Abstract
The objective of the present study was to investigate the presence of interleukin (IL)-27 in pleural effusions and to evaluate the diagnostic significance of pleural IL-27. The concentrations of IL-27 were determined in pleural fluids and sera from 68 patients with tuberculous pleural effusion, 63 malignant pleural effusion, 22 infectious pleural effusion, and 21 transudative pleural effusion. Flow cytometry was used to identify which pleural cell types expressed IL-27. It was found that the concentrations of pleural IL-27 in tuberculous group were significantly higher than those in malignant, infectious, and transudative groups, respectively. Pleural CD4+ T cells, CD8+ T cells, NK cells, NKT cells, B cells, monocytes, macrophages, and mesothelial cells might be the cell sources for IL-27. IL-27 levels could be used for diagnostic purpose for tuberculous pleural effusion, with the cut off value of 1,007 ng/L, IL-27 had a sensitivity of 92.7% and specificity of 99.1% for differential diagnosing tuberculous pleural effusion from non-tuberculous pleural effusions. Therefore, compared to non-tuberculous pleural effusions, IL-27 appeared to be increased in tuberculous pleural effusion. IL-27 in pleural fluid is a sensitive and specific biomarker for the differential diagnosing tuberculous pleural effusion from pleural effusions with the other causes.
Introduction
Pleural effusions (PEs) are a frequent clinical problem with more than 50 recognized causes including diseases local to the pleura or underlying lung, organ dysfunction, systemic conditions and drugs [1]. The development of PE is often associated with the accumulation of fluid enriched in proteins and cells in the pleural space [2]. There are many causes of PEs, and the precise pathophysiology of fluid accumulation varies according to underlying etiologies. It has been well documented that tuberculosis and cancer represent the two most frequent causes of exudative PEs with predominantly lymphocytes in pleural fluid; whereas infectious PEs, including empyema and parapneumonic effusion, are typically associated with an influx of neutrophils [3], [4].
Interleukin (IL)-27, a member of IL-12 family, is a recently discovered heterodimeric cytokine consisting of Epstein-Barr virus-induced gene protein 3 and p28 subunits [5], and is mainly produced by activated antigen-presenting cells [6]. IL-27 has been found to be involved in malignancy [7] and in infection, including severe sepsis, bacteraemia, as well as tuberculosis [8], [9]. This raises the possibility that IL-27 may play a role in the pathogenesis of PE, and may further be of diagnostic significance. We therefore performed the present study to: 1) determine whether PE IL-27 is produced in the pleural space; 2) identify the cell origins of pleural IL-27; 3) evaluate the diagnostic value of IL-27 in PEs.
Materials and Methods
Subjects
The study protocol was approved by our institutional review board for human studies of Tongji Medical College, China, and informed written consent was obtained from all subjects. One hundred and ninety-three consecutive patients with PEs of unknown causes were hospitalized for diagnostic investigation. Of these patients, 12 were excluded because they had received anti-tuberculosis treatment (n = 5) or anti-cancer chemotherapy (n = 7) prior to study, four were excluded because they had received corticosteroids or other nonsteroid anti-inflammatory drugs, and three were discharged from the hospital without a definitive diagnosis of PEs. Eventually, 174 patients were included in the current study (Table 1).
Table 1. Biochemical and cytological characteristics in pleural effusions*.
| Pleural effusions | ||||
| Tuberculous | Malignant | Infectious | Transudative | |
| Patients, No. | 68 | 63 | 22 | 21 |
| Sex, male/female, No. | 41/27 | 38/25 | 13/9 | 12/9 |
| Age, yr | 39.8±2.0† | 54.9±1.8 | 53.5±2.7 | 57.9±3.6 |
| Protein, g/L | 47.3±3.6‡ | 44.5±2.8‡ | 48.7±2.5‡ | 16.6±9.6 |
| Lactate dehydrogenase, IU/L | 593.7±80.4‡ | 464.5±108.6‡ | 648.4±81.3‡ | 102.3±42.5 |
| Total cell counts, × 109/L | 4.10±0.63‡ | 2.55±0.36‡ | 8.24±0.76† | 0.44±0.06 |
| Differential cell counts, % | ||||
| Lymphocytes | 78.5±1.8† | 52.5±1.1 | 13.3±2.1† | 34.4±3.3 |
| Neutrophils | 7.5±0.8 | 4.7±0.9 | 81.2±2.2† | 8.3±1.2 |
| Macrophages | 11.8±0.6† | 33.4±1.6† | 5.1±1.0† | 44.7±3.3 |
| Mesothelial cells | 2.1±0.4† | 6.4±0.8† | 0.3±0.2† | 12.7±1.8 |
| Malignant cells | – | 3.1±1.6 | – | – |
Values are presented as mean ± SEM.
p<0.01 compared with each of the other three groups; ‡ p<0.05 compared with transudative group. The comparisons were.
determined by one-way analysis of variance.
Sixty-eight anti-HIV Ab negative patients (age range: 16 to 76 yr) were proven to have tuberculous PE with, as evidenced by 1) presence of acid fast bacilli in pleural fluid specimen, growth of Mycobacterium tuberculosis (MTB) from pleural fluid, or demonstration of granulomatous pleurisy on closed pleural biopsy specimen in the absence of any evidence of other granulomatous diseases (n = 59); 2) an exudative lymphocytic effusion with an adenosine deaminase level of >40 U/L, along with a positive purified protein derivative skin test result and the exclusion of any other potential causes of pleurisy; after anti-tuberculosis chemotherapy, the resolution of pleural effusion and clinical symptoms was observed (n = 9).
Malignant PE was collected from 63 patients (age range: 37 to 84 yr) with newly diagnosed lung cancer with PE. Histologically, 44 cases were adenocarcinoma and 19 were squamous cell carcinoma. A diagnosis of malignant PE was established by demonstration of malignant cells in PE and/or on closed pleural biopsy specimen.
Twenty-two PE patients (age range: 38 to 74 yr) were classified as infectious PE (including 16 empyema and six parapneumonic effusion). Empyema was defined as an effusion that met one or more of the following criteria: presence of frank pus in the pleural space, purulent fluid on macroscopic examination, positive Gram stain and/or growth of organisms in culture, and PE pH <7.2 or glucose <3.3 mmol/L in association with pneumonia. Parapneumonic effusion was those with a glucose concentration >3.3 mmol/L and pH >7.2, and no organisms seen on Gram stain or found on PE culture in a patient with pneumonia.
Twenty-one patients (age range: 36 to 81 yr) with PE were classified as transudates on the basis of Light’s criteria [10].
Sample Collection and Processing
The PE samples were collected in heparin-treated tubes from each subject, using a standard thoracocentesis technique within 24 h after hospitalization. Twenty milliliters of venous blood were drawn simultaneously. The PE specimens were immersed in ice immediately and were then centrifuged at 1,200 g for five min. The cell-free supernatants of PE and sera were frozen at –80°C immediately after centrifuge for later determining concentrations of IL-27, lactate dehydrogenase, and protein. Analyses of PEs for total nucleated cell and differentials counts were performed. Biochemical and cytological characteristics in PEs are summarized in Table in webappendix.
Flow Cytometry
The intracellular expression of IL-27 on various pleural cell types were determined by flow cytometry after surface or intracellular staining with anti-human-specific Abs conjugated with FITC, phycoerythrin, PerCP-cy5.5, or eFluor 660. These human Abs included anti–CD3, –CD4, –CD8, –CD14, –CD19, –CD56, –CD68, and –IL-27 mAbs, which were purchased from BD Biosciences (Franklin Lakes, NJ), eBioscience (San Diego, CA), or R&D systems (Minneapolis, MN). Intracellular staining for IL-27 was performed on pleural cells stimulated with phorbol myristate acetate (50 ng/ml; Sigma-Aldrich St. Louis, MO) and ionomycin (1 µM; Sigma-Aldrich) in the presence of GolgiStop (BD Biosciences) for 4 h, and then stained with anti–IL-27 mAb conjugated with phycoerythrin. To identify pleural mesothelial cells, intracellular staining for calretinin was performed. Fixed and permeabilized mesothelial cells were primarily stained with mouse anti–human calretinin mAb (BD Biosciences), and then stained with FITC goat anti–mouse Igs (BD Biosciences). Appropriate species matched Abs served as isotype control. Flow cytometry was performed on a FACS Canto II (BD Biosciences) and analyzed using BD FCSDiva Software and FCS Epress 4 software (De Novo Software, Los Angeles, CA).
Cell Culture
We isolated peripheral blood mononuclear cells (PBMCs) from five anti-HIV Ab negative subjects with latent tuberculosis infection using Ficoll-Hypaque gradient centrifugation (Pharmacia, Uppsala, Sweden) and cultured them in RPMI-1640 supplemented with penicillin (100 U/ml), streptomycin (100 µg/ml), L-glutamine (2 mM), HEPES (10 mM), 10% fetal bovine serum in flat bottomed 48-well plates (105 cells/0.5 ml) for designated time points. In some experiments, designated doses such as 2.5 µg/ml, 5 µg/ml, 10 µg/ml or 15 µg/ml of MTB-specific peptides of early secretory antigenic target-6 kDa/culture filtrate protein-10 (ESAT-6/CFP-10) (State Key Laboratory of Agricultural Microbiology, Huazhong Agricultural University, Wuhan, China) were added into the culture. At the various time points of 4 h, 12 h, 48 h or 72 h, supernatants were harvested for determining IL-27 concentrations.
Measurement of IL-27
The concentrations of IL-27 in PEs, culture supernatants, and sera were measured by ELISA kit according to the manufacturer’s protocol (BioLegend Inc., San Diego, CA, USA). The minimum detectable concentration of IL-27 was 11.0 ng/L. All samples were assayed in duplicate.
Statistical Analysis
Data were expressed as mean ± SEM. Changes of IL-27 in PE were adjusted to that in serum by calculating either the IL-27 concentration ratio between PE and serum (PE/serum IL-27 ratio) or the difference between the concentrations of IL-27 between the two media (PE–serum IL-27Δ). Parametric tests were used since IL-27 data were normally distributed as determined by a normality test. Comparisons of data between different groups were performed using one-way analysis of variance or Student’s t test. Comparisons of IL-27 levels in PEs and in corresponding sera were made using paired t test. The correlations between variables were determined by Pearson correlation coefficients. Receiver operating characteristic curve analyses were used to evaluate the capacity of IL-27 to differentiate tuberculous PE from PEs with the other etiologies. Analysis was done using SPSS version 16.0 Statistical Software (Chicago, IL, USA), a p-value <0.05 was considered as statistically significant.
Results
Characteristics in PEs
Biochemical and cytological characteristics in PEs are illustrated in Table 1. Subjects with lung cancer showed a large proportion of lymphocytes and macrophages in PE. Subjects with tuberculosis showed a marked elevation of total cell counts, and a large proportion of these cells were lymphocytes, with some neutrophils and macrophages. Absolute lymphocyte counts evidenced the highest values in tuberculous PE, showing a significant increase in comparison with malignant (p = 0.012), infectious (p = 0.009), transudative (p<0.001) PE, respectively. Also as expected, total cell counts in infectious PE were the greatest among four groups, and neutrophil was the most predominant cell type, the numbers of this kind of white cells were significantly greater than those in the PEs with any other causes (all p<0.001).
Concentrations of IL-27 in PEs
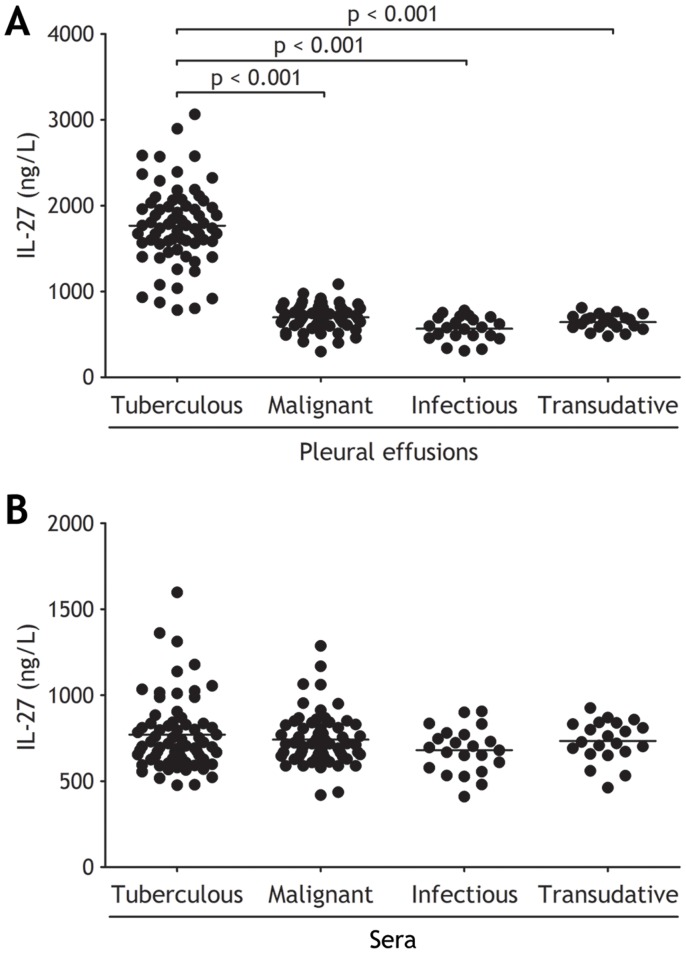
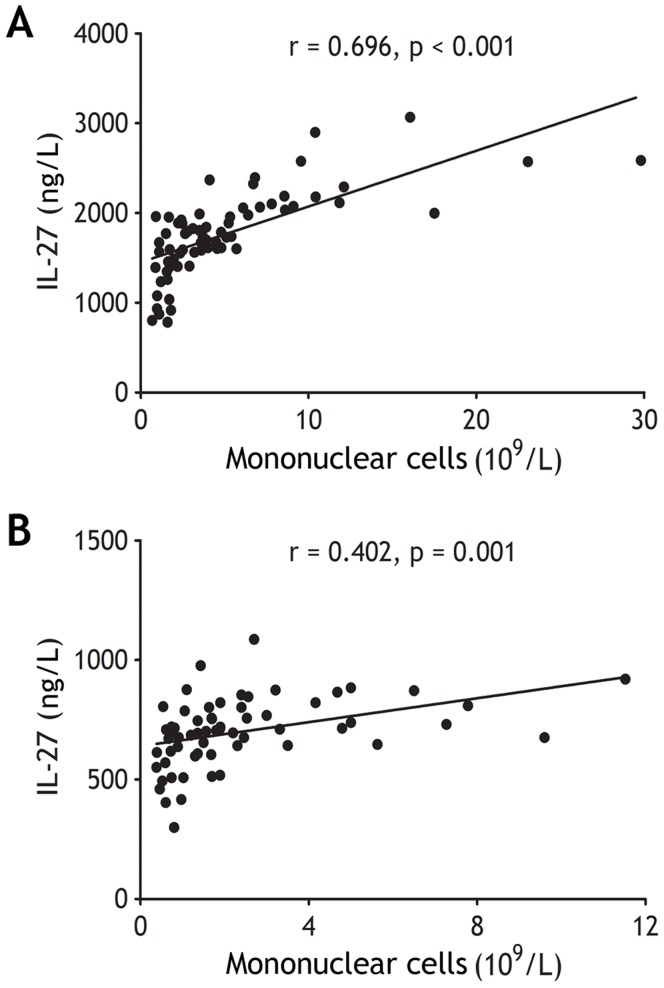
The concentrations of IL-27 in tuberculous PE were significantly higher than those in malignant, infectious, and transudative PE (all p<0.001) (Figure 1A and Table 2). The difference also emerged from both PE/serum IL-27 ratio and PE–serum IL-27Δ that were much higher in tuberculous PE than in non-tuberculous PEs (all p<0.001) (Table 2). On the other hand, concentrations of IL-27 in sera were not different with one another among four groups (all p>0.05) (Figure 1B and Table 2). In addition, concentrations of IL-27 in both tuberculous and malignant PE were correlated positively with numbers of pleural mononuclear cells, including lymphocytes, macrophages, and mesothelial cells (r = 0.696; p<0.001 and 0.402; p = 0.001, respectively) (Figure 2), suggested that PE IL-27 might be produced by these local mononuclear cells.
Figure 1. Comparisons of interleukin (IL)-27 concentrations in pleural effusions (A) and sera (B) in patients with tuberculous (n = 68), malignant (n = 63), infectious (n = 22), and transudative (n = 21) pleural effusion.
Horizontal bars indicate means. Statistical analysis was done by one-way analysis of variance.
Table 2. Concentrations of IL-27 in pleural effusions and sera.
| Pleural effusions | ||||
| Tuberculous | Malignant | Infectious | Transudative | |
| Patients, No. | 68 | 63 | 22 | 21 |
| PE IL-27, ng/L | 1,767.6±56.3† ‡ | 698.9±17.8 | 566.7±29.5‡ | 643.0±19.4‡ |
| Serum IL-27, ng/L | 770.6±25.8 | 741.9±19.1 | 697.6±21.7 | 787.5±23.9 |
| PE/serumIL-27 ratio | 2.35±0.07† | 0.97±0.03 | 0.85±0.05 | 0.90±0.04 |
| PE–serumIL-27Δ, ng/L | 997.0±52.0† | −44.6±24.5 | −113.3±30.4 | −91.1±31.1 |
Values are presented as mean ± SEM; PE = pleural effusion.
p<0.001 compared with each of the other groups determined by one-way analysis of variance.
p<0.01 compared with the corresponding compartments in sera, determined by paired t test.
Figure 2. The concentrations of interleukin (IL)-27 correlated with the numbers of mononuclear cells in tuberculous (A, n = 68) and malignant pleural effusion (B, n = 63).
Correlations were determined by Pearson correlation coefficients.
The comparisons of IL-27 concentrations in PEs and sera in each group are also shown in Table 2. IL-27 concentrations were much higher in tuberculous PE than in the corresponding serum (p<0.001); there was no difference in IL-27 concentrations between PE and serum in malignant group (p = 0.091). IL-27 concentrations were lower in PEs than in sera from patients with infectious (p = 0.001), and transudative (p = 0.008) PE, respectively.
Cell origins of Pleural IL-27
Having known that IL-27 was significantly increased in tuberculous PE, and that PE IL-27 concentrations were correlated positively with numbers of pleural mononuclear cells, we then used flow cytometry to identify which pleural cell types could be the cell origins for IL-27 in PEs. The representative flow cytometric dot-plots are presented Figure 3, and the summary data of percentages of pleural cells expressing IL-27 are presented in Table 3. Our data showed that CD4+ T cells, CD8+ T cells, NK cells, NKT cells, B cells, monocytes, macrophages, and mesothelial cells from both tuberculous and malignant PE expressed intracellular IL-27 at different extents, and that all the above pleural cells, but not NK cells, in tuberculous PE expressed much higher levels of IL-27 than their corresponding compartments in malignant PE did.
Figure 3. The representative flow cytometric dot-plots showing intracellular expressions of interleukin (IL)-27 in CD3+CD4+ T cells, CD3+CD8+ T cells, CD3−CD56+ NK cells, CD3+CD56+ NKT cells, CD3−CD19+ B cells, CD14+ monocytes, CD68+ macrophages, and calretinin-positive mesothelial cells in tuberculous pleural effusion (TPE) and malignant pleural effusion (MPE).
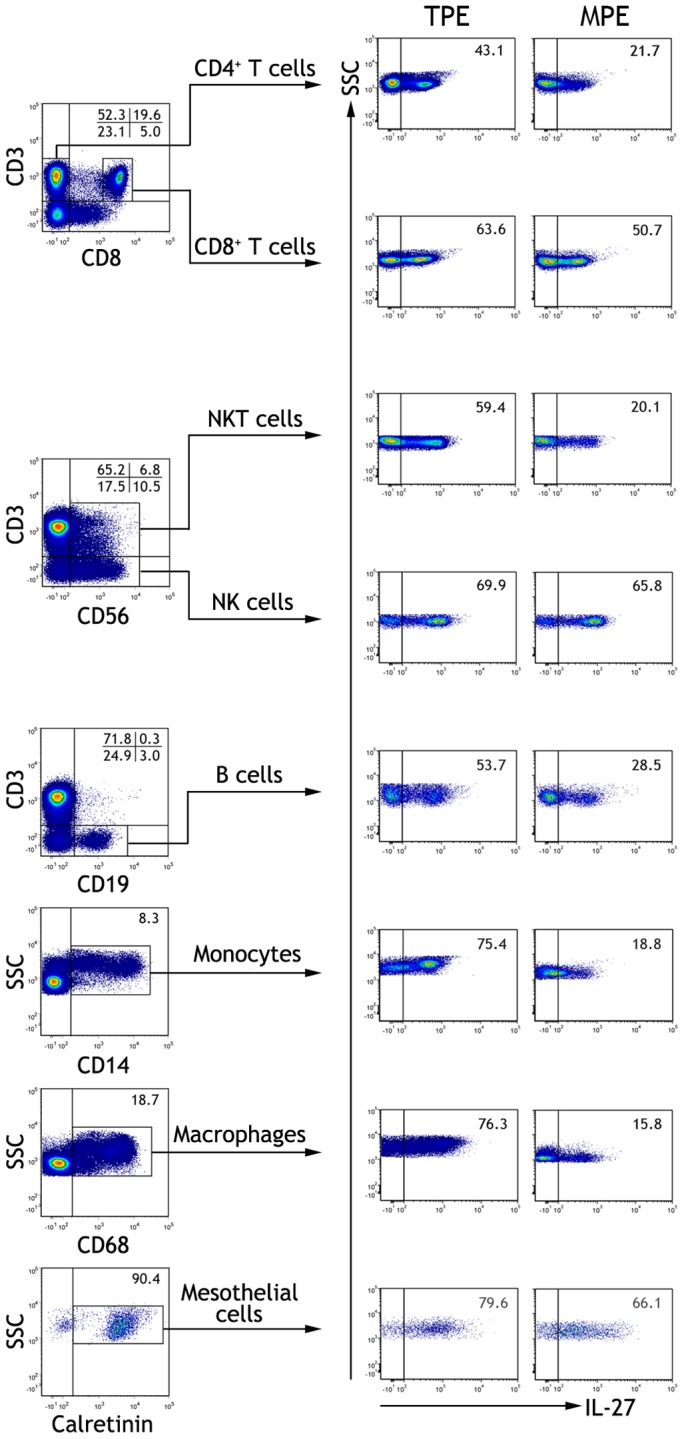
Table 3. Cell origins of IL-27 in tuberculous and malignant pleural effusion.
| IL-27+ cells, (%) | Pleural effusions | ||
| Tuberculous(n = 13) | Malignant(n = 12) | p values | |
| CD4+ T cells | 46.8±2.4 | 20.8±2.0 | <0.001 |
| CD8+ T cells | 70.2±3.7 | 52.9±3.6 | <0.001 |
| NK cells | 73.6±4.1 | 64.8±3.4 | 0.134 |
| NKT cells | 58.7±3.0 | 19.6±0.8 | <0.001 |
| B cells | 56.8±3.7 | 27.5±3.1 | <0.001 |
| Monocytes | 79.3±4.0 | 19.3±1.7 | <0.001 |
| Macrophages | 76.0±5.8 | 16.1±1.0 | <0.001 |
| Mesothelial cells | 81.3±7.3 | 63.2±5.8 | 0.002 |
Values are presented as mean ± SEM. Comparisons were performed by Student’s t test.
To explored effect of MTB antigen on IL-27 production, we isolated PBMCs from subjects with latent tuberculosis infection and cultured them in vitro in the presence of exogenous ESAT-6/CFP-10. As shown in Figure 4, ESAT-6/CFP-10 is capable of inducing IL-27 production from PBMCs in a time- and dose-dependent manner.
Figure 4. Production of interleukin (IL)-27 by peripheral blood mononuclear cells isolated from subjects with latent tuberculosis infection were cultured in vitro in the presence of escalating doses of tuberculosis-specific peptides of early secretory antigenic target-6 kDa/culture filtrate protein-10 at designated time points.
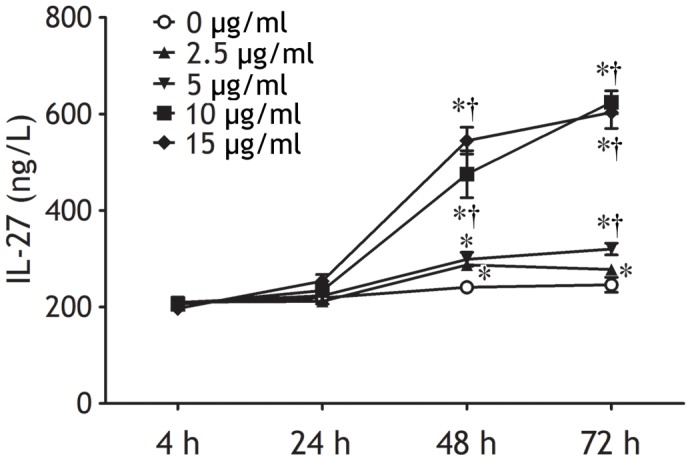
IL-27 concentrations in the culture supernatants were determined by ELISA. The results are reported as mean ± SEM from five independent experiments. The comparisons were determined by one-way analysis of variance. *p<0.05 compared with 4 h within each time-dependent experiment; †p<0.05 compared with medium alone at the same time points within each dose-dependent experiment.
Diagnostic Value of IL-27
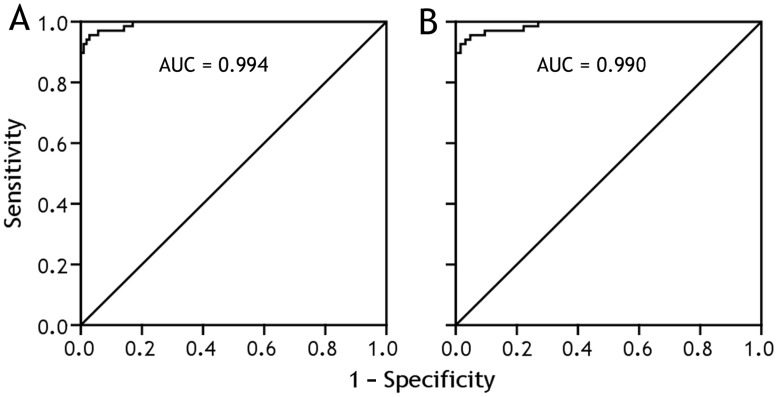
The capacity of IL-27 to differentiate tuberculous PE from PEs with the other etiologies was assessed with receiver operating characteristic curve analyses (Figure 5). The area under curve when IL-27 was used to differentiate tuberculous from non-tuberculous PEs (including malignant, infectious, and transudative PE) was 0.994 (95% confidence internal, 0.986 − 1.001; p<0.001). With a cut-off value of 1,007 ng/L, sensitivity, specificity, positive likelihood ratios, negative likelihood ratio, were 92.7%, 99.1%, 98.21, 0.07, respectively (Table 4). We also noted that the diagnostic performance of PE/serum IL-27 ratio or PE−serum IL-27Δ was almost as high as that of PE IL-27 (Table 4). Also as shown in Table 4, very similar results were observed when we evaluated the diagnostic significance of IL-27, PE/serum IL-27 ratio and PE−serum IL-27Δ for differentiating tuberculous from malignant PE, both are lymphocytic PEs.
Figure 5. Receiver operating characteristic curves of interleukin 27 for differential diagnosis of tuberculous (n = 68) versus non-tuberculous pleural effusions (n = 106) (A), and of tuberculous (n = 68) versus malignant pleural effusion (n = 63). AUC = area under curve.
Table 4. Diagnostic accuracy of IL-27 for tuberculous pleural effusion.
| Tuberculous versus non-tuberculous PEs | Tuberculous versus malignant PE | |||||
| PE IL-27 | PE/serum IL-27 ratio | PE–serum IL-27Δ | PE IL-27 | PE/serum IL-27 ratio | PE–serum IL-27Δ | |
| Cut off value | 1,007 ng/L | 1.464 | 269.1 ng/L | 1,007 ng/L | 1.463 | 269.1 ng/L |
| Area under curve(95% confidence interval) | 0.994(0.986–1.001) | 0.993(0.985–1.002) | 0.996(0.991–1.001) | 0.990(0.978–1.002) | 0.991(0.979–1.002) | 0.995(0.988–1.002) |
| p value | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
| Sensitivity, % | 92.7 | 94.1 | 97.1 | 92.7 | 94.1 | 97.1 |
| Specificity, % | 99.1 | 98.1 | 99.1 | 98.4 | 98.4 | 98.4 |
| Positive likelihood ratio | 98.21 | 99.76 | 102.88 | 58.37 | 59.29 | 61.15 |
| Negative likelihood ratio | 0.07 | 0.06 | 0.03 | 0.07 | 0.06 | 0.03 |
PE = pleural effusion.
Discussion
In the previous studies, we have demonstrated that regulatory T cells and IL-17−producing CD4+ T cells play important immunoregulatory roles in the pathogenesis of lymphocytic PEs [11]–[14]. It has been reported that IL-27 controls the development of regulatory T cells and IL-17−producing CD4+ T cells [15]–[17]. We therefore speculated that IL-27 might be involved in the development of PE.
The first identified biological function for IL-27 was its ability to increase the proliferation of naïve CD4+ T cells [18]. Initial studies with IL-27 receptor deficient mice indicated that IL-27 can promote the generation of Th1-cell responses [19], [20]. It is well accepted that IL-27 regulates various immune diseases through its dual proinflammatory and anti-inflammatory effects on immune responses [21]. In the present study, our data have shown for the first time that IL-27 could be detected in PEs, and that IL-27 concentrations in tuberculous PE were significantly higher than those in malignant, infectious, and transudative PE, suggesting that more pleural sources of IL-27 exist in tuberculous patients. Our results also favored the concept of a local production of IL-27 in tuberculous PE, rather than a passive diffusion of this cytokine from plasma to the pleural compartment, since IL-27 concentration in serum was much lower than that in tuberculous PE.
The fact that increased IL-27 was found in tuberculous PE compared with malignant, infectious, or transudative PE suggested that IL-27 might be of particular interest in tuberculosis. Animal studies have demonstrated that mice deficient in IL-27 or its receptor chains are able to reduce microbial burdens during MTB infection; on the other hand, IL-27 limits the pathological sequelae of chronic inflammation [22], [23]. Moreover, IL-27 can enhance the ability of MTB-specific Th1 cells to inhibit intracellular mycobacterial growth in human macrophages [24]. The precise pathophysiological roles of IL-27 in tuberculous PE need further investigation.
Although few studies have directly addressed the events that lead to the production of IL-27, it is known that IL-27 is mainly produced by activated antigen-presenting cells [6]. To reveal the cell origins of PE IL-27, flow cytometry was performed to determine the expression of IL-27 on pleural cells. Our data showed that CD4+ T cells, CD8+ T cells, NK cells, NKT cells, B cells, monocytes, macrophages, and mesothelial cells from both tuberculous and malignant PE expressed intracellular IL-27, and that all these pleural cell types, but not NK cells, in tuberculous PE expressed much higher IL-27 than their corresponding compartments in malignant PE. We therefore inferred that PE IL-27 might be derived from pleural CD4+ T cells, CD8+ T cells, NK cells, NKT cells, B cells, monocytes, macrophages, and mesothelial cells, and that all these pleural cell types, but not NK cells, were further responsible for the increase in IL-27 in tuberculous PE. The mechanism by which pleural cells expressed more IL-27 in tuberculous than in malignant PE might be associated with MTB antigen stimulation, since we observed in the present study that MTB antigen stimulated IL-27 production from PBMCs in vitro in a time- and dose-dependent manner.
The presence of MTB antigens in pleural space elicits an intense immune response, initially by macrophages and neutrophils [25], [26], followed by Th1 cells [27], resulting in a lymphocyte-predominant exudative effusion. Accompanying the infiltrate of immune cells is the accumulation of cytokines, chemokines, growth factors, and other soluble mediators in PEs, some of them have been proposed to be helpful in diagnosis of PEs [28]. Of all the cytokines, interferon-γ has been the most studied. The evidence for use of pleural interferon-γ for diagnosis of tuberculous PE has been reviewed in our previous meta-analysis [29]. Our analysis showed that the summary estimate of sensitivity of interferon-γ was 89% (95% confidence interval, 87–91%) and of specificity was 97% (96–98%) [30], which seemed to be somehow better than the diagnostic accuracy of adenosine deaminase [30].
To our knowledge, the current study was the first one to investigate the diagnostic value of IL-27 in differential diagnosing tuberculous PE from PEs with the other etiologies (Panel). Using the receiver operating characteristic curve, we noted that the area under curve was 0.994 for IL-27 to diagnose tuberculous PE. The cut off value for IL-27 was selected on the basis of the highest diagnostic accuracy (true positive + true negative/true positive + true negative + false positive + false negative) with the highest sensitivity, which was determined to be 1,007 ng/L. With this cut off, sensitivity and specificity of IL-27 for tuberculous PE were 92.7% and 99.1%, respectively. Our data also revealed a high positive likelihood ratio of 98.21 and a low negative likelihood ratio of 0.07, further confirming IL-27 was a good diagnostic parameter. It should be noted that although IL-27 has a good diagnostic performance, it is a biomarker of the inflammatory process in the pleural space and does not confirm the etiologic agent.
An obvious limitation of this study was the relatively low diversity of PE etiologies. For example, we failed to recruit patients with exudates secondary to collagen vascular diseases, abdominal inflammatory disorders, drugs, etc. When a new marker is considered for diagnosing PE, one always needs to include patients with exudates of different origins. On the other hand, the receiver operating characteristic analysis in a first study such as this might always be more sensitive/specific than seen in real practice, as the cut off was derived from the subjects assessed. It should therefore be mentioned that further prospective studies are necessary to verify the diagnostic value of IL-27 in a larger number of patients with PEs of diverse etiologies.
In conclusion, our data showed that compared to non-tuberculous PEs, IL-27 was increased in tuberculous PE, and that pleural CD4+ T cells, CD8+ T cells, NK cells, NKT cells, B cells, monocytes, macrophages, and mesothelial cells were cell sources of PE IL-27. We further described for the first time that IL-27 appeared to distinguish well between tuberculous and non-tuberculous PEs, especially malignant PE.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: This work was supported in part by a grant from National Basic Research Program of China (973 Program 2012CB518706); in part by a grant from National Science Fund for Distinguished Young Scholars (No. 30925032) of China; and in part by a grant from the 12th Five-Year National Science and Technology Program of Social Development, Ministry of Science and Technology, China (No. 2012BAI05B02). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Sahn SA, Heffner JE. Light RW, Lee YCG, editors. Pleural fluid analysis. 2008. Textbook of pleural diseases. 2nd edn. London: Arnold Press, 209–226.
- 2.Light RW. Clinical practice. Pleural effusion. N Engl J Med. 2002;346:1971–1977. doi: 10.1056/NEJMcp010731. [DOI] [PubMed] [Google Scholar]
- 3.Villena V, López Encuentra A, Echave-Sustaeta J, Alvarez Martínez C, Martín Escribano P. Prospective study of 1,000 consecutive patients with pleural effusion. Etiology of the effusion and characteristics of the patients (Spanish). Arch Bronconeumol. 2002;38:21–26. doi: 10.1016/s0300-2896(02)75142-9. [DOI] [PubMed] [Google Scholar]
- 4.Hamm H, Light RW. Parapneumonic effusion and empyema. Eur Respir J. 1997;10:1150–1156. doi: 10.1183/09031936.97.10051150. [DOI] [PubMed] [Google Scholar]
- 5.Pflanz S, Timans JC, Cheung J, Rosales R, Kanzler H, et al. IL-27, a heterodimeric cytokine composed of EBI3 and p28 protein, induces proliferation of naive CD4+ T cells. Immunity. 2002;16:779–790. doi: 10.1016/s1074-7613(02)00324-2. [DOI] [PubMed] [Google Scholar]
- 6.Batten M, Ghilardi N. The biology and therapeutic potential of interleukin 27. J Mol Med. 2007;85:661–672. doi: 10.1007/s00109-007-0164-7. [DOI] [PubMed] [Google Scholar]
- 7.Hisada M, Kamiya S, Fujita K, Belladonna ML, Aoki T, et al. Potent antitumor activity of interleukin-27. Cancer Res. 2004;64:1152–1156. doi: 10.1158/0008-5472.can-03-2084. [DOI] [PubMed] [Google Scholar]
- 8.O'Dwyer MJ, Mankan AK, White M, Lawless MW, Stordeur P, et al. The human response to infection is associated with distinct patterns of interleukin 23 and interleukin 27 expression. Intensive Care Med. 2008;34:683–691. doi: 10.1007/s00134-007-0968-5. [DOI] [PubMed] [Google Scholar]
- 9.Robinson CM, Nau GJ. Interleukin-12 and interleukin-27 regulate macrophage control of Mycobacterium tuberculosis. J Infect Dis. 2008;198:359–366. doi: 10.1086/589774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Light RW, Macgregor MI, Luchsinger PC, Ball WC., Jr Pleural effusions: the diagnostic separation of transudates and exudates. Ann Intern Med. 1972;77:507–513. doi: 10.7326/0003-4819-77-4-507. [DOI] [PubMed] [Google Scholar]
- 11.Qin XJ, Shi HZ, Liang QL, Liu GN, Jiang J, et al. CCL22 recruits CD4-positive CD25-positive regulatory T cells into malignant pleural effusion. Clin Cancer Res. 2009;15:2231–2237. doi: 10.1158/1078-0432.CCR-08-2641. [DOI] [PubMed] [Google Scholar]
- 12.Wu C, Zhou Q, Qin XJ, Qin SM, Shi HZ. CCL22 is involved in the recruitment of CD4+CD25high T cells into tuberculous pleural effusions. Respirology. 2010;15:522–529. doi: 10.1111/j.1440-1843.2010.01719.x. [DOI] [PubMed] [Google Scholar]
- 13.Ye ZJ, Zhou Q, Gu YY, Qin SM, Ma WL, et al. Generation and differentiation of interleukin-17-producing CD4+ T cells in malignant pleural effusion. J Immunol. 2010;185:6348–6354. doi: 10.4049/jimmunol.1001728. [DOI] [PubMed] [Google Scholar]
- 14.Ye ZJ, Zhou Q, Du RH, Li X, Huang B, et al. The imbalance of Th17 cells and regulatory T cells in tuberculous pleural effusion. Clin Vaccine Immunol. 2011;18:1608–1615. doi: 10.1128/CVI.05214-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wojno ED, Hosken N, Stumhofer JS, O'Hara AC, Mauldin E, et al. A role for IL-27 in limiting T regulatory cell populations. J Immunol. 2011;187:266–273. doi: 10.4049/jimmunol.1004182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stumhofer JS, Laurence A, Wilson EH, Huang E, Tato CM, et al. Interleukin 27 negatively regulates the development of interleukin 17-producing T helper cells during chronic inflammation of the central nervous system. Nat Immunol. 2006;7:937–945. doi: 10.1038/ni1376. [DOI] [PubMed] [Google Scholar]
- 17.Neufert C, Becker C, Wirtz S, Fantini MC, Weigmann B, et al. IL-27 controls the development of inducible regulatory T cells and Th17 cells via differential effects on STAT1. Eur J Immunol. 2007;37:1809–1816. doi: 10.1002/eji.200636896. [DOI] [PubMed] [Google Scholar]
- 18.Hunter CA. New IL-12-family members: IL-23 and IL-27, cytokines with divergent functions. Nat Rev Immunol. 2005;5:521–531. doi: 10.1038/nri1648. [DOI] [PubMed] [Google Scholar]
- 19.Chen Q, Ghilardi N, Wang H, Baker T, Xie MH, et al. Development of Th1-type immune responses requires the type I cytokine receptor TCCR. Nature. 2000;407:916–920. doi: 10.1038/35038103. [DOI] [PubMed] [Google Scholar]
- 20.Yoshida H, Hamano S, Senaldi G, Covey T, Faggioni R, et al. WSX-1 is required for the initiation of Th1 responses and resistance to L. major infection. Immunity. 2001;15:569–578. doi: 10.1016/s1074-7613(01)00206-0. [DOI] [PubMed] [Google Scholar]
- 21.Yoshida H, Nakaya M, Miyazaki Y. Interleukin 27: a double-edged sword for offense and defense. J Leukoc Biol. 2009;86:1295–1303. doi: 10.1189/jlb.0609445. [DOI] [PubMed] [Google Scholar]
- 22.Pearl JE, Khader SA, Solache A, Gilmartin L, Ghilardi N, et al. IL-27 signaling compromises control of bacterial growth in mycobacteria-infected mice. J Immunol. 2004;173:7490–7496. doi: 10.4049/jimmunol.173.12.7490. [DOI] [PubMed] [Google Scholar]
- 23.Hölscher C, Hölscher A, Rückerl D, Yoshimoto T, Yoshida H, et al. The IL-27 receptor chain WSX-1 differentially regulates antibacterial immunity and survival during experimental tuberculosis. J Immunol. 2005;174:3534–3544. doi: 10.4049/jimmunol.174.6.3534. [DOI] [PubMed] [Google Scholar]
- 24.Zhang J, Qian X, Ning H, Eickhoff CS, Hoft DF, et al. Transcriptional suppression of IL-27 production by Mycobacterium tuberculosis-activated p38 MAPK via inhibition of AP-1 binding. J Immunol. 2011;186:5885–5895. doi: 10.4049/jimmunol.1003447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Antony VB, Repine JE, Harada RN, Good JT, Jr, Sahn SA. Inflammatory responses in experimental tuberculosis pleurisy. Acta Cytol. 1983;27:355–361. [PubMed] [Google Scholar]
- 26.Antony VB, Sahn SA, Antony AC, Repine JE. Bacillus Calmette Guérin-stimulated neutrophils release chemotaxins for monocytes in rabbit pleural spaces and in vitro. J Clin Invest. 1985;76:1514–1521. doi: 10.1172/JCI112131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Barnes PF, Mistry SD, Cooper CL, Pirmez C, Rea TH, et al. Compartmentalization of a CD4+ T lymphocyte subpopulation in tuberculous pleuritis. J Immunol. 1989;142:1114–1119. [PubMed] [Google Scholar]
- 28.Trajman A, Pai M, Dheda K, van Zyl Smit R, Zwerling AA, et al. Novel tests for diagnosing tuberculous pleural effusion: what works and what does not? Eur Respir J. 2008;31:1098–1106. doi: 10.1183/09031936.00147507. [DOI] [PubMed] [Google Scholar]
- 29.Jiang J, Shi HZ, Liang QL, Qin SM, Qin XJ. Diagnostic value of interferon-γ in tuberculous pleurisy: a metaanalysis. Chest. 2007;131:1133–1141. doi: 10.1378/chest.06-2273. [DOI] [PubMed] [Google Scholar]
- 30.Liang QL, Shi HZ, Wang K, Qin SM, Qin XJ. Diagnostic precision of adenosine deaminase in tuberculous pleurisy: a meta-analysis. Respir Med. 2008;102:744–754. doi: 10.1016/j.rmed.2007.12.007. [DOI] [PubMed] [Google Scholar]