxylase inhibitor 3-hydroxybenzylhydrazine dihydrochloride (NSD 1015).32 (100 mg/kg i.p.) to inhibit 5-HT synthesis, 30 min before sacrifice. Mice were anesthetized with ketamine/xylazine, decapitated, brains removed, and samples of frontal cortex were dissected, frozen on dry ice and stored at −80°C for high-performance liquid chromatography (HPLC) analyses.
Human tissue
Punches and blocks from discrete regions of human brains were collected at autopsy by Miklos Palkovits in the Laboratory of Neuromorphology, Semmelweis University (Budapest, Hungary). The protocol of tissue sampling and retrospective assessments was approved by the Institutional Review Board of the Semmelweis University, Budapest. Microdissected regions from patients (ages 24–66) were taken 1–6 h postmortem. The tissue was immediately frozen on dry ice and stored at −80°C. Human postmortem tissue blocks were used from the same source to cut frozen sections for ICC staining.
TPH2 antibody generation
Two N-terminal peptides corresponding to TPH2 10–24: SKYWARRGLSLDSAV and TPH2 26–39: EDHQLLGSLTQNKA were synthesized (Biosource International Custom Antibody Production Services, Hopkinton, MA, USA) and used as immunogens to produce antibodies in rabbits. Two rabbits were immunized per peptide. Crude sera were harvested and analyzed by enzyme-linked immunosorbant assay to the original peptide and by western Blot techniques. Two polyclonal sera that recognize TPH2 were identified and affinity purified: TPH2-6361, which was generated against TPH2 10–24, and TPH2-6362, which was generated against TPH2 26–39. Based on the results of immunoblot and immunohistochemical studies, data presented herein were obtained using TPH2-6361.
Western blot analyses
Frozen tissue was homogenized on ice in homogenization buffer (50 mm Tris-HCl, 150 mm NaCl, 1% Triton X-100, 2 mm ethylenediaminetetraacetic acid (EDTA), 0.05% sodium dodecylsulfate (SDS), 10 mg/ml Pepstatin, and ×2 concentration of complete protease inhibitor cocktail (Roche Applied Science, Indianapolis, IN, USA), using a FastPrep 120 (Q Biogene, Irvine, CA, USA), and clarified by centrifugation. COS cells transfected with the expression plasmid pcDNA 3.1 (Invitrogen, Carlsbad, CA, USA) containing the TPH1 or TPH2 coding region were lysed in homogenization buffer and prepared as described previously. Total protein was determined using a modified Lowry method (BioRad DC Protein Assay, BioRad, Hercules, CA, USA). Total protein for tissue and cell homogenates (see individual experiments for amounts loaded), in Laemmli reducing sample buffer was loaded in a 12.5% Tris-HCl SDS-polyacrylamide gel (SDS-PAGE Criterion System, BioRad) and fractionated by electrophoresis. Samples were transferred to 0.2 µm nitrocellulose membranes by electroelution. Membranes were blocked with TBS + 1% Casein + 0.05% Tween 20 (Pierce, Rockland, IL, USA). Blocked membranes were incubated overnight at 4°C with a 1/10 000 dilution of affinity-purified rabbit anti-TPH-2 antibody 6361 in TBS +0.05% Casein + 0.05% Tween 20. After washing (six changes over 40 min) in TBS + 0.1% Tween 20 (Sigma, St Louis, MO, USA), membranes were incubated in TBS + 0.05% Casein + 0.05% Tween 20 containing 1/75 000 dilution of horseradish peroxidase-conjugated Goat anti-rabbit IgG (Santa Cruz Biotechnology, Santa Cruz, CA, USA) for 4 h at room temperature. Membranes were washed (six changes over 40 min) in TBS + 0.1% Tween 20 and then incubated in SuperSignal West Dura Extended (Pierce) duration enhanced chemiluminescent substrate for 5 min. Antigens were visualized by exposing the blot to Kodak Biomax MR X-Ray film (Kodak, Rochester, NY, USA) for 6 s.
To determine differences in TPH2 protein between vehicle and steroid-treated tissue samples, homogenates were prepared, fractionated by SDS-PAGE and blotted to nitrocellulose membranes as described above (see individual experiments in Results and Figure Legends for treatment paradigms). Membranes were incubated with TPH2-6361, developed with SuperSignal West Dura Extended and apposed to films. Films were scanned using BioRad GS 800 Calibrated Densitometer. Images were analyzed using AlphaEaseFC Imaging Software (Alpha Innotech, San Leandro, CA, USA). Using the spot density tools, an integrated density value (IDV) was determined for each sample. The values represent mean ± s.e.m. for five individual animals and are presented as fold change as compared to vehicle control. Raw IDV values for each experiment were analyzed using a one-way analysis of variance before conversion of data to fold changes.
Immunocytochemistry
Staining with polyclonal antiserum TPH2-6361 was performed on perfused mouse and rat brains and fresh frozen human brain sections (12 µm). Rodents were perfusion fixed with 4% buffered paraformaldehyde. The brains were dissected, cryoprotected in an increasing concentration of sucrose, frozen and sectioned in a cryostat. All sections were blocked in Power Block (BioGenex, San Ramon, CA, USA) for 10 min at room temperature before immunostaining. The slides containing the rodent sections were incubated in TPH2-6361 (1:10 000) at 4°C overnight, and visualized with anti-rabbit Alexa-594 (1:1000; Molecular Probes, Carlsbad, CA, USA) for 1 h at room temperature. Human fresh frozen brain sections were fixed with 8% formaldehyde in PBS for 10 min at room temperature and then incubated in TPH2 antibody (1:30 000) at 4°C overnight. Anti-rabbit Alexa-594 (1:1000) was used for 1 h at room temperature to visualize the immunostaining. For human pineal body, anti-rabbit SuperPicTure (Zymed, Invitrogen, Carlsbad, CA, USA) was used, followed by Alexa-594-conjugated tyramide to enhance sensitivity. In all sections, nuclei were stained with 4’,6-Diamidino-2-phenylindole and in the human brain tissue, Sudan black was used to block lipofucsin-induced autofluorescence. TPH2-6361 was preabsorbed by incubating 1mg of peptide antigen with 0.5 mg of affinity-purified antibody overnight at 4°C. The solution was briefly centrifuged and used to stain tissue sections as described above. No immunoreactivity (IR) was observed when tissue was incubated without TPH2-6361, without secondary antibody or with preabsorbed antibody.
Determination of cortical 5-HT synthesis
Cortical 5-HT synthesis was determined by measuring the rate of accumulation of 5-HTP 30 min after inhibition of 5-HT synthesis by the DOPA decarboxylase inhibitor NSD 101532 (100 mg/kg i.p.). Cortical tissue (n = 6 for each treatment group) was removed from −80°C storage, weighed and homogenized in 10 volumes of homogenization buffer (0.4 m perchloric acid, 0.02% l-cysteine, 0.02% ascorbic acid, 0.0035% sodium EDTA). Homogenates were centrifuged at 10 000 g and supernatants frozen and stored at −80°C until analysis. Samples were analyzed by HPLC with electrochemical detection essentially as described by Barton and Hutson.32
Results
Western blot validation studies with TPH2 polyclonal antiserum
TPH2-6361 detected a band of approximately 50 kDa in murine raphe and forebrain homogenates (Figure 1). In COS cells overexpressing TPH2, the antiserum detected a dominant band of approximately 55 kDa and a minor band of approximately 47 kDa (Figure 1). The antiserum did not detect bands in vector-transfected COS cells, COS cells overexpressing TPH1 or in P815 cells, a murine mastocytoma cell line that expresses TPH1 exclusively (Figure 1).
Figure 1.
Immunoblotting with TPH2-specific antiserum. Rodent brain tissue samples (40 µg), P815 whole-cell homogenate (30 µg) and COS whole-cell homogenates (1 µg) were run on sodium dodecylsulfate-polyacrylamide gel electrophoresis and transferred to nitrocellulose for immunoblotting. The TPH2-specific antiserum TPH2-6361 detected a dominant ~55 kDa and a minor ~47.5 kDa band in whole-cell homogenates from COS cells overexpressing TPH2 (TPH2-COS). The antiserum detected an ~51 kDa band in total protein from a murine midbrain slice containing raphe nuclei (R) and murine forebrain (Fb). The antiserum did not detect bands in whole-cell homogenates from pcDNA 3.1-transfected COS cells (Vector), COS cells overexpressing TPH1 (TPH1-COS) or in P815 cells, a murine mastocytoma cell line that expresses TPH1 exclusively. TPH, tryptophan hydroxylase.
TPH2-6361 immunoreactivity in rodent and human brain
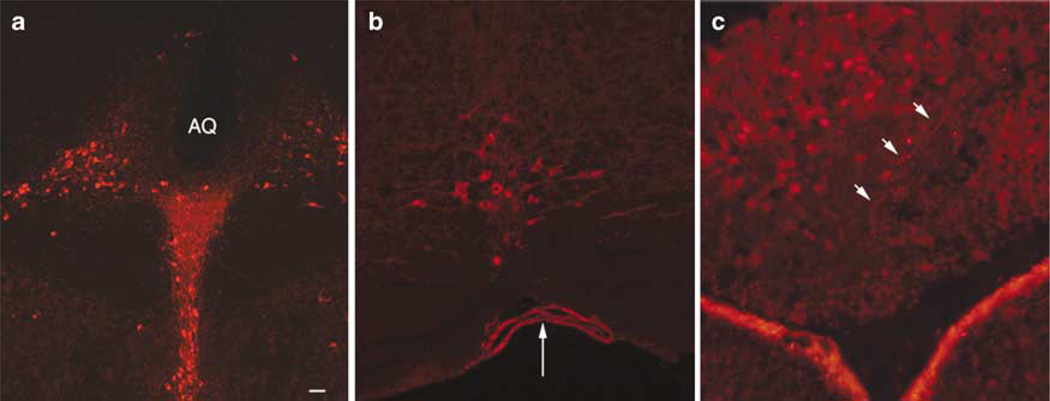
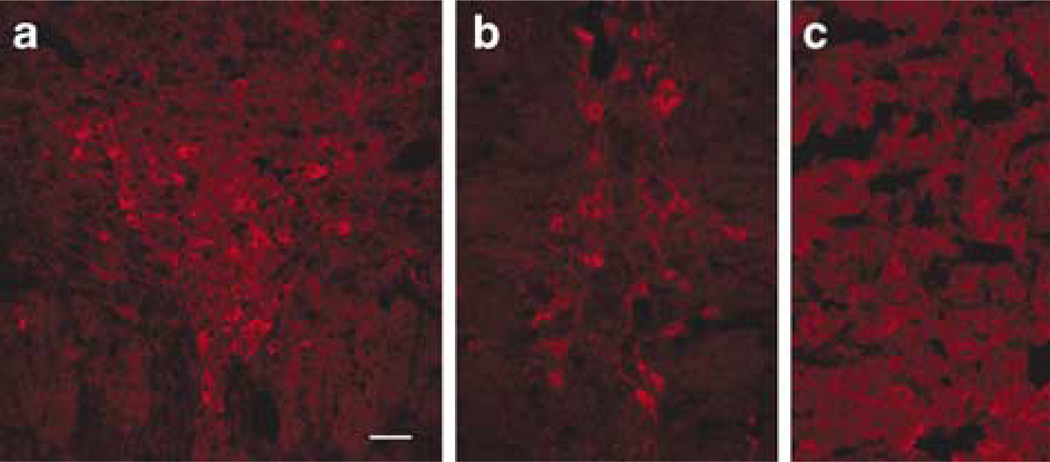
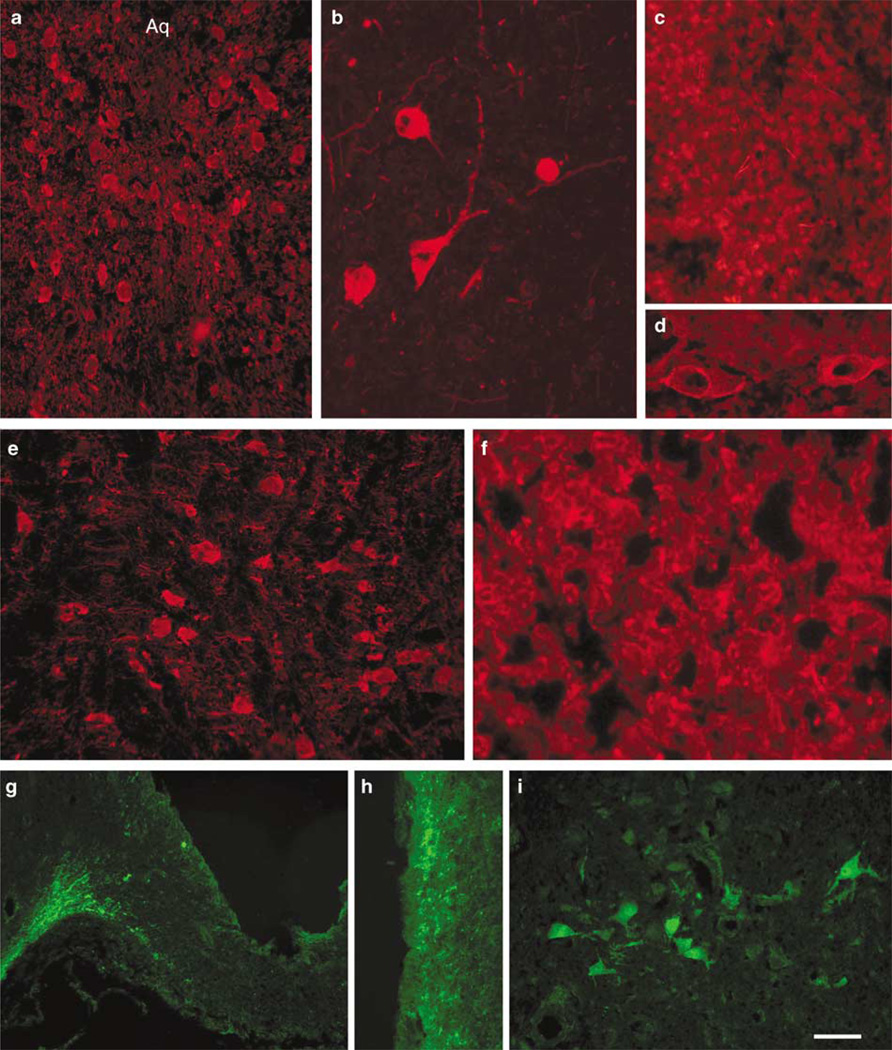
TPH2-IR was detected in raphe nuclei from mouse, rat and human. In rodent brain, TPH2-6361 IR was found across the full extent of the raphe nuclei from the midbrain throughout the brainstem. TPH2-6361-IR was seen in the dorsal raphe (Figures 2a, 3a, 4a and b), raphe magnus (Figures 2b and 4e) and in the rat caudal linear midbrain raphe (Figure 3b). Large arteries in human (Figure 4d) brain showed pronounced TPH2-6361-IR. Rodent and human pineal body exhibited TPH2-6361-IR in cell bodies and in beaded fibers (Figures 2c, 3c and 4c). Neuronal cell bodies in human lateral hypothalamus (Figure 4i) and cells in the anterior pituitary (Figure 4f) exhibited TPH2-6361-IR.
Figure 2.
Immunocytochemical staining of murine central nervous system tissue with TPH2-6361. TPH2-6361 IR was found in neuronal cell bodies in murine dorsal raphe nucleus (a), raphe magnus (b) and pineal gland (c). Arrows point to TPH2 immunoreactivity in the basal artery at the pontine level (b) and in beaded fibers in the pineal body (c) AQ, aqueduct; Bar = 100 µm (a) and 25 µm (b and c). IR, immunoreactivity; TPH, tryptophan hydroxylase.
Figure 3.
Immunocytochemical staining of rat central nervous system tissue with TPH2-6361. TPH2-6361 immunoreactivity was found along the raphe nuclei in the midbrain and brainstem. Neuronal cell bodies in rat dorsal raphe nucleus (a), linear raphe (b) and pineal body (c) are shown. Bar = 50 µm. TPH, tryptophan hydroxylase.
Figure 4.
Immunocytochemical staining of human central nervous system tissue with TPH2-6361. TPH2-6361 IR in human dorsal raphe neurons at low (a) and high (b) magnification. Panels show TPH2-6361 IR in fibers and cells in the pineal body (c), and pituitary gland (f), blood vessels (d), neurons in the pontine raphe (e) and lateral hypothalamus (i), as well as a fiber network in the median eminence (g) and periventricular zone (h). The fluorochrome used was AlexaFluor-594 (a–f) or AlexaFluor-488 (g–i) Aq, aqueduct. Bar = 25 µm (b), 50 µm (a, e, i) and 100 µm (c, d, f–h). IR, immunoreactivity; TPH, tryptophan hydroxylase.
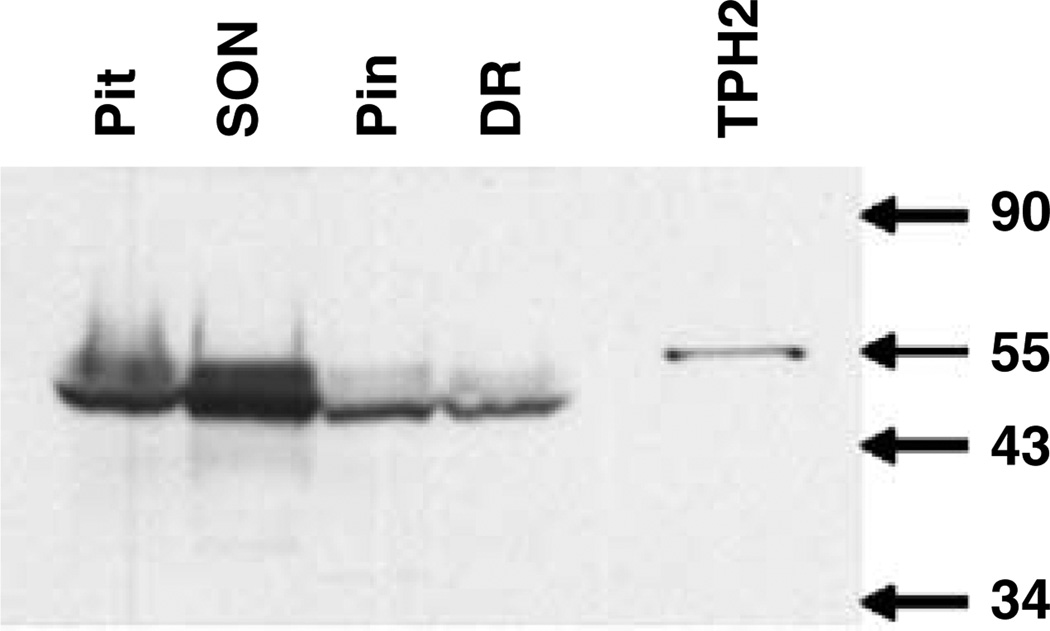
Western blot analyses of microdissected human brain tissue samples revealed TPH2-6361 reactive bands in pituitary, dorsal raphe, supraoptic and pineal samples (Figure 5). A band of approximately 55 kDa was detected in COS cells expressing the TPH2 coding region; however, the prominent band in the human tissue samples was approximately 47 kDa in size and was found in the pituitary, dorsal raphe, supraoptic nucleus and pineal body. A second band of approximately 50 kDa was detected in both the human dorsal raphe and pineal body samples.
Figure 5.
Immunoblotting of tryptophan hydroxylase (TPH)2 in microdissected human brain regions. Total protein from human brain microdissected regions (100 µg) and COS whole-cell homogenate (0.5 µg) were run on sodium dodecylsulfate-polyacrylamide gel electrophoresis and transferred to nitrocellulose for immunoblotting. TPH2-6361 detected a single band of 55 kDa in whole-cell homogenate from COS cells overexpressing TPH2. A predominant band of 47 kDa and a weaker band of 51 kDa was found in total protein from pituitary, supraoptic nucleus, pineal body and dorsal raphe. DR, dorsal raphe; Pin, pineal body; Pit, pituitary; SON supraoptic nucleus; TPH2, COS cell lysate overexpressing TPH2.
Glucocorticoid modulation of TPH2 protein in murine raphe nuclei and in vivo 5-HT synthesis in frontal cortex
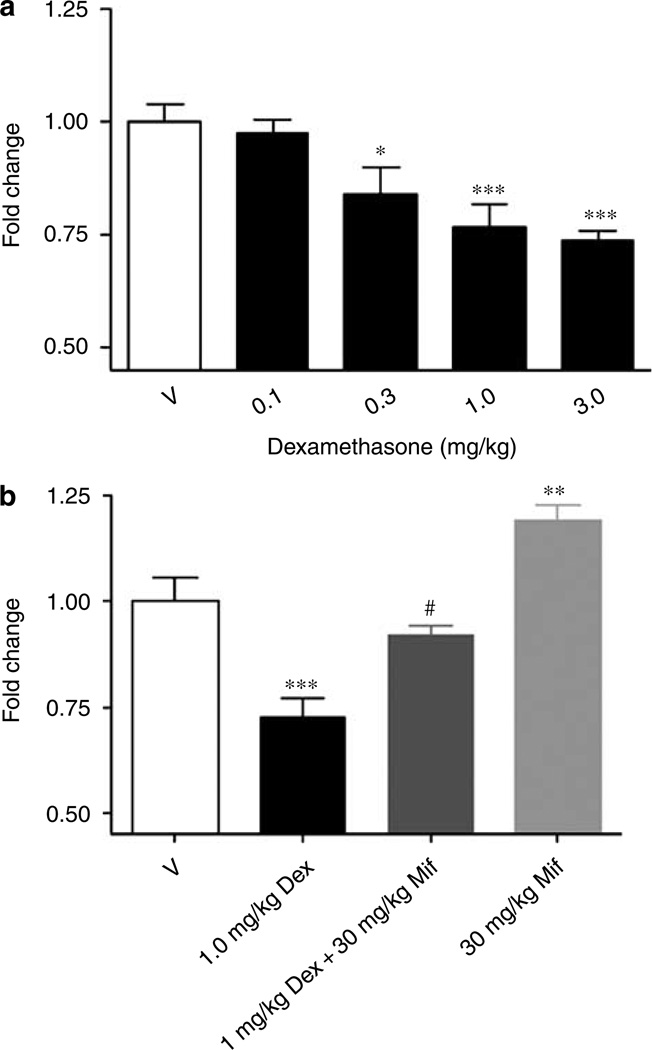
Daily s.c. administration of dexamethasone at doses from 0.3 to 3 mg/kg for 4 days in mice decreased TPH2 protein levels in the raphe by 23–30% (0.3 mg/kg, P < 0.01 and 3 mg/kg, P ≤ 0.001, respectively; Figure 6a). In a separate experiment, administration of 1.0 mg/kg dexamethasone once daily for 4 days resulted in a 27% reduction in TPH2 protein levels (P ≤ 0.001) in the raphe (Figure 6b) and co-administration of dexamethasone (1 mg/kg) and mifepristone (30 mg/kg) reversed the glucocorticoid effect (Figure 6b). Co-administration of dexamethasone and mifepristone reduced TPH2 protein levels by 8%; this change was statistically different from the dexamethasone effect (P < 0.005), but not from the vehicle control value (Figure 6b). Treatment with mifepristone alone caused a small, but statistically significant increase in TPH2 protein levels (P < 0.005; Figure 6b).
Figure 6.
Regulation of TPH2 protein by dexamethasone in murine pontine raphe as measured by western blot analyses. Administration (s.c.) of dexamethasone (Dex) at doses from 0.1 to 3.0 mg/kg once daily for 4 days resulted in a reduction in TPH2 protein levels from 23% at 0.3 mg/kg to 30% at 3 mg/kg in the murine raphe slice preparation (a). Administration of 1 mg/kg dexamethasone once daily for 4 days resulted in a 27% decrease in TPH2 protein in the murine raphe slice (b). Co-administration of 1 mg/kg dexamethasone and 30 mg/kg mifepristone (Mif) resulted in protein levels that were not statistically different from vehicle control levels, but were statistically different from the effect of 1 mg/kg dexamethasone alone (b). A 30 mg/kg portion of mifepristone caused a small, but statistically significant increase in TPH2 protein levels (b). *P < 0.01, **P < 0.005, and ***P ≤ 0.001 with respect to vehicle control; #P < 0.005 with respect to 1 mg/kg dexamethasone, one-way analysis of variance. TPH, tryptophan hydroxylase.
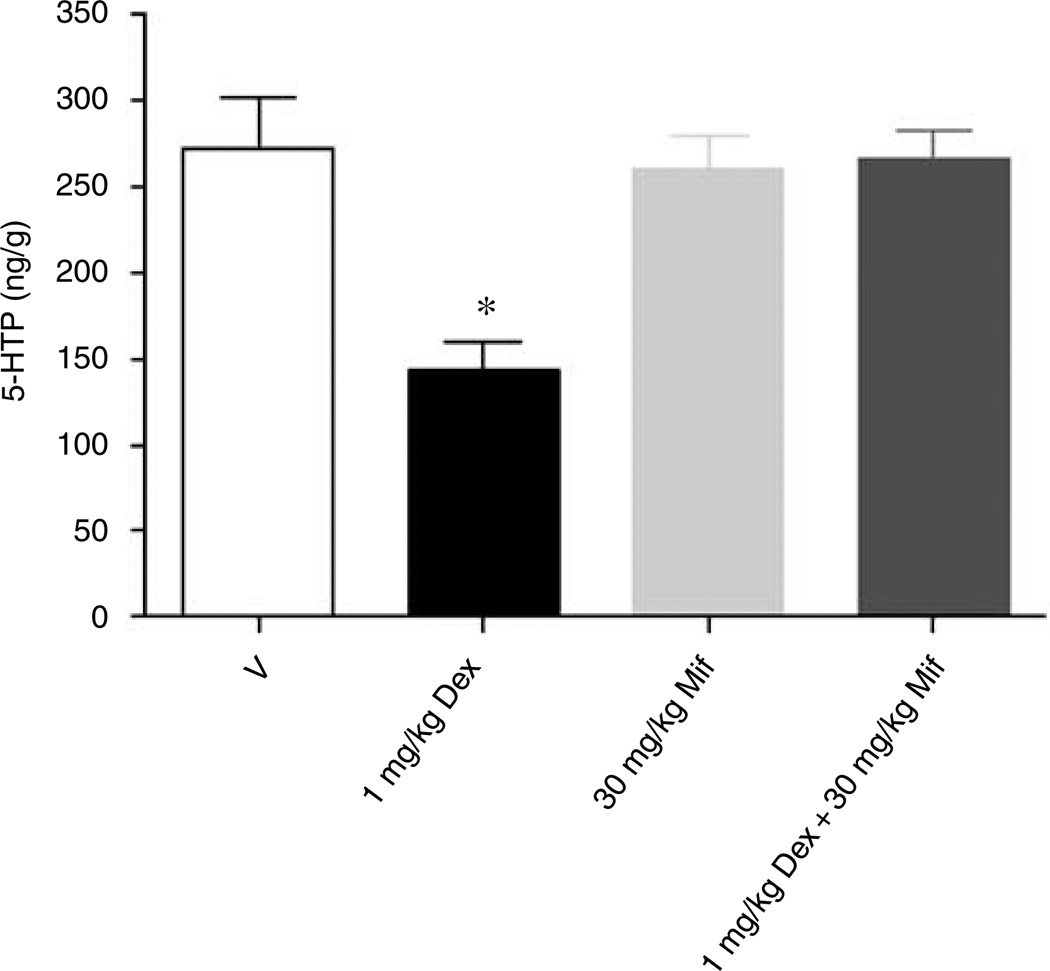
Concentrations of 5-HTP in frontal cortex were reduced by 54% (P < 0.01) in response to once daily dosing with 1.0 mg/kg dexamethasone (Figure 7). Co-administration of mifepristone (30 mg/kg) completely blocked the effect of dexamethasone (1 mg/kg) on cortical 5-HTP levels. Administration of mifepristone alone had no significant effect on 5-HTP concentrations.
Figure 7.
In vivo 5-HT synthesis is modulated by dexamethasone in murine frontal cortex. Accumulation of 5-HTP in vivo in murine frontal cortex was reduced by 54% in response to once daily dosing with 1.0 mg/kg dexamethasone for 4 days. Co-administration of mifepristone (30 mg/kg) blocked the effect of dexamethasone (1 mg/kg) on cortical 5-HTP levels and administration of mifepristone alone had no significant effect. *P < 0.01 in comparison to vehicle, one-way analysis of variance + Dunnett’s.
Discussion
A TPH2-specific antiserum TPH2-6361 was developed to a peptide antigen in the N terminus of the protein, a location with no homology to TPH1. TPH2-6361 is specific for the TPH2 isoform; it reacted with protein extracts from TPH2-expressing COS cells, but not from TPH1-expressing COS cells or P815 mastocytoma cells, which express only TPH1. TPH2-6361 retained its reactivity and specificity in immunoblotting and ICC applications, demonstrating its usefulness in examining the distribution of TPH2 protein in the CNS and suggesting its potential usefulness in detecting modulatory changes in TPH2 protein expression. TPH2-6361 showed reactivity against the respective antigen in both rodent and human tissues and has been used to examine the distribution of TPH2 protein in CNS tissue. TPH2 protein was found in both pineal gland and raphe nuclei of rodent and human brain. To date, study of TPH2 mRNA distribution has focused on the pineal gland and raphe nuclei. These studies have shown low, but detectable levels of TPH2 message in the pineal gland, which do not vary with time of day, in contrast to the circadian expression of TPH1 mRNA in this tissue.5,8 TPH2 mRNA is robustly expressed in median and dorsal raphe nuclei and its expression has been shown to exhibit a circadian rhythmicity in these brain regions.33 These data suggest that serotonin rhythmicity in the circadian system eminates from transcriptional regulation of TPH2 in the raphe nuclei.
Immunoblot analyses of human dorsal raphe and pineal tissues with TPH2-6361 revealed a doublet of 47 and 50 kDa, while a dense band of 55 kDa and a minor band of 47 kDa were detected in protein extracts from COS cells expressing murine TPH2. It is possible that the difference in the size of the human and murine TPH2 proteins is due to differences in post-translational modifications made in different cell types or tissues from different species. With regard to the doublet detected in some human CNS samples, Sakowski et al.34 have generated a TPH2-specific antiserum to an overlapping region of the TPH2 sequence and have reported the detection of a similar doublet, when immunoblotting protein extracts from certain rodent CNS tissues. Further study is required to understand the size differences detected in certain tissue and cell types and the significance of the doublet.
Sakowski et al.34 have recently reported the use of their TPH isoform-specific antiserum in immunoblot analyses to study the tissue distribution of TPH1 and TPH2. The TPH2-specific antiserum (αTPH2) detected proteins of approximately 55 kDa in murine mesencephalic tegmental, striatal and hippocampal samples.34 No TPH2 protein was detected in the pineal gland with αTPH2 by immunoblot. These findings stand in contrast to our data, which show fiber and cell body staining with TPH2-6361 in rodent and human pineal gland. It is unlikely that the discrepancy in our findings is due to the different antiserum, because the antigens used to generate these antisera are overlapping peptides in the N-terminal sequence of TPH2. It is possible that the immunoblot with mouse αTPH2 lacked the sensitivity necessary to detect the TPH2 band in pineal gland. It will be of interest to perform ICC studies with αTPH2 to determine whether the presence of immunoreactive fibers or cell bodies in the pineal gland can be demonstrated with the αTPH2 antisera and a technique of greater sensitivity.
TPH2-6361 IR in human raphe nuclei, pineal gland, pituitary, hypothalamus and CNS vasculature was robust using immuncytochemical staining and the presence of TPH2 IR was confirmed in pituitary, pineal gland and dorsal raphe by immunoblot analyses. Although the vast majority of CNS serotonin is known to originate from the brainstem raphe nuclei, several groups reported data suggesting the presence of serotonin-producing cells outside the raphe nuclei. Over two decades ago Friedman and coworkers used biochemical measurements in combination with surgeries to demonstrate that the intermediate lobe of the pituitary gland is innervated by serotonin fibers that originate within the hypothalamus.35–37 Hypothalamic serotonin-producing neurons have also been reported by other groups, following pharmacological manipulations.38,39 While no TPH1 was previously described in the hypothalamus, we now report the presence of TPH2 in hypothalamic areas in both rodent and humans.
TPH2 expression in human CNS has been studied in postmortem tissue from normal controls and individuals with psychiatric disease.9,40,41 Quantitative real-time reverse-transcription-polymerase chain reaction with TPH2-specific primers and probe detected TPH2 mRNA in human cortex, hypothalamus, thalamus, hippocampus, amygdala, cerebellum and raphe nuclei.41 Studies of postmortem raphe and cortex show that TPH2 message is increased in suicide and major depression subjects.9,40 In the CNS, migraine headaches have long been associated with serotonin-induced vasodilation.42 The presence of TPH2 IR in large CNS vasculature in the human brain may underlie serotonin regulation of cerebral vasculature and possibly contribute to migraine pathology. With the availability of TPH2-specific antiserum (TPH2-6361; αTPH234; TPH2 antibody43), it is now possible to determine the protein status of TPH2 in these and other disease states.
Data presented in this study extend previous work by our group, showing that glucocorticoids regulate TPH2 mRNA expression in murine raphe nuclei and that this regulation is glucocorticoid receptor specific.31 We have now used TPH2-6361 to show glucocorticoid-specific regulation of TPH2 protein in murine raphe nuclei. These data represent the first to show isoform-specific glucocorticoid modulation of TPH protein in any species. Earlier work by several groups has shown that TPH mRNA, protein and activity can be modulated by hormones in raphe nuclei of various species21–25,27–30,32 and thus may affect serotonin biosynthesis. It will be important to revisit these studies using isoform-specific antibodies such as TPH2-6361 to determine the specific role of each isoform in each species.
The data presented herein have extended our findings on glucocorticoid modulation of TPH2 protein to show that glucocorticoids modulate serotonin biosynthesis in vivo, most probably, through regulation of the expression of TPH2 protein in raphe nuclei. It has been previously shown that glucocorticoids do not effect TPH1 mRNA expression in murine raphe nuclei31 and so the glucocorticoid-mediated changes in serotonin biosynthesis observed here are most likely due to changes in expression levels of TPH2 protein, although a glucocorticoid effect on TPH1 protein turnover cannot be entirely ruled out. Steroid modulation of TPH2 protein is specific to the glucocorticoid receptor as co-administration of the glucocorticoid antagonist mifepristone blocked the effects of dexamethasone. A small but statistically significant increase in TPH2 protein was observed with administration of mifepristone alone; this may be due to the fact that mifepristone is both a glucocorticoid and progesterone receptor antagonist. Ovx mice have low levels of progesterone therefore the effects of mifepristone on TPH2 are not likely to be due to engagement of this receptor. Instead, it is more likely that the effect of mifepristone on TPH2 protein expression is due to antagonism of endogenous glucocorticoids. It is more likely that the effect of mifepristone on TPH2 protein expression is due to antagonism of endogenous glucocorticoids as ovx mice should have low levels of progesterone. This report is the first to show that modulation of serotonin biosynthesis through changes in TPH2 expression may contribute to glucocorticoid modulation of central serotonergic neurotransmission in the mouse. The potential relevance of the connection between glucocorticoids and modulatory changes in the serotonergic system were recently illustrated by Chen et al.,20 who identified several polymorphisms in the 3′-UTR of rhesus monkey TPH2 that modulate HPA axis function, most likely by modulating serotonin biosynthesis. It will be of interest to determine the reactivity of TPH2-6361 in monkey tissue, as it would be a useful tool in understanding the interplay between HPA axis function and serotonin biosynthesis.
In summary, we have generated a TPH2-specific antiserum with broad reactivity in several species that is useful for both immunoblotting and ICC studies. We have detected TPH2-6361 IR in human and rodent raphe and pineal tissues and in human pituitary, hypothalamus and CNS vasculature. TPH2-6361 was used to show glucocorticoid modulation of TPH2 protein expression in murine raphe nuclei that was blocked by co-administration of the glucocorticoid antagonist mifepristone. Modulation of TPH2 protein in raphe nuclei is accompanied by a reduction in serotonin biosynthesis in the frontal cortex and this change in synthesis is also blocked by co-administration of the glucocorticoid antagonist mifepristone. There is considerable interplay between the serotonergic and HPA systems and evidence suggests that dysfunction in one of these systems could lead to impairment in the other.44,45 Glucocorticoid regulation of TPH2 expression and the resulting changes in serotonin biosynthesis may contribute to the serotonergic response to stressful stimuli and may have relevance to the etiology of psychiatric disease accompanied by HPA axis dysregulation,46,47 as well as psychiatric illness that can be triggered by glucocorticoid therapy.
Acknowledgments
We thank S Adamski for her technical expertise, S Alves and S Rohrer for their support, and M Palkovits for generous provision of the human tissue samples. S Key, I Szalayova and E Mezey are supported by the NIH intramural program, NIDCR.
References
- 1.Grahame-Smith DG. Tryptophan hydroxylation in brain. Biochem Biophys Res Commun. 1964;16:586–592. doi: 10.1016/0006-291x(64)90197-4. [DOI] [PubMed] [Google Scholar]
- 2.Lovenberg W, Jequier E, Sjoerdsma A. Tryptophan hydroxylation: measurement in pineal gland, brainstem, and carcinoid tumor. Science. 1967;155:217–219. doi: 10.1126/science.155.3759.217. [DOI] [PubMed] [Google Scholar]
- 3.Jequier E, Robinson DS, Lovenberg W, Sjoerdsma A. Further studies on tryptophan hydroxylase in rat brainstem and beef pineal. Biochem Pharmacol. 1969;18:1071–1081. doi: 10.1016/0006-2952(69)90111-7. [DOI] [PubMed] [Google Scholar]
- 4.Walther DJ, Peter J-U, Bashammakh S, Hortnagl H, Voits M, Fink H, et al. Synthesis of serotonin by a second tryptophan hydroxylase isoform. Science. 2003;299:76. doi: 10.1126/science.1078197. [DOI] [PubMed] [Google Scholar]
- 5.Patel PD, Pontrello C, Burke S. Robust and tissue-specific expression of TPH2 versus TPH1 in rat raphe and pineal gland. Biol Psychiatry. 2004;55:428–433. doi: 10.1016/j.biopsych.2003.09.002. [DOI] [PubMed] [Google Scholar]
- 6.Clark MS, McDevitt RA, Neumaier JF. Quantitative mapping of tryptophan hydroxylase-2,5-HT1A, 5-HT1B, and serotonin transporter expression across the anteroposterior axis of the rat dorsal and median raphe nuclei. J Comp Neurol. 2006;498:611–623. doi: 10.1002/cne.21073. [DOI] [PubMed] [Google Scholar]
- 7.Gundlah C, Clark JA, Alves SE, Pai L-Y, Schaeffer JM, Rohrer SP. Estrogen receptor-beta regulates tryptophan hydroxylase expression in the murine midbrain raphe. Biol Psychiatry. 2005;57:938–942. doi: 10.1016/j.biopsych.2005.01.014. [DOI] [PubMed] [Google Scholar]
- 8.Sugden D. Comparison of circadian expression of tryptophan hydroxylase isoform mRNAs in the rat pineal gland using real-time PCR. J Neurochem. 2003;86:1308–1311. doi: 10.1046/j.1471-4159.2003.01959.x. [DOI] [PubMed] [Google Scholar]
- 9.De Luca V, Likhodi O, Van Tol HH, Kennedy JL, Wong AHC. Gene expression of tryptophan hydroxylase 2 in post-mortem brain of suicide subjects. Int J Neuropsychopharmacol. 2006;9:21–25. doi: 10.1017/S1461145705005572. [DOI] [PubMed] [Google Scholar]
- 10.Peters EJ, Slager SL, McGrath PJ, Knowles JA, Hamilton SP. Investigation of serotonin-related genes in antidepressant response. Mol Psychiatry. 2004;9:879–889. doi: 10.1038/sj.mp.4001502. [DOI] [PubMed] [Google Scholar]
- 11.Van Den Bogaert A, De Zutter S, Heyrman L, Mendlewicz J, Adolfsson R, Van Broekhoven C, et al. Response to Zhang et al. (2005) loss-of-function mutation in tryptophan hydroxylase-2 identified in unipolar major depression. Neuron 45, 11–16. Neuron. 2005;48:704. doi: 10.1016/j.neuron.2005.11.017. [DOI] [PubMed] [Google Scholar]
- 12.Glatt CE, Carlson E, RTaylor TR, Risch N, Reus VI, Schaefer CA. Response to Zhang et al. (2005) loss-of-function mutation in tryptophan hydroxylase-2 identified in unipolar major depression. Neuron 2005; 45, 11–16. Neuron. 2005;48:704–705. doi: 10.1016/j.neuron.2005.11.019. [DOI] [PubMed] [Google Scholar]
- 13.Zhou Z, Peters EJ, Hamilton SP, McMahon F, Thomas C, McGrath PJ, et al. Response to Zhang et al. (2005) loss-of-function mutation in tryptophan hydroxylase-2 identified in unipolar major depression. Neuron 45, 11–16. Neuron. 2005;48:702–703. doi: 10.1016/j.neuron.2005.11.018. [DOI] [PubMed] [Google Scholar]
- 14.Zill P, Baghai TC, Zwanger P, Schule C, Eser D, Rupprecht R, et al. SNP and haplotype analysis of a novel tryptophan hydroxylase isoform (TPH2) gene provide evidence for association with major depression. Mol Psychiatry. 2004;9:1030–1036. doi: 10.1038/sj.mp.4001525. [DOI] [PubMed] [Google Scholar]
- 15.Sheehan K, Lowe N, Kirley A, Mullins C, Fitzgerald M, Gill M, et al. Tryptophan hydroxylase-2 (TPH2) gene variants associated with ADHD. Mol Psychiatry. 2005;10:944–949. doi: 10.1038/sj.mp.4001698. [DOI] [PubMed] [Google Scholar]
- 16.Mossner R, Walitza S, Geller F, Scherag A, Gutknecht L, Jacob C, et al. Transmission disequilibrium of polymorphic variants in the tryptophan hydroxylase-2 gene in children and adolescents with obsessive-compulsive disorder. Int J Neuropsychophramacol. 2005;9:1–6. doi: 10.1017/S1461145705005997. [DOI] [PubMed] [Google Scholar]
- 17.Brookes K, Xu X, Chen W, Zhou K, Neale B, Lowe N, et al. The analysis of 51 genes in DSM-IV combined type attention deficit hyperactivity disorder: association signals in DRD4, DAT1 and 16 other genes. Mol Psychiatry. 2006;11:934–953. doi: 10.1038/sj.mp.4001869. [DOI] [PubMed] [Google Scholar]
- 18.Coon H, Dunn D, Lainhart J, Miller J, Hamil C, Battaglia A, et al. Possible association between autism and variants in the brain-expressed tryptophan hydroxylase gene (TPH2) Am J Med Gen Part B. 2005;135B:42–46. doi: 10.1002/ajmg.b.30168. [DOI] [PubMed] [Google Scholar]
- 19.Harvey M, Shink E, Tremblay M, Gagne B, Raymond C, Labbe M, et al. Support for the involvement of TPH2 gene in affective disorders. Mol Psychiatry. 2004;9:980–981. doi: 10.1038/sj.mp.4001557. [DOI] [PubMed] [Google Scholar]
- 20.Chen G-L, Novak MA, Hakim S, Xie Z, Miller GM. Tryptophan hydroxylase-2 gene polymorphisms in rhesus monkeys: association with hypothalamic-pituitary-adrenal axis function and in vitro gene expression. Mol Psychiatry. 2006;11:914–928. doi: 10.1038/sj.mp.4001870. [DOI] [PubMed] [Google Scholar]
- 21.Azmitia EC, Jr, Liao B, Chen Y. Increase of tryptophan hydroxylase enzyme protein by dexamethasone in adrenalectomized rat midbrain. J Neurosci. 1993;13:5041–5055. doi: 10.1523/JNEUROSCI.13-12-05041.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Azmitia EC, Jr, McEwen BS. Corticosterone regulation of tryptophan hydroxylase in midbrain of the rat. Science. 1969;166:1274–1276. doi: 10.1126/science.166.3910.1274. [DOI] [PubMed] [Google Scholar]
- 23.Azmitia EC, Jr, McEwen BS. Adrenalcortical influence on rat brain tryptophan hydroxylase activity. Brain Res. 1974;78:291–302. doi: 10.1016/0006-8993(74)90553-8. [DOI] [PubMed] [Google Scholar]
- 24.Bethea CL, Mirkes SJ, Shively CA, Adams MR. Steroid regulation of tryptophan hydroxylase protein in the dorsal raphe of Macaques. Biol Psychiatry. 2000;47:562–576. doi: 10.1016/s0006-3223(99)00156-0. [DOI] [PubMed] [Google Scholar]
- 25.Clark MS, Russo AF. Tissue-specific glucocorticoid regulation of tryptophan hydroxylase mRNA levels. Mol Brain Res. 1997;48:346–354. doi: 10.1016/s0169-328x(97)00106-x. [DOI] [PubMed] [Google Scholar]
- 26.Lu NZ, Shlaes TA, Gundlah C, Dziennis SE, Lyle RE, Bethea CL. Ovarian steroid action on tryptophan hydroxylase protein and serotonin compared to localization of ovarian steroid receptors in midbrain of guniea pigs. Endocrine. 1999;11:257–267. doi: 10.1385/ENDO:11:3:257. [DOI] [PubMed] [Google Scholar]
- 27.Park DH, Paivarinta H, Joh TH. Tryptophan hydroxylase activity in hypothalamus and brainstem of neonatal and adult rats treated with hydrocortisone or parachlorophenylalanine. Neurosci Res. 1989;7:76–80. doi: 10.1016/0168-0102(89)90039-4. [DOI] [PubMed] [Google Scholar]
- 28.Pecins-Thompson M, Brown NA, Kohama SG, Bethea CL. Ovarian steroid regulation of tryptophan hydroxylase mRNA expression in Rhesus macaques. J Neurosci. 1996;16:7021–7029. doi: 10.1523/JNEUROSCI.16-21-07021.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rastogi RB, Singhal RL. Adrenocorticoids control 5-hydroxytryptamine metabolism in rat brain. J Neural Trasm. 1978;42:63–71. doi: 10.1007/BF01262730. [DOI] [PubMed] [Google Scholar]
- 30.Sze PY, Neckers L, Towle AC. Glucocorticoids as a regulatory factor for brain tryptophan hydroxylase. J Neurochem. 1976;26:169–173. [PubMed] [Google Scholar]
- 31.Clark JA, Pai L-Y, Flick RB, Rohrer SP. Differential hormonal regulation of tryptophan hydroxylase-2 mRNA in the murine dorsal raphe nucleus. Biol Psychiatry. 2005;57:943–946. doi: 10.1016/j.biopsych.2005.01.013. [DOI] [PubMed] [Google Scholar]
- 32.Barton CL, Hutson PH. Inhibition of hippocampal 5-HT synthesis by fluoxetine and paroxetine: evidence for the involvement of both 5-HT1A and 5-HT1B/D autoreceptors. Synapse. 1999;31:13–19. doi: 10.1002/(SICI)1098-2396(199901)31:1<13::AID-SYN3>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 33.Malek ZS, Dardente H, Pevet P, Raison S. Tissue-specific expression of tryptophan hydroxylase mRNAs in the rat midbrain: anatomical evidence and daily profiles. Eur J Neurosci. 2005;22:895–901. doi: 10.1111/j.1460-9568.2005.04264.x. [DOI] [PubMed] [Google Scholar]
- 34.Sakowski SA, Geddes TJ, Thomas DM, Levi E, Hatfield JS, Kuhn DM. Differential tissue distribution of tryptophan hydroxylase isoforms 1 and 2 as revealed with monospecific antibodies. Brain Res. 2006;1085:11–18. doi: 10.1016/j.brainres.2006.02.047. [DOI] [PubMed] [Google Scholar]
- 35.Friedman E, Krieger DT, Mezey E, Leranth C, Brownstein MJ, Palkovits M. Serotonergic innervation of the rat pituitary intermediate lobe: decrease after stalk section. Endocrinology. 1983;112:1943–1947. doi: 10.1210/endo-112-6-1943. [DOI] [PubMed] [Google Scholar]
- 36.Mezey E, Leranth C, Brownstein MJ, Friedman E, Krieger DT, Palkovits M. On the origin of the serotonergic input to the intermediate lobe of the rat pituitary. Brain Res. 1984;294:231–237. doi: 10.1016/0006-8993(84)91034-5. [DOI] [PubMed] [Google Scholar]
- 37.Palkovits M, Mezey E, Chiueh CG, Krieger DT, Gallatz K, Brownstein MJ. Serotonin-containing elements of the rat pituitary intermediate lobe. Neuroendocrinology. 1986;43:526–532. doi: 10.1159/000124497. [DOI] [PubMed] [Google Scholar]
- 38.Frankfurt M, Lauder JM, Azmitia EC., Jr The immunocytochemical localization of serotonergic neurons in the rat hypothalamus. Neurosci Letts. 1981;24:227–232. doi: 10.1016/0304-3940(81)90161-0. [DOI] [PubMed] [Google Scholar]
- 39.Arezki F, Bosler O, Steinbusch HW. Morphological evidence that serotonin-immunoreactive neurons in the nucleus dorsomedialis hypothalami could be under catecholaminergic influence. Neurosci Letts. 1985;56:161–166. doi: 10.1016/0304-3940(85)90123-5. [DOI] [PubMed] [Google Scholar]
- 40.Bach-Mizrachi H, Underwood MD, Kassir SA, Bakalian MJ, Sibille E, Tamir H, et al. Neuronal tryptophan hydroxylase mRNA expression in the human dorsal and median raphe nuclei: major depression and suicide. Neuropsychopharmacology. 2006;31:814–824. doi: 10.1038/sj.npp.1300897. [DOI] [PubMed] [Google Scholar]
- 41.Zill P, Buttner A, Eisenmenger W, Moller H-J, Ackenheil M, Bondy B. Analysis of tryptophan hydroxylase I and II mRNA expression in the human brain: a post-mortem study. J Psych Res. 2007;41:168–173. doi: 10.1016/j.jpsychires.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 42.Arulmozhi DK, Veeranjaneyulu A, Bodhankar SL. Migraine: current therapeutic targets and future avenues. Curr Vasc Pharmacol. 2006;4:117–128. doi: 10.2174/157016106776359853. [DOI] [PubMed] [Google Scholar]
- 43.Kerman IA, Shabrang C, Taylor L, Akil H, Watson SJ. Relationship of presympathetic-premotor neurons to the serotonergic transmitter system in the rat brainstem. J Comp Neurol. 2006;499:882–896. doi: 10.1002/cne.21129. [DOI] [PubMed] [Google Scholar]
- 44.Dinan TG. Glucocorticoids and the genesis of depressive illness: a psychobiological model. Br J Psychiatry. 1994;164:365–371. doi: 10.1192/bjp.164.3.365. [DOI] [PubMed] [Google Scholar]
- 45.McAllister-Williams RH, Ferrier IN, Young AH. Mood and neuropsychological function in depression: the role of corticosteroids and serotonin. Psychol Med. 1998;28:573–584. doi: 10.1017/s0033291798006680. [DOI] [PubMed] [Google Scholar]
- 46.Young AH. Antiglucocorticoid treatments for depression. Austr New Zealand J Psych. 2006;40:402–405. doi: 10.1080/j.1440-1614.2006.01813.x. [DOI] [PubMed] [Google Scholar]
- 47.Strohle A, Holsboer F. Stress responsive neurohormones in depression and anxiety. Pharmacopsychiatry. 2003;36:S207–S214. doi: 10.1055/s-2003-45132. [DOI] [PubMed] [Google Scholar]