Abstract
Coordination of transport steps between intracellular compartments is important for ensuring unobstructed traffic flow while maintaining compartment size. Small GTPases from the Rab, Arf and Rho families, which regulate individual transport steps, have also emerged as coordinators of these steps. Here, I summarize evidence supporting the existence of GTPase-dependent transport step coordination at three levels: maturation of two cellular sorting compartments, Golgi and endosomes; coupling of vesicular transport sub-steps between donor and acceptor compartments; and integration of transport steps into whole pathways. The mechanisms proposed for GTPase-mediated transport-step coordination depend on the ability of single GTPases to interact with multiple effectors and on interactions of multiple GTPases through common accessory factors.
Introduction
Proteins and membranes shuttle between cellular compartments, and between the cell and its milieu, through the exocytic and endocytic pathways (Figure 1). Each pathway has multiple transport steps. In each transport step, membrane-bound motile carriers, vesicles or tubules, deliver material from a donor to an acceptor compartment in a process termed vesicular transport.
Figure 1. Intracellular trafficking: compartments and pathways.
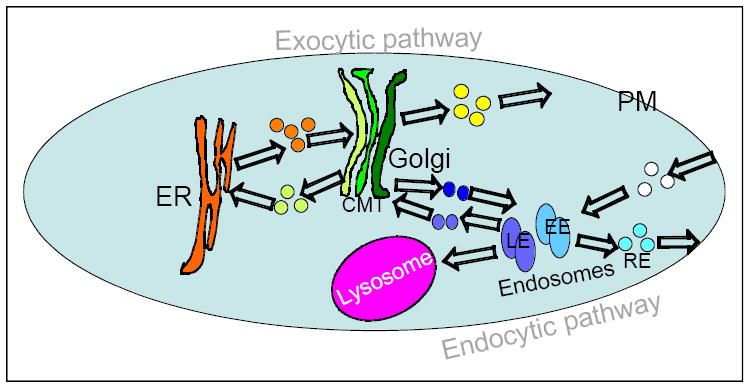
In the exocytic pathway, proteins and membranes are transported from the endoplasmic reticulum (ER), through the Golgi cistrnae – cis, medial and trans (CMT) – to the plasmam membrane (PM). In the endocytic pathway, proteins and membranes are transported from the PM through early and late endosomes (EE and LE, respectively), to the lysosomes. Retrograde trafficking delivers material from the Golgi to the ER and from recycling endosomes (RE) to the PM. Transport between the Golgi and endosomes delivers biosynthetic enzymes to endocytic compartments and returns their receptors back to the Golgi.
Each pathway has a sorting compartment in which cargo slated for delivery to different destinations is sorted into separate vesicles. The sorting compartment of the exocytic pathway is the Golgi apparatus. The Golgi is comprised of three cisternae: cis, medial and trans, which differ in their internal enzyme repertoire. Sorting of cargo occurs at the two extreme Golgi cisternae, cis and trans. At the cis-Golgi, proteins arriving from the endoplasmic reticulum (ER) are sorted for forward delivery towards the trans-Golgi or for retrograde delivery back to the ER. At the trans-Golgi, proteins are sorted for delivery to the plasma membrane (PM) or endocytic compartments, the endosome or lysosome. The sorting compartment of the endocytic pathway is the endosome. Early endosomes (EE) are the entry station for proteins arriving from the PM or the trans-Golgi. From EE, proteins can move forward to late-endosomes (LE) or recycle through recycling endosomes (RE) back to the PM. From LE, proteins are sorted for transport to the lysosome or to the Golgi (Tokarev et al., 2009).
While mobile carriers connect separate compartments, vesicular transport probably does not play a role in cargo transport within the two major sorting compartments of the cell, the Golgi and endosomes. Forward progress of cargo through the Golgi cistrnae and from early to late endosomes happens by cisternal maturation. The mechanism by which maturation of these compartments occurs is still controversial. Golgi cisternal maturation may be driven by delivery of resident enzymes, either via vesicles that recycle material from late to early cisternae or through connecting tubules (Nakano and Luini, 2010). Endosomes may mature by Rab conversion (Rink et al., 2005).
Small GTPases that belong to the Ypt/Rab, Arf and Rho families are key regulators of vesicular transport. These GTPases switch between the GTP-bound “on” state and the GDP-bound “off” state. This switching is catalyzed by upstream regulators: guanine nucleotide exchange factors (GEFs) stimulate the GDP-to-GTP switch, whereas GTPase activating proteins (GAPs) stimulate the GTP-to-GDP switch (Bos et al., 2007). GTPases also cycle between membranes on which they function and the cytoplasm. Members of the Rab and Rho families are extracted from membranes when in the GDP-bound form by the GDP-dissociation inhibitor, GDI (Seabra and Wasmeier, 2004; DerMardirossian and Bokoch, 2005). Recruitment of GDI-bound GTPases from the cytoplasm to specific membranes is probably mediated by specific receptors (Ali and Seabra, 2005). The association of Arf proteins with membranes is also coupled to their nucleotide cycling. When in the GTP-bound form, Arf association with membrane is enhanced, whereas in the GDP-bound form Arf dissociate from membranes without the need for a GDI (Kahn, 2009).
When in the “on” state, all GTPases interact with downstream effectors that mediate the different vesicle transport steps: from vesicle formation and motility, to their targeting and fusion with the acceptor compartment (Segev, 2001). When regulating individual vesicular transport steps, GTPases function as membrane organizers by occupying membrane domains and recruiting their specific multiple effectors to these domains (Pfeffer, 2005).
Coordination of transport steps would ensure unobstructed flow of material between cellular compartments while maintaining their size. For example, entry into a compartment without exit can cause expansion of the compartment, whereas exit without entry can result in its disappearance. An emerging theme in the intracellular trafficking field is that GTPases regulate not only individual transport steps, but also coordinate them. Here I discuss what is known about GTPases regulation of cisternal maturation, coordination of vesicular transport sub-steps and integration of transport steps into whole pathways.
I. Rab Conversion in Compartment Maturation
Current models suggest that cisternae of the two sorting cellular compartments, Golgi and endosomes, change by maturation. Thus, cargo is probably not transported between the cisternae inside vesicles. Rather, cargo stays within a cisterna and the cisterna itself matures. Maturation of the Golgi cisternae is defined by a change in the content inside the cisternae, whereas maturation of endosomes is defined by a change of proteins on the membrane that surrounds the compartment.
The mechanism, by which the content of Golgi cisternae matures from cis, through medial, to trans, is still not clear. It is thought that cisterna-specific luminal enzymes are transported from a later cisterna either by retrograde vesicles or through bridging tubules that can be gated (Nakano and Luini, 2010). The conversion from early-to-late endosome occurs as result of a change in the Rabs attached to the cytoplasmic side of the membrane surrounding the compartment (Rink et al., 2005). Because each Rab recruits its own specific set of effectors, Rab conversion results in a change of the compartment-specific membrane-associated proteins. It is not known if Rab conversion happens during Golgi maturation, or how the luminal change in pH and enzyme content occurs during endosome maturation.
However, it is tempting to speculate that maturation of the two compartments is similar. Thus, Rab conversion occurs on the Golgi membrane during its maturation similarly to how it occurs during endosomes maturation, and content change inside endosomes occurs in a way similar to the content change that occurs during Golgi maturation. The fact that in yeast different Ypt/Rabs, Ypt1 and Ypt31/32, reside on the cis and trans Golgi, respectively (Jedd et al., 1995; Jedd et al., 1997), and conversion of Ypt1 compartments over time into Ypt32 compartments (Rivera-Molina and Novick, 2009), support the idea that Ypt/Rab conversion may occur during Golgi maturation. Moreover, it is possible that Rab conversion on the membrane surrounding the compartment drives the content change inside the compartment by allowing vesicles or tubules from a later compartment to fuse or bridge with an earlier compartment. In this model, Rab exchange drives maturation of both Golgi and endosome cisternae.
There are two requirements for driving compartment maturation by GTPase exchange. First, the early GTPase should bring the later GTPase to the compartment. Second, the later GTPase should cause the early GTPase to leave the compartment, like a “cut-out fuse” that serves to break a portion of an electric circuit. For the early GTPase to leave a compartment, the later GTPase should turn it off, because GTPases can leave membranes only when they are in the GDP-bound form (Del Conte-Zerial et al., 2008). Evidence supporting each of these requirements exists: a mechanism for Rab conversion was proposed for endosome maturation, and a mechanism of a “cut-out switch” was suggested for Golgi maturation (Figure 2).
Figure 2. Rab-dependent cisternal maturation.
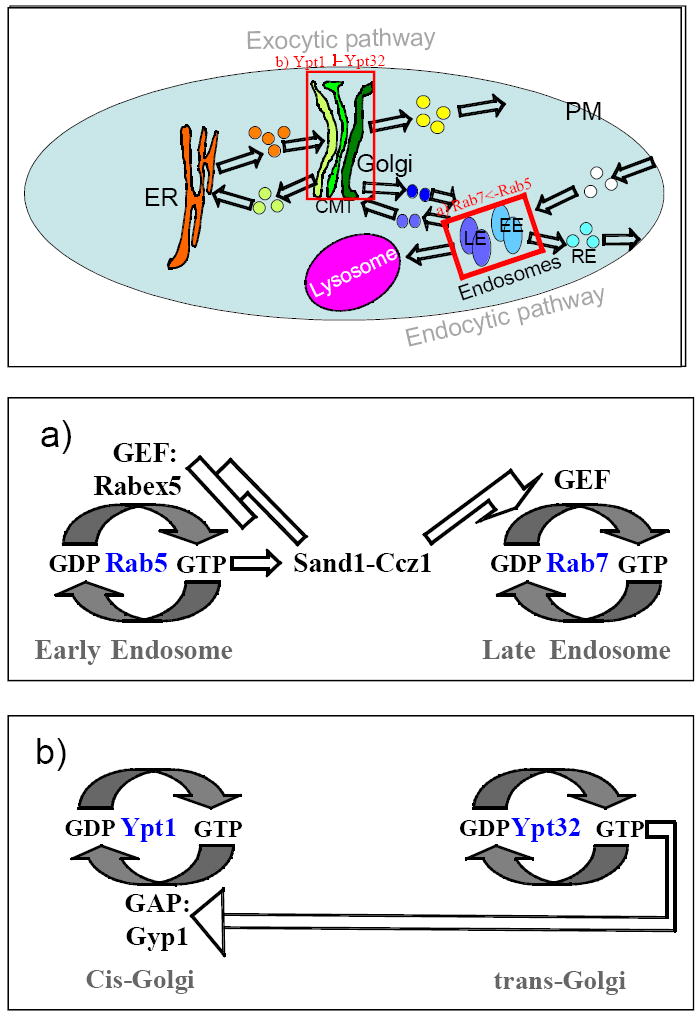
Models that depend on Ypt/Rab GTPases were proposed for the maturation of the two sorting compartments of the cell, endosome and Golgi. a) Endosome maturation was observed as Rab5-to-Rab7 conversion, which is dependent on recruitment of Rab5 effectors that act as positive switches for Rab7 recruitment. b) A model was proposed for keeping only one Ypt active at the trans-Golgi. In this model, Ypt32, which functions at the trans-Golgi, recruits a GAP that serves as a negative switch for the cis-Golgi Ypt, Ypt1. The consequence of this recruitment is that Ypt32 would be the only Ypt active in the late Golgi (see text for details). Arrows depict recruitment or activation; flat arrows represent displacement or inhibition.
Ia. Rab conversion in endosome maturation
Rab5-to-Rab7 conversion. Rab5 is the EE membrane organizer whereas Rab7 is a LE membrane organizer. Exchange of Rab5 to Rab7 on cargo-filled carriers was documented using live-cell imaging (Rink et al., 2005).
There are three suggested mechanisms for EE-to-LE conversion by Rab exchange, which are not mutually exclusive. The first mechanism suggests that Rab5 interaction with a GEF for Rab7, the HOPS complex, is required for Rab5-to-Rab7 exchange. This idea is based on the findings that Rab5 in its GTP-bound form interacts with HOPS, and that HOPS function is required for Rab7 nucleotide exchange (Rink et al., 2005). However, recently the role of mVps39 and HOPS as a Rab7 GEF has been disputed, leaving this mechanism questionable (Peralta et al., 2010). Another suggested mechanism entails that Sand1/Mon1 serves as a switch for the Rab exchange (Kinchen and Ravichandran, 2010; Poteryaev et al., 2010). Sand1/Mon1 is a Rab5 effector. When in a complex with another protein, Ccz1, it interacts with Rab7 and can positively affect its activation (Kinchen and Ravichandran, 2010). A third mechanism suggests that recruitment of Sand1/Mon1 is correlated with displacement of the Rab5 GEF, Rabex5, from membranes, thus ensuring that Rab5 is not re-activated on these membranes (Poteryaev et al., 2010). A mechanism for Rab5 inactivation and extraction from the EE membrane has not been elucidated yet (Cabrera and Ungermann, 2010).
Ib. Ypt/Rab “cut-out fuse” in Golgi maturation
Ypt32-GAP-Ypt1 cascade. GTPase inactivation requires a switch from GTP-bound form to the GDP-bound form. This occurs by stimulation of GTP hydrolysis by a GAP. When in the GDP-bound form, the GTPase cannot interact with its effectors, and can be extracted from the membrane by GDI. Therefore, one way for a later GTPase to replace an earlier GTPase on a compartment is to recruit a GAP for the earlier GTPase.
Recruitment of a GAP for an earlier GTPase by a later GTPase has been observed in the yeast Golgi. In yeast, Ypt1 is a resident of the cis Golgi, whereas Ypt31/32 localize to the trans Golgi (Segev et al., 1988; Jedd et al., 1995; Jedd et al., 1997). Two observations suggest a Rab GAP cascade in the Golgi. First, Gyp1, a GAP of Ypt1, can act as an effector of Ypt32 and depends on Ypt32 for its cellular localization. In addition, whereas in wild-type cells Ypt1 compartments convert to Ypt32 compartments, in cells deleted for Gyp1 there is an increase in the number of compartments that contain both Ypts. Based on these findings, it was suggested that Ypt32 recruits Gyp1 to ensure that Ypt1 is in the GDP-bound “off” state at the trans-Golgi where Ypt32 acts (Rivera-Molina and Novick, 2009). In this model, the Ypt1 in its GDP-bound form would be extracted from the trans-Golgi membrane by GDI (Nottingham and Pfeffer, 2009). While attractive, this model needs further investigation especially because of the following two reasons: First, in cells deleted for Gyp1, not only the Ypts, but also other Golgi proteins, change their localization, raising a doubt about the nature of this abberant compartment. Second, the effect of Ypt32 on the GTP-to-GDP switch of Ypt1 is inferred from lower co-localization of the Ypts in Gyp1-depleted cells, not from direct evidence.
In summary, the phenomenon of Rab switching was shown for endosome maturation, and interactions of Rabs with proteins that can act as positive and negative switches support the existence of a Rab-dependent coordination mechanism in both Golgi and endosome maturation. However, more investigation is needed to identify the physiological consequences of these interactions.
II. Coordination of Vesicular Transport Sub-Steps by GTPases
There are at least seven transport steps mediated by vesicular transport in eukaryotic cells (Figure 1). Coordination of vesicular transport sub-steps would ensure that vesicles formed at a specific compartment and loaded with a specific cargo, would be targeted and fuse with the right compartment. Coordination of vesicular transport sub-steps by GTPases was first suggested to exist in yeast based on genetic evidence. Here, over-expression analysis suggested a Ypt-Arf GEF cascade in the regulation of the exocytic pathway (Jones et al., 1999). Since then, multiple examples of such coordination by GTPases have been proposed (Figure 3). The mechanisms proposed for coordination of vesicular transport sub-steps rely on two basic principles: First, the ability of a single GTPase to interact with multiple effectors; and second, interaction between different GTPases via common accessory proteins.
Figure 3. GTPase-dependent coordination of vesicular transport sub-steps.
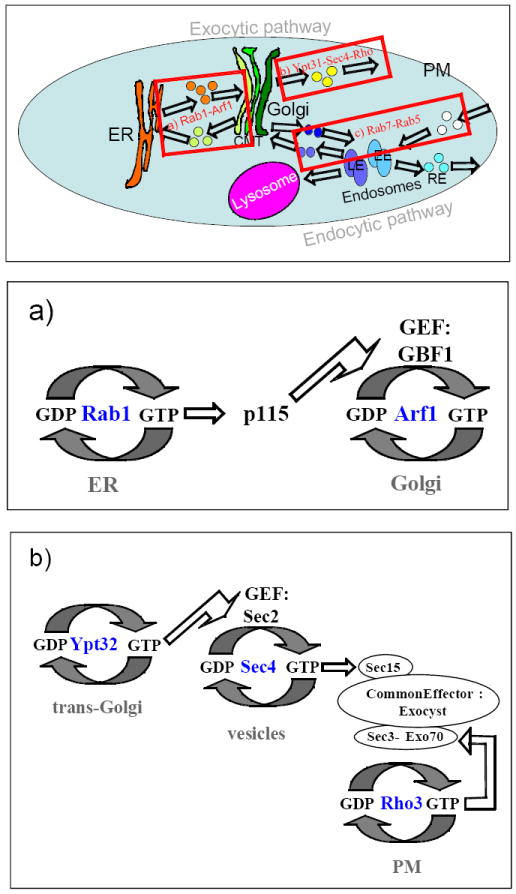
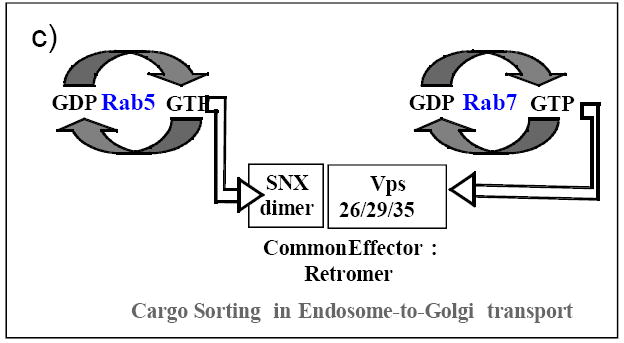
Coordination of individual vesicular transport sub-steps of a transport step by GTPases and their accessory proteins has been proposed: a) Formation of ER-vesicles is coupled to their targeting to the cis-Golgi by interaction of Ypt/Rabs with Arf GEFs; cascades of interactions of Rab1-p115-GBF1-Arf1 and Rab1-GM130/p115-syntaxin regulate ER vesicle targeting and fusion with the cis-Golgi. b) Trans-Golgi vesicle formation, tethering and fusion are coordinated by a cascade of GTPase interactions: Ypt31/32-Myo2/Sec2-Sec4-exocyst-Rho-SNAREs. c) Successive interactions of Rab5 with PI regulators and FYVE-domain proteins regulates entry into the EE, and Rab5-retromer-Rab7 interaction mediates sorting cargo for LE-to-Golgi transport (see text for details).
IIa. Recruitment of sequentially acting effectors by a single GTPase
The ability of a single GTPase to recruit multiple effectors could serve to coordinate vesicular transport steps if the recruitment is done sequentially. Examples suggesting that such coordination exists are described below.
Rab5 in its GTP-bound form can bind multiple effectors and many of them were shown to have a role in early endosome fusion (Christoforidis et al., 1999a). A number of these effectors regulate the phosphoinositide (PI) content in the endosomal membrane. Recruitment of other Rab5 effectors that contain a FYVE domain is, in turn, dependent on the PI content of endosomal membranes (Christoforidis et al., 1999b; Nielsen et al., 2000; Shin et al., 2005). Thus, by recruiting multiple effectors, Rab5 coordinates membrane composition, with endosome tethering and fusion. Moreover, sequential recruitment of Rab5 effecotrs is achieved through the regulation of membrane content.
Effectors of Rab1, which regulates ER-to-Golgi transport, provide another example of possible sequential effector activity. Two Rab1 effectors, p115 and GM130, act as membrane tethers, which mediate vesicle docking (Short et al., 2005). Recently it was shown that GM130 interacts with syntaxin 5, a t-SNARE required for vesicle fusion, and this interaction is inhibited by p115. Thus, the interaction of Rab1 with two effectors, P115 and GM130, was suggested to couple vesicle tethering and fusion (Diao et al., 2008).
Another example involves Rab6, a GTPase that regulates forward and retrograde transport in the Golgi. Recently, interaction of Rab6 with two molecular motors was suggested to drive vesicle biogenesis. Thus, successive interactions of Rab6 with kinesin-1, a microtubular motor and myosin II, an actin motor, were suggested to drive membrane elongation and vesicle fission, respectively (Miserey-Lenkei et al., 2010). The mechanism by which the myosin II motor mediates vesicle fission is still unknown and it is probably not the only fission factor that functions in the Golgi (Valente et al., 2010).
In yeast, the functional pair of Ypt31 and Ypt32 GTPases acts at the trans-Golgi (Jedd et al., 1997). Interaction of Ypt31/32 with at least two effectors has been shown. One Ypt31/32 effector is Myo2, a myosin V motor, which is required for trans-Golgi vesicle motility (Lipatova et al., 2008). Another Ypt32 effector is Sec2, the GEF for Sec4 (Ortiz et al., 2002). Thus, by interacting with different effectors Ypt31/32 can coordinate Myo2-dependent motility with Sec4-dependent targeting of trans-Golgi vesicles.
In summary, a number of examples suggest that sequential recruitment of multiple effectors by a single Ypt/Rab coordinate vesicular transport sub-steps. For most examples it is still unclear how interactions of a single Rab with its multiple effectors are regulated so that they occur in the order required for their sequential function. In the Rab5 example, the effectors themselves determine the sequential order of their interactions. Thus, recruitment of FYVE-domain proteins requires a change in PI membrane composition, which is dependent on earlier interactions of Rab5 with effetors that chage the PI content of the endosome membrane.
IIb. Interaction of different GTPases via common accessory proteins
Interaction of different GTPases with a common protein was proposed to serve in vesicular transport sub-step coordination.
One suggested coordination mechanism entails an effector of one GTPase acting as a GEF for another. If these GTPases regulate different vesicle transport sub-steps, the interactions could coordinate the sub-steps. A cascade of Ypt-Arf GEF interactions was suggested to coordinate the steps of the exocytic pathway in yeast. In this cascade, three Arf-GEFs that act in the Golgi, Gea2, Sec7 and Syt1, alternate with the Golgi Ypts, Ypt1 and Ypt31/32 as follows: Gea2-Ypt1-Sec7-Ypt31/32-Syt1. This suggestion is based on genetic analyses in which over-expression of each gene suppresses phenotypes of mutations in an upstream gene, and enhances phenotypes of mutations in a downstream gene. The order of the genetic interactions is in agreement with the localization of the proteins (Jones et al., 1999). However, the molecular mechanisms underlying this cascade are not known. One specific Rab-Arf GEF module was suggested to coordinate ER-to-Golgi transport in mammalian cells. Here, an effector of Rab1, p115, interacts with GBF1, a GEF for Arf (Garcia-Mata and Sztul, 2003). In this example Rab1, through its effector p115, recruits an Arf-GEF to coordinate sequential function of Rab1 and Arf. An example of an effector/GEF was also described in the endocytic pathway. Here, Rab22 in its GTP-bound form recruits its effector Rabex-5 to early endosomes, where is acts as a GEF for Rab5 (Zhu et al., 2009).
Another coordination mechanism is when different GTPases interact with a common effector. For example: The retromer complex mediates sorting of cargo for endosome-to-Golgi transport. Here, two Rabs, Rab5 and Rab7, are required for the recruitment of the two sub-complexes of retromer to endosomes, SNX and VPS, respectively (Rojas et al., 2008). In the trans-Golgi, several proteins that contain a GRIP domain can bind multiple Rabs and the Arf-like protein Arl1. These proteins might serve as tethers that ensure capturing of GTPase-decorated membrane carriers and/or stacking of GTPase-bearing Golgi cisternae (Sinka et al., 2008; Hayes et al., 2009)
Interactions of Arf and Rab GTPases through both a GEF and a common effector were proposed to regulate formation of cilia. First, coordination of cargo packaging and vesicle formation by two GTPases that bind a common effector complex was suggested. Specifically, a cargo-sorting motif binds Arf4, which is found in a complex with Rab11 and their effortors, ASAP1 and FIP3, respectively (Mazelova et al., 2009). Second, coordination of transport from the TGN, through recycling endosomes (RE), to the PM was also suggested. In this case, Rab11-GTP at the TGN/RE binds Rabin8 as an effcector and activates it. Rabin8 acts as a GEF for Rab8, which regulates targeting and fusion of RE vesicles with PM (Knodler et al., 2010). Thus, formation, targeting and fusion of vesicles that deliver cargo to primary cilium involve coordination of Rab and Arf function.
In yeast, a cascade of interactions that involves Ypt/Rab and Rho GTPases is suggested to coordinate trans-Golgi vesicle targeting, tethering and fusion. Ypt31/32 mediate trans-Golgi vesicle formation and targeting (Jedd et al., 1997; Lipatova et al., 2008). The interaction of Ypt32 with Sec2, the GEF for Sec4 was suggested to recruit Sec2 to these vesicles, which in turn might recruit Sec4 to trans-Golgi vesicles (Ortiz et al., 2002). Activated Sec4 recruits the eight-subunit tethering complex, exocyst, by interaction with the Sec15 subunit (He and Guo, 2009). Two other exocyst subunits, Sec3 and Exo70, interact with Rho GTPases, Rho3 and Cdc42 (Zhang et al., 2008; Wu et al., 2010), which were suggested to regulate vesicle fusion (Wu et al., 2008). Thus, this is an example where multiple GTPases interact through a GEF and a common effector complex to coordinate all the sub-steps of one vesicular transport step, trans-Golgi to the PM.
III. Transport Steps Integration by Rab GTPases
Individual transport steps must be coordinated to ensure unobstructed transport flow through a pathway and maintenance of compartment size (Figure 4). Based on their interactions, Rab GTPases were proposed to integrate individual transport steps into whole pathways.
Figure 4. Rab-dependent transport-step integration.
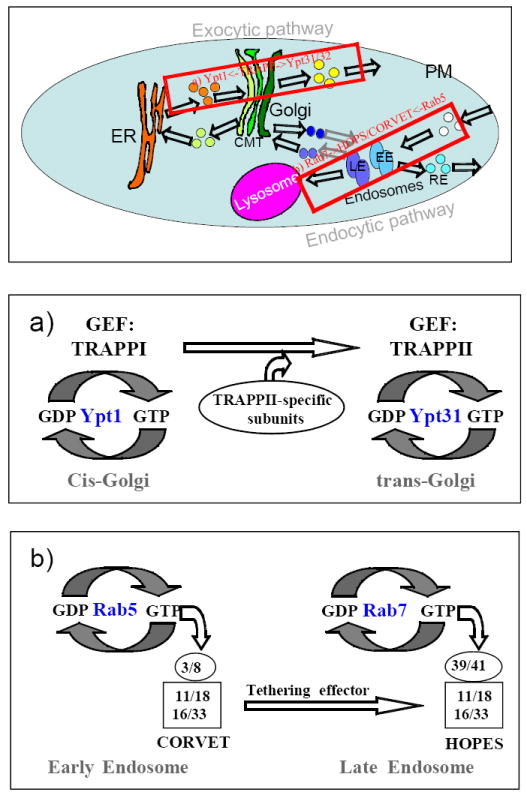
Integration of individual transport steps into a whole pathway has been proposed: a) Ypt1-dependent entry into the Golgi and Ypt31/32-dependent exit from the Golgi are coordinated by sequential activation by their modular GEF complex TRAPP. b) Rab5-dependent entry into EE and Rab7-dependent LE-to-Golgi transport are coordinated by the two Vps-C tethering effectors CORVET and HOPS.
The Ypt/Rab GTPases Ypt1 and Ypt31/32 regulate the two main steps of the yeast exocytic pathway, ER-to-Golgi and Golgi-to-PM, respectively (Segev et al., 1988; Jedd et al., 1997). The modular complex TRAPP acts a GEF for these three Ypts (Jones et al., 2000). TRAPP can form at least two complexes, TRAPP I, which is comprised of five essential subunits, and TRAPP II, which includes all the TRAPP I subunits plus two additional essential subunits (Sacher et al., 2008). TRAPP I localizes to the cis-Golgi and acts as a GEF for Ypt1 whereas TRAPP II localizes to the trans-Golgi and acts as a GEF for Ypt31/32. Based on these findings, the switch of the GEF complex TRAPP, from TRAPP I to TRAPP II, was proposed to coordinate Ypt1-mediated Golgi entry with Ypt31/32-mediated Golgi exit, thus integrating these two steps into one pathway (Morozova et al., 2006).
In yeast, entry into EE is regulated by the Rab5 ortholog Vps21, whereas LE-to-lysosome transport is regulated by the Rab7 ortholog Ypt7. Two Vps-C tethering complexes that share core subunits and organization serve as effectors for the two Rabs. The two complexes share four core subunits, Vps11, Vps16, Vps18 and Vps33, the latter being a Sec1-like protein that interacts with SNAREs. The other end of the core complex, Vsp11 and Vps18, interact with the Rab-binding subunits: Vps3-Vps8 in CORVET, and Vps39-Vps41 in HOPS (Ostrowicz et al., 2010). In EE, the CORVET complex acts as an effector of Vps21, while in LE the HOPS complex acts as an effector of Ypt7. Thus, a Rab-dependent dynamic subunit exchange of the core effector complex from CORVET to HOPS might integrate the two major steps of the endocytic pathway (Nickerson et al., 2009).
In summary, existing evidence suggest a Rab-dependent transport-step integration in both the exocytic and the endocytic pathway.
Conclusions and Perspectives
The idea that coordination of intra-cellular transport steps must exist and that GTPases and their accessory factors play a role in such coordination is very attractive. This past decade has seen the emergence of examples for GTPase roles in transport step coordination at three different levels: maturation of a compartment, multiple vesicular transport sub-steps of a transport step, and multiple transport steps of a pathway. Rab-driven cisternal maturation mechanisms have been proposed for explaining how an early Rab GTPase, Rab5, brings the next Rab, Rab7, in endosome maturation, and how a later Ypt GTPase, Ypt32, turns off an earlier Ypt, Ypt1, in Golgi maturation. Multiple examples exist for coordination of vesicular transport sub-steps by GTPases, and a role for GTPases in integration of transport steps into whole pathways has been proposed in both the exocytic and endocytic pathways.
The field now faces two major challenges. First, proving the physiological relevance of transport-step coordination by GTPases requires disrupting coordination without affecting individual transport steps or sub-steps. Because all the proposed players are required for mediation of individual transport steps and sub-steps, it is difficult to disrupt coordination without disrupting individual transport steps. Another challenge is to clarify the mechanism that determines the order of interactions, allowing completion of one step before the next interaction occurs. For example, when Rab5 switches with Rab7 to drive early-to-late endosome maturation, we do not know what mechanism ensures that all the Rab5 interactions required for early-endosome function occur before Rab7 is recruited.
Transport step coordination reflects fine-tuning of intra-cellular trafficking. Such fine-tuning should be important for efficient functioning of all cells, and defects in such adjustments are expected to result in disease. Further insight into GTPase-dependent coordination of intra-cellular trafficking steps is likely in the future, helping to unravel the underlying molecular mechanisms involved.
Acknowledgments
I thank A. Shah for critical reading and suggestions and acknowledge support from the National Institutes of Health GM45-444.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ali BR, Seabra MC. Targeting of Rab GTPases to cellular membranes. Biochemical Society transactions. 2005;33:652–656. doi: 10.1042/BST0330652. [DOI] [PubMed] [Google Scholar]
- Bos JL, Rehmann H, Wittinghofer A. GEFs and GAPs: critical elements in the control of small G proteins. Cell. 2007;129:865–877. doi: 10.1016/j.cell.2007.05.018. [DOI] [PubMed] [Google Scholar]
- Cabrera M, Ungermann C. Guiding endosomal maturation. Cell. 2010;141:404–406. doi: 10.1016/j.cell.2010.04.013. [DOI] [PubMed] [Google Scholar]
- Christoforidis S, McBride HM, Burgoyne RD, Zerial M. The Rab5 effector EEA1 is a core component of endosome docking. Nature. 1999a;397:621–625. doi: 10.1038/17618. [DOI] [PubMed] [Google Scholar]
- Christoforidis S, Miaczynska M, Ashman K, Wilm M, Zhao L, Yip SC, Waterfield MD, Backer JM, Zerial M. Phosphatidylinositol-3-OH kinases are Rab5 effectors. Nature cell biology. 1999b;1:249–252. doi: 10.1038/12075. [DOI] [PubMed] [Google Scholar]
- Del Conte-Zerial P, Brusch L, Rink JC, Collinet C, Kalaidzidis Y, Zerial M, Deutsch A. Membrane identity and GTPase cascades regulated by toggle and cut-out switches. Molecular systems biology. 2008;4:206. doi: 10.1038/msb.2008.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DerMardirossian C, Bokoch GM. GDIs: central regulatory molecules in Rho GTPase activation. Trends in cell biology. 2005;15:356–363. doi: 10.1016/j.tcb.2005.05.001. [DOI] [PubMed] [Google Scholar]
- Diao A, Frost L, Morohashi Y, Lowe M. Coordination of golgin tethering and SNARE assembly: GM130 binds syntaxin 5 in a p115-regulated manner. The Journal of biological chemistry. 2008;283:6957–6967. doi: 10.1074/jbc.M708401200. [DOI] [PubMed] [Google Scholar]
- Garcia-Mata R, Sztul E. The membrane-tethering protein p115 interacts with GBF1, an ARF guanine-nucleotide-exchange factor. EMBO reports. 2003;4:320–325. doi: 10.1038/sj.embor.embor762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes GL, Brown FC, Haas AK, Nottingham RM, Barr FA, Pfeffer SR. Multiple Rab GTPase binding sites in GCC185 suggest a model for vesicle tethering at the trans-Golgi. Molecular biology of the cell. 2009;20:209–217. doi: 10.1091/mbc.E08-07-0740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He B, Guo W. The exocyst complex in polarized exocytosis. Current opinion in cell biology. 2009;21:537–542. doi: 10.1016/j.ceb.2009.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jedd G, Mulholland J, Segev N. Two new Ypt GTPases are required for exit from the yeast trans-Golgi compartment. The Journal of cell biology. 1997;137:563–580. doi: 10.1083/jcb.137.3.563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jedd G, Richardson C, Litt R, Segev N. The Ypt1 GTPase is essential for the first two steps of the yeast secretory pathway. The Journal of cell biology. 1995;131:583–590. doi: 10.1083/jcb.131.3.583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones S, Jedd G, Kahn RA, Franzusoff A, Bartolini F, Segev N. Genetic interactions in yeast between Ypt GTPases and Arf guanine nucleotide exchangers. Genetics. 1999;152:1543–1556. doi: 10.1093/genetics/152.4.1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones S, Newman C, Liu F, Segev N. The TRAPP complex is a nucleotide exchanger for Ypt1 and Ypt31/32. Molecular biology of the cell. 2000;11:4403–4411. doi: 10.1091/mbc.11.12.4403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahn RA. Toward a model for Arf GTPases as regulators of traffic at the Golgi. FEBS letters. 2009;583:3872–3879. doi: 10.1016/j.febslet.2009.10.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinchen JM, Ravichandran KS. Identification of two evolutionarily conserved genes regulating processing of engulfed apoptotic cells. Nature. 2010;464:778–782. doi: 10.1038/nature08853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knodler A, Feng S, Zhang J, Zhang X, Das A, Peranen J, Guo W. Coordination of Rab8 and Rab11 in primary ciliogenesis. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:6346–6351. doi: 10.1073/pnas.1002401107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipatova Z, Tokarev AA, Jin Y, Mulholland J, Weisman LS, Segev N. Direct interaction between a myosin V motor and the Rab GTPases Ypt31/32 is required for polarized secretion. Molecular biology of the cell. 2008;19:4177–4187. doi: 10.1091/mbc.E08-02-0220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazelova J, Astuto-Gribble L, Inoue H, Tam BM, Schonteich E, Prekeris R, Moritz OL, Randazzo PA, Deretic D. Ciliary targeting motif VxPx directs assembly of a trafficking module through Arf4. The EMBO journal. 2009;28:183–192. doi: 10.1038/emboj.2008.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miserey-Lenkei S, Chalancon G, Bardin S, Formstecher E, Goud B, Echard A. Rab and actomyosin-dependent fission of transport vesicles at the Golgi complex. Nature cell biology. 2010;12:645–654. doi: 10.1038/ncb2067. [DOI] [PubMed] [Google Scholar]
- Morozova N, Liang Y, Tokarev AA, Chen SH, Cox R, Andrejic J, Lipatova Z, Sciorra VA, Emr SD, Segev N. TRAPPII subunits are required for the specificity switch of a Ypt-Rab GEF. Nature cell biology. 2006;8:1263–1269. doi: 10.1038/ncb1489. [DOI] [PubMed] [Google Scholar]
- Nakano A, Luini A. Passage through the Golgi. Current opinion in cell biology. 2010;22:471–478. doi: 10.1016/j.ceb.2010.05.003. [DOI] [PubMed] [Google Scholar]
- Nickerson DP, Brett CL, Merz AJ. Vps-C complexes: gatekeepers of endolysosomal traffic. Current opinion in cell biology. 2009;21:543–551. doi: 10.1016/j.ceb.2009.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen E, Christoforidis S, Uttenweiler-Joseph S, Miaczynska M, Dewitte F, Wilm M, Hoflack B, Zerial M. Rabenosyn-5, a novel Rab5 effector, is complexed with hVPS45 and recruited to endosomes through a FYVE finger domain. The Journal of cell biology. 2000;151:601–612. doi: 10.1083/jcb.151.3.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nottingham RM, Pfeffer SR. Defining the boundaries: Rab GEFs and GAPs. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:14185–14186. doi: 10.1073/pnas.0907725106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortiz D, Medkova M, Walch-Solimena C, Novick P. Ypt32 recruits the Sec4p guanine nucleotide exchange factor, Sec2p, to secretory vesicles; evidence for a Rab cascade in yeast. The Journal of cell biology. 2002;157:1005–1015. doi: 10.1083/jcb.200201003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostrowicz CW, Brocker C, Ahnert F, Nordmann M, Lachmann J, Peplowska K, Perz A, Auffarth K, Engelbrecht-Vandre S, Ungermann C. Defined Subunit Arrangement and Rab Interactions Are Required for Functionality of the HOPS Tethering Complex. Traffic (Copenhagen, Denmark) 2010 doi: 10.1111/j.1600-0854.2010.01097.x. [DOI] [PubMed] [Google Scholar]
- Peralta ER, Martin BC, Edinger AL. Differential effects of TBC1D15 and mammalian Vps39 on Rab7 activation state, lysosomal morphology, and growth factor dependence. The Journal of biological chemistry. 2010;285:16814–16821. doi: 10.1074/jbc.M110.111633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeffer S. A model for Rab GTPase localization. Biochemical Society transactions. 2005;33:627–630. doi: 10.1042/BST0330627. [DOI] [PubMed] [Google Scholar]
- Poteryaev D, Datta S, Ackema K, Zerial M, Spang A. Identification of the switch in early-to-late endosome transition. Cell. 2010;141:497–508. doi: 10.1016/j.cell.2010.03.011. [DOI] [PubMed] [Google Scholar]
- Rink J, Ghigo E, Kalaidzidis Y, Zerial M. Rab conversion as a mechanism of progression from early to late endosomes. Cell. 2005;122:735–749. doi: 10.1016/j.cell.2005.06.043. [DOI] [PubMed] [Google Scholar]
- Rivera-Molina FE, Novick PJ. A Rab GAP cascade defines the boundary between two Rab GTPases on the secretory pathway. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:14408–14413. doi: 10.1073/pnas.0906536106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rojas R, van Vlijmen T, Mardones GA, Prabhu Y, Rojas AL, Mohammed S, Heck AJ, Raposo G, van der Sluijs P, Bonifacino JS. Regulation of retromer recruitment to endosomes by sequential action of Rab5 and Rab7. The Journal of cell biology. 2008;183:513–526. doi: 10.1083/jcb.200804048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacher M, Kim YG, Lavie A, Oh BH, Segev N. The TRAPP complex: insights into its architecture and function. Traffic (Copenhagen, Denmark) 2008;9:2032–2042. doi: 10.1111/j.1600-0854.2008.00833.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seabra MC, Wasmeier C. Controlling the location and activation of Rab GTPases. Current opinion in cell biology. 2004;16:451–457. doi: 10.1016/j.ceb.2004.06.014. [DOI] [PubMed] [Google Scholar]
- Segev N. Ypt and Rab GTPases: insight into functions through novel interactions. Current opinion in cell biology. 2001;13:500–511. doi: 10.1016/s0955-0674(00)00242-8. [DOI] [PubMed] [Google Scholar]
- Segev N, Mulholland J, Botstein D. The yeast GTP-binding YPT1 protein and a mammalian counterpart are associated with the secretion machinery. Cell. 1988;52:915–924. doi: 10.1016/0092-8674(88)90433-3. [DOI] [PubMed] [Google Scholar]
- Shin HW, Hayashi M, Christoforidis S, Lacas-Gervais S, Hoepfner S, Wenk MR, Modregger J, Uttenweiler-Joseph S, Wilm M, Nystuen A, Frankel WN, Solimena M, De Camilli P, Zerial M. An enzymatic cascade of Rab5 effectors regulates phosphoinositide turnover in the endocytic pathway. The Journal of cell biology. 2005;170:607–618. doi: 10.1083/jcb.200505128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Short B, Haas A, Barr FA. Golgins and GTPases, giving identity and structure to the Golgi apparatus. Biochimica et biophysica acta. 2005;1744:383–395. doi: 10.1016/j.bbamcr.2005.02.001. [DOI] [PubMed] [Google Scholar]
- Sinka R, Gillingham AK, Kondylis V, Munro S. Golgi coiled-coil proteins contain multiple binding sites for Rab family G proteins. The Journal of cell biology. 2008;183:607–615. doi: 10.1083/jcb.200808018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokarev AA, Alfonso A, Segev N. Overview of Intracellular Compartments and Trafficking Pathways. In: Segev N, editor. Trafficking Inside Cells: Pathways, Mechanisms and Regulation. Landes Bioscience and Springer Science+Business Media; 2009. [Google Scholar]
- Valente C, Polishchuk R, De Matteis MA. Rab6 and myosin II at the cutting edge of membrane fission. Nature cell biology. 2010;12:635–638. doi: 10.1038/ncb0710-635. [DOI] [PubMed] [Google Scholar]
- Wu H, Rossi G, Brennwald P. The ghost in the machine: small GTPases as spatial regulators of exocytosis. Trends in cell biology. 2008;18:397–404. doi: 10.1016/j.tcb.2008.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu H, Turner C, Gardner J, Temple B, Brennwald P. The Exo70 subunit of the exocyst is an effector for both Cdc42 and Rho3 function in polarized exocytosis. Molecular biology of the cell. 2010;21:430–442. doi: 10.1091/mbc.E09-06-0501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Orlando K, He B, Xi F, Zhang J, Zajac A, Guo W. Membrane association and functional regulation of Sec3 by phospholipids and Cdc42. The Journal of cell biology. 2008;180:145–158. doi: 10.1083/jcb.200704128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu H, Liang Z, Li G. Rabex-5 is a Rab22 effector and mediates a Rab22-Rab5 signaling cascade in endocytosis. Molecular biology of the cell. 2009;20:4720–4729. doi: 10.1091/mbc.E09-06-0453. [DOI] [PMC free article] [PubMed] [Google Scholar]


