Abstract
Background
The role of the placenta in fetal programming has been recognized as a highly significant, yet often neglected area of study. We investigated placental size in relation to psychopathology, in particular attention deficit hyperactivity disorder (ADHD) symptoms, in children at 8 years of age, and later as adolescents at 16 years.
Methodology/Principal Findings
Prospective data were obtained from The Northern Finland Birth Cohort (NFBC) 1986. Placental weight, surface area and birth weight were measured according to standard procedures, within 30 minutes after birth. ADHD symptoms, probable psychiatric disturbance, antisocial disorder and neurotic disorder were assessed at 8 years (n = 8101), and ADHD symptoms were assessed again at 16 years (n = 6607), by teachers and parents respectively. We used logistic regression analyses to investigate the association between placental size and mental health outcomes, and controlled for gestational age, birth weight, socio-demographic factors and medical factors, during gestation. There were significant positive associations between placental size (weight, surface area and placental-to-birth-weight ratio) and mental health problems in boys at 8 and 16 years of age. Increased placental weight was linked with overall probable psychiatric disturbance (at 8y, OR = 1.14 [95% CI = 1.04–1.25]), antisocial behavior (at 8 y, OR = 1.14 [95% CI = 1.03–1.27]) and ADHD symptoms (inattention-hyperactivity at 16y, OR = 1.19 [95% CI = 1.02–1.38]). No significant associations were detected among girls.
Conclusions/Significance
Compensatory placental growth may occur in response to prenatal insults. Such overgrowth may affect fetal development, including brain development, and ultimately contribute to psychopathology.
Introduction
Mental health problems, in particular attention deficit hyperactivity disorder (ADHD) symptoms, are a significant cause of functional disability in children and adolescents [1], [2]. While genetic and childhood environmental factors have been studied extensively in relation to the development of psychiatric disorders, accumulating evidence has revealed that prenatal factors are potentially another powerful source of influence [3], in accordance with fetal programming [4], [5]. Indeed, an adverse intrauterine environment has been associated with a range of mental health problems in children, including ADHD symptoms, anxiety and antisocial disorder [6]. However, our understanding of the mechanisms linking prenatal exposures to later health outcomes is very limited. It has recently been suggested that the placenta may be a significant component in translating maternal influences during pregnancy, consequently affecting fetal development and thereby adult health [7], [8]. The placenta, providing an interface between the mother and fetus, appears to be in a key position to mediate fetal programming. Evidence from recent studies indicates that the placenta responds to disturbances in the maternal environment with a range of structural and functional adaptations, including changes in placental growth [8], [9]. Abnormal placental growth is associated with altered fetal nutrient and hormone supply [5], which in turn may induce adaptations in the fetus, thereby programming an increased risk of developing disease in adult life [8]. In light of this, it is surprising that the role of the placenta in the programming of psychopathology has received limited research attention. To our knowledge, only one previous study has examined the relation between placental size and mental health, which found that small placental weight was associated with schizotypal traits in women at 31 years of age [10]. To date, it is not known whether placental size is related to mental health in children and adolescents.
Here we analysed prospective data from a large, longitudinal cohort to investigate whether characteristics related to placental size – placental weight, surface area and placental-to-birth-weight ratio, are associated with mental health, in particular ADHD symptoms, in childhood and adolescence. We investigated sexual dimorphism, due to well-established sex differences in behavior and the placenta. Besides the higher prevalence of behavioral problems among boys compared to girls [11], male placentas are more vulnerable to maternal undernutrition, and more readily undergo compensatory placental growth in response to such insults [12]. We hypothesise that placental size will be related to psychopathology, in particular ADHD symptoms, during childhood (8 years) and later in adolescence (16 years), after controlling for relevant confounders.
Methods
Ethics Statement
The ethics committee of Northern Ostrobotnia Hospital District approved the study, and both parents and adolescents gave written informed consent.
Cohort
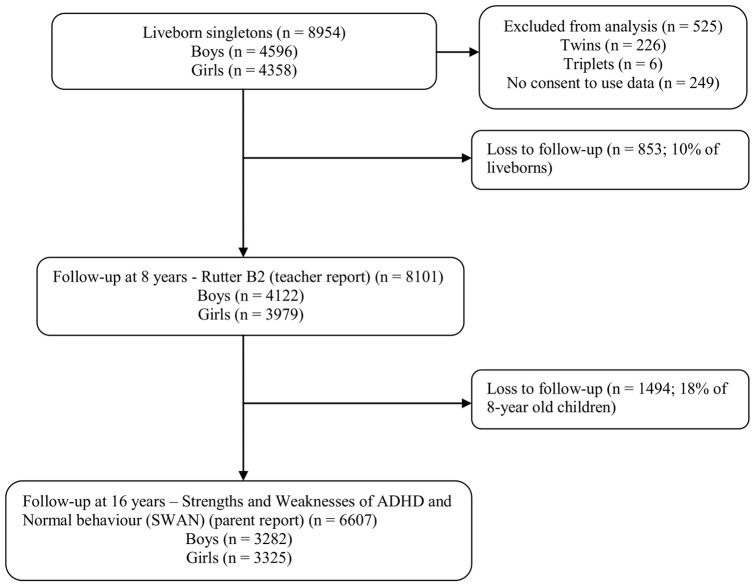
Data were obtained from The Northern Finland Birth Cohort (NFBC) 1986, which consists of 9479 children born in Oulu and Lapland provinces, who were studied prospectively from early pregnancy until 16 years of age (Figure 1). Children with an expected date of birth between July 1, 1985 and June 30, 1986 were eligible; 99% of eligible births in the study area were included.
Figure 1. Flowchart of study participants.
Pregnant women were recruited at maternity health centres, at their first antenatal visit (approximately gestational week 12), and provided background information using structured self-report questionnaires, which were returned by gestational week 24, if still pregnant. Antenatal clinical and birth outcome data were obtained from maternity health centres and hospital medical records (completed by midwives during pregnancy and at birth), and abstracted onto study forms.
After excluding multiple birth children (226 twins and 6 triplets) and those who had not consented use of their data (n = 249), data from 8954 liveborns (4596 boys) were available for analysis (Figure 1). When children were 8 years of age, teachers were asked to complete questionnaires regarding child behavior. Of children still residing in Finland, 90% (n = 8101) of the teachers completed the questionnaire. At 16 years of age, 74% (n = 6607) of families still residing in Finland provided information on adolescent behavior.
Variables
Placental Characteristics
Within 30 minutes following delivery, placentas were washed with water, cleaned from blood clots, and then weighed to the nearest gram. Placental weight included membranes and the umbilical cord, cut approximately 5 cm from the neonate. Whilst spread out on a plane, placental breadth and length were used to measure placental surface area (maternal side) in centimetres squared. The placental-to-birth-weight ratio was calculated by dividing placental weight by birth weight, and then multiplying by 100 to produce a percentage of placental weight relative to birth weight.
Child and Adolescent Behavior
Teachers assessed child behavior at the age of 8 years using the Rutter B2 scale [13], a well-validated screener for childhood psychopathology. Each of the 26 items is rated as either it ‘certainly applies’ (scored = 2), ‘applies somewhat’ (scored = 1) or ‘does not apply’ (scored = 0); yielding a total score between 0 to 52. According to the screening criteria, a total score of ≥9 indicates probable psychiatric disturbance in general. The questionnaire generates three sub-scores: the neurotic sub-score (the sum of four items – often worried, miserable, fearful and tears on arrival at school), the antisocial sub-score (the sum of six items – destructive, fights, disobedient, lies, steals and bullies) and the inattention-hyperactivity sub-score (the sum of three items – restless, squirmy and fidgety, and poor concentration ). Probable positive screen for neurotic disorder is identified by a total score of ≥9 and a higher score on neurotic items versus antisocial items, whereas antisocial disorder is identified by a total score of ≥9 and a higher score on antisocial items versus neurotic items. Probable combined inattention-hyperactivity disorder is defined as a total score of ≥9 and sum of all three inattention-hyperactivity items ≥3. Additionally, we examined the core ADHD symptoms individually i.e. inattention (item ‘poor concentration’ ≥1) and hyperactivity (sum of items ‘restless’ and ‘squirmy and fidgety’ ≥3).
At 16 years of age, parents reported adolescents' behavior using the Strengths and Weaknesses of ADHD symptoms and Normal behavior (SWAN) scale [14]. The SWAN is an 18-item scale, based on the symptoms of ADHD listed in the DSM-IV. As this scale measures both weaknesses (scored as 3, 2 and 1) and strengths (scored as −1, −2 and −3), along with average behavior (scored as 0), it is expected to produce a normal distribution of behavioral scores, thereby reducing the risk of over-identifying youths as screening positive for ADHD. The inattentive subscale and hyperactive-impulsivity subscale each comprise 9 items, and the combined subscale contains all items. The 95th percentile of the distribution of mean scores on each subscale was used as a cut-off point to identify adolescents with ADHD symptoms as probable clinical cases. The cut-off values for the inattention, hyperactive-impulsivity and combined subscales were respectively 0.625, 0.125 and 0.277, previously described in detail [15].
Confounders
We considered potential confounders which may be related to placental size and mental health outcomes, as indicated by our descriptive analysis as well as the literature. These were gestational age (weeks) [16], birth weight (grams) [17] as well as maternal socio-demographic and medical factors [16], [18], [19], [20], all of which were collected prospectively. The socio-demographic factors were maternal age (years), education (either <11 years of education or ≥11 years of education, coded as 0 or 1, respectively), family structure (either married/co-habiting or single/widowed/divorced, coded as 0 or 1, respectively) and social class (by occupation; either class 1 (professional, upper/lower white collar or farmer ≥8 hectares of land) or class 2 (unskilled worker or farmer <8 hectares of land), coded as 0 or 1, respectively). This dichotomized social class variable was based on paternal occupation (or maternal occupation, if missing data or single mother), which was transformed to an indicator of social class according to a national system of classifications and standards in Finland [21]. The medical factors controlled for were parity (included as a continuous variable), pre-pregnancy body mass index (BMI) (pre-pregnancy weight [kg]/height2 [m2]) (included as a continuous variable), gestational weight gain (weight at last antenatal appointment [kg] – pre-pregnancy weight [kg]), and smoking during pregnancy (either no or yes, coded as 0 and 1, respectively).
Statistical Analyses
We initially performed correlation and ANOVA analyses to identify any relations between placental size and potential confounders. Logistic regression analyses were used to investigate the association between placental size (placental weight, surface area and placental-to-birth-weight ratio) and mental health outcomes in children and adolescents. The predictors – the placental characteristics, were included as continuous variables. Placental weight and surface area were initially analysed continuously in 1 g and 1 cm2 increments, respectively. To facilitate clinical interpretation of the results, the main analysis included placental weight and surface area as continuous variables in 100 g and 10 cm2 increments, respectively. All the mental health outcomes were dichotomized, coded as 1 to indicate the presence of mental health problems (and 0 if absent), according to the criteria of each screening instrument. We controlled for gestational age, birth weight, socio-demographic factors (maternal age, education, family structure and social class), and medical factors (parity, pre-pregnancy BMI, gestational weight gain and smoking during pregnancy).
To investigate non-linear associations, we repeated the analysis with stratified placental data (8 groups: <400 g, 400–499 g, 500–599, 600–699 g, 700–799 g, 800–899 g, 900–999 g and ≥1000 g). Furthermore, to assess frequency distributions for descriptive purposes, placental weight was stratified according to its distribution in the study population, such that three categories were formed: <550 g (represents <25th percentile), 550–719 g (represents 25th–75th percentiles) and >720 g (represents >75th percentile).
Using SPSS 17.0, all analyses were conducted separately for males and females, due to sex differences in placentas and behavioral traits.
Results
Table 1 shows birth and child/adolescent behavioural outcomes. For the three categories of placental weight, <550 g, 550–719 g and >720 g, the weight ranges from the mean were 422–530 g, 578–676 g and 726–902 g, respectively, for the entire study sample.
Table 1. Birth and child/adolescent characteristics presented as means ± SD or n (%).
| Characteristic | Mean ± SD or n (%) | n | ||
| All | Male | Female | ||
| Birth outcomes | ||||
| Sex | 4596 (51.3) | 4358 (48.7) | 8954 | |
| Birth weight (g) | 3575±534 | 3638±537 | 3509±523 | 8954 |
| <2500 | 2044±449 | 2025±459 | 2059±441 | 248 |
| 2500–4499 | 3578±424 | 3626±420 | 3528±422 | 8388 |
| ≥4500 | 4704±194 | 4707±197 | 4697±190 | 318 |
| Gestational age at birth (weeks) | 39.4±1.6 | 39.4±1.6 | 39.5±1.6 | 8950 |
| Preterm birth (<37 weeks) | 34.4±2.3 | 34.4±2.3 | 34.3±2.3 | 366 |
| Term birth (≥37 weeks) | 39.6±1.2 | 40.0±1.2 | 40.0±1.2 | 8584 |
| Placental weight (g) | 646±132 | 652±133 | 640±131 | 8934 |
| <550 | 874 (48.6) | 924 (51.4) | 1798 | |
| 550–719 | 2425 (50.8) | 2349 (49.2) | 4774 | |
| ≥720 | 1287 (54.5) | 1075 (45.5) | 2362 | |
| Placental surface area (cm2) | 335±70 | 337±70 | 334±69 | 8821 |
| Placental-to-birth-weight ratio | 18.2+3.1 | 18.0±3.1 | 18.3±3.0 | 8934 |
| Behavioral outcomes | ||||
| 8-y-olds (teacher report) | ||||
| Rutter a | ||||
| Probable psychiatric disturbance | 1140 (14.1) | 801 (19.5) | 339 (8.6) | 8065 |
| Antisocial disorder | 725 (9.0) | 570 (13.9) | 155 (4.0) | 8065 |
| Neurotic disorder | 328 (4.1) | 171 (4.2) | 157 (4.0) | 8065 |
| Inattention-hyperactivity | 756 (9.4) | 576 (14.0) | 180 (4.5) | 8080 |
| Inattentionb | 1705 (21.1) | 1220 (30.0) | 485 (12.2) | 8088 |
| Hyperactivityc | 575 (7.1) | 468 (11.4) | 107 (2.7) | 8086 |
| 16-y-olds (parent report) | ||||
| SWAN a | ||||
| Combined ADHD | 349 (5.3) | 231 (7.0) | 118 (3.5) | 6607 |
| Inattentiond | 332 (5.1) | 230 (7.1) | 102 (3.1) | 6500 |
| Hyperactivity-impulsivitye | 306 (4.7) | 197 (6.1) | 109 (3.3) | 6467 |
assessment of symptoms based on fulfilment of criteria according to the Rutter B2/Strengths and Weaknesses of ADHD symptoms and Normal behavior (SWAN) scale.
assessment based on Rutter item number 16.
assessment based on sum of Rutter items 1 and 3.
SWAN subscale consisting of sum of 9 items.
SWAN subscale consisting of sum of 9 items.
Correlation analyses showed that placental size was significantly associated with the potential confounders. Placental weight significantly correlated with gestational age (r = .23, p<.01), birth weight (r = .65, p<.01), maternal socio-demographic factors (except for education): age (r = .08, p<.01), family structure at birth (r = −.05, p<.01), social class by occupation (r = .02, p<.05), and medical factors: parity (r = .11, p<.01), pre-pregnancy BMI (r = .18, p<.01), gestational weight gain (r = .14, p<.01) and smoking during pregnancy (r = −.05, p<.01). Table 2 shows significant associations between mean placental weight and each of the potential confounders (except for maternal education). Mean placental weight differed significantly between the dichotomized mental health outcomes specifying antisocial disorder (males and entire sample) and neurotic disorder (females) at 8 years, and inattention symptoms (entire sample) at 16 years.
Table 2. Mean placental weight according to potential confounders and child/adolescent mental health outcomes.
| Potential Confounders and Mental Health Outcomes | Mean Placental Weight (g) (SD) | |||||
| n (%) | All | n (%) | Male | n (%) | Female | |
| Potential Confounders | ||||||
| Birth weight (g) | 8934 | 4586 | 4348 | |||
| <2500 | 247 (2.8) | 448 (106) | 110 (2.4) | 455 (108) | 137 (3.2) | 443 (105) |
| 2500–4499 | 8370 (93.7) | 644 (121) | 4259 (92.9) | 646 (121) | 4111 (94.5) | 641 (121) |
| ≥4500 | 317 (3.5) | 860 (138) | 217 (4.7) | 837 (139) | 100 (2.3) | 869 (136) |
| pa | .000 | .000 | .000 | |||
| Gestational age at birth (weeks) | 8930 | 4582 | 4348 | |||
| <37 | 363 (4.1) | 524 (163) | 189 (4.1) | 555 (171) | 174 (4.0) | 528 (154) |
| 37–41 | 8226 (92.1) | 650 (129) | 4213 (92) | 655 (130) | 4013 (92.3) | 644 (127) |
| ≥42 | 341 (3.8) | 600 (127) | 180 (3.9) | 663 (124) | 161 (3.7) | 656 (131) |
| pa | .000 | .000 | .000 | |||
| Maternal age (years) | 8934 | 4586 | 4348 | |||
| <20 | 655 (7.3) | 636 (125) | 336 (7.3) | 645 (120) | 319 (7.3) | 626 (129) |
| 20–34 | 7105 (79.5) | 645 (131) | 3635 (79.3) | 651 (133) | 3470 (79.8) | 639 (129) |
| ≥35 | 1174 (13.1) | 654 (142) | 615 (13.4) | 657 (143) | 559 (12.9) | 652 (141) |
| pa | .012 | .381 | .019 | |||
| Maternal education (years) | 7855 | 3999 | 3856 | |||
| <11 | 2377 (30.3) | 651 (138) | 1184 (29.6) | 657 (136) | 1193 (30.9) | 645 (139) |
| ≥11 | 5478 (69.7) | 645 (127) | 2815 (70.4) | 651 (129) | 2663 (69.1) | 640 (125) |
| pa | .089 | .172 | .265 | |||
| Family structure | 8908 | 4570 | 4338 | |||
| Married/cohabiting | 8453 (94.9) | 647 (132) | 4347 (95.1) | 654 (133) | 4106 (94.7) | 641 (131) |
| Single/widowed/divorced | 455 (5.1) | 620 (127) | 223 (4.9) | 614 (123) | 232 (5.3) | 625 (130) |
| pa | .000 | .000 | .069 | |||
| Family social class (by occupation) | 8646 | 4438 | 4208 | |||
| I | ||||||
| Professional | 521 (6.0) | 647 (132) | 265 (6.0) | 647 (142) | 256 (6.1) | 647 (121) |
| Upper white collar | 1723 (20.0) | 648 (127) | 853 (19.2) | 659 (131) | 870 (20.7) | 638 (122) |
| Lower white collar | 3476 (40.2) | 644 (131) | 1802 (40.6) | 651 (130) | 1674 (39.8) | 638 (131) |
| Farmer ≥8 hectares | 348 (4.0) | 666 (146) | 178 (4.0) | 676 (154) | 170 (4.0) | 655 (136) |
| II | ||||||
| Unskilled worker | 2525 (29.2) | 642 (133) | 1319 (29.7) | 645 (131) | 1206 (28.7) | 640 (135) |
| Farmer <8 hectares | 53 (.6) | 672 (178) | 21 (.5) | 731 (202) | 32 (.7) | 633 (151) |
| pa | .025 | .001 | .565 | |||
| Parity | 8895 | 4565 | 4330 | |||
| 0 | 3029 (34.1) | 623 (128) | 1551 (34) | 629 (128) | 1478 (34.1) | 617 (128) |
| 1 | 2969 (33.4) | 653 (132) | 1485 (32.5) | 658 (134) | 1484 (34.3) | 647 (130) |
| 2 | 1604 (18.0) | 660 (134) | 863 (18.9) | 669 (136) | 741 (17.1) | 650 (126) |
| ≥3 | 1293 (14.5) | 666 (132) | 666 (14.6) | 666 (133) | 627 (14.5) | 666 (136) |
| pa | .000 | .000 | .000 | |||
| Pre-pregnancy BMI (kg/m2) | 8708 | 4478 | 4230 | |||
| <20 | 2136 (24.5) | 612 (121) | 1138 (25.4) | 618 (120) | 998 (23.6) | 606 (123) |
| 20–24.99 | 5078 (58.3) | 649 (130) | 2576 (57.5) | 655 (132) | 2502 (59.1) | 643 (127) |
| ≥25 | 1494 (17.2) | 683 (142) | 764 (17.1) | 688 (142) | 730 (17.3) | 677 (141) |
| pa | .000 | .000 | .000 | |||
| Gestational weight gain (kg) | 8262 | 4244 | 4018 | |||
| <11 | 1911 (23.1) | 620 (132) | 909 (21.4) | 623 (137) | 1002 (25.0) | 617 (128) |
| 11–16.99 | 4310 (52.2) | 645 (126) | 2223 (52.4) | 650 (125) | 2087 (51.9) | 640 (126) |
| ≥17 | 2041 (24.7) | 673 (136) | 1112 (26.2) | 679 (136) | 929 (23.1) | 666 (130) |
| pa | .000 | .000 | .000 | |||
| Smoking during pregnancy | 8696 | 4455 | 4241 | |||
| No | 6989 (80.4) | 649 (131) | 3564 (80.0) | 654 (133) | 3425 (80.8) | 644 (130) |
| Yes | 1707 (19.6) | 633 (130) | 891 (20.0) | 638 (131) | 816 (19.2) | 627 (129) |
| pa | .000 | .001 | .001 | |||
| Number of cigarettes (per day) | 939 | 500 | 439 | |||
| <10 cigarettes | 556 (59.2) | 630 (121) | 293 (58.6) | 634 (116) | 263 (59.9) | 626 (127) |
| ≥10 cigarettes | 383 (40.8) | 631 (138) | 207 (41.4) | 641 (138) | 176 (40.1) | 618 (137) |
| pa | .968 | .539 | .544 | |||
| Mental Health Outcomes | ||||||
| 8-y-olds Rutter (teacher report) b | ||||||
| Probable psychiatric disturbance | 8046 | 4097 | 3949 | |||
| Yes | 1139 (14.2) | 651 (138) | 800 (19.5) | 660 (141) | 339 (8.6) | 632 (130) |
| No | 6907 (85.8) | 646 (129) | 3297 (80.5) | 651 (127) | 3610 (91.4) | 642 (129) |
| pa | .245 | .089 | .145 | |||
| Antisocial disorder | 8046 | 4097 | 3949 | |||
| Yes | 724 (9.0) | 659 (142) | 569 (13.9) | 663 (144) | 155 (3.9) | 646 (135) |
| No | 7322 (91.0) | 646 (129) | 3528 (86.1) | 651 (128) | 3794 (96.1) | 641 (129) |
| pa | .010 | .046 | .683 | |||
| Neurotic disorder | 8046 | 4097 | 3949 | |||
| Yes | 328 (4.1) | 641 (133) | 171 (4.2) | 660 (137) | 157 (4.0) | 621 (126) |
| No | 7718 (95.9) | 647 (130) | 3926 (95.8) | 652 (130) | 3792 (96.0) | 642 (130) |
| pa | .419 | .417 | .041 | |||
| Inattention- hyperactivity | 8061 | 4103 | 3958 | |||
| Yes | 755 (9.4) | 650 (138) | 575 (14.0) | 656 (136) | 180 (4.5) | 628 (140) |
| No | 7306 (90.6) | 647 (129) | 3528 (86.0) | 652 (129) | 3778 (95.5) | 642 (129) |
| pa | .568 | .442 | .163 | |||
| Inattentionc | 8069 | 4108 | 3961 | |||
| Yes | 1703 (21.1) | 650 (135) | 1218 (29.6) | 655 (135) | 485 (12.2) | 639 (132) |
| No | 6366 (78.9) | 646 (129) | 2890 (70.4) | 652 (128) | 3476 (87.8) | 642 (129) |
| pa | .281 | .496 | .625 | |||
| Hyperactivityd | 8067 | 4105 | 3962 | |||
| Yes | 573 (7.1) | 654 (137) | 466 (11.4) | 655 (138) | 107 (2.7) | 653 (132) |
| No | 7494 (92.9) | 646 (129) | 3639 (88.6) | 652 (129) | 3855 (97.3) | 641 (130) |
| pa | .159 | .677 | .354 | |||
| 16-y-olds SWAN (parent report) b | ||||||
| Combined ADHD | 6594 | 3275 | 3319 | |||
| Yes | 349 (5.3) | 659 (138) | 231 (7.1) | 668 (144) | 118 (3.6) | 641 (123) |
| No | 6245 (94.7) | 647 (129) | 3044 (92.9) | 652 (128) | 3201 (96.4) | 642 (129) |
| pa | .098 | .076 | .930 | |||
| Inattentione | 6488 | 3227 | 3261 | |||
| Yes | 332 (5.1) | 663 (138) | 230 (7.1) | 666 (140) | 102 (3.1) | 656 (132) |
| No | 6156 (94.9) | 646 (128) | 2997 (92.9) | 652 (127) | 3159 (96.9) | 641 (129) |
| pa | .024 | .110 | .256 | |||
| Hyperactivity-impulsivityf | 6455 | 3206 | 3249 | |||
| Yes | 306 (4.7) | 650 (135) | 197 (6.1) | 660 (135) | 109 (3.4) | 632 (133) |
| No | 6149 (95.3) | 647 (129) | 3009 (93.9) | 652 (129) | 3140 (96.6) | 642 (128) |
| pa | .677 | .402 | .427 | |||
for heterogeneity, analysis of variance.
assessment of symptoms based on fulfilment of criteria according to the Rutter B2/Strengths and Weaknesses of ADHD symptoms and Normal behavior (SWAN) scale.
assessment based on Rutter item number 16.
assessment based on sum of Rutter items 1 and 3.
SWAN subscale consisting of sum of 9 items.
SWAN subscale consisting of sum of 9 items.
Both unadjusted and adjusted logistic regression analyses revealed positive associations between placental size (placental weight, surface area and placental-to-birth-weight ratio – as continuous variables) and mental health outcomes in boys at 8 and 16 years of age, as shown in Table 3. The adjusted analyses largely show stronger and more significant associations between male placental size and mental health outcomes, including overall probable psychiatric disturbance, ADHD symptoms and antisocial disorder in 8-year olds, and ADHD symptoms in 16-year olds. For example, for every 100 g increase in placental weight, the risk for probable psychiatric disturbance at 8 years and combined inattention-hyperactivity at 16 years increased by 14% and 19%, respectively. Furthermore, the adjusted results indicate that compared to placental surface area (10 cm2) and placental-to-birth-weight ratio, increased placental weight (100 g) was the strongest predictor of mental health problems in boys at both 8 and 16 years of age. Linear analyses indicated that a respective increase of 1 g and 1 cm2 in placental weight and surface area were significantly associated with mental health problems in boys. A 1 g increase in placental weight was associated with probable psychiatric disturbance at 8 years (OR = 1.001 [95% CI = 1.000–1.002]) and combined inattention-hyperactivity at 16 years (OR = 1.002 [95% CI = 1.000–1.003]), and a 1 cm2 increase in placental surface area was associated with inattention-hyperactivity symptoms at 8 years (OR = 1.002 [95% CI = 1.001–1.004]) and inattention symptoms at 16 years (OR = 1.003 [95% CI = 1.000–1.005]). A 10 g increase in placental weight i.e. within 1 SD from the mean, was positively associated with mental health problems in boys (for probable psychiatric disturbance at 8 years, OR = 1.013 [95% CI = 1.004–1.023]). In girls, no significant associations were detected between placental size and mental health outcomes at either 8 years or 16 years of age, as shown in Table 4.
Table 3. Logistic regression results for the association between male placental size (weight, surface area and placental-to-birth-weight ratio) and mental health outcomes.
| Behavior | Male Placental Weight (100g) | Male Placental Surface Area (10cm2) | Male Placental-to-Birth-Weight Ratio | ||||||||||||
| Unadjusted | Adjusteda | Unadjusted | Adjusteda | Unadjusted | Adjustedb | ||||||||||
| OR | 95% CI | n | OR | 95% CI | OR | 95% CI | n | OR | 95% CI | OR | 95% CI | n | OR | 95% CI | |
| 8-y-olds Rutter (teacher report) | |||||||||||||||
| Probable psychiatric disturbance | 1.05 | .99–1.12 | 3276 | 1.14** | 1.04–1.25 | 1.01 | 1.00–1.02 | 3236 | 1.01 | 1.00–1.03 | 1.04** | 1.01–1.07 | 3276 | 1.04* | 1.01–1.08 |
| Antisocial disorder | 1.07* | 1.00–1.15 | 3276 | 1.14* | 1.03–1.27 | 1.01 | 1.00–1.02 | 3236 | 1.02* | 1.00–1.04 | 1.05** | 1.02–1.08 | 3276 | 1.04* | 1.01–1.08 |
| Neurotic disorder | 1.05 | .93–1.18 | 3276 | 1.19 | .99–1.42 | 1.00 | .98–1.02 | 3236 | 1.00 | .97–1.03 | 1.04 | .98–1.09 | 3276 | 1.06 | .99–1.13 |
| Inattention-hyperactivity | 1.03 | .96–1.10 | 3282 | 1.11* | 1.00–1.24 | 1.01 | 1.00–1.02 | 3242 | 1.02** | 1.01–1.04 | 1.03 | 1.00–1.07 | 3282 | 1.03 | .99–1.07 |
| Inattention | 1.02 | .97–1.07 | 3286 | 1.11* | 1.02–1.20 | 1.00 | .99–1.01 | 3246 | 1.01 | 1.00–1.03 | 1.03** | 1.01–1.06 | 3286 | 1.03* | 1.00–1.06 |
| Hyperactivity | 1.02 | .94–1.09 | 3283 | 1.12* | 1.00–1.26 | 1.01 | 1.00–1.02 | 3243 | 1.03** | 1.01–1.05 | 1.03 | .99–1.06 | 3283 | 1.04 | .99–1.08 |
| 16-y-olds SWAN (parent report) | |||||||||||||||
| Combined ADHD | 1.10 | .99–1.21 | 2754 | 1.19* | 1.02–1.38 | 1.01 | .99–1.03 | 2724 | 1.02 | 1.00–1.05 | 1.06* | 1.01–1.11 | 2754 | 1.06* | 1.00–1.11 |
| Inattention | 1.09 | .98–1.20 | 2720 | 1.17* | 1.00–1.37 | 1.01 | .99–1.03 | 2691 | 1.03* | 1.01–1.06 | 1.06* | 1.01–1.11 | 2720 | 1.05 | 1.00–1.11 |
| Hyperactivity-impulsivity | 1.05 | .94–1.17 | 2701 | 1.11 | .94–1.31 | 1.01 | .99–1.03 | 2672 | 1.02 | 1.00–1.05 | 1.03 | 1.00–1.08 | 2701 | 1.03 | .97–1.09 |
adjusted for gestational age, birth weight, socio-demographic factors (maternal age, family structure, education and social class) and medical factors (smoking during pregnancy, parity, pre-pregnancy BMI and gestational weight gain).
adjusted as above, except for birth weight.
p<.05; **p<.01.
Table 4. Logistic regression results for the association between female placental size (weight, surface area and placental-to-birth-weight ratio) and mental health outcomes.
| Behavior | Female Placental Weight (100g) | Female Placental Surface Area (10cm2) | Female Placental-to-Birth-Weight Ratio | ||||||||||||
| Unadjusted | Adjusteda | Unadjusted | Adjusteda | Unadjusted | Adjustedb | ||||||||||
| OR | 95% CI | n | OR | 95% CI | OR | 95% CI | n | OR | 95% CI | OR | 95% CI | n | OR | 95% CI | |
| 8-y-olds Rutter (teacher report) | |||||||||||||||
| Probable psychiatric disturbance | .94 | .86–1.02 | 3176 | .91 | .79–1.04 | 1.00 | .98–1.01 | 3140 | 1.00 | .97–1.02 | .99 | .95–1.03 | 3176 | .97 | .92–1.01 |
| Antisocial disorder | 1.03 | .91–1.16 | 3176 | .97 | .80–1.20 | 1.01 | .99–1.03 | 3140 | 1.01 | .99–1.04 | 1.00 | .95–1.06 | 3176 | .98 | .92–1.05 |
| Neurotic disorder | .88 | .77–.99 | 3176 | .88 | .72–1.10 | .99 | .97–1.02 | 3140 | 1.00 | .96–1.02 | .97 | .92–1.03 | 3176 | .96 | .89–1.02 |
| Inattention-hyperactivity | .92 | .82–1.04 | 3185 | .90 | .75–1.1 | .99 | .97–1.01 | 3149 | .99 | .96–1.02 | .98 | .93–1.03 | 3185 | .95 | .90–1.02 |
| Inattention | .98 | .91–1.10 | 3187 | 1.01 | .90–1.13 | 1.00 | .99–1.01 | 3151 | 1.01 | .99–1.03 | 1.02 | .99–1.10 | 3187 | 1.00 | .96–1.04 |
| Hyperactivity | 1.07 | .93–1.24 | 3189 | .95 | .76–1.19 | 1.00 | .97–1.03 | 3153 | .99 | .95–1.03 | .99 | .93–1.06 | 3189 | .98 | .90–1.06 |
| 16-y-olds SWAN (parent report) | |||||||||||||||
| Combined ADHD | .99 | .86–1.15 | 2779 | .92 | .74–1.15 | 1.01 | .98–1.04 | 2745 | 1.01 | .98–1.05 | .99 | .93–1.05 | 2779 | .97 | .90–1.05 |
| Inattention | 1.09 | .94–1.27 | 2737 | 1.20 | .95–1.50 | 1.01 | .98–1.04 | 2702 | 1.02 | .98–1.06 | 1.05 | .98–1.11 | 2737 | 1.06 | .98–1.14 |
| Hyperactivity-impulsivity | .94 | .81–1.10 | 2725 | .91 | .73–1.15 | 1.00 | .97–1.03 | 2690 | 1.01 | .98–1.05 | .98 | .92–1.05 | 2725 | .96 | .89–1.04 |
adjusted for gestational age, birth weight, socio-demographic factors (maternal age, family structure, education and social class) and medical factors (smoking during pregnancy, parity, pre-pregnancy BMI and gestational weight gain).
adjusted as above, except for birth weight.
After repeating the analysis using stratified placental data, we did not find evidence for a U-shaped association, as small placental size was unrelated to the mental health outcomes.
Discussion
This is the first study to investigate the relation between placental size and mental health outcomes in children and adolescents. We identified a positive association between placental size (placental weight, surface area and placental-to-birth-weight ratio) and mental health problems in boys; increased placental size was linked with overall probable psychiatric disturbance, ADHD symptoms and antisocial disorder at 8 years of age, and ADHD symptoms at 16 years, after adjusting for known confounders. It is important to note that the magnitude of the associations were small; however, because psychiatric disorders are highly complex – in terms of both etiology and diagnosis, it is expected that any single variable will contribute a small portion of the variance. Our results suggest that variation in placental size may play a role in the causal pathway leading from prenatal exposures to later mental health problems, but further work is required to determine causality.
Placental overgrowth may occur as a compensatory mechanism in response to various maternal prenatal insults [9], [22], [23], [24]. It has been theorized that an enlarged placenta may reduce its supply of nutrients to the fetus [5]; consequent fetal adaptations may lead to permanent structural and physiological changes to developing organs, programming an increased risk of disease. Our results are in line with this, such that we may speculate that increased placental size – a possible consequence of an adverse maternal environment, may alter fetal nutrient supply, which in turn may affect normal brain development [25], [26], increasing the risk of psychiatric problems later in life. In support of this potential mechanism, prenatal psychosocial stress, a common environmental insult, has been associated with increased placental weight [9], and has been directly and independently linked with atypical cerebral laterality [27], [28], and an increased risk of psychiatric problems in children [29], [30]. Furthermore, atypical cerebral lateralization, which has been linked to child and adolescent mental health problems (including ADHD [31], [32]), was detected in 8–9 year old children who had a moderately low birth weight and disproportionately large placenta [33]. On the surface, it may seem that the suggested mechanism implies that an increased placental-to-birth weight ratio would be the strongest predictor of psychiatric outcomes. However, whilst possibly reducing nutrient supply to the fetus, compensatory mechanisms in the expanding placenta may counteract any intrauterine deficiencies, so that a normal birth weight is still achieved despite suboptimal conditions [7]. This concept could explain why we found increased placental weight was the strongest predictor of psychopathology, compared to the other placental characteristics which we studied. Thus, an enlarged placenta may represent an important link between disturbances in the maternal environment and perturbed fetal brain development, with ensuing mental health problems in children and adolescents.
Variation in the size of the placenta affects aspects of its function, in particular the ability to transfer nutrients to the fetus via changes in the exchange surface area [34]; in general, small placentas are associated with small fetuses [35]. Placental size is affected by maternal factors, such as BMI, gestational weight gain and smoking [16], as well as various other medical and socio-demographic factors, as demonstrated by our own results, indicating that the placenta is receptive to the maternal environment, and undergoes changes in size in an effort to maintain fetal development under suboptimal conditions [7]. For example, in response to maternal undernutrition, the placenta may undergo compensatory enlargement [22]; although this adaptation may improve the overall nutrient supply to the fetus, ensuring a normal birth weight is achieved, the relative contribution of specific nutrients to fetal organs may be altered, resulting in the programming of developing organs [7]. Thus, placental compensatory mechanisms may often ensure a normal birth weight is achieved in adverse circumstances, whilst the placenta itself may be markedly affected [36] – reflecting the physiological stresses which occurred during development. Therefore, compared to birth weight and other common indices used to identify suboptimal intrauterine conditions, placental phenotype can provide additional insight into the intrauterine environment and improve our understanding of the processes underlying fetal programming. Furthermore, placental size may enhance the ability to predict later disease, as several lines of evidence suggest [36], [37], [38].
It is interesting to note that placental size was related to a range of mental health problems (including general probable psychiatric disturbance) in this study. This may correspond to the understanding that placental size is sensitive to various maternal influences, and thus possibly represents an archive of gestational insults, which could affect the developing brain non-specifically.
While there is evidence for a U-shaped relation between placental size and various health outcomes [39], here we report a positive linear association, which may be explained by factors related to the direction of placental-fetal growth disproportion, including maternal nutritional status and the timing of prenatal insults. A study by Barker et al. found that in the offspring of tall, middle-class mothers, who were likely to be well nourished, hypertension was related to large placental size [40]. As Finland is a high income country, it is likely that the general nutritional standard of the nation is good. Furthermore, Finland has an exemplary antenatal care system, along with very low infant and maternal mortality rates [41]. Thus, it is likely that women in the NFBC 1986 experienced compensatory placental growth in response to adverse prenatal conditions. Extreme maternal undernutrition at early and late stages of gestation have respectively been associated with increased and reduced placental size [22]. We speculate that prenatal insults related to socio-demographic factors, which tend to be chronic, may induce a trajectory for increased placental size from the start of pregnancy. Acute gestational insults in late pregnancy may alone reduce placental size; however such insults are less common than perhaps chronic stressors, and so the effect of late gestational insults on placental size may be diminished, thereby masking a potential relationship between small placental size and mental health problems.
In the present study, large placental size was associated with psychiatric problems only in boys. Male placentas may be more sensitive to prenatal insults, and more readily undergo compensatory growth in response to such disturbances [12]. Compared to girls, boys grow faster throughout gestation [42], are usually longer at any placental weight and have a smaller placental-to-birth-weight ratio [43]; it has thus been postulated that male placentas are more efficient but have less reserve capacity, causing them to be more vulnerable to undernutrition [12], and presumably to other forms of physiological stress. One study found that in response to maternal asthma during pregnancy, there was no change in the activity of the male placental hydroxysteroid (11-beta) dehydrogenase 2 (HSD11B2) enzyme – the fetoplacental barrier to maternal cortisol, and the fetus continued to grow [44]. In contrast, females showed reduced placental HSD11B2 activity and decreased fetal growth. The authors suggested that the lack of response by the male placenta may contribute to the increased risk of morbidity and mortality of the male fetus. Furthermore, a study in mice demonstrated that male placentas were more vulnerable to prenatal stress, exhibiting an increase in the expression of placental genes related to growth factors [45], which may lead to increased male placental size.
The present study has a number of strengths, including use of prospective data, derived from a large, longitudinal, population-based cohort. Placental measurements were performed according to standard procedures, by medical personnel at the time of birth. Furthermore, mental health outcomes were assessed twice over an 8-year period, using validated screening instruments. Nevertheless, the findings should be viewed in light of the following limitations. First, placental weight included membranes and the umbilical cord, and since these components are not involved in nutrient exchange, this may in particular affect measurement of the placental-to-birth weight ratio. Second, placental size provides only limited insight into the role of the placenta in fetal programming [8]. Recent work has found that the expression of placental HSD11B2 mRNA is decreased in anxious pregnant women [46]; since increased fetal exposure to cortisol has been associated with neurodevelopmental disorders later in life [30], it is possible that reduced placental HSD11B2 activity provides a link between altered placental function and fetal programming [47]. In animals, reduced HSD11B2 levels have been associated with decreased placental weight [48]; it is of interest to investigate whether this is also the case in humans, which should provide further insight into how placental size relates to function. Third, we only examined ADHD symptoms in adolescence due to limited data availability. Fourth, we did not assess mental health diagnosis, but rather whether children/adolescents screened positive for probable diagnosis based on symptoms. This may help explain why the frequencies of mental health problems in this study appear somewhat high, in particular at 8 years, but are typical at the symptom level for children of this age.
In conclusion, this study shows that placental size was associated with mental health problems in boys during childhood and adolescence. Placental enlargement may occur in response to chronic adverse intrauterine conditions, and could lead to altered fetal brain development, with long-term effects on mental health. Future work is required to determine whether deviation in placental size is causal or lies on the causal pathway linking prenatal exposures to child psychopathology.
Acknowledgments
The authors wish to thank Professor Paula Rantakallio (launch of NFBC 1986). Part of this work was conducted while Dr Rodriguez was at Uppsala University, Sweden.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: Academy of Finland (103451); Sigrid Juselius Foundation, Finland; Thule Institute; University of Oulu, Finland; the National Institute of Mental Health (MH63706); and EURO-BLCS. Dr Rodriguez received support from VINNMER (P32925-1). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Rodriguez A, Jarvelin M-R, Obel C, Taanila A, Miettunen J, et al. Do inattention and hyperactivity symptoms equal scholastic impairment? evidence from three European cohorts. BMC Public Health 7. 2007;327:10.1186/1471–2458-7-327. doi: 10.1186/1471-2458-7-327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Meltzer H, Gatward R, Goodman R, Ford T. Mental health of children and adolescents in Great Britain. International Review of Psychiatry 15. 2003;185–187:10.1080/0954026021000046155. doi: 10.1080/0954026021000046155. [DOI] [PubMed] [Google Scholar]
- 3.Schlotz W, Phillips DIW. Fetal origins of mental health: evidence and mechanisms. Brain, Behavior, and Immunity 23. 2009;905–916:10.1016/j.bbi.2009.02.001. doi: 10.1016/j.bbi.2009.02.001. [DOI] [PubMed] [Google Scholar]
- 4.Gluckman PD, Hanson MA. Living with the past: evolution, development, and patterns of disease. Science 305. 2004;1733–1736:10.1126/science.1095292. doi: 10.1126/science.1095292. [DOI] [PubMed] [Google Scholar]
- 5.Godfrey KM, Barker DJ. Fetal programming and adult health. Public Health Nutrition 4. 2001;611–624:10.1079/PHN2001145. doi: 10.1079/phn2001145. [DOI] [PubMed] [Google Scholar]
- 6.Glover V. Annual Research Review: Prenatal stress and the origins of psychopathology: an evolutionary perspective. Journal of Child Psychology and Psychiatry 52: 356–367. 2011;10(1111/j.1469-7610.2011.02371):x. doi: 10.1111/j.1469-7610.2011.02371.x. [DOI] [PubMed] [Google Scholar]
- 7.Fowden AL, Forhead AJ, Coan PM, Burton GJ. The placenta and intrauterine programming. Journal of Neuroendocrinology 20: 439–450. 2008;10(1111/j.1365-2826.2008.01663):x. doi: 10.1111/j.1365-2826.2008.01663.x. [DOI] [PubMed] [Google Scholar]
- 8.Lewis RM, Poore KR, Godfrey KM. The role of the placenta in the developmental origins of health and disease–implications for practice. Reviews in Gynaecological and Perinatal Practice 6. 2006;70–79:10.1016/j.rigapp.2005.12.001. [Google Scholar]
- 9.Tegethoff M, Greene N, Olsen J, Meyer AH, Meinlschmidt G. Maternal psychosocial stress during pregnancy and placenta weight: evidence from a national cohort study. PLoS ONE 5. 2010;e14478:10.1371/journal.pone.0014478. doi: 10.1371/journal.pone.0014478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lahti J, Raïkkönen K, Sovio U, Miettunen J, Hartikainen A-L, et al. Early-life origins of schizotypal traits in adulthood. The British Journal of Psychiatry 195. 2009;132–137:10.1192/bjp.bp.108.054387. doi: 10.1192/bjp.bp.108.054387. [DOI] [PubMed] [Google Scholar]
- 11.Faraone SV, Sergeant J, Gillberg C, Biederman J. The worldwide prevalence of ADHD: is it an American condition? World psychiatry: official journal of the World Psychiatric Association (WPA) 2: 104–113. citeulike. 2003. 2427139. [PMC free article] [PubMed]
- 12.Eriksson JG, Kajantie E, Osmond C, Thornburg K, Barker DJ. Boys live dangerously in the womb. American Journal of Human Biology 22. 2010;330–335:10.1002/ajhb.20995. doi: 10.1002/ajhb.20995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rutter M. A children's behaviour questionnaire for completion by teachers: preliminary findings. Journal of Child Psychology and Psychiatry 8: 1–11. 1967;10(1111/j.1469-7610.1967.tb02175):x. doi: 10.1111/j.1469-7610.1967.tb02175.x. [DOI] [PubMed] [Google Scholar]
- 14.Swanson J, Schuck S, Mann M, Carlson C, Hartman K, et al. Categorical and dimensional definitions and evaluations of symptoms of ADHD: the SNAP and SWAN ratings scales. ADHD.net website. Available: http://www.adhd.net/SNAP_SWAN.pdf. Accessed 2012 June 13. [PMC free article] [PubMed]
- 15.Smalley SL, McGough JJ, Moilanen IK, Loo SK, Taanila A, et al. Prevalence and psychiatric comorbidity of attention-deficit/hyperactivity disorder in an adolescent Finnish population. Journal of the American Academy of Child and Adolescent Psychiatry 46. 2007;1575–1583:10.1097/chi.0b013e3181573137. doi: 10.1097/chi.0b013e3181573137. [DOI] [PubMed] [Google Scholar]
- 16.L'Abée C, Vrieze I, Kluck T, Erwich JJHM, Stolk RP, et al. Parental factors affecting the weights of the placenta and the offspring. Journal of Perinatal Medicine 39. 2010;27–34:10.1515/jpm.2010.119. doi: 10.1515/jpm.2010.119. [DOI] [PubMed] [Google Scholar]
- 17.Aarnoudse-Moens CSH, Weisglas-Kuperus N, van Goudoever JB, Oosterlaan J. Meta-Analysis of neurobehavioral outcomes in very preterm and/or very low birth weight children. Pediatrics 124. 2009;717–728:10.15421peds.2008–2816. doi: 10.1542/peds.2008-2816. [DOI] [PubMed] [Google Scholar]
- 18.Baptiste-Roberts K, Salafia C, Nicholson W, Duggan A, Wang N-Y, et al. Maternal risk factors for abnormal placental growth: the national collaborative perinatal project. BMC Pregnancy and Childbirth 8. 2008;44:10.1186/1471–2393-8-44. doi: 10.1186/1471-2393-8-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rodriguez A, Olsen J, Kotimaa AJ, Kaakinen M, Moilanen I, et al. Is prenatal alcohol exposure related to inattention and hyperactivity symptoms in children? Disentangling the effects of social adversity. Journal of Child Psychology and Psychiatry 50: 1073–1083. 2009;10(1111/j.1469-7610.2009.02071):x. doi: 10.1111/j.1469-7610.2009.02071.x. [DOI] [PubMed] [Google Scholar]
- 20.Rodriguez A, Miettunen J, Henriksen TB, Olsen J, Obel C, et al. Maternal adiposity prior to pregnancy is associated with ADHD symptoms in offspring: evidence from three prospective pregnancy cohorts. International Journal of Obesity 32. 2007;550–557:10.1038/sj.ijo.0803741. doi: 10.1038/sj.ijo.0803741. [DOI] [PubMed] [Google Scholar]
- 21.Gissler M, Meriläinen J, Vuori E, Hemminki E. Register based monitoring shows decreasing socioeconomic differences in Finnish perinatal health. Journal of Epidemiology and Community Health 57. 2003;433–439:10.1136/jech.57.6.433. doi: 10.1136/jech.57.6.433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lumey LH. Compensatory placental growth after restricted maternal nutrition in early pregnancy. Placenta 19. 1998;105–111:10.1016/S0143–4004(98)90105-9. doi: 10.1016/s0143-4004(98)90105-9. [DOI] [PubMed] [Google Scholar]
- 23.Hindmarsh PC, Geary MPP, Rodeck CH, Jackson MR, Kingdom JCP. Effect of early maternal iron stores on placental weight and structure. The Lancet 356. 2000;719–723:10.1016/S0140–6736(00)02630-1. doi: 10.1016/s0140-6736(00)02630-1. [DOI] [PubMed] [Google Scholar]
- 24.Kruger H, Arias-Stella J. The placenta and the newborn infant at high altitudes. American Journal of Obstetrics and Gynecology. 1970;106:586–591. doi: 10.1016/0002-9378(70)90045-1. [DOI] [PubMed] [Google Scholar]
- 25.Rees S, Harding R. Brain development during fetal life: influences of the intra-uterine environment. Neuroscience Letters 361. 2004;111–114:10.10161.j.neulet.2004.02.002. doi: 10.1016/j.neulet.2004.02.002. [DOI] [PubMed] [Google Scholar]
- 26.Kyle UG, Pichard C. The Dutch Famine of 1944–1945: a pathophysiological model of long-term consequences of wasting disease. Current Opinion in Clinical Nutrition and Metabolic Care 9. 2006;388–394:10.1097/01.mco.0000232898.74415.42. doi: 10.1097/01.mco.0000232898.74415.42. [DOI] [PubMed] [Google Scholar]
- 27.Obel C, Hedegaard M, Brink T, Secher NJ, Olsen J. Psychological factors in pregnancy and mixed-handedness in the offspring. Developmental Medicine and Child Neurology 45. 2003;557–561:10.1111/j.1469–8749.2003.tb00956. doi: 10.1017/s0012162203001014. [DOI] [PubMed] [Google Scholar]
- 28.Glover V, O'Connor TG, Heron J, Golding J. Antenatal maternal anxiety is linked with atypical handedness in the child. Early Human Development 79. 2004;107–118:10.1016/j.earlhumdev.2004.04.012. doi: 10.1016/j.earlhumdev.2004.04.012. [DOI] [PubMed] [Google Scholar]
- 29.Rodriguez A, Bohlin G. Are maternal smoking and stress during pregnancy related to ADHD symptoms in children? Journal of Child Psychology and Psychiatry 46: 246–254. 2005;10(1111/j.1469-7610.2004.00359):x. doi: 10.1111/j.1469-7610.2004.00359.x. [DOI] [PubMed] [Google Scholar]
- 30.Talge NM, Neal C, Glover V, the Early Stress Translational Research and Prevention Science Network: Fetal and Neonatal Experience on Child and Adolescent Mental Health. Antenatal maternal stress and long-term effects on child neurodevelopment: how and why? Journal of Child Psychology and Psychiatry 48: 245–261. 2007;10(1111/j.1469-7610.2006.01714):x. doi: 10.1111/j.1469-7610.2006.01714.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rodriguez A, Kaakinen M, Moilanen I, Taanila A, McGough JJ, et al. Mixed-handedness is linked to mental health problems in children and adolescents. Pediatrics 125. 2010;e340–348:10.1542/peds.2009–1165. doi: 10.1542/peds.2009-1165. [DOI] [PubMed] [Google Scholar]
- 32.Rodriguez A, Waldenström U. Fetal origins of child non-right-handedness and mental health. Journal of Child Psychology and Psychiatry 49: 967–976. 2008;10(1111/j.1469-7610.2008.01923):x. doi: 10.1111/j.1469-7610.2008.01923.x. [DOI] [PubMed] [Google Scholar]
- 33.Jones A, Osmond C, Godfrey KM, Phillips DIW. Evidence for developmental programming of cerebral laterality in humans. PLoS ONE 6. 2011;e17071:10.1371/journal.pone.0017071. doi: 10.1371/journal.pone.0017071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fowden AL, Ward JW, Wooding FPB, Forhead AJ, Constancia M. Programming placental nutrient transport capacity. The Journal of Physiology 572. 2006;5–15:10.1113/jphysiol.2005.104141. doi: 10.1113/jphysiol.2005.104141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Roseboom TJ, Painter RC, de RooijSR, van Abeelen AFM, Veenendaal MVE, et al. Effects of famine on placental size and efficiency. Placenta 32. 2011;395–399:10.1016/j.placenta.2011.03.001. doi: 10.1016/j.placenta.2011.03.001. [DOI] [PubMed] [Google Scholar]
- 36.Jansson T, Powell TL. Role of the placenta in fetal programming: underlying mechanisms and potential interventional approaches. Clinical Science 113. 2007;1–13:10.1042/cs20060339. doi: 10.1042/CS20060339. [DOI] [PubMed] [Google Scholar]
- 37.Barker DJP, Thornburg KL, Osmond C, Kajantie E, Eriksson JG. Beyond birthweight: the maternal and placental origins of chronic disease. Journal of Developmental Origins of Health and Disease 1. 2010;360–364:10.1017/S2040174410000280. doi: 10.1017/S2040174410000280. [DOI] [PubMed] [Google Scholar]
- 38.Sibley CP, Turner MA, Cetin I, Ayuk P, Boyd CAR, et al. Placental phenotypes of intrauterine growth. Pediatric Research 58. 2005;827–832:10.1203/01.PDR.0000181381.82856.23. doi: 10.1203/01.PDR.0000181381.82856.23. [DOI] [PubMed] [Google Scholar]
- 39.Godfrey KM. The Role of the placenta in fetal programming–a review. Placenta 23. 2002;S20–S27:10.1016/j.rigapp.2005.12.001. doi: 10.1053/plac.2002.0773. [DOI] [PubMed] [Google Scholar]
- 40.Barker DJP, Thornburg KL, Osmond C, Kajantie E, Eriksson JG. The surface area of the placenta and hypertension in the offspring in later life. International Journal of Developmental Biology 54. 2010;525–530:10.1387/ijdb.082760db. doi: 10.1387/ijdb.082760db. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Raatikainen K, Heiskanen N, Heinonen S. Under-attending free antenatal care is associated with adverse pregnancy outcomes. BMC Public Health 7. 2007;268:10.1186/1471–2458-7-268. doi: 10.1186/1471-2458-7-268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pedersen JF. Ultrasound evidence of sexual difference in fetal size in first trimester. Obstetrical and Gynecological Survey. 1981;36:305–306. doi: 10.1136/bmj.281.6250.1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Forsén T, Eriksson JG, Tuomilehto J, Osmond C, Barker DJP. Growth in utero and during childhood among women who develop coronary heart disease: longitudinal study. British Medical Journal 319. 1999;1403–1407:10.1136/bmj.319.7222.1403. doi: 10.1136/bmj.319.7222.1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Clifton V. Sexually dimorphic effects of maternal asthma during pregnancy on placental glucocorticoid metabolism and fetal growth. Cell and Tissue Research 322. 2005;63–71:10.1007/s00441–005-1117-5. doi: 10.1007/s00441-005-1117-5. [DOI] [PubMed] [Google Scholar]
- 45.Mueller BR, Bale TL. Sex-specific programming of offspring emotionality after stress early in pregnancy. The Journal of Neuroscience 28. 2008;9055–9065:10.1523/JNEUROSCI.1424–08.2008. doi: 10.1523/JNEUROSCI.1424-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.O'Donnell KJ, Bugge Jensen A, Freeman L, Khalife N, O'Connor TG, et al. Maternal prenatal anxiety and downregulation of placental 11β-HSD2. Psychoneuroendocrinology. 10.1016/j.psyneuen.2011.09.014. [DOI] [PubMed]
- 47.O'Donnell K, O'Connor TG, Glover V. Prenatal stress and neurodevelopment of the child: focus on the HPA axis and role of the placenta. Developmental Neuroscience 31. 2009;285–292:10.1159/000216539. doi: 10.1159/000216539. [DOI] [PubMed] [Google Scholar]
- 48.Wyrwoll CS, Seckl JR, Holmes MC. Altered placental function of 11{beta}-hydroxysteroid dehydrogenase 2 knockout mice. Endocrinology 150. 2009;1287–1293:10.1210/en.2008–1100. doi: 10.1210/en.2008-1100. [DOI] [PMC free article] [PubMed] [Google Scholar]