Abstract
Objective & Background
Inflammation is a pivotal process in the progression of atherosclerosis, which can be non-invasively imaged by 18F-fluorodeoxyglucose positron emission tomography (FDG-PET). In this study, the impact of non-insulin dependent type-2 diabetes on carotid wall FDG uptake in patients with documented or suspected cardiovascular disease was evaluated.
Methods
Carotid artery wall FDG uptake was quantified in 134 patients (age 60.2±9.7 years; diabetic subjects: n=43). The pre-scan glucose (gluc) level corrected mean of the maximum standardized uptake value (SUV) values (meanSUVgluc), mean of the maximum target-to-background ratio (meanTBRgluc), and Single Hottest Segment (SHSgluc) of FDG uptake in the artery wall were calculated. Associations between FDG uptake, the presence of risk factors for atherosclerosis, and diabetes were then assessed by multiple regression analysis with backward elimination.
Results
We demonstrated a significant association between diabetes and FDG uptake in the arterial wall (diabetes: meanSUVgluc; β=0.324, meanTBRgluc; β=0.317, and SHSgluc; β=0.298; for all: p<0.0001, respectively). In addition, in diabetic patients, both body mass index (BMI) ≥30 kg/m2 (BMI ≥30 kg/m2: meanSUVgluc; β=0.4, meanTBRgluc; β=0.357, and SHSgluc; β=0.388; for all: p<0.015) and smoking (smoking: meanTBRgluc; β=0.312, SHSgluc; β=0.324; for all: p<0.04) were significantly associated with FDG uptake.
Conclusions
Type-2 diabetes was significantly associated with carotid wall FDG uptake in patients with known or suspected cardiovascular disease. In diabetic patients, obesity and smoking add to the risk of increased FDG uptake values. Furthermore, the degree of carotid wall FDG uptake increases with increments of fasting glucose levels in diabetic patients.
Keywords: FDG-PET, Inflammation, Atherosclerosis, Diabetes, Carotid Arteries
Introduction
Cardiovascular disease remains one of the leading causes of death in the United States, accounting for 1 in every 2.8 deaths [1]. It is also known that diabetes is associated with a high risk of cardiovascular disease. Atherosclerotic plaque inflammation plays a central role in atherosclerotic plaque progression, vulnerability and thrombogenicity. The exact mechanisms underlying the association between diabetes and atherosclerotic disease are not well known [2]. Epidemiological studies suggest that type 2 diabetes mellitus is not merely an independent risk factor for atherosclerotic cardiovascular disease but is also associated with increased levels of inflammatory biomarkers [3]. In line with these findings, efforts have been made to use non-invasive imaging to quantify vessel wall inflammation and to provide further evidence of the impact of clinical risk factors such as diabetes on atherosclerosis.
Carotid 18F-fluordeoxyglucose positron emission tomography (FDG-PET) has been shown to reflect the metabolic rate of glucose, a process known to be enhanced in inflamed tissue. FDG uptake has been shown to be significantly associated with both the degree of macrophage infiltration and the levels of inflammatory gene expression in plaques [4, 5, 6]. Several clinical cardiovascular disease (CVD) risk factors have also been shown to exhibit a significant association with carotid wall inflammation [7, 8, 9]. However, data regarding the impact of diabetic disease on vessel wall inflammation remains inconclusive. Some trials failed to show any association between diabetes and atherosclerosis as assessed by FDG-PET [8, 9], while others have observed a significant association between diabetic disease and vascular inflammation [7, 10]. Tumor PET studies have also shown that FDG accumulation is diminished during hyperglycemia [11, 12, 13]. This effect however, has not been evaluated in vascular PET imaging.
The aim of the current study was to assess the impact of non-insulin dependent type-2 diabetes on carotid wall FDG uptake whilst seeking to avoid some of the limitations of previous studies. We therefore designed a cross-sectional study in a larger sample population and performed FDG-PET imaging with protocols optimized for vessel wall visualization/analysis [7, 9, 14].
METHODS
Study Design
This was a cross-sectional study, investigating the impact of non-insulin dependent type-2 diabetes on the prevalence of carotid wall inflammation assessed by FDG-PET. This study, approved by the institutional review board, was conducted from February 2006 until October 2010 at the Mount Sinai School of Medicine, New York, U.S.A. All subjects gave written informed consent.
Inclusion criteria were as follows: Males and females with a diagnosis of CVD or individuals with multiple CVD risk factors were recruited. CVD was defined as a previous myocardial infarction, stroke, transient ischemic attack [TIA], history of peripheral artery disease; or a history of a coronary revascularization procedure. Patients with insulin-dependent type-2 diabetes and those with type-1 diabetic disease were excluded from the study, as were subjects with fasting glucose levels ≥ 11.1 mmol/l or previous carotid surgery.
Questionnaire, Biometric and Biochemical Measurements
Presence of cardiovascular risk factors, use of medication, and family history of CVD, were assessed by a questionnaire. Presence of hypertension was defined as a history systolic blood pressure > 140 mmHg, or a diastolic blood pressure > 90 mmHg. Diabetes was defined as documented diagnosis of type-2 diabetic disease and the use of anti-diabetic treatment (diet or oral, no insulin treatment). Weight and height were measured to calculate body mass index (BMI). Smoking was defined as smoking at least one cigarette on a daily basis. Fasting glucose levels were obtained by finger stick blood glucose measurements (Accu-Chek™ Advantage™, Roche Diagnostics; Indianapolis, Indiana) prior to FDG administration.
FDG-PET/CT Imaging
FDG-PET/CT was performed after an overnight fast using a General Electric Healthcare (Milwaukee, Wisconsin) Lightspeed discovery™ ST 16-slice PET/CT scanner. FDG was administered intravenously (557.6 ± 84.0 MBq), and patients rested comfortably for 97 to 193 min (136.7 ± 21.2 min) before the scan of the neck was started. Subjects were placed into a head holder for imaging of the carotids. A low dose CT scan (140 kV, 80 mA, and 4.25 mm slice thickness) was performed for attenuation correction and co-registration. Images from one bed position (15.5 cm) with coverage extending inferior to the internal auditory meatus were acquired in 3D mode using a 128 × 128 pixel matrix for 15 minutes. No CT contrast agent was administered. The total radiation dose from participating into this study was approximately 12 mSv.
Image Analysis
Image analysis was performed on a dedicated commercially available workstation (Extended Brilliance™ Workspace V4.0.0.3206; Philips Medical Systems Inc.; Cleveland, Ohio). An experienced reader (J. B.) analyzed all scans. Methodology for analysis and reproducibility of the measurements have been previously reported [15].
Briefly, arterial FDG uptake was quantified by manually drawing a region of interest (ROI) around each artery (common carotid arteries) on every slice of the co-registered transaxial PET/CT images. Next, the maximum arterial standardized uptake value (SUV) (highest pixel activity within the region of interest) was determined. The SUV is the decay-corrected tissue concentration of FDG in kBq/ml, adjusted for the injected FDG dose and the body weight of the patient. By averaging the maximum SUV values of all arterial slices of the left and right carotid artery, a meanSUV value was derived for the carotid arteries.
meanSUV values were also corrected for patient’s fasting pre-scan glucose levels to account for a competitive impact of glucose (gluc) and FDG using an established formula [16]. The measured glucose content was normalized for an overall population average of 5.0 mmol/l [16]:
The arterial target-to-background ratio (TBRgluc) was calculated by normalizing the SUVgluc for blood pool activity by dividing the SUVgluc value in the artery by the average blood mean SUV estimated from both jugular veins (JV). The TBRgluc is a blood-normalized arterial SUV, considered to be a reflection of arterial FDG uptake and reflective of underlying macrophage activity [14]. For evaluation of the FDG blood pool activity, at least six 3-4 mm ROIs were placed in consecutive slices of both JVs and averaged.
The arterial TBR values obtained were then averaged in order to derive a meanTBRgluc for both carotid arteries. Additionally, we identified the glucose-corrected Single Hottest Slice (SHSgluc), defined as the highest TBRgluc value of the carotid arteries.
Statistical Analysis
All continuous variables are expressed as mean ± standard deviation and categorical data as absolute numbers and percentages throughout this manuscript. In order to also assess a potential relation between continuous BMI values of the patients and the different FDG uptake parameters instead of assessing the impact of increased BMI values ≥ 30 kg/m2, Pearson or Spearman’s rho correlation coefficients (r) were calculated depending on normal distribution between the BMI values between the uncorrected- and glucose corrected FDG uptake parameters in the entire study population as well as in the two subgroups of patients with and without type II diabetes. In general, normal distribution of data was tested for all of the different statistical calculations using the Kolmogorov-Smirnov-Test.
Subgroup Analysis
The study population was divided into two subgroups of patients i.e. with and without diabetes. Furthermore, in order to test for the effect of anti-diabetic medication on the degree of carotid wall inflammation, we compared the different FDG uptake parameters between patient with oral anti-diabetic drugs and those with dietary treatment only. For all comparisons of subgroups of patients, student’s t-test or Mann U Whitney test was performed to compare continuous variables depending on normal distribution. Categorical variables were compared between diabetic and non-diabetic patients by using the Fisher’s Exact Test.
Multiple Regression with Backward Elimination and Linear Regression with ENTER Method (significant variables of the Backward Elimination entered a consecutive Linear Regression Model in a block in a single step):
In order to look at the relationship between each of more than one independent variable on one dependent variable, multiple regression analyses were used in the present study.
In the present study, a multiple linear regression analysis with backward elimination was used to assess the association between the cardiovascular risk factors and glucose-corrected FDG uptake parameters (meanSUVgluc, meanTBRgluc, and SHSgluc) in the entire study population as well as in both subgroups of patients with and without diabetes [17, 18]. FDG uptake parameters were treated as the response variables (dependent) and cardiovascular risk factors as the explanatory (independent) variables for the regression analysis. The explanatory variables included were as follows: age > 65 years, male gender, body mass index (BMI) ≥ 30 kg/m2, statin use, type-2 diabetes, history of cardiovascular disease, smoking, alcohol use, hypertension, and family history of cardiovascular disease. In order to evaluate a potential beneficial effect of exercise on carotid wall inflammation, exercise was one of the explanatory variables in the regression analyses. Following this, the ENTER regression was used to determine independent predictors of the response variables. For this method, all of the explanatory variables of the backward elimination model that showed a significant association with the FDG uptake value were retained and entered the regression model in a block in a single step. This entry method was preferred over the forward selection of variables since after excluding all of the explanatory variables without a significant association with the different carotid wall FDG uptake values, only few significant variables were left for a relatively low number of cases. A similar analysis approach was also followed for the FDG uptake values obtained without the applied glucose correction (meanSUV, meanTBR, SHS) [16]. Throughout the manuscript, all results of the multiregression models were given with the standardized regression coefficient (β), the 95% confidence interval, and the p-value for the estimate of the statistical significance.
Tertile Analysis
The patients were divided into tertiles based on their FDG-PET uptake parameters. Pearson χ2 tests were performed to compare the prevalence of clinical variables across the groups of patients classified by tertiles of the different FDG uptake parameters (meanSUVgluc, meanTBRgluc, and SHSgluc).
ANOVA with the appropriate adjustment for multiple comparisons was performed to compare FDG uptake values between different levels of fasting glucose in non-diabetics and patients with diabetes (according to the recommendations by The International Diabetes Federation IGF/IGT consensus statement [19]). Post hoc analyses were performed using the Tukey test.
All statistical analyses were performed using SPSS™ statistical package 16.0 (SPSS Inc.; Chicago, Illinois).
RESULTS
Population Characteristics
One hundred thirty-four patients were included in the study. In 3 of the patients, FDG-PET analysis could not be performed due to high FDG uptake in the thyroid, affecting the visualization and analysis of FDG uptake within the carotid arteries, leaving 131 eligible patients for image analysis. On average, 7.56 ± 2.49 slices for the left common carotid artery and 7.36 ± 2.5 slices for the right common carotid artery were analyzed to derive the FDG uptake parameters. Table 1a shows characteristics of the whole population, both diabetic and non-diabetic subjects. There were some differences in demographics between the diabetic and non-diabetic group (history of percutaneous coronary intervention and use of beta blockers being higher in diabetic patients, exercise, family history of cardiovascular disease, and cigarettes per day in current smokers all being higher in non-diabetics); otherwise the two groups were similar. As expected, fasting glucose was higher in the diabetic population compared to non-diabetics.
Table 1.
a. Characteristics of Study Population.
| Total | Diabetic Subjects | Non-Diabetic Subjects | ||
|---|---|---|---|---|
| n (%) | 134 | 43 (32) | 91 (68) | |
| Characteristics | p-value | |||
| Age (years) | 60.2 ± 10.1 | 61.3 ± 10.0 | 59.6 ± 10.2 | 0.37 |
| • Age > 65 years (n, %) | 46 (34) | 16 (37) | 30 (33) | 0.7 |
| Gender | ||||
| • Male (n, %) | 96 (72) | 33 (77) | 63 (69) | |
| • Female (n, %) | 38 (28) | 10 (23) | 28 (31) | 0.42 |
| Body Mass Index (BMI; kg/m2) | 28.9 ± 5.7 | 29.6 ± 4.8 | 28.6 ± 6.1 | 0.34 |
| • BMI < 25 (n, %) | 32 (24) | 8 (19) | 24 (26) | 0.39 |
| • BMI ≥ 25 < 30 (n, %) | 54 (40) | 17 (40) | 37 (41) | 1.0 |
| • BMI ≥ 30 (n, %) | 48 (36) | 18 (42) | 30 (33) | 0.34 |
| Lifestyle | ||||
| Smoking | ||||
| • Never (n, %) | 58 (43) | 19 (44) | 40 (44) | 1.0 |
| • Former (n, %) | 57 (43) | 21 (49) | 35 (39) | 0.35 |
| • Cigarettes per day (Range) | 24.7 ± 14.6 (2 - 80) | 24.4 ± 15.8 (2 - 60) | 24.8 ± 14.3 (3 - 80) | 0.82 |
| • Current (n, %) | 19 (14) | 3 (7) | 16 (18) | 0.12 |
| • Cigarettes per day (Range) | 12.6 ± 11.9 (2 - 40) | 3.0 ± 1.0 (2 - 4) | 14.6 ± 12.2 (3 - 40) | 0.004 |
| Alcohol Users (n, %) | 50 (37) | 12 (28) | 38 (42) | 0.13 |
| Exercisers (n, %) | 72 (54) | 16 (37) | 56 (62) | 0.010 |
| • Times per week | 4.46 ± 2.08 | 5.0 ± 2.4 | 4.31 ± 2.0 | 0.34 |
| • Time per session (min) | 46.7 ± 30.0 | 41.2 ± 22.2 | 48.1 ± 31.7 | 0.65 |
| Medical history | ||||
| Cardiovascular Disease | ||||
| • Myocardial Infarction (n, %) | 27 (21) | 8 (19) | 19 (21) | 1.0 |
| • Percutaneous Coronary Intervention (n, %) | 60 (46) | 25 (58) | 35 (39) | 0.041 |
| • Coronary Artery Bypass Surgery (n, %) | 22 (17) | 11 (26) | 11 (12) | 0.078 |
| • Stroke/Transient Ischemic Attack (TIA) | 11 (8) | 2 (5) | 9 (10) | 0.5 |
| • Peripheral Artery Disease | 5 (4) | 0 (0) | 5 (6) | 0.18 |
| Family History of Cardiovascular Disease (n, %) | 80 (60) | 19 (44) | 61 (67) | 0.015 |
| Hypertension (n, %) | 90 (67) | 32 (74) | 58 (64) | 0.24 |
| • Duration of Hypertension (months) | 129.9±119.7 | 110.5 ± 96.9 | 142.8 ± 132.4 | 0.3 |
| Diabetes Type II | 43 (32) | 43 (100) | Ø | |
| • Duration of Diabetes (years) (Range) | 7.25 ± 7.35 | 7.25 ± 7.35 | Ø Ø | |
| Fasting Glucose (mmol/l) | (0.5 - 34.0) | (0.5 - 34.0) | 5.4 ± 0.8 | <0.000 1 |
| • < 6.1 mmol/l (n, %) | 5.9 ± 1.3 | 6.8 ± 1.7 | 73 (80) | <0.000 1 |
| • ≥ 6.1 - < 7.0 mmol/l (n, %) | 89 (66) | 16 (37) | 15 (17) | 0.81 |
| • ≥ 7.0 mmol/l (n, %) | 23 (17) | 8 (19) | 3 (3) | 0.030 |
| • ≥ 7.8 mmol/l (n, %) | 9 (7) | 6 (14) | 0 (0) | <0.000 1 |
| 13 (10) | 13 (30) | |||
| Medication | ||||
| Statin (n, %) | 104 (78) | 37 (86) | 67 (74) | 0.13 |
| • Duration (months) | 51.6 ± 64.4 | 44.3 ± 56.3 | 54.5 ± 67.7 | 0.49 |
| Beta-blockers (n, %) | 63 (48) | 26 (61) | 37 (41) | 0.041 |
| Calcium Channel Blockers (n, %) | 21 (16) | 8 (19) | 13 (14) | 0.61 |
| ACE Inhibitors (n, %) | 43 (33) | 17 (40) | 26 (29) | 0.24 |
| AT II Blockers (n, %) | 20 (16) | 6 (14) | 14 (15) | 1.0 |
| Nitrates (n, %) | 7 (5) | 2 (5) | 5 (6) | 1.0 |
| Diuretics (n, %) | 20 (15) | 6 (14) | 14 (15) | 1.0 |
| Aspirin (n, %) | 91 (70) | 30 (70) | 61 (67) | 0.84 |
| Clopidrogrel (n, %) | 61 (47) | 23 (54) | 38 (42) | 0.27 |
| Oral Anti-Diabetics (n, %) | 36 (27) | 36 (84) | Ø | Ø |
| b. FDG-PET Imaging Results. | ||||
| Total | Diabetic Subjects | Non-Diabetic Subjects | ||
| n (%) | 131 | 42 (32) | 89 (68) | |
| FDG-PET / CT | p-value | |||
| FDG uptake time (time difference between FDG injection and starting time of data acquisition; min) | 136.7 ± 21.2 | 136.2 ± 21.6 | 137.5 ± 20.5 | 0.71 |
| meanSUV | 2.17 ± 0.35 | 2.12 ± 0.29 | 2.20 ± 0.37 | 0.21 |
| meanSUVgluc | 2.53 ± 0.64 | 2.84 ± 0.71 | 2.01 ± 0.33 | <0.0001 |
| meanTBR | 1.99 ± 0.32 | 1.95 ± 0.3 | 2.17 ± 0.48 | 0.34 |
| meanTBRgluc | 2.31 ± 0.56 | 2.6 ± 0.62 | 2.31 ± 0.43 | < 0.0001 |
| SHS | 2.30 ± 0.44 | 2.29 ± 0.48 | 2.50 ± 0.61 | 0.42 |
| SHSgluc | 2.68 ± 0.76 | 3.06 ± 0.9 | < 0.0001 | |
| Blood Pool Activity (left and right jugular vein; meanSUV of the mean) | 1.11 ± 0.2 | 1.09 ± 0.11 | 1.11 ± 0.2 | 0.59 |
| Glucose-corrected FDG uptake parameters depending on patient’s fasting glucose levels (median 5.5 mmol / l) | ||||
| Group 1 (2.8 - 5.5 mmol / l): n (%) | 65 | 12 (18.5) | 53 (81.5) | |
| meanSUVgluc | 2.17 ± 0.46 | 2.21 ± 0.38 | 2.16 ± 0.48 | 0.77 |
| meanTBRgluc | 2.01 ±0.41 | 2.13 ± 0.45 | 1.98 ± 0.4 | 0.26 |
| SHSgluc | 2.32 ± 0.51 | 2.55 ± 0.71 | 2.27 ± 0.44 | 0.09 |
| Group 2 (5.6 - 10.6 mmol / l): n (%) | 66 | 30 (45.5) | 36 (54.5) | |
| meanSUVgluc | 2.89 ± 0.59 | 3.09 ± 0.65 | 2.72 ± 0.48 | 0.011 |
| meanTBRgluc | 2.61 ± 0.54 | 2.78 ± 0.58 | 2.46 ± 0.46 | 0.013 |
| SHSgluc | 3.03 ± 0.8 | 3.26 ± 0.9 | 2.84 ± 0.66 | 0.032 |
Values are indicated as mean ± SD and categorical data is indicated as absolute numbers and percentages. Significant differences were found with regard to exercise and family history of cardiovascular disease being higher in non-diabetic patients as well as for percutaneous coronary intervention and medication with beta-blockers being higher for subjects with diabetes. As expected, mean fasting glucose levels as well was increased levels of the classified glucose values were significantly higher in diabetic patients whereas glucose values within the normal range were significantly more often found in non-diabetic subjects. No significant differences between both groups were observed for slightly increased glucose values ≥ 6.1 - < 7.0 mmol/l.
Values are indicated as mean ± SD and categorical data is indicated as absolute numbers and percentages. By averaging the maximum SUV values of all arterial slices of the left and right carotid artery, a meanSUV value was derived for the carotid arteries. By averaging the mean SUV values of all analyzed slices of the left and right jugular vein the meanSUV for the FDG bloodpool activity was calculated. TBR is the Target-to-Background-ratio, SHS is the Single Hottest Segment. No statistically significant differences were found between patients with and without diabetic disease with regard to the FDG uptake time. Whereas all of the glucose-corrected FDG uptake parameters were significantly higher in diabetic patients no significant differences between the groups were found for the uncorrected parameters. Dividing the patients into two groups of different pre-scan glucose levels by using the median of all glucose values revealed statistically significant differences between patients with type II diabetes and patients without diabetes for all glucose-corrected FDG uptake values in the group of patients with higher pre-scan glucose levels.
FDG-PET Imaging Results
The imaging analyses of the groups (Table 1b), however showed that glucose-corrected FDG-PET parameters (meanSUVgluc, meanTBRgluc, SHSgluc) were significantly higher in the diabetic group compared to the non-diabetics. This difference was not observed in FDG-PET parameters that were not corrected for pre-scan glucose. Dividing patients into two groups according to their pre-scan glucose values by using the median of these values, we also found significantly higher values for all glucose-corrected FDG uptake parameters in diabetic patients in the group of higher (5.6 - 10.6 mmol/l) pre-scan glucose levels. In the group of patients with lower pre-scan glucose levels (2.8 - 5.5 mmol/l) all glucose-corrected FDG uptake parameters were also found to be higher in diabetic patients, however, these differences failed to be statistically significant (Table 1b). In diabetic patients, no significant differences were observed for all glucose-corrected FDG parameters between those patients treated with oral anti-diabetic drugs and those on dietary treatment only (meanSUVgluc: p = 0.313, meanTBRgluc: p = 0.314, SHSgluc: p = 0.122).
FDG-PET Imaging and CVD Risk Factors
Table 2a shows the results of the multiple linear regression analysis with backward elimination to identify clinical risk factors associated with glucose-corrected FDG-PET uptake parameters (i.e. measures of plaque inflammation). Diabetes showed the strongest relationship with all FDG-PET uptake parameters (meanSUVgluc, meanTBRgluc, SHSgluc) (standardized regression coefficient β = 0.38, p < 0.0001) followed by BMI > 30 kg/m2 (β = 0.25, p < 0.001). Only risk factors that had a p < 0.10 were retained in the model for the ENTER regression (all significant and therefore retained variables entered this model in a block in a single step) and are shown in the Table. Following the ENTER regression, it was found that diabetes, BMI > 30 kg/m2, and alcohol use (except of meanSUVgluc values) were independent predictors of plaque inflammation as measured by FDG-PET imaging (Figures 1, 2, and 3).
Table 2.
a: Multiple Linear Regression Analyses with Backward Elimination to Identify Clinical Risk Factors of Carotid Vessel Wall Inflammation as Depicted by Glucose- Corrected FDG Uptake Parameters in the Whole Study Population.
| Standardized Coefficient β | 95 % Confidence Interval | Adjusted R2 | Significance | p - value | |
|---|---|---|---|---|---|
| Glucose-Corrected FDG Uptake Parameters | |||||
| meanSUVgluc | 0.201 | <0.0001 | |||
| Diabetes | 0.324 | 0.227 - 0.656 | <0.0001 | ||
| BMI ≥ 30 kg/m2 | 0.282 | 0.168 - 0.587 | 0.001 | ||
| Alcohol | 0.157 | -0.001 - 0.411 | 0.051 | ||
| meanTBRgluc | 0.229 | <0.0001 | |||
| Diabetes | 0.317 | 0.191 - 0.570 | <0.0001 | ||
| BMI ≥ 30 kg/m2 | 0.219 | 0.077 - 0.440 | 0.006 | ||
| Family History of Cardiovascular Disease | -0.2 | -0.408 - -0.051 | 0.012 | ||
| Alcohol | 0.197 | 0.049 - 0.406 | 0.013 | ||
| SHSgluc | 0.234 | <0.0001 | |||
| Diabetes | 0.298 | 0.228 - 0.737 | <0.0001 | ||
| BMI ≥ 30 kg/m2 | 0.259 | 0.168 - 0.656 | 0.001 | ||
| Family History Cardiovascular Disease | -0.21 | -0.564 - -0.084 | 0.009 | ||
| Alcohol | 0.163 | 0.014 - 0.494 | 0.038 |
meanSUVgluc, mean Target-to-Background Ratio (meanTBRgluc), and Single Hottest Segment (SHSgluc) respectively, were the response variables and the cardiovascular risk factors age > 65 years, male gender, body mass index (BMI) ≥ 30 kg/m2, statin medication, history of cardiovascular disease, smoking, alcohol use, exercise, hypertension, diabetes, and family history of cardiovascular disease were the explanatory variables. Variables were retained in the model when p < 0.10. β is the standardized regression coefficient. Diabetes was the most significant predictor for carotid wall inflammation as depicted by all of the FDG uptake parameters followed by obesity as depicted by BMI values ≥ 30 kg/m2. Notably, the results were highly consistent among the different FDG uptake values.
Figure 1. Clinical Risk Factors of Carotid Vessel Wall Inflammation.
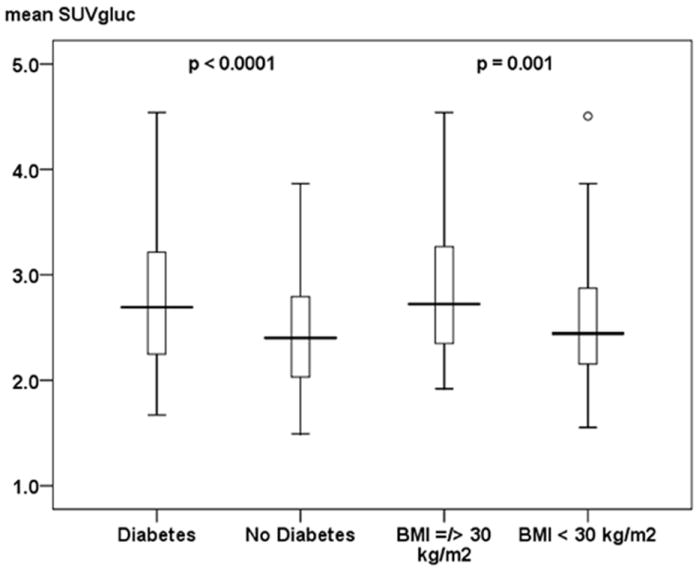
This figure shows differences in meanSUVgluc values in patients with and without diabetes and with and without BMI values ≥ 30 kg/m2. Both variables were identified as significant (p < 0.05) independent predictors for carotid wall inflammation as depicted by meanSUVgluc values. Data is presented as median (bolded line), 25th - 75th percentile (box), 5th - 95th percentile (whiskers). Circles represent outliers. The p-value for each of the given independent predictors for carotid wall inflammation as depicted by meanSUVgluc values is adjusted for the other significant variable given in Table 2a (diabetes, BMI ≥ 30 kg/m2, and alcohol). Alcohol failed to show a statistical significant association (p < 0.05) with the meanSUVgluc values in the ENTER regression model and is therefore not shown as an independent predictor in this figure.
Figure 2. Clinical Risk Factors of Carotid Vessel Wall Inflammation.
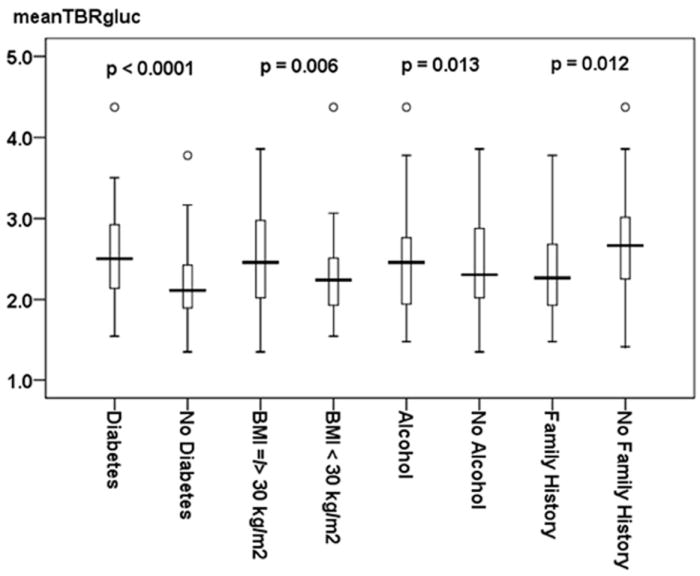
This figure shows differences in meanTBRgluc values in patients with and without diabetes, BMI ≥ 30 kg/m2, and alcohol. All variables were identified as significant (p < 0.05) independent predictors for carotid wall inflammation as depicted by the mean Target-to-Background-Ratio (meanTBRgluc). Family history is independently associated with a decreased risk of carotid wall inflammation as revealed by significantly lower meanTBRgluc values in patients with a family history of cardiovascular disease. Data is presented as median (bolded line), 25th - 75th percentile (box), 5th - 95th percentile (whiskers). Circles represent outliers. The p-value for each of the given independent predictors for carotid wall inflammation as depicted by meanTBRgluc values is adjusted for the other significant variables given in Table 2a (diabetes, BMI ≥ 30 kg/m2, alcohol, and family history of cardiovascular disease).
Figure 3. Clinical Risk Factors of Carotid Vessel Wall Inflammation.
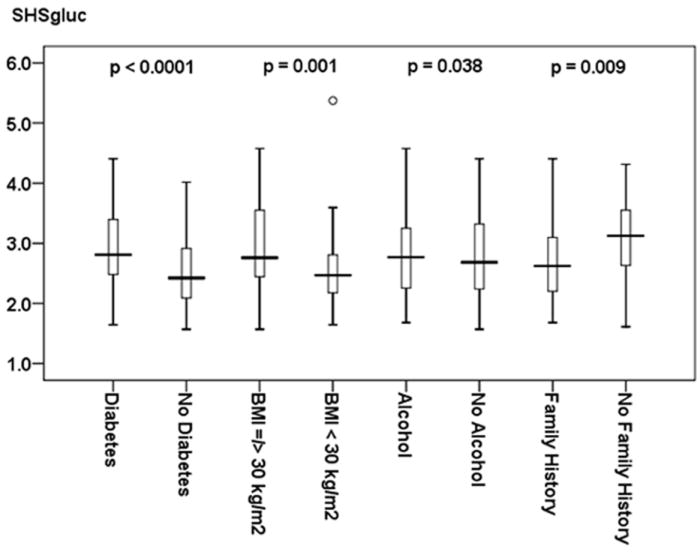
This figure shows differences in SHSgluc values in patients with and without diabetes, BMI ≥ 30 kg/m2, and alcohol. All variables were identified as significant (p < 0.05) independent predictors for carotid wall inflammation as depicted by the Single Hottest Segment (SHSgluc). Family history is independently associated with a decreased risk of carotid wall inflammation as revealed by significantly lower SHSgluc values in patients with a family history of cardiovascular disease. Data is presented as median (bolded line), 25th - 75th percentile (box), 5th - 95th percentile (whiskers). Circles represent outliers. The p-value for each of the given independent predictors for carotid wall inflammation as depicted by SHSgluc values is adjusted for the other significant variables given in Table 2a (diabetes, BMI ≥ 30 kg/m2, alcohol, and family history of cardiovascular disease).
ENTER regression also showed that meanTBRgluc and SHSgluc were inversely associated with a family history of cardiovascular disease (Figure 2 and 3).
The multiple regression analysis for non-glucose corrected FDG-PET data is presented in the Appendix (Table 2b).
Multiple Regressions in Subgroups
Multiple linear regression analyses in the subgroup of patients with and without diabetic disease is presented in Table 3a following a similar procedure as described previously. In diabetic subjects, BMI ≥ 30 kg/m2 continued to be a significantly associated with all of the three glucose-corrected FDG-PET uptake parameters (meanSUVgluc: β = 0.571, p = 0.009, meanTBRgluc: β = 0.443, p = 0.014, SHSgluc: β = 0.706, p = 0.007). In non-diabetic subjects, hypertension showed the strongest association with the FDG-PET uptake parameters (meanSUVgluc: β = 0.371, p = 0.002, meanTBRgluc: β = 0.361, p < 0.0001, SHSgluc: β = 0.434, p = 0.001). These and other associations are shown in Table 3a.
Table 3.
a: Multiple Linear Regression Analyses with Backward Elimination to Identify Clinical Risk Factors of Carotid Vessel Wall Inflammation as Depicted by Glucose-Corrected FDG Uptake Parameters in Diabetic- and Non-Diabetic Subjects.
| Standardized Coefficient β | 95 % Confidence Interval | Adjusted R2 | Significance | p-value | |
|---|---|---|---|---|---|
| Glucose-Corrected FDG Uptake Parameters | |||||
| meanSUVgluc | |||||
| Diabetic Subjects | 0.139 | 0.009 | |||
| BMI ≥ 30 kg/m2 | 0.4 | 0.153 - 0.989 | 0.009 | ||
| Non-Diabetic Subjects | 0.153 | <0.0001 | |||
| Hypertension | 0.325 | 0.146 - 0.597 | 0.002 | ||
| BMI ≥ 30 kg/m2 | 0.212 | 0.017 - 0.483 | 0.036 | ||
| meanTBRgluc | |||||
| Diabetic Subjects | 0.22 | 0.003 | |||
| BMI ≥ 30 kg/m2 | 0.357 | 0.093 - 0.793 | 0.014 | ||
| Smoking | 0.312 | 0.072 - 1.405 | 0.031 | ||
| Non-Diabetic Subjects | 0.185 | <0.0001 | |||
| Hypertension | 0.361 | 0.167 - 0.555 | <0.0001 | ||
| SHSgluc | |||||
| Diabetic Subjects | 0.256 | 0.001 | |||
| BMI ≥ 30 kg/m2 | 0.388 | 0.205 - 1.208 | 0.007 | ||
| Smoking | 0.324 | 0.169 - 2.081 | 0.022 | ||
| Non-Diabetic Subjects | 0.109 | 0.001 | |||
| Hypertension | 0.345 | 0.182 - 0.685 | 0.001 |
meanSUVgluc, mean Target-to-background ratio (meanTBRgluc) and Single Hottest Segment (SHSgluc), were the response variables and the cardiovascular risk factors age > 65 years, male gender, body mass index (BMI) ≥ 30 kg/m2, statin medication, history of cardiovascular disease, smoking, alcohol use, exercise, hypertension, and family history of cardiovascular disease were the explanatory variables. Variables were retained in the model when p < 0.05. The best model is shown. β is the standardized regression coefficient. Same models were calculated in diabetic- and non-diabetic patients. BMI ≥ 30 kg/m2 and smoking were significantly associated with all FDG uptake parameters (except of smoking with regard to meanSUVgluc) in diabetic subjects. In non-diabetic subjects, hypertension was consistently associated with all FDG uptake parameters.
Again, results for the regression analysis for non-glucose corrected FDG-PET data is presented in the Appendix (Table 3b).
Tertile Analysis
Table 4 (Appendix) shows clinical characteristics stratified by tertiles of meanSUVgluc, meanTBRgluc, and SHSgluc. The prevalence of type 2 diabetes and BMI ≥ 30 kg/m2 were both significantly higher at higher tertiles of the three glucose-corrected FDG-PET uptake parameters.
Correlation between Continuous BMI Values and the FDG Uptake Parameters in the Entire Study Population and the Subgroups
Positive significant correlations were found between the continuous BMI values and all of the uncorrected- and glucose-corrected FDG uptake parameters in the entire study population and the two subgroups (r > 0.25, p < 0.02 for all) except of TBRmax values in the total study population and in non-diabetic patients, SUVmax values in diabetic patients as well as TBRmaxgluc and SHSgluc values in non-diabetic patients.
Distribution of the FDG-PET Uptake Parameters According to the Pre-Scan Glucose Levels
Figures 4 (Appendix) depict significantly increasing FDG uptake parameters (meanSUVgluc, meanTBRgluc, and SHSgluc) by increments of fasting glucose levels in patients with type 2 diabetes. Remarkably, FDG uptake values in non-diabetic subjects were similar (SHSgluc) or even slightly, but not significantly, higher (meanSUVgluc, meanTBRgluc) compared to diabetic patients with fasting glucose levels within the normal range (< 6.1 mmol/l, Figures 4; Appendix).
DISCUSSION
The aim of our study was to determine if the presence of type 2 diabetes was related to carotid wall FDG uptake. This relation might therefore represent a link between diabetic disease and carotid wall inflammation in a population of patients with known cardiovascular disease or multiple risk factors for it. We used a cross sectional study design in a larger sample population than previous studies [7, 8, 9, 10] and performed FDG-PET imaging with protocols optimized for vessel wall FDG uptake [9, 14]. Our results demonstrate that diabetes was significantly associated with the FDG uptake in the carotid wall. Additionally, we showed that obesity was also related to carotid wall inflammation as depicted by FDG-PET. In the non-diabetic group, hypertension was the leading variable associated with inflammation measured by FDG-PET uptake. We also identified increasing fasting glucose levels in diabetic patients to be significantly associated with increments of the FDG uptake, which might be indicative of a higher propensity for carotid wall inflammation with increasing degrees of hyperglycemia.
FDG-PET/CT Methodology
The rationale for choosing to perform glucose correction of the FDG uptake is based on several oncology studies, which suggest that elevated pre-scan glucose levels can influence significantly the tumor’s uptake of FDG during PET imaging [12, 20, 21]. One potential pitfall to using a glucose correction however is a resultant increase in the variability of the SUV measurements [22]. The role of glucose correction of FDG uptake in non-cancer lesions is not well understood. However, we felt that since the mechanism of uptake of FDG into inflammatory cells is the same as for tumor cells, that the same correction should be applied. In accordance to the European Association of Nuclear Medicine procedure guidelines for tumor PET imaging, we have also presented the results without glucose correction (Appendix) [16].
Multivariate regression analyses revealed an unexpected negative association between diabetes and the uncorrected meanSUV values of the carotids in our study. This finding is in contrast to the well-known clinical impact of diabetes on cardiovascular disease. However, future studies still need to be performed to investigate whether the corrected- or uncorrected FDG uptake values are more sensitive surrogate markers for carotid wall inflammation by correlating both FDG uptake parameters with the histological assessment of vascular inflammation.
Impact of Circulation Time on FDG-Uptake
The optimal circulation time before imaging plaque inflammation has still not been definitively established. Typically, a circulation time between one and three hours is used by most groups [4, 5, 23, 24]. In order to exclude an impact of the FDG circulation time on the FDG uptake in patients with and without diabetes, we compared the FDG circulation time between both groups of patients. As we did not find a statistically significant difference, the differences of the FDG uptake between diabetic and non-diabetic patients cannot be explained by different FDG circulation times in both groups.
Type-2 Diabetes and Carotid Wall Inflammation
We observed that type-2 diabetes and obesity (BMI) were independently associated with increased FDG-PET uptake values. In patients with type-2 diabetes, obesity and smoking added additional risk for increased FDG uptake in the carotid wall. In non-diabetics however, only hypertension was found to be significantly associated with carotid wall inflammation as depicted by all FDG uptake parameters.
Our results are in agreement with some of the previously published studies evaluating the association between cardiovascular risk factors and vessel wall inflammation. In a case-control study of patients with type-2 diabetes, impaired glucose tolerance, and controls, Kim et al. reported higher TBRmax values in both study groups compared to controls [10]. As in the current study, they also observed increasing prevalence of diabetes with increments of maximum TBR values as depicted by tertiles. However, glucose correction was not used in their study, and this may have resulted in an underestimation of FDG-PET uptake values.
Previous studies have demonstrated the link between circulating insulin levels and its effect on the over expression of GLUT transporter types [10, 11]. Tahara et al. have also shown that carotid inflammation was associated with several cardiovascular risk factors including a homeostasis model assessment of insulin resistance [7]. They found obesity, as assessed by waist circumference and use of hypertensive medication to be significantly associated with carotid wall inflammation. However, fewer subjects in their population had cardiovascular disease and the cardiovascular risk profile for their population was much lower primarily because their retrospective analysis was done in cancer patients. In our study, we found a relationship between hypertension and carotid wall inflammation measured by FDG-PET in the non-diabetic group and a relationship between BMI and carotid wall FDG uptake both diabetics and non-diabetics.
Relationship between Fasting Glucose Levels and Carotid Wall FDG-Uptake
Studies have shown that hyperglycemia leads to increased oxidative stress producing endothelial dysfunction [25]. Several observational studies have shown an association between levels of glycemia and macrovascular events in patients with diabetes [26, 27]. Early data from the UKPDS suggested a protective effect of improved glucose control on cardiovascular disease incidence and mortality [26 29]. Results from our study seem to support these findings, as we found similar meanTBRgluc values in patients with diabetes and fasting glucose levels < 6.1 mmol/l compared to non-diabetic patients (p = 0.985). We also observed higher FDG uptake parameters with increasingly poorer glycemic control.
Relationship between Hypertension and Carotid Wall FDG-Uptake
Two recently published trials prospectively investigated the impact of hypertension on cardiovascular risk in patients with and without diabetic disease [28, 29]. Both trials found hypertension to be associated with a higher risk of cardiovascular disease in diabetic patients but failed to show a significant interaction between diabetes and increased blood pressure. We found that hypertension showed a significant association with carotid wall FDG uptake only in the non-diabetic subgroup.
Limitations
There are several limitations to our study that need to be addressed. Firstly, we did not obtain serum lipid levels, markers of glucose metabolism or serum inflammatory markers. Secondly, this is a cross-sectional study. Therefore we could not address whether there was a causal relationship between the presence of diabetic disease and vessel wall inflammation. Thirdly, it is unknown if vessel wall FDG uptake is predictive of progression of disease or future cardiovascular events in diabetic patients. Longitudinal studies are currently underway to establish such a relation. Finally, in the present study image analyses was performed by only one reader. This might reduce statistical noise related to inter-observer variation but might raise concerns regarding intra-observer bias. However, previous reports demonstrated that this method has good inter- and intra-observer reproducibility [15].
CONCLUSION
In the current study, we show that type-2 diabetes has a significant impact on the FDG uptake in the wall of the carotid arteries. Obesity (BMI ≥ 30 kg/m2) and smoking are also significantly associated with FDG-PET uptake parameters in diabetic patients. In non-diabetics, hypertension was significantly associated with carotid wall inflammation. Furthermore, the degree of the carotid wall FDG uptake increases with increments of fasting glucose levels in diabetic patients. Whether the glucose-corrected FDG uptake parameters are indicative of vessel wall inflammation has to be determined by future studies.
Supplementary Material
Acknowledgments
The authors wish to thank Ash Rafique, RT, BS, CNMT for his assistance with the image acquisition.
Work in this paper was partly supported by the NIHR Cambridge Biomedical Research Centre (J. H. F. R.).
Partial support was provided by: NIH/NHLBI R01 HL071021 (Z. A. F.) and NIH/NHLBI R01 HL078667 (Z. A. F and M. E. F.).
Abbreviations
- FDG-PET
18F-Fluordeoxyglucose Positron Emission Tomography
- SUV
Standardized Uptake Value
- TBR
Target-to-Background Ratio
- SHS
Single Hottest Segment
- JV
Jugular Vein
- CVD
Cardiovascular Disease
- TIA
Transient Ischemic Attack
- BMI
Body Mass Index
- GLUT
Glucose Transporter Protein
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Roger VL, Go AS, Lloyd-Jones DM, Roger VL, Turner MB, et al. on behalf of the American Heart Association Statistics Committee and Stroke Statistics Subcommittee and On behalf of the American Heart Association Heart Disease and Stroke Statistics Writing Group. Heart disease and stroke statistics 2011 update: A report from the American Heart Association. Circulation. 2011;123:e18–e209. doi: 10.1161/CIR.0b013e3182009701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Libby P, Ridker PM, Maseri A. Inflammation and atherosclerosis. Circulation. 2002;105:1135–43. doi: 10.1161/hc0902.104353. [DOI] [PubMed] [Google Scholar]
- 3.Pickup JC. Inflammatory markers and type 2 diabetes. Diabetes Technol Ther. 2006;8:1–6. doi: 10.1089/dia.2006.8.1. [DOI] [PubMed] [Google Scholar]
- 4.Tawakol A, Migrino RQ, Bashian GG, et al. In vivo 18F-fluorodeoxyglucose positron emission tomography imaging provides a noninvasive measure of carotid plaque inflammation in patients. J Am Coll Cardiol. 2006;48:1818–24. doi: 10.1016/j.jacc.2006.05.076. [DOI] [PubMed] [Google Scholar]
- 5.Rudd JH, Warburton EA, Fryer TD, et al. Imaging atherosclerotic plaque inflammation with [18F]-fluorodeoxyglucose positron emission tomography. Circulation. 2002;105:2708–11. doi: 10.1161/01.cir.0000020548.60110.76. [DOI] [PubMed] [Google Scholar]
- 6.Pedersen SF, Graebe M, Fisker Hag AM, Højgaard L, Sillesen H, Kjaer A. Gene expression and 18FDG uptake in atherosclerotic carotid plaques. Nucl Med Commun. 2010;31:423–9. doi: 10.1097/MNM.0b013e32833767e0. [DOI] [PubMed] [Google Scholar]
- 7.Tahara N, Kai H, Yamagishi S, et al. Vascular inflammation evaluated by [18F]-fluorodeoxyglucose positron emission tomography is associated with the metabolic syndrome. J Am Coll Cardiol. 2007;49:1533–9. doi: 10.1016/j.jacc.2006.11.046. [DOI] [PubMed] [Google Scholar]
- 8.Rominger A, Saam T, Wolpers S, et al. 18F-FDG PET/CT identifies patients at risk for future vascular events in an otherwise asymptomatic cohort with neoplastic disease. J Nucl Med. 2009;50:1611–20. doi: 10.2967/jnumed.109.065151. [DOI] [PubMed] [Google Scholar]
- 9.Rudd JH, Myers KS, Bansilal S, et al. Relationships among regional arterial inflammation, calcification, risk factors, and biomarkers: a prospective fluorodeoxyglucose positron-emission tomography/computed tomography imaging study. Circ Cardiovasc Imaging. 2009;2:107–15. doi: 10.1161/CIRCIMAGING.108.811752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim TN, Kim S, Yang SJ, et al. Vascular inflammation in patients with impaired glucose tolerance and type 2 diabetes: analysis with 18F-fluorodeoxyglucose positron emission tomography. Circ Cardiovasc Imaging. 2010;3:142–8. doi: 10.1161/CIRCIMAGING.109.888909. [DOI] [PubMed] [Google Scholar]
- 11.Shepherd PR, Kahn BB. Glucose transporters and insulin action - implications for insulin resistance and diabetes mellitus. N Engl J Med. 1999;341:248–57. doi: 10.1056/NEJM199907223410406. [DOI] [PubMed] [Google Scholar]
- 12.Wahl RL, Henry CA, Ethier SP. Serum glucose: effects on tumor and normal tissue accumulation of 2-[F-18]-fluoro-2-deoxy-D-glucose in rodents with mammary carcinoma. Radiology. 1992;183:643–7. doi: 10.1148/radiology.183.3.1584912. [DOI] [PubMed] [Google Scholar]
- 13.Deichen JT, Prante O, Gack M, Schmiedehause K, Kuwert T. Uptake of [(18)F]fluorodeoxyglucose in human monocyte-macrophages in vitro. Eur J Nucl Med Mol Imaging. 2003;30:267–73. doi: 10.1007/s00259-002-1018-8. [DOI] [PubMed] [Google Scholar]
- 14.Tawakol A, Migrino RQ, Hoffmann U, et al. Noninvasive in vivo measurement of vascular inflammation with F-18 fluorodeoxyglucose positron emission tomography. J Nucl Cardiol. 2005;12:294–301. doi: 10.1016/j.nuclcard.2005.03.002. [DOI] [PubMed] [Google Scholar]
- 15.Rudd JHF, Myers KS, Bansilal S, Machac J, Pinto CA, Tong C, Rafique A, Hargeaves R, Farkouh M, Fuster V, Fayad ZA. Atherosclerosis inflammation imaging with 18F-FDG PET: Carotid, iliac, and femoral uptake reproducibility, quantification methods, and recommendations. J Nucl Med. 2008;49:871–8. doi: 10.2967/jnumed.107.050294. [DOI] [PubMed] [Google Scholar]
- 16.Boellaard R, O’Doherty MJ, Weber WA, et al. FDG PET and PET/CT: EANM procedure guidelines for tumour PET imaging: version 1.0. Eur J Nucl Med Mol Imaging. 2010;37:181–200. doi: 10.1007/s00259-009-1297-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hocking RR. The analysis and selection of variables in linear regression. Biometrics. 1976;32:1–49. [Google Scholar]
- 18.Draper N, Smith H. Applied Regression Analysis. 2. New York: John Wiley & Sons, Inc; 1981. [Google Scholar]
- 19.Unwin N, Shaw J, Zimmet P, Alberti KG. Impaired glucose tolerance and impaired fasting glycaemia: the current status on definition and intervention. Diabet Med. 2002;19:708–23. doi: 10.1046/j.1464-5491.2002.00835.x. [DOI] [PubMed] [Google Scholar]
- 20.Langen KJ, Braun U, Rota Kops E, et al. The influence of plasma glucose levels on fluorine-18-fluorodeoxyglucose uptake in bronchial carcinomas. J Nucl Med. 1993;34:355–9. [PubMed] [Google Scholar]
- 21.Lindholm P, Minn H, Leskinen-Kallio S, Bergman J, Ruotsalainen U, Joensuu H. Influence of the blood glucose concentration on FDG uptake in cancer - a PET study. J Nucl Med. 1993;34:1–6. [PubMed] [Google Scholar]
- 22.Hadi M, Bacharach SL, Whatley M, et al. Glucose and insulin variations in patients during the time course of a FDG-PET study and implications for the “glucose-corrected” SUV. Nucl Med Biol. 2008;35:441–5. doi: 10.1016/j.nucmedbio.2008.02.007. [DOI] [PubMed] [Google Scholar]
- 23.Menezes LJ, Kotze CW, Hutton BF, et al. Vascular inflammation imaging with 18F-FDG PET/CT: when to image? J Nucl Med. 2009;50:854–7. doi: 10.2967/jnumed.108.061432. [DOI] [PubMed] [Google Scholar]
- 24.Rudd JH, Elkhawad M, Fayad ZA. Vascular imaging with 18F-FDG PET/CT: optimal 18F-FDG circulation time? J Nucl Med. 2009;50:1560. doi: 10.2967/jnumed.109.066456. [DOI] [PubMed] [Google Scholar]
- 25.Ceriello A. New insights on oxidative stress and diabetic complications may lead to a ‘causal’ antioxidant therapy. Diabetes Care. 2003;26:1589–96. doi: 10.2337/diacare.26.5.1589. [DOI] [PubMed] [Google Scholar]
- 26.Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type diabetes (UKPDS 33) UK Prospective Diabetes Study (UKPDS) Group. Lancet. 1998;352:837–53. [PubMed] [Google Scholar]
- 27.Wild SH, Dunn CJ, McKeigue PM, Comte S. Glycemic control and cardiovascular disease in type 2 diabetes: a review. Diabetes Metab Res Rev. 1999;15:197–204. doi: 10.1002/(sici)1520-7560(199905/06)15:3<197::aid-dmrr28>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 28.Sehestedt T, Hansen TW, Li Y, et al. Are blood pressure and diabetes additive or synergistic risk factors? Outcome in 8494 subjects randomly recruited from 10 populations. Hypertens Res. 2011;34:714–21. doi: 10.1038/hr.2011.6. [DOI] [PubMed] [Google Scholar]
- 29.Eguchi K, Pickering TG, Hoshide S, et al. Ambulatory blood pressure is a better marker than clinic blood pressure in predicting cardiovascular events in patients with/without type 2 diabetes. Am J Hypertens. 2008;21:443–50. doi: 10.1038/ajh.2008.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


