Abstract
Background
This study evaluated brain volumes in healthy older subjects without dementia who presented with memory complaints. The objective was to examine cortical volumes in relation to cognitive performance among patients who do not have dementia, but who do have mild cognitive deficits.
Methods
Fifteen participants were evaluated (mean age = 71.8 ± 6.2). Brain structure was measured via high-resolution magnetic resonance imaging to quantify gray and white matter volumes. Volumetric measures were assessed relative to cognitive function in separate regression models controlling for total cerebral volume. Reported here are measures of global cognitive performance using the Mattis Dementia Rating Scale (DRS) in relation to volumetric measures.
Results
Baseline MMSE scores ranged from 27 to 30 (mean = 29.3; SD = 0.9). After controlling for total cerebral volume, we observed that lower white matter volume in the temporal lobe [F(1,14) = 5.72, p = 0.03] was associated with lower performance on the Mattis Dementia Rating Scale (DRS).
Conclusions
Structural imaging may help provide useful clinical information in the context of mild cognitive decline. Currently, the diagnosis of dementia relies on longitudinal measures of cognition. Future studies will help determine whether the addition of brain imaging may enhance diagnostic certainty as well as predict long-term outcome.
Keywords: Cognition, Imaging, Aging, Memory
INTRODUCTION
Structural brain imaging in older adults is increasingly used in clinical practice to assist in excluding treatable causes of cognitive decline (e.g., normopressure hydrocephalus or CNS neoplastic disease) as well as to assist in the diagnostic process when a neurodegenerative dementia such as Alzheimer’s disease is suspected. However, in the early stages of cognitive aging, the delineation of normal aging and pathologic change remains difficult to define. Structural neuroimaging studies in dementia have shown that atrophy of the cerebral cortex appears to occur on a continuum with increasing age (1). Even earlier in this continuum, Visser et al. (2) demonstrated that among patients with mild cognitive impairment, reduced temporal lobe volume predicted subsequent dementia, suggesting that imaging may be informative prior to onset of overt decline. However the same group also found that in healthy older adults, reduced total temporal volume as well as reduced regional hippocampal volumes were associated with increasing age, but they were not able to detect an association with cognitive measures (3).
Overall, the choice to use magnetic resonance imaging (MRI) as a diagnostic tool in early memory decline lacks a clear evidence base, but nonetheless it is a fairly common practice. Given that Alzheimer’s dementia is characterized largely by memory loss with temporoparietal atrophy, studies addressing potential prognostic significance have focused on the temporal lobe. Along these lines, it has been suggested that specifically temporal lobe decrements predict crossover of mild cognitive impairment to Alzheimer’s dementia (4). This does appear to potentially occur across a spectrum of mild impairments. For example, one study longitudinally followed persons with “minor cognitive impairments” over a 2 year period, and noted that smaller temporal lobe volume was associated with progression to Alzheimer’s disease (5).
Among healthy adults without dementia, structural changes in the temporal lobe may be difficult to separate from the continuum of brain changes that occur with normal aging, such that mapping of the transition from normal aging to overt dementia remains an evolving area of important research. One interesting recent study detected reduced anterotemporal lobe volumes among a sample of older persons without impairments who went on to have mild cognitive decline, suggesting that there may indeed be a continuum of temporal lobe changes even between the normal aging and mild deficit range (6).
This area of detecting early prognostic features from a cognitive perspective is also rapidly growing, in an effort to push the detection of dementia earlier and earlier in the process. For example, Saykin et al. (7) recently reported on a sample of subjects identified only by the subjective experience of “cognitive complaints” in the absence of detectable deficits. In a cross-sectional analysis, they compared this group to a group with mild cognitive impairment (MCI) as well as healthy controls. They found that the “cognitive complaints” group was similar to the MCI group in that they had lower brain volumes particularly in the medial temporal lobe and frontotemporal regions when compared to healthy controls.
Given that MRI is a readily assessable technique available clinically, it is helpful in clinical practice to understand the extent to which structural measures relate to cognitive testing. On the other hand, one might argue that if cognitive function is largely intact, then imaging measures have little clinical meaning for the patient. Furthermore, given that not all mild impairments progress to overt dementia, one might even view it as concerning to over-emphasize the predictive value of brain imaging in the absence of a clear diagnosis based on cognitive testing. This concern brings up potential ethical issues, as has been discussed by Illes et al. (8) in a recent paper.
In an effort to help clarify some of these issues, the findings reported here seek to provide some insights regarding the relationship between brain structural measures and cognitive function among a group of older adults who do not have a diagnosis of dementia, but do complain of self-perceived memory impairment.
METHODS
Fifteen older adults with mild cognitive deficits were studied after written informed consent was obtained in accordance with the University of Iowa Institutional Review Board. Participants were all independent community dwelling individuals who responded to an advertisement seeking persons who were interested in participating in a clinical trial for persons with mild memory problems. Cognitive impairment was objectively assessed with several neuropsychological measures, including the Mini Mental Status Examination (MMSE) (9) and the Hopkins Verbal Learning Test–Revised (HVLT-R) (10). All participants had complaints of memory problems and demonstrated objective memory deficits on at least one test of learning and memory. Subjects were not enrolled if they had a diagnosis of dementia and no subjects reported impairment in activities of daily living. All subjects were over 62 years of age. Subjects were excluded if they had a history of major neurological, metabolic, or psychiatric disorders, cardiovascular disease, cerebrovascular event, substance abuse or other significant medical problems.
Neuropsychological Testing
Neuropsychological testing was administered by a trained research assistant under the supervision of faculty neuropsychologists. All participants were initially evaluated during a screening visit with a brief battery of cognitive tests and were determined to have mild objective memory deficits, defined as scores > 1.5 standard deviation below expectations based on normative values on at least one memory test from the following measures: Mattis Dementia Rating Scale, Memory subscale (11); Brief Visuospatial Memory Test–Revised (BVMT-R) (12); Wechsler Memory Scale–III (13). Each participant’s overall level of cognitive functioning was not impaired (mean MMSE = 29.3 ± 1.0, range = 27–30). General cognitive function was assessed using the Mattis Dementia Rating Scale (11). The Mattis DRS has a total score of 144, encompassing domains of attention, initiation/perseveration, construction, conceptualization and memory.
Imaging Methods
The MR scans were acquired using a multimodal imaging protocol consisting of T1 and T2 weighted sequences on a GE 1.5T CV/i scanner. The T1 sequence was obtained in the coronal plane using a spoiled GRASS sequence with the following parameters: TE = 6 ms, TR = 20 ms, flip angle = 30°, FOV = 160×160×192 mm, matrix = 256 × 256 × 124, NEX = 2. The T2 images were acquired using a 2D fast spin-echo sequence in the coronal plane with the following parameters: TE = 85 ms, TR=4800 ms, slice thickness/gap=1.8/0.0 mm, FOV = 160 × 160 mm, matrix = 256 × 256, NEX = 3, number of echoes = 8, number of slices = 124. The MR data were transferred to the Department of Radiology Image Processing Laboratory for analysis.
The anatomical images were processed using the locally developed software BRAINS2 (Brain Research: Analysis of Images, Networks, and Systems) as previously described (14-16). A standard analysis pipeline was applied to all of the scans that included spatial alignment, tissue classification and automated segmentation. Briefly, the T1 images were realigned in a standard orientation to correct for head rotation, with the interhemispheric fissure determining alignment in axial and coronal planes and the anterior-posterior commissure line determining the horizontal in the sagittal plane. The T2 images were aligned to the spatially normalized T1 image using the automated image registration program (17,18). A Talairach-based atlas coordinate system was overlaid onto each individual brain, aligning with anatomical landmarks of that brain without normalization to a standardized brain size. These coordinates were then used to generate automated measurements of frontal, temporal, parietal, and occipital lobes, subcortical, brainstem, and cerebellum (14). This method permits morphological measurements to be made in non-normalized or “raw” space.
To classify tissue volumes into gray matter, white matter, and cerebrospinal fluid, a discriminant analysis method of tissue segmentation based on automated training class selection was used with data from the T1 and T2 sequences (15). The tissue classification generates a continuous (fuzzy) classification of the brain tissue. The resulting image contained the percentage of gray matter, white matter and cerebrospinal fluid (CSF) at each voxel. Subsequently, these continuous tissue classification images were input into a neural network to define the brain (16).
Intracranial volume was divided into grey matter, white matter and cerebrospinal fluid (CSF) based on the neural network definition of the brain and the tissue classified image. Brain tissue was then subdivided into the cerebrum and cerebellum based on the automated regional definitions as described above. Cerebral gray matter volume represents the volume of the cortex as the subcortical structures are excluded from this measure and is therefore referred to as cortical gray matter volume.
DATA ANALYSIS
The Mattis Dementia Rating Scale score was used to measure cognitive function in relation to structural measures. The total scores were ranked, as the data were not normally distributed. A linear regression analysis was used to assess whether specific regional volume decrements predicted impairment in the Mattis DRS as a dependent measure. This analysis used a regression model that was conducted by entering the regional volumes as independent measures in separate regression analyses for each regional brain volume with the cognitive scores on the Mattis Dementia Rating Scale total score as the dependent variables. The total cerebral volume was included in the regression model, entered as an independent measure to account for variance attributable to individual differences in total brain size. Total cerebral volume was calculated by summing the volumes of total gray matter, white matter and cerebrospinal fluid with cerebellum and brainstem excluded
RESULTS
The sample was comprised of 5 men and 10 women, all participants were Caucasian. The mean age of the sample was 71.8 (SD = 6.2) years. The recruited sample resulted from approximately 40 responses to an advertisement for a study involving imaging (19,20). Of those screened, approximately half did not want to undergo brain imaging and were excluded and the remaining group was not recruited due to an exclusionary medical condition. Mean years of education were 15.6 (SD = 3.0). The mean MMSE score was 29.3; SD = 0.9. A composite score for memory was determined for each subject by summing age-corrected scale scores (mean = 10, SD = 3 for each subtest) from four related subtests, i.e., the sum of the BVMT-R Delayed Recall, WMS-III Logical Memory II, WMS-III Word Lists II, DRS Memory. The mean of this composite score was 36.7 (SD = 8.2). The mean Mattis DRS total score was 137.7 SD = 4.9), representing a group with very mild deficits. Individual subscale means were as follows: Attention 35.7 (SD = 2.6), Initiation 36.1 (SD = 1.8), Construction 6 (SD = 0), Conceptualization 36.1 (SD = 2.7), Memory 23.1 SD = 1.6).
In the separate regression models including regional volumes as well as total cerebral volume, there was no significant association of any specific regional volume with cognitive function, with the exception of temporal lobe white matter volume. Please refer to Table 1 for the findings regarding total cerebral volume and individual regional volumes for gray and white matter volumes in the frontal, temporal and parietal lobes. Table 2 depicts the regression results of each regional volume relative to Mattis DRS total score. Figure 1 demonstrates the relationship between temporal lobe white matter volume and Mattis DRS scores to illustrate the direction of the association, i.e., persons with lower volumes of temporal lobe white matter performed more poorly on the Mattis DRS, reflected in a lower total score. This finding was present in both the right and left temporal lobe, although the combined volume is represented in Figure 1.
Table 1.
Regional MRI Brain Volumes in Subjects with Mild Cognitive Deficits
| Region | Volume, cm3 (SD) |
|---|---|
| Total Cerebral Volumea | 1108 (136) |
| Frontal Gray Matter | 193 (22) |
| Frontal White Matter | 173 (31) |
| Temporal Gray Matter | 142 (17) |
| Temporal White Matter | 67 (14) |
| Parietal Gray Matter | 123 (14) |
| Parietal White Matter | 88 (16) |
Gray matter, white matter and cerebrospinal fluid with cerebellum and brainstem excluded.
Table 2.
Association of Regional Volumes with Mattis Dementia Rating Scale Scores*
| Frontal Gray Matter | F(1, 14) = 0.45; p = 0.52 |
|---|---|
| Frontal White Matter | F(1, 14) = 0.65; p = 0.65 |
| Temporal Gray Matter | F(1, 14) = 2.86; p = 0.12 |
| Temporal White Matter | F(1, 14) = 5.72; p = 0.03 |
| Parietal Gray Matter | F(1, 14) = 1.93; p = 0.19 |
| Parietal White Matter | F(1, 14) = 2.34; p = 0.15 |
After controlling for total cerebral volume.
Figure 1.
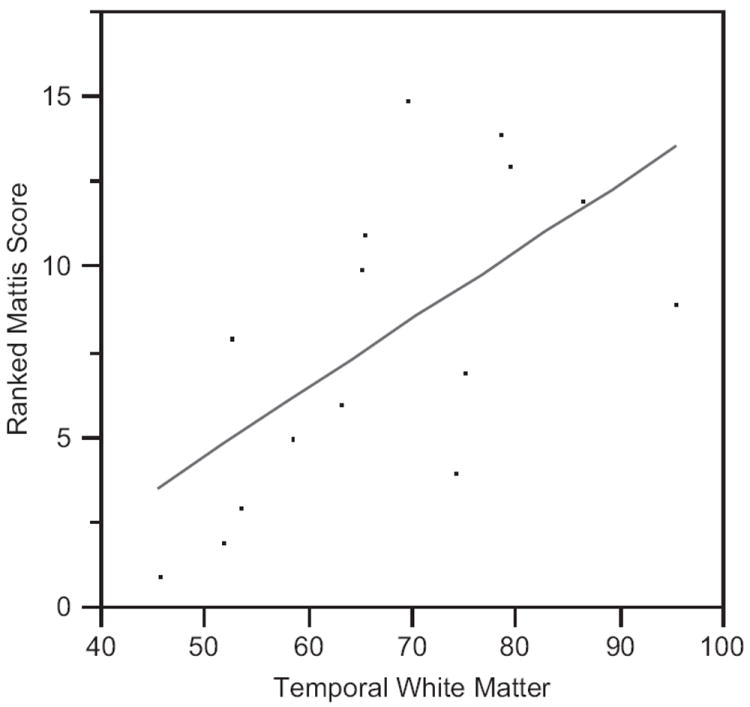
This figure represents the regression analysis demonstrating lower temporal lobe white volume associated with lower Mattis Dementia Rating Scale total scores.
DISCUSSION
As imaging procedures are increasingly utilized in clinical practice, the ability to gain information from magnetic resonance findings may eventually be incorporated into clinical diagnostic criteria for early dementia. This study supports the possibility that over and above the typical reports of “diffuse atrophy” seen in clinical radiology results, more specialized regional analyses may potentially yield important diagnostic information.
One might have expected that temporal lobe measures would have been more specifically related to memory sub-scales within the Mattis DRS, however, we did not find a relationship between individual subscales. This may well have been due to the heterogenous nature of our sample, as the subjects had only mild deficits in a number of domains which might have diffused the specificity of the underlying process. That is, our sample may have included subjects who were at risk for Alzheimer’s dementia, but there may also have been other preclinical pathoetiologies underway that may ultimately manifest as vascular dementia, Lewy body disease or frontotemporal dementia. It is also of note that our sample had a relatively high educational level, with a mean of 15.6 years of education, hence the relationship between structural measures and memory performance observed in this group may not be entirely representative of the population at large.
While the Mattis DRS is only a screening tool, it is precisely the type of measure that could be obtained feasibly in clinical practice, where access and resources may be limited for more comprehensive neuropsychological testing. Studies have suggested that the Mattis DRS may indeed serve just such a function; for example, Paul et al. (21) showed that the total score on the Mattis DRS was significantly related to whole brain volume in a group of patients with vascular dementia. Furthermore, Smith et al. (22) conducted proportional hazards modeling of Mattis scores from 221 newly diagnosed dementia patients and 53 patients with mild cognitive impairment, and found that an initial lower Mattis DRS total score was a significant predictor of longitudinal institutionalization and mortality outcomes.
Yet another limitation to this study is the small sample size of only 15 older adults. Most certainly larger scale studies may help clearly identify the diagnostic value of imaging that includes not only a greater sample size, but also includes the important aspect of longitudinal follow-up to ultimately determine the long term dementia outcomes. Finally, our lack of a completely normal comparison group is another significant limitation to this analysis, as the results reported here could be the same relationships one would observe in normal age-related changes in brain volume. In fact one might argue that some or all of the participants in this sample are essentially experiencing normal aging, given that previous studies have shown that not all patients with mild deficits proceed to overt dementia. However, given that these subjects were enrolled based on cognitive deficits that were outside of age-adjusted normative values in their screening testing, they likely do represent a group that verges toward the impaired side on this continuum of late-life changes. The challenge for the future is to better demarcate this clinical “gray zone” by defining the boundaries between normal aging and true clinical deficits. With these caveats, then, this report offers some evidence for clinical value in exploring changes in temporal lobe white matter as one potential marker that may have a relationship with cognitive deficits in this mildly impaired group. It is important for current studies to begin to combine various methods in estimating the prognosis for different patient scenarios. For example, Devanand et al. (23) have recently reported new findings showing that by combining hippocampal volume changes with cognitive measures, the predictive diagnostic accuracy for Alzheimer’s dementia is maximized.
We are approaching an era where many older adults are acutely aware of their risk for cognitive decline and actively seek information and potential interventions. The future of brain imaging research will very likely focus on specialized functional techniques (e.g., positron emission tomography and functional magnetic resonance imaging) that may have much greater predictive value than structural magnetic resonance imaging (MRI). Yet until functional imaging is readily available clinically to the general population, the ability for the general practitioner to have a more informative interpretation of structural MRI may also be important.
Acknowledgments
This work was supported by the National Institute of Health grants 5R21MH061801-03 (Schultz, PI) and 5R01CA 22934 (Schultz, PI).
Footnotes
Publisher's Disclaimer: Full terms and conditions of use: http://www.informaworld.com/terms-and-conditions-of-access.pdf
This article may be used for research, teaching and private study purposes. Any substantial or systematic reproduction, re-distribution, re-selling, loan or sub-licensing, systematic supply or distribution in any form to anyone is expressly forbidden.
The publisher does not give any warranty express or implied or make any representation that the contents will be complete or accurate or up to date. The accuracy of any instructions, formulae and drug doses should be independently verified with primary sources. The publisher shall not be liable for any loss, actions, claims, proceedings, demand or costs or damages whatsoever or howsoever caused arising directly or indirectly in connection with or arising out of the use of this material.
Contributor Information
SUSAN K. SCHULTZ, University of Iowa Department of Psychiatry, Carver College of Medicine, Iowa City, IA, USA.
VINCENT MAGNOTTA, University of Iowa Department of Radiology, Carver College of Medicine, Iowa City, IA, USA.
KEVIN DUFF, University of Iowa Department of Psychiatry, Carver College of Medicine, Iowa City, IA, USA.
LAURIE L. BOLES PONTO, University of Iowa Department of Radiology, Carver College of Medicine, Iowa City, IA, USA.
DAVID J. MOSER, University of Iowa Department of Psychiatry, Carver College of Medicine, Iowa City, IA, USA.
References
- 1.Rapoport SI. Discriminant analysis of brain imaging data identifies subjects with early Alzheimer’s disease. Int Psychogeriatr. 1997;9(Suppl 1):229–235. doi: 10.1017/s1041610297004936. [DOI] [PubMed] [Google Scholar]
- 2.Visser PJ, Scheltens P, Verhey FR. Medial temporal lobe atrophy and memory dysfunction as predictors for dementia in subjects with mild cognitive impairment. J Neurol. 1999;6:477–485. doi: 10.1007/s004150050387. [DOI] [PubMed] [Google Scholar]
- 3.Tisserand DJ, Visser PJ, van Boxtel MP, Jolles J. The relation between global and limbic brain volumes on MRI and cognitive performance in healthy individuals across the age range. Neurobiol Aging. 2000;4:569–576. doi: 10.1016/s0197-4580(00)00133-0. [DOI] [PubMed] [Google Scholar]
- 4.Jack CR, Petersen RC, Xu YC, O’Brien PC, Smith GE, Ivnik RJ, Boeve BF, Waring SC, Tangalos EG, Kokmen E. Prediction of AD with MRI-based hippocampal volume in mild cognitive impairment. Neurology. 1999;7:1397–1403. doi: 10.1212/wnl.52.7.1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Visser PJ, Verhey FR, Hofman PA, Scheltens P, Jolles J. Medial temporal lobe atrophy predicts Alzheimer’s disease in patients with minor cognitive impairment. J Neurol Neurosurg Psychiatry. 2002;4:491–497. doi: 10.1136/jnnp.72.4.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Smith CD, Chebrolu H, Wekstein DR, Schmitt FA, Jicha GA, Cooper G, Markesbery WR. Brain structural alterations before mild cognitive impairment. Neurology. 2007;16:1268–1273. doi: 10.1212/01.wnl.0000259542.54830.34. [DOI] [PubMed] [Google Scholar]
- 7.Saykin AJ, Wishart HA, Rabin LA, Santulli RB, Flashman LA, West JD, McHugh TL, Mamourian AC. Older adults with cognitive complaints show brain atrophy similar to that of amnestic MCI. Neurology. 2006;5:834–842. doi: 10.1212/01.wnl.0000234032.77541.a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Illes J, Rosen A, Greicius M, Racine E. Prospects for prediction: ethics analysis of neuroimaging in Alzheimer’s disease. Ann N Y Acad Sci. 2007;7:278–295. doi: 10.1196/annals.1379.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Folstein M, Folstein S, McHugh P. Mini-mental State: A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 10.Benedict RHB, Schretlen D, Groninger L, Brandt J. Hopkins Verbal Learning Test - Revised: Normative data and analysis of inter-form and test-retest reliability. Clin Neuropsychol. 1998;12:43–55. [Google Scholar]
- 11.Lucas JA, Ivnik RJ, Smith GE, Bohac DL, Tangalos EG, Kokmen E, Graff-Radford NR, Petersen RC. Normative data for the Mattis Dementia Rating Scale. J Clin Exp Neuropsychol. 1998;20:536–547. doi: 10.1076/jcen.20.4.536.1469. [DOI] [PubMed] [Google Scholar]
- 12.Benedict RHB. Brief Visuospatial Memory Test-Revised. Odessa, FL: Psychological Assessment Resources, Inc; 1997. [Google Scholar]
- 13.Wechsler D. Wechsler Adult Intelligence Scale-III (WAIS-III) San Antonio, Texas: Psychological Corporation; 1997. [Google Scholar]
- 14.Andreasen NC, Rajarethinam R, Cizadlo T. Automatic atlas-based volume estimation of human brain regions from MR images. J Comput Assist Tomogr. 1996;20:98–106. doi: 10.1097/00004728-199601000-00018. [DOI] [PubMed] [Google Scholar]
- 15.Harris G, Andreasen NC, Cizadlo T. Improving tissue classification in MRI: a three-dimensional multispectral discriminant analysis method with automated training class selection. J Comput Assist Tomogr. 1999;23:144–154. doi: 10.1097/00004728-199901000-00030. [DOI] [PubMed] [Google Scholar]
- 16.Magnotta VA, Heckel D, Andreasen NC. Measurement of brain structures with artificial neural networks: two- and three-dimensional applications. Radiology. 1999;211:781–790. doi: 10.1148/radiology.211.3.r99ma07781. [DOI] [PubMed] [Google Scholar]
- 17.Woods RP, Cherry SR, Mazziotta JC. Rapid automated algorithm for aligning and reslicing PET images. J Comput Assist Tomogr. 1992;16:620–633. doi: 10.1097/00004728-199207000-00024. [DOI] [PubMed] [Google Scholar]
- 18.Woods RP, Grafton ST, Holmes CJ, Cherry SR, Mazziotta JC. Automated image registration: I. General methods and intra-subject, intramodality validation. J Comput Assist Tomogr. 1998;22:139–152. doi: 10.1097/00004728-199801000-00027. [DOI] [PubMed] [Google Scholar]
- 19.Boles Ponto LL, Magnotta VA, Moser DJ, Duff KM, Schultz SK. Global cerebral blood flow in relation to cognitive performance and reserve in subjects with mild memory deficits. Mol Imaging Biol. 2006;8:363–372. doi: 10.1007/s11307-006-0066-z. [DOI] [PubMed] [Google Scholar]
- 20.Chen X, Magnotta VA, Duff K, Boles Ponto LL, Schultz SK. Donepezil effects on cerebral blood flow in older adults with mild cognitive deficits. J Neuropsychiatry Clin Neurosci. 2006;18:178–185. doi: 10.1176/jnp.2006.18.2.178. [DOI] [PubMed] [Google Scholar]
- 21.Paul RH, Cohen RA, Moser D, Ott BR, Zawacki T, Gordon N, Bell S, Stone W. Performance on the Mattis Dementia Rating Scale in patients with vascular dementia, relationship to neuroimaging findings. J Geriatr Psychiatry Neurol. 2001;1:33–136. doi: 10.1177/089198870101400108. [DOI] [PubMed] [Google Scholar]
- 22.Smith G, Ivnik RJ, Malec JF, Kokmen E, Tangalos E, Petersen RC. Psychometric properties of the Mattis Dementia Rating Scale. Assessment. 1994;1:123–131. doi: 10.1177/1073191194001002002. [DOI] [PubMed] [Google Scholar]
- 23.Devanand DP, Pradhaban G, Liu X, Khandji A, De Santi S, Segal S, Rusinek H, Pelton GH, Honig LS, Mayeux R, Stern Y, Tabert MH, de Leon MJ. Hippocampal and entorhinal atrophy in mild cognitive impairment: prediction of Alzheimer disease. Neurology. 2007;68:828–836. doi: 10.1212/01.wnl.0000256697.20968.d7. [DOI] [PubMed] [Google Scholar]


