Abstract
Aim
Previous studies have demonstrated relationships between sleep and both obesity and diabetes. Additionally, exercise may improve sleep and daytime function, in addition to weight and metabolic function. The present study extends these findings by examining how general sleep-related complaints are associated with body mass index (BMI), diabetes diagnosis, and exercise in a large, nationally representative sample.
Subject and methods
Participants were respondents to the Behavioral Risk Factor Surveillance System (BRFSS). Sleep complaint (SC) was measured with “Over the last 2 weeks, how many days have you had trouble falling asleep or staying asleep or sleeping too much?” Daytime complaint (DC) was measured with “Over the last 2 weeks, how many days have you felt tired or had little energy?” Responses were dichotomized, with ≥6 days indicating complaint. Covariates included age, race/ethnicity, income, and education.
Results
Being overweight was associated with DC in women only. Obesity was significantly associated with SC and DC in women, and DC in men. Diabetes was associated with SC and DC in both genders. Any exercise in the past 30 days did not attenuate any BMI or diabetes relationships, but was independently associated with a decrease in SC and DC in both men and women.
Conclusion
These results suggest that for both men and women diabetes is a significant predictor of sleep and daytime complaints, and there is a relationship between obesity and sleep and complaints for women to a greater extent than men. Finally, exercise was associated with much fewer sleep and daytime complaints in both genders.
Keywords: Sleep, Sleep disturbance, Obesity, Diabetes, Exercise
Introduction
Rates of obesity and diabetes are increasing and constitute a major public health issue. The rise of these conditions has been attributed to profound changes in societal and behavioral patterns, notably diet and exercise. Consonant with the growing obesity epidemic, there has been a growing concern about the relationships of sleep disturbances to a number of health outcomes (Grandner et al. 2010b; Grandner et al. 2010a; Colten et al. 2006). Sleep disturbances are significantly associated with obesity (Grandner et al. 2010b; Watanabe et al. 2010; Chaput et al. 2009a) as well as morbidity and mortality (Gallicchio and Kalesan 2009; Grandner et al. 2010d; Grandner et al. 2010b).
One aspect of sleep disturbance, sleep deprivation, has been explored in a number of ways with respect to obesity. Subjective habitual short sleep is associated with increased body mass index (BMI) (Watanabe et al. 2010; Thomas et al. 2009; Di Milia and Mummery 2009; Lauderdale et al. 2009; Cappuccio et al. 2008; Patel et al. 2008), less habitual sleep is associated with a higher-fat diet (Grandner et al. 2010a), and experimental sleep deprivation is associated with physiological disruptions associated with obesity risk such as alterations in leptin and ghrelin (Mullington et al. 2009; Gangwisch 2009; Punjabi 2009; Hall et al. 2008; Van Cauter et al. 2008). In addition, decreased sleep duration is also a clinical feature of diabetes (Beihl et al. 2009; Chaput et al. 2009a; Tasali et al. 2009; Idris et al. 2009) and predicts its development (Beihl et al. 2009; Chaput et al. 2009a). While several explanations for the relationships between sleep and both obesity and diabetes have been postulated, the precise mechanisms of action are still under investigation.
Proposed mechanisms that have received the most attention include alterations in glucose tolerance, insulin resistance, and the metabolic hormones leptin and ghrelin. Current evidence from laboratory studies indicates that sleep deprivation is associated with impaired glucose tolerance and insulin resistance (Van Cauter et al. 2008; Van Cauter et al. 2007; Knutson et al. 2007). Additionally, sleep loss may alter the ability of leptin and ghrelin to accurately signal caloric need and may lead to increased food intake (Knutson et al. 2007). Epidemiological studies also demonstrate that short habitual sleep duration is associated with impaired glucose tolerance and insulin resistance (Gangwisch 2009; Spiegel et al. 2005; Schultes et al. 2005), as well as alterations of leptin and ghrelin secretion (Gangwisch 2009; Spiegel et al. 2005; Chaput et al. 2007). Together, these findings suggest that short sleep duration is an independent risk factor for the development of both obesity (Watanabe et al. 2010; Chaput et al. 2009b; Salihu et al. 2009) and diabetes (Tuomilehto et al. 2009; Knutson and Van Cauter 2008; Gangwisch et al. 2007), and that it may play a role in their development (Grandner and Patel 2009; Lévy et al. 2009; Schuster 2009; Gimble et al. 2009; Gangwisch 2009; Grandner et al. 2010b).
Although a major assumption of this work is that it is the sleep problems that are causally related to the health outcomes, it is likely that reciprocal relationships exist as well. Most of the studies in this domain are correlational and would reflect effects in both directions. Other studies have shown that intervening on risk factors for obesity and diabetes can have effects on sleep as well (Brand et al. 2010; Tuomilehto et al. 2009). Thus, although many previous studies target obesity and diabetes factors as outcomes with sleep as predictor, it is possible to explore relative influence of obesity and diabetes on sleep as an outcome, as it is likely that these relationships are, at least in part, bidirectional.
Adequate healthy sleep requires both sufficient sleep duration and good sleep quality; sleep disturbance may be due to habitual short sleep duration or chronic sleep deprivation, sleep fragmentation or sleep disruption, or even excessive sleep (Grandner et al. 2010c; Grandner and Patel 2009; Grandner et al. 2009; Lauderdale et al. 2008). Yet, as reviewed above, sleep duration has been the primary sleep variable in epidemiological surveys of obesity and diabetes and experimental sleep deprivation paradigms typically vary sleep duration rather than sleep quality.
Furthermore, physical activity is an important factor in not only obesity and diabetes, but it is also related to sleep (Brand et al. 2010; Reynolds et al. 2010; Ueno et al. 2009; Oliver et al. 2009). It is unclear, however, whether the degree of physical activity necessary for a significant impact on BMI or diabetes risk is necessary for a significant impact on sleep-related complaints (Gerber et al. 2010). It may be the case that a relatively small amount of physical activity may be insufficient to significantly impact obesity or diabetes risk directly, but may improve sleep and thus indirectly play a role.
Although it is well known that obesity is associated with diabetes and physical inactivity, and that all of these factors affect sleep, this study extends the existing literature in a few important ways: First, this study examines general sleep disturbance and fatigue, whereas previous studies have examined the sleep disturbance resulting from sleep disorders (e.g., sleep apnea), sleep in artificial laboratory settings (e.g., sleep deprivation), or other aspects of sleep (e.g., sleep duration). Second, this study explores the unique contributions of obesity and diabetes to sleep disturbance and fatigue. Third, this study supports previous findings in a large, diverse sample.
The present study was designed to investigate whether obesity and diabetes diagnosis predict sleep disturbance and daytime fatigue in the American population and to what extent, if any, that exercise attenuates these relationships. Specifically, our hypotheses included the following:
Adjusting for all covariates (including diabetes), obesity is a significant predictor of increased sleep disturbance and daytime fatigue.
Adjusting for all covariates (including obesity), diabetes diagnosis is a significant predictor of increased sleep disturbance and daytime fatigue.
When exercise is added to the model, the relationship between both sleep disturbance and daytime fatigue will remain significant.
Exercise is independently associated with decreased sleep disturbance and daytime fatigue.
To address these hypotheses, we conducted an analysis using the Behavioral Risk Factor Surveillance System (BRFSS) for the year 2006.
Methods
Data source
Data were obtained from the 2006 BRFSS (Centers for Disease Control 2007). The BRFSS is a state-based, random-digit-dialed telephone interview survey of adults aged ≥18 years from all over the USA conducted every year. It is the world's largest telephone survey, designed to monitor health-related behaviors in the general population. For this study, participants were respondents who had answered a question on sleep complaint (SC): “Over the last 2 weeks, how many days have you had trouble falling asleep or staying asleep or sleeping too much?” To assess daytime complaint (DC), respondents answered the question: “Over the last 2 weeks, how many days have you felt tired or had little energy?” Answers for both questions ranged from 0 to 14. However, the distributions were bimodal, with highest peaks at 0 and 14 and few responses of 3–11 days; this non-normal distribution thus precludes analysis as a continuous variable. Thus, SC and DC were dichotomized into two categories: those who report complaints ≥6 days and those who report complaints <6 days. This is consistent with other classification approaches where a frequency of 3 or more events per week has been used [such as in insomnia where 3 nights per week or more of poor sleep is used (Perlis et al. 2010; American Academy of Sleep Medicine et al. 2006)].
Covariates used to estimate socioeconomic factors included education (less than high school, high school graduate, some college, college graduate) and income level (<US $10,000 pretax income per year, US $10,000–$15,000, US $15,000–$20,000, US $20,000–$25,000, US $25,000–$35,000, US $35,000–$50,000, US $50,000–$75,000, and > US $75,000). Previous analyses have found that education and income are significant predictors of SC in this sample (Grandner et al. 2010c; 2009).
Obesity was estimated by calculating BMI based on self-reported estimates of weight and height and categorizing volunteers as normal weight (BMI<25), over-weight (BMI=25−29.9), or obese (BMI≥30)—categories widely used in research and practice. Although calculation of BMI based on self-report may be problematic, BMI data from the BRFSS are accepted estimates in other studies (Fairley et al. 2010; Heo et al. 2010; Jia and Lubetkin 2010). Diabetes diagnosis history was obtained via self-report.
The presence of exercise was assessed by asking participants the following question: “During the past month, other than your regular job, did you participate in any physical activities or exercises such as running, calisthenics, golf, gardening, or walking for exercise?” (coded “yes” or “no”). This item, in particular, was chosen for a number of reasons. Numerous studies have shown that increased exercise is associated with decreased risk for diabetes and decreased BMI. However, the amount of moderate to vigorous activity (generally required for significant effects on BMI and diabetes risk) in the general population is probably very low (Hubácek 2009; Macfarlane and Thomas 2010). Additionally, subjective estimates of moderate and vigorous exercise (especially those requiring precision) are known to be unreliable. The chosen item reflects these limitations by assessing exercise with minimal precision (with no need to estimate intensity or duration, which would likely be inaccurate) and allowing for an amount of activity (minimal) that would more likely be attainable by the majority of the population. In addition to these benefits, there are a number of problems with this item. For example, interpretation of results may be difficult. If no significant effect is found, it would be unclear whether a significant effect could have been found with a measure that was related to the higher levels of exercise previously reported to be associated with reductions in BMI and diabetes risk. If a significant effect is found, the measure is so broad that it is unclear what level of exercise would be required to show the effect. Despite these limitations, this item was chosen because of its novelty and ability to reflect minimal exercise in a way that may be more reliable than other estimates.
Statistical analyses
Complete-case analysis was implemented for both SC and DC; thus, only participants who provided complete data were included for each analysis. Percentages of respondents indicating SC and DC across variables were calculated and differences in reported SC and DC among groups were compared using Rao-Scott chi-square tests using SAS software (SAS Institute 2008) with the PROC SURVEYFREQ procedure. Data were also reviewed graphically to assess distribution and relationship to sleep disturbance.
The logit of odds of the categorical SC and DC was modeled using generalized linear models for each individual variable and all combined variables both for all and separated by gender. Age was considered as a continuous variable while others were considered as categorical variables, to best model how the data were originally collected and to maximize resolution. All sampling was weighted appropriately for representativeness, using weighting scores specifically developed for BRFSS 2006 (Centers for Disease Control 2007). The odds ratios (ORs) and 95% confidence intervals (CIs) were estimated among groups relative to a preselected reference. Analyses were performed using SAS software (SAS Institute 2008) with the PROC SURVEYLOGISTIC procedure. All statistical tests were two-tailed. Statistical significance was set at the p<0.05 level unless otherwise indicated. Interactions for all variables with race/ethnicity were computed separately for men and women.
For men and women, for both SC and DC, three models were computed: (1) BMI + diabetes, (2) BMI + diabetes + covariates (age, education, income), and (3) BMI + diabetes + covariates + exercise. The first model explores unadjusted effects, and the second model explores the contribution of age, education, and income to the unadjusted findings. The final model includes exercise, to determine its value as an independent predictor and to evaluate the impact of exercise on the degree of the relationship between SC/DC and both BMI and diabetes.
Results
Subject characteristics
A total of n=156,252 participants provided complete data for the SC analyses and n=155,761 participants provided complete data for the DC analyses. Characteristics of the samples are reported in Table 1 (SC) and Table 2 (DC). Based on this classification, 19.37% of the sample (21.76% of women and 15.87% of men) were classified as reporting SC (SC+) and 22.81% of the sample (25.78% of women and 18.47% of men) were classified as reporting DC.
Table 1.
Characteristics of participants who responded to the SC question, separated by groupa
| Women (n=92,924) | Men (n=63,328) | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| Category | SC− (%) | SC+ (%) | Total (%) | SC− (%) | SC+ (%) | Total (%) |
| Age | ||||||
| Mean | 53.06 | 51.43 | 53.18 | 51.71 | 51.12 | 52.15 |
| Education | ||||||
| Less than high school | 6.62 | 2.93 | 9.55 | 7.58 | 2.34 | 9.92 |
| High school | 22.73 | 7.29 | 30.02 | 23.81 | 5.21 | 29.02 |
| Some college | 21.82 | 6.56 | 28.38 | 20.76 | 4.21 | 24.97 |
| College graduate | 27.07 | 4.98 | 32.04 | 31.98 | 4.11 | 36.09 |
| Income | ||||||
| < US $10,000 | 4.67 | 2.64 | 7.31 | 2.71 | 1.40 | 4.10 |
| US $10,000–$15,000 | 5.13 | 2.36 | 7.49 | 3.32 | 1.44 | 4.76 |
| US $15,000–$20,000 | 6.51 | 2.45 | 8.95 | 4.82 | 1.44 | 4.76 |
| US $20,000–$25,000 | 7.66 | 2.54 | 10.20 | 6.66 | 1.74 | 8.40 |
| US $25,000–$35,000 | 10.51 | 2.90 | 13.41 | 10.71 | 2.22 | 12.92 |
| US $35,000–$50,000 | 12.99 | 3.13 | 16.12 | 14.74 | 2.48 | 17.22 |
| US $50,000–$75,000 | 13.27 | 2.72 | 15.99 | 16.58 | 2.31 | 18.89 |
| US $75,000+ | 17.50 | 3.03 | 20.53 | 24.61 | 2.84 | 27.45 |
| BMI | ||||||
| Normal | 34.46 | 7.86 | 42.31 | 23.38 | 4.34 | 27.72 |
| Overweight | 24.55 | 6.55 | 31.10 | 38.78 | 6.33 | 45.11 |
| Obese | 19.24 | 7.35 | 26.58 | 21.98 | 5.19 | 27.17 |
| Diabetes | ||||||
| No | 69.70 | 18.24 | 87.94 | 74.71 | 13.15 | 87.87 |
| Yes | 8.55 | 3.51 | 12.06 | 9.42 | 2.71 | 12.13 |
| Exercise | ||||||
| No | 18.27 | 7.99 | 26.25 | 17.14 | 5.35 | 22.49 |
| Yes | 59.98 | 13.77 | 73.75 | 67.00 | 10.52 | 77.51 |
SC+ indicates that difficulties were reported ≥6 days across 2 weeks; SC- indicates that difficulties were reported ≤6 days across 2 weeks
Table 2.
Characteristics of participants who responded to the DC question, separated by groupa
| Women (n=92,645) | Men (n=63,116) | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| Category | DC− (%) | DC+ (%) | Total (%) | DC− (%) | DC+ (%) | Total (%) |
| Age | ||||||
| Mean | 52.96 | 51.54 | 52.61 | 51.43 | 52.08 | 51.54 |
| Education | ||||||
| Less than high school | 5.96 | 3.55 | 9.51 | 7.12 | 2.74 | 9.87 |
| High school | 21.46 | 8.53 | 29.99 | 22.66 | 6.38 | 29.04 |
| Some college | 20.76 | 7.66 | 28.42 | 20.15 | 4.85 | 25.00 |
| College graduate | 26.03 | 6.04 | 32.08 | 31.59 | 4.50 | 36.09 |
| Income | ||||||
| < US $10,000 | 4.15 | 3.16 | 7.30 | 2.56 | 1.52 | 4.08 |
| US $10,000–$15,000 | 4.54 | 2.90 | 7.44 | 3.08 | 1.70 | 4.77 |
| US $15,000–$20,000 | 6.06 | 2.90 | 8.96 | 4.47 | 1.81 | 6.29 |
| US $20,000–$25,000 | 7.14 | 3.05 | 10.19 | 6.34 | 2.04 | 8.38 |
| US $25,000–$35,000 | 9.92 | 3.48 | 13.40 | 10.26 | 2.64 | 12.90 |
| US $35,000–$50,000 | 12.43 | 3.70 | 16.13 | 14.27 | 2.95 | 17.22 |
| US $50,000–$75,000 | 12.79 | 3.23 | 16.01 | 16.17 | 2.73 | 18.91 |
| $75,000+ | 17.19 | 3.38 | 20.57 | 24.37 | 3.08 | 27.45 |
| BMI | ||||||
| Normal | 33.42 | 8.88 | 42.29 | 22.78 | 4.95 | 27.73 |
| Overweight | 23.43 | 7.65 | 31.08 | 37.91 | 7.21 | 45.17 |
| Obese | 17.36 | 9.27 | 26.63 | 20.83 | 6.32 | 27.15 |
| Diabetes | ||||||
| No | 66.65 | 21.30 | 87.96 | 72.82 | 15.05 | 87.87 |
| Yes | 7.56 | 4.49 | 12.04 | 8.71 | 3.42 | 12.13 |
| Exercise | ||||||
| No | 15.53 | 10.72 | 26.26 | 15.46 | 6.96 | 22.42 |
| Yes | 58.67 | 15.07 | 73.74 | 66.07 | 11.51 | 77.58 |
DC+ indicates that difficulties were reported ≥6 days across 2 weeks; DC- indicates that difficulties were reported ≤6 days across 2 weeks
Regression results
Results of unadjusted analyses are reported in Table 3 for SC and Table 4 for DC when considering BMI and diabetes (model 1). For women, overweight, obesity, and diabetes were significantly associated with an increased odds of both SC and DC. For men, the relationships varied: diabetes diagnosis was significantly associated with an increased OR for both SC and DC; obesity status was significantly associated with an increased OR for DC only (not SC); and overweight status was associated with a reduced odds risk for both SC and DC.
Table 3.
OR and 95% CI for SC
| Variable | Women | Men | ||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| OR | CI | p | OR | CI | p | |||
| Model 1: BMI + diabetes | ||||||||
| BMI (reference = normal) | ||||||||
| Overweight | 1.117 | 1.029 | 1.212 | 0.0082 | 0.833 | 0.734 | 0.946 | 0.0049 |
| Obese | 1.556 | 1.430 | 1.694 | <0.0001 | 1.039 | 0.916 | 1.178 | 0.2035 |
| Diabetes (reference = no diagnosis) | ||||||||
| Yes | 1.488 | 1.348 | 1.641 | <0.0001 | 1.459 | 1.295 | 1.643 | <0.0001 |
| Model 2: BMI + diabetes + age + education + income | ||||||||
| BMI (reference = normal) | ||||||||
| Overweight | 1.079 | 0.993 | 1.173 | 0.0718 | 0.919 | 0.807 | 1.047 | 0.2035 |
| Obese | 1.408 | 1.293 | 1.534 | <0.0001 | 1.090 | 0.959 | 1.238 | 0.1873 |
| Diabetes (reference = no diagnosis) | ||||||||
| Yes | 1.396 | 1.265 | 1.541 | <0.0001 | 1.409 | 1.238 | 1.603 | <0.0001 |
| Model 3: BMI + diabetes + age + education + income + exercise | ||||||||
| BMI (reference = normal) | ||||||||
| Overweight | 1.068 | 0.982 | 1.162 | 0.122 | 0.913 | 0.802 | 1.040 | 0.1723 |
| Obese | 1.346 | 1.235 | 1.467 | <0.0001 | 1.053 | 0.927 | 1.197 | 0.4297 |
| Diabetes (reference = no diagnosis) | ||||||||
| Yes | 1.375 | 1.244 | 1.519 | <0.0001 | 1.388 | 1.218 | 1.583 | <0.0001 |
| Exercise (reference = no exercise last 30 days) | ||||||||
| Yes | 0.678 | 0.627 | 0.734 | <0.0001 | 0.643 | 0.573 | 0.720 | <0.0001 |
Table 4.
OR and 95% CI for DC
| Variable | Women | Men | ||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| OR | CI | p | OR | CI | p | |||
| Model 1: BMI + diabetes | ||||||||
| BMI (reference = normal) | ||||||||
| Overweight | 1.209 | 1.117 | 1.310 | <0.0001 | 0.863 | 0.772 | 0.966 | 0.0101 |
| Obese | 1.846 | 1.703 | 2.000 | <0.0001 | 1.208 | 1.077 | 1.355 | 0.0013 |
| Diabetes (reference = no diagnosis) | ||||||||
| Yes | 1.580 | 1.439 | 1.736 | <0.0001 | 1.653 | 1.476 | 1.850 | <0.0001 |
| Model 2: BMI + diabetes + age + education + income | ||||||||
| BMI (reference = normal) | ||||||||
| Overweight | 1.191 | 1.097 | 1.293 | <0.0001 | 0.931 | 0.828 | 1.046 | 0.2262 |
| Obese | 1.696 | 1.562 | 1.841 | <0.0001 | 1.247 | 1.108 | 1.404 | 0.0003 |
| Diabetes (reference = no diagnosis) | ||||||||
| Yes | 1.534 | 1.393 | 1.688 | <0.0001 | 1.516 | 1.346 | 1.708 | <0.0001 |
| Model 3: BMI + diabetes + age + education + income + exercise | ||||||||
| BMI (reference = normal) | ||||||||
| Overweight | 1.171 | 1.077 | 1.273 | 0.0002 | 0.922 | 0.820 | 1.037 | 0.1754 |
| Obese | 1.568 | 1.441 | 1.706 | <0.0001 | 1.187 | 1.054 | 1.338 | 0.0048 |
| Diabetes (reference = no diagnosis) | ||||||||
| Yes | 1.496 | 1.358 | 1.648 | <0.0001 | 1.490 | 1.318 | 1.685 | <0.0001 |
| Exercise (reference = no exercise last 30 days) | ||||||||
| Yes | 0.486 | 0.450 | 0.524 | <0.0001 | 0.533 | 0.482 | 0.589 | <0.0001 |
Results of adjusted analyses (model 2: BMI + diabetes + covariates of age, education, and income) are presented in Table 3 for SC and Table 4 for DC. After adjusting for age, income, and education, overweight status no longer is significantly associated with an increased OR for SC in women and a decreased OR for SC and DC in men. All other relationships are generally maintained: diabetes diagnosis predicting both SC and DC in men and women and obesity was associated with both SC and DC in women, and only DC in men.
Results of adjusted analyses including exercise (model 3: BMI + diabetes + exercise + covariates of age, education, and income) are presented Table 3 for SC and Table 4 for DC. Though slightly attenuated, all of the significant relationships found in the previous models were maintained after adjusting for exercise. This is despite the finding that exercise itself was significantly associated with SC and DC in both men and women, such that individuals who reported any exercise were about half as likely to report DC and about a third less likely to report SC. Figure 1 represents the findings of this model for SC, and Fig. 2 represents the findings for DC.
Fig. 1.
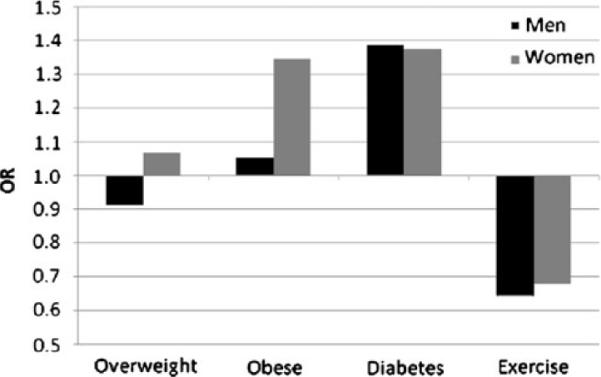
Graphical representation of ORs for SC from multivariate analysis that included BMI categories, diabetes diagnosis, and report of exercise in the past 30 days, adjusted for age, income, and education (model 3). Reference categories were normal weight (BMI), no diagnosis (diabetes), and no exercise (exercise)
Fig. 2.
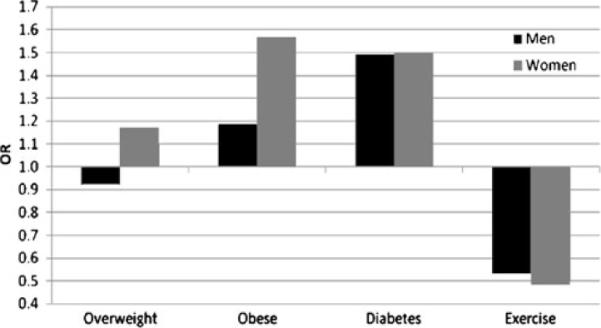
Graphical representation of ORs for DC from multivariate analysis that included BMI categories, diabetes diagnosis, and report of exercise in the past 30 days, adjusted for age, income, and education (model 3). Reference categories were normal weight (BMI), no diagnosis (diabetes), and no exercise (exercise)
Because the pattern of findings for SC and DC were so similar, we explored whether these two variables were measuring the same construct. However, although there was a significant correlation between SC and DC (r=0.32, p<0.0005), each of these two variables only explain approximately 10% of the variance in the other (r2=0.1024). Thus, they represent two very different constructs that, though related, are not collinear.
Discussion
The present study evaluated BMI and diabetes diagnosis as predictors of sleep disturbance in a large representative sample of the American population. This was explored using 3 models: model 1 (BMI + diabetes), model 2 (BMI + diabetes + covariates), and model 3 (BMI + diabetes + covariates + exercise). The findings suggest a significant relationship between DC and both overweight and obesity status in women and obese (but not overweight) men. In contrast, only in women was obesity significantly associated with SC. Diabetes was significantly associated with both SC and DC in men and women. Any exercise in the past 30 days was associated with reduced levels of both SC and DC, though the ORs for the other relationships were not notably affected, suggesting that the variance explained by exercise is different than that explained by obesity or diabetes.
It is important to note that previous studies have tended to view sleep disturbance (however measured) as an independent variable, whereas the analyses in the present study view sleep as a dependent variable influenced by obesity, diabetes, and exercise. This is not meant to imply a unidirectional relationship where sleep problems are caused by obesity/diabetes/exercise. Rather, we designed our statistical analyses around the notion that obesity, diabetes, and exercise are related constructs, each linked to sleep. Using this approach, the relative variance contributions to sleep-related complaints of these related constructs can be evaluated simultaneously.
Sleep disturbance is associated with diabetes risk independent of obesity
Several previous studies have shown a relationship between diabetes and sleep deprivation (Gangwisch 2009; Knutson and Van Cauter 2008). Fewer studies have investigated the association with sleep disruption, daytime sleepiness, and fatigue. Nonetheless, significant relationships have been reported (Knutson et al. 2006). Several studies of sleep apnea patients (who experience sleep disruption) have demonstrated that this condition is a significant predictor of diabetes diagnosis (and vice versa) (Ronksley et al. 2009). This study extends those previous studies to a much larger and more representative sample. That the relationship of diabetes to SC and DC was independent of covariates, BMI, and exercise, and nearly identical in men and women, suggests that there is something specific to the pathophysiology of diabetes itself that confers risk. The specific nature of this risk still needs to be explored, but it may have to do with alterations in insulin sensitivity and glucose metabolism (Barf et al. 2010; Lui and Ip 2010; Buxton et al. 2010) associated with sleep disruption. Additionally, other factors associated with diabetes that have been independently associated with sleep disturbance over and above effects of BMI may be implicated, such as atherogenic lipoprotein profile (Grandner et al. 2010a; Gangwisch et al. 2010; Kaneita et al. 2008; Mackiewicz et al. 2008; Mackiewicz et al. 2007; Williams et al. 2007), inflammatory factors (Jain and Mills 2007; Opp et al. 2007; Irwin 2002; Meier-Ewert et al. 2004; Okun et al. 2009), and the hormones leptin and ghrelin (Knutson and Van Cauter 2008; Van Cauter et al. 2008; Chaput et al. 2007; Littman et al. 2007; Knutson et al. 2007; Gangwisch 2009; Copinschi 2005; Spiegel et al. 1999; Spiegel et al. 2004a, b).
It is possible that sleep apnea may also play a role in the link between diabetes and SC or DC. The fact that diabetes remained a risk factor even after controlling for obesity/overweight status suggests that there are additional factors associated with diabetes. It is possible that the presence of diabetes increases the risk of sleep apnea beyond that associated with a specific BMI category, or that diabetes itself causes SC or DC through other pathology.
It is unclear whether the relationship between sleep and diabetes is unidirectional or bidirectional. Increasing evidence suggests that this relationship is complex (Eastwood et al. 2010; Bopparaju and Surani 2010; Celen et al. 2010; Leow 2010; Lam et al. 2010; Zizi et al. 2010; Barone and Menna-Barreto 2010). These findings support the proposition that maintaining healthy sleep can be included in strategies for diabetes prevention as well as the suggestion that diabetes preventive activities should incorporate an aim of reducing sleep disturbance. For example, the recent IMAGE guidelines for preventing type 2 diabetes includes a discussion of sleep (Lindström et al. 2010).
Sleep complaint and daytime tiredness are associated with obesity
Numerous studies have found that short sleep duration is a significant predictor of BMI (Grandner et al. 2010b; Chaput et al. 2009b; Must and Parisi 2009; Grandner and Patel 2009), and the findings from laboratory studies have also supported this (Nedeltcheva et al. 2009). Studies in the sleep apnea literature have found significant relationships between sleep fragmentation (due to respiratory events) and daytime sleepiness and fatigue (Yue et al. 2009). However, previous studies have not examined this issue more broadly by looking for a more general link between obesity and sleep quality. Of note, being overweight was significantly related to both SC and DC in men and women in unadjusted analyses, but the inclusion of age and socioeconomic factors attenuate this relationship significantly, suggesting that this relationship may have been related to a general unhealthy lifestyle, to which overweight is related as well as age and socioeconomic factors, rather than BMI. It should be noted that the findings were more robust in women than men, replicating previous findings regarding sleep duration and BMI (Kripke et al. 2002) and cardiovascular risk (Cappuccio et al. 2007). Not all studies have found this pattern, though (Watanabe et al. 2010)
Presence of physical activity is important
Although a significant relationship among obesity, diabetes, and poor sleep has been reported frequently in the literature, few studies have examined the role of exercise. Self-report measures of exercise are difficult because responses depend on social desirability and demand characteristics of the individual study (Youngstedt and Kline 2006). Thus, we chose a variable that presumably would have high reliability, despite low resolution. Including a simple measure of any exercise in the past 30 days into the model resulted in two notable findings: (1) the ORs for obesity and diabetes were attenuated only very slightly, even though (2) this simple measure of exercise was strongly, inversely related to both SC and DC.
That there was little attenuation of the obesity and diabetes relationships in the presence of exercise suggests that exercise does not directly alter the relationship of sleep, diabetes, and obesity. The mechanism by which diabetes and obesity are associated with SC or DC may not be impacted by exercise as measured in this study. Previous studies have found that health outcomes of obesity and diabetes are affected by exercise (Macfarlane and Thomas 2010), and this large study raises some questions about how this is involved in sleep and tiredness.
The finding that exercise had beneficial effects on SC and DC in the larger adjusted model is encouraging. It suggests that even in the presence of obesity or diabetes, and irrespective of age or socioeconomic factors, exercise can have a beneficial effect on SC and DC. Previous studies have found beneficial effects of exercise on sleep (Brand et al. 2010). However, these studies often found relatively modest effects, with careful measurement of physical activity. That such a general measure was so highly predictive, given its imprecise measurement, is surprising. Perhaps, for general sleep quality, it is not the intensity or duration of exercise that is important—merely its presence (or the subjective judgment of its presence) is sufficient to categorically improve sleep quality and daytime tiredness.
Limitations
This study has a number of limitations. First, the SC and DC items are not specific for particular symptoms. This limits our ability to use responses to this item to describe symptoms or syndromes (e.g., insufficient sleep, long sleep, insomnia, sleep apnea, daytime sleepiness). The broad nature of the SC question in particular captures a gamut of etiologies for suboptimal sleep including acute and chronic partial sleep deprivation, insomnia, poor sleep quality, and excessive sleep. SC is not a typical metric that has been employed in epidemiological analyses of sleep in the population. However, it provides a valuable and sensitive estimate of suboptimal sleep, for whatever reason, in the general population. We purport that the item is sensitive because almost any problem associated with sleep—especially the most common (sleep insufficiency, sleep fragmentation, insomnia, sleep apnea, restless legs, etc.)—could be captured by this question. However, this question has not been validated against standard measures of subjective and objective sleep. This is a major limitation of these items. However, we still believe that they are useful, as they provide an overall population-level indicator of the presence of sleep complaints, with almost none of the limitations that would be applied if specific symptoms had been targeted. This general level of complaint demonstrates a great deal of face validity and, more importantly, external validity, as it represents the vague complaints presented when people discuss sleep problems (Cole et al. 2007; Grandner et al. 2006). This is pertinent as recognition for the importance of sleep and sleep disorders in public health continues to rise (Colten et al. 2006).
Second, cross-sectional analyses limit our ability to comment on causality. Thus, SC and DC ORs may reflect other causes as well.
A third limitation is the self-report nature of the survey. Diabetes may be underreported. Also, self-reports of BMI are known to have limited reliability and validity, though they have been useful in documenting population trends (Fairley et al. 2010; Heo et al. 2010; Strine et al. 2005; Richardson et al. 2008). Regarding the exercise question, subjective self-reports of exercise frequency and severity are known to be very unreliable and may not accurately reflect actual activity. This question avoids this problem by not asking details—any exercise in the past 30 days is all that is asked. As would be expected, most individuals endorsed this item, and it is probably not related to much, if any, moderate or strenuous activity. This may explain why the addition of this item to the model did not affect the ORs for diabetes and obesity, although physical activity is a well-known factor in these conditions; the sort of physical activity captured by this question is probably insufficient to have an impact on these. However, that there was such a strong relationship with SC and DC is rendered even more surprising then, as these findings suggest that even very small amounts of activity might have profound impacts on sleep and tiredness.
Conclusions
The large representative sample size and broad sleep measures allow us to posit important population-level observations about sleep in the population. Based on these data, we conclude that obesity and diabetes are significant independent predictors of sleep disturbance and daytime complaints, though the relationship with obesity is notably stronger in women than men. A broad measure of exercise (any at all in the past 30 days) was a significant predictor of lack of both sleep disturbance and daytime complaints, with those reporting exercise one third less likely to report sleep problems and half as likely to report daytime tiredness. Analyses of smaller sample sizes reporting diabetes and obesity effects on sleep are supported by this analysis.
Regarding future directions, the biological pathways linking diabetes, sleep disturbances, and daytime sequelae of poor sleep remain to be fully elucidated. From a public health perspective, this study highlights the importance of sleep in public health campaigns targeting obesity (and diabetes) and the potential benefits of exercise.
Acknowledgements
This work was supported by T32HL007713, as well as funding for Biostatistical Support from the University of Pennsylvania Center for Sleep and Respiratory Neurobiology. We wish to thank Dr. Allan Pack MB ChB PhD for guidance and other support. Also, we wish to thank the Centers for Disease Control and Prevention for collecting these data and making it available and the BRFSS participants for providing data.
Footnotes
Conflicts of interest The authors declare that they have no conflict of interest.
References
- American Academy of Sleep Medicine. Winkelman JW, Kotagal S, Olson CM, Scammell T, Schenck C, Spielman A. The International Classification of Sleep Disorders. 2nd edn. American Academy of Sleep Medicine; Westchester: 2006. [Google Scholar]
- Barf RP, Meerlo P, Scheurink AJ. Chronic sleep disturbance impairs glucose homeostasis in rats. Int J Endocrinol. 2010;2010:819414. doi: 10.1155/2010/819414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barone MT, Menna-Barreto L. Diabetes and sleep: a complex cause-and-effect relationship. Diabetes Res Clin Pract. 2010 doi: 10.1016/j.diabres.2010.07.011. [DOI] [PubMed] [Google Scholar]
- Beihl DA, Liese AD, Haffner SM. Sleep duration as a risk factor for incident type 2 diabetes in a multiethnic cohort. Ann Epidemiol. 2009;19(5):351–357. doi: 10.1016/j.annepidem.2008.12.001. [DOI] [PubMed] [Google Scholar]
- Bopparaju S, Surani S. Sleep and diabetes. Int J Endocrinol. 2010;2010:759509. doi: 10.1155/2010/759509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brand S, Gerber M, Beck J, Hatzinger M, Pühse U, Holsboer-Trachsler E. High exercise levels are related to favorable sleep patterns and psychological functioning in adolescents: a comparison of athletes and controls. J Adolesc Health. 2010;46(2):133–141. doi: 10.1016/j.jadohealth.2009.06.018. [DOI] [PubMed] [Google Scholar]
- Buxton OM, Pavlova M, Reid EW, Wang W, Simonson DC, Adler GK. Sleep restriction for 1 week reduces insulin sensitivity in healthy men. Diabetes. 2010;59(9):2126–2133. doi: 10.2337/db09-0699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cappuccio FP, Stranges S, Kandala NB, Miller MA, Taggart FM, Kumari M, Ferrie JE, Shipley MJ, Brunner EJ, Marmot MG. Gender-specific associations of short sleep duration with prevalent and incident hypertension: the Whitehall II Study. Hypertension. 2007;50(4):693–700. doi: 10.1161/HYPERTENSIONAHA.107.095471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cappuccio FP, Taggart FM, Kandala NB, Currie A, Peile E, Stranges S, Miller MA. Meta-analysis of short sleep duration and obesity in children and adults. Sleep. 2008;31(5):619–626. doi: 10.1093/sleep/31.5.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celen YT, Hedner J, Carlson J, Peker Y. Impact of gender on incident diabetes mellitus in obstructive sleep apnea: a 16-year follow-up. J Clin Sleep Med. 2010;6(3):244–250. [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control . Behavioral Risk Factor Surveillance System. Department of Health and Human Services, Centers for Disease Control and Prevention; Atlanta: 2007. [Google Scholar]
- Chaput JP, Després JP, Bouchard C, Tremblay A. Short sleep duration is associated with reduced leptin levels and increased adiposity: results from the Quebec family study. Obesity (Silver Spring) 2007;15(1):253–261. doi: 10.1038/oby.2007.512. [DOI] [PubMed] [Google Scholar]
- Chaput JP, Després JP, Bouchard C, Astrup A, Tremblay A. Sleep duration as a risk factor for the development of type 2 diabetes or impaired glucose tolerance: analyses of the Quebec Family Study. Sleep Med. 2009a;10:919–924. doi: 10.1016/j.sleep.2008.09.016. [DOI] [PubMed] [Google Scholar]
- Chaput JP, Leblanc C, Pérusse L, Després JP, Bouchard C, Tremblay A. Risk factors for adult overweight and obesity in the Quebec Family Study: have we been barking up the wrong tree? Obesity (Silver Spring) 2009b;17:1964–1970. doi: 10.1038/oby.2009.116. [DOI] [PubMed] [Google Scholar]
- Cole JC, Dubois D, Kosinski M. Use of patient-reported sleep measures in clinical trials of pain treatment: a literature review and synthesis of current sleep measures and a conceptual model of sleep disturbance in pain. Clin Ther. 2007;29(Suppl):2580–2588. doi: 10.1016/j.clinthera.2007.12.005. [DOI] [PubMed] [Google Scholar]
- Colten HR, Altevogt BM, Institute of Medicine Committee on Sleep Medicine and Research . Sleep disorders and sleep deprivation: an unmet public health problem. Institute of Medicine; National Academies Press; Washington: 2006. [PubMed] [Google Scholar]
- Copinschi G. Metabolic and endocrine effects of sleep deprivation. Essent Psychopharmacol. 2005;6(6):341–347. [PubMed] [Google Scholar]
- Di Milia L, Mummery K. The association between job related factors, short sleep and obesity. Ind Health. 2009;47(4):363–368. doi: 10.2486/indhealth.47.363. [DOI] [PubMed] [Google Scholar]
- Eastwood PR, Malhotra A, Palmer LJ, Kezirian EJ, Horner RL, Ip MS, Thurnheer R, Antic NA, Hillman DR. Obstructive sleep apnoea: from pathogenesis to treatment: current controversies and future directions. Respirology. 2010;15:587–595. doi: 10.1111/j.1440-1843.2009.01699.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fairley TL, Hawk H, Pierre S. Health behaviors and quality of life of cancer survivors in Massachusetts, 2006: data use for comprehensive cancer control. Prev Chronic Dis. 2010;7(1):A09. [PMC free article] [PubMed] [Google Scholar]
- Gallicchio L, Kalesan B. Sleep duration and mortality: a systematic review and meta-analysis. J Sleep Res. 2009;18(2):148–158. doi: 10.1111/j.1365-2869.2008.00732.x. [DOI] [PubMed] [Google Scholar]
- Gangwisch JE. Epidemiological evidence for the links between sleep, circadian rhythms and metabolism. Obes Rev. 2009;10(Suppl 2):37–45. doi: 10.1111/j.1467-789X.2009.00663.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gangwisch JE, Heymsfield SB, Boden-Albala B, Buijs RM, Kreier F, Pickering TG, Rundle AG, Zammit GK, Malaspina D. Sleep duration as a risk factor for diabetes incidence in a large U. S. sample. Sleep. 2007;30(12):1667–1673. doi: 10.1093/sleep/30.12.1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gangwisch JE, Malaspina D, Babiss LA, Opler MG, Posner K, Shen S, Turner JB, Zammit GK, Ginsberg HN. Short sleep duration as a risk factor for hypercholesterolemia: analyses of the National Longitudinal Study of Adolescent Health. Sleep. 2010;33(7):956–961. doi: 10.1093/sleep/33.7.956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerber M, Brand S, Holsboer-Trachsler E, Pühse U. Fitness and exercise as correlates of sleep complaints: is it all in our minds? Med Sci Sports Exerc. 2010;42:893–901. doi: 10.1249/MSS.0b013e3181c0ea8c. [DOI] [PubMed] [Google Scholar]
- Gimble JM, Bray MS, Young A. Circadian biology and sleep: missing links in obesity and metabolism? Obes Rev. 2009;10(Suppl 2):1–5. doi: 10.1111/j.1467-789X.2009.00672.x. [DOI] [PubMed] [Google Scholar]
- Grandner MA, Patel NP. From sleep duration to mortality: implications of meta-analysis and future directions. J Sleep Res. 2009;18(2):145–147. doi: 10.1111/j.1365-2869.2009.00753.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grandner MA, Kripke DF, Yoon IY, Youngstedt SD. Criterion validity of the Pittsburgh Sleep Quality Index: investigation in a non-clinical sample. Sleep Biol Rhythms. 2006;4:129–136. doi: 10.1111/j.1479-8425.2006.00207.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grandner MA, Patel NP, Gehrman PR, Xie D, Sha D, Weaver T, Gooneratne N. Who sleeps better? Socioeconomic differences in reports of sleep disturbance. Sleep. 2009;32(Abstract Supplement):A422–A423. [Google Scholar]
- Grandner MA, Kripke DF, Naidoo N, Langer RD. Relationships among dietary nutrients and subjective sleep, objective sleep, and napping in women. Sleep Med. 2010a;11(2):180–184. doi: 10.1016/j.sleep.2009.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grandner MA, Patel NP, Gehrman PR, Perlis ML, Pack AI. Problems associated with short sleep: bridging the gap between laboratory and epidemiological studies. Sleep Med Rev. 2010b;14:239–247. doi: 10.1016/j.smrv.2009.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grandner MA, Patel NP, Gehrman PR, Xie D, Sha D, Weaver T, Gooneratne N. Who gets the best sleep? Ethnic and socioeconomic factors related to sleep complaints. Sleep Med. 2010c;11:470–479. doi: 10.1016/j.sleep.2009.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grandner MA, Patel NP, Hale L, Moore M. Mortality associated with sleep duration: the evidence, the possible mechanisms, and the future. Sleep Med Rev. 2010d;14:191–203. doi: 10.1016/j.smrv.2009.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall MH, Muldoon MF, Jennings JR, Buysse DJ, Flory JD, Manuck SB. Self-reported sleep duration is associated with the metabolic syndrome in midlife adults. Sleep. 2008;31(5):635–643. doi: 10.1093/sleep/31.5.635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heo M, Pietrobelli A, Wang D, Heymsfield SB, Faith MS. Obesity and functional impairment: influence of comorbidity, joint pain, and mental health. Obesity (Silver Spring) 2010;18:2030–2038. doi: 10.1038/oby.2009.400. [DOI] [PubMed] [Google Scholar]
- Hubácek JA. Eat less and exercise more—is it really enough to knock down the obesity pandemia? Physiol Res. 2009;58(Suppl 1):S1–S6. doi: 10.33549/physiolres.931855. [DOI] [PubMed] [Google Scholar]
- Idris I, Hall AP, O'Reilly J, Barnett A, Allen M, Andrews R, Grunstein P, Lewis K, Goenka N, Wilding JP. Obstructive sleep apnoea in patients with type 2 diabetes: aetiology and implications for clinical care. Diabetes Obes Metab. 2009;11:733–741. doi: 10.1111/j.1463-1326.2009.01045.x. [DOI] [PubMed] [Google Scholar]
- Irwin M. Effects of sleep and sleep loss on immunity and cytokines. Brain Behav Immun. 2002;16(5):503–512. doi: 10.1016/s0889-1591(02)00003-x. [DOI] [PubMed] [Google Scholar]
- Jain S, Mills PJ. Cytokines, chronic stress, and fatigue. In: Fink G, editor. Encyclopedia of stress. 2nd edn. vol 1. Academic; Oxford: 2007. pp. 698–704. [Google Scholar]
- Jia H, Lubetkin EI. Trends in quality-adjusted life-years lost contributed by smoking and obesity. Am J Prev Med. 2010;38(2):138–144. doi: 10.1016/j.amepre.2009.09.043. [DOI] [PubMed] [Google Scholar]
- Kaneita Y, Uchiyama M, Yoshiike N, Ohida T. Associations of usual sleep duration with serum lipid and lipoprotein levels. Sleep. 2008;31(5):645–652. doi: 10.1093/sleep/31.5.645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knutson KL, Van Cauter E. Associations between sleep loss and increased risk of obesity and diabetes. Ann N Y Acad Sci. 2008;1129:287–304. doi: 10.1196/annals.1417.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knutson KL, Ryden AM, Mander BA, Van Cauter E. Role of sleep duration and quality in the risk and severity of type 2 diabetes mellitus. Arch Intern Med. 2006;166(16):1768–1774. doi: 10.1001/archinte.166.16.1768. [DOI] [PubMed] [Google Scholar]
- Knutson KL, Spiegel K, Penev P, Van Cauter E. The metabolic consequences of sleep deprivation. Sleep Med Rev. 2007;11(3):163–178. doi: 10.1016/j.smrv.2007.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kripke DF, Garfinkel L, Wingard DL, Klauber MR, Marler MR. Mortality associated with sleep duration and insomnia. Arch Gen Psychiatry. 2002;59(2):131–136. doi: 10.1001/archpsyc.59.2.131. [DOI] [PubMed] [Google Scholar]
- Lam KB, Jiang CQ, Thomas GN, Arora T, Zhang WS, Taheri S, Adab P, Lam TH, Cheng KK. Napping is associated with increased risk of type 2 diabetes: the Guangzhou Biobank Cohort Study. Sleep. 2010;33(3):402–407. doi: 10.1093/sleep/33.3.402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauderdale DS, Knutson KL, Yan LL, Liu K, Rathouz PJ. Self-reported and measured sleep duration: how similar are they? Epidemiology. 2008;19(6):838–845. doi: 10.1097/EDE.0b013e318187a7b0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauderdale DS, Knutson KL, Rathouz PJ, Yan LL, Hulley SB, Liu K. Cross-sectional and longitudinal associations between objectively measured sleep duration and body mass index: the CARDIA Sleep Study. Am J Epidemiol. 2009;170:805–813. doi: 10.1093/aje/kwp230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leow MK. Obstructive sleep apnoea and the orexigenic pathway in type 2 diabetes. Diabetologia. 2010;53(8):1807–1808. doi: 10.1007/s00125-010-1778-9. [DOI] [PubMed] [Google Scholar]
- Lévy P, Bonsignore MR, Eckel J. Sleep, sleep-disordered breathing and metabolic consequences. Eur Respir J. 2009;34(1):243–260. doi: 10.1183/09031936.00166808. [DOI] [PubMed] [Google Scholar]
- Lindström J, Neumann A, Sheppard KE, Gilis-Januszewska A, Greaves CJ, Handke U, Pajunen P, Puhl S, Pölönen A, Rissanen A, Roden M, Stemper T, Telle-Hjellset V, Tuomilehto J, Velickiene D, Schwarz PE, Acosta T, Adler M, AlKerwi A, Barengo N, Barengo R, Boavida JM, Charlesworth K, Christov V, Claussen B, Cos X, Cosson E, Deceukelier S, Dimitrijevic-Sreckovic V, Djordjevic P, Evans P, Felton AM, Fischer M, Gabriel-Sanchez R, Goldfracht M, Gomez JL, Hall M, Hauner H, Herbst J, Hermanns N, Herrebrugh L, Huber C, Hühmer U, Huttunen J, Jotic A, Kamenov Z, Karadeniz S, Katsilambros N, Khalangot M, Kissimova-Skarbek K, Köhler D, Kopp V, Kronsbein P, Kulzer B, Kyne-Grzebalski D, Lalic K, Lalic N, Landgraf R, Lee-Barkey YH, Liatis S, Makrilakis K, McIntosh C, McKee M, Mesquita AC, Misina D, Muylle F, Paiva AC, Paulweber B, Peltonen M, Perrenoud L, Pfeiffer A, Raposo F, Reinehr T, Robinson C, Rothe U, Saaristo T, Scholl J, Spiers S, Stratmann B, Szendroedi J, Szybinski Z, Tankova T, Terry G, Tolks D, Toti F, Undeutsch A, Valadas C, Valensi P, Vermunt P, Weiss R, Wens J, Yilmaz T. Take action to prevent diabetes-the IMAGE toolkit for the prevention of type 2 diabetes in Europe. Horm Metab Res. 2010;42(Suppl 1):S37–S55. doi: 10.1055/s-0029-1240975. [DOI] [PubMed] [Google Scholar]
- Littman AJ, Vitiello MV, Foster-Schubert K, Ulrich CM, Tworoger SS, Potter JD, Weigle DS, McTiernan A. Sleep, ghrelin, leptin and changes in body weight during a 1-year moderate-intensity physical activity intervention. Int J Obes (Lond) 2007;31(3):466–475. doi: 10.1038/sj.ijo.0803438. [DOI] [PubMed] [Google Scholar]
- Lui MM, Ip MS. Disorders of glucose metabolism in sleep-disordered breathing. Clin Chest Med. 2010;31(2):271–285. doi: 10.1016/j.ccm.2010.02.001. [DOI] [PubMed] [Google Scholar]
- Macfarlane DJ, Thomas N. Exercise and diet in weight management: updating what works. Br J Sports Med. 2010;44:1197–1201. doi: 10.1136/bjsm.2009.065235. [DOI] [PubMed] [Google Scholar]
- Mackiewicz M, Shockley KR, Romer MA, Galante RJ, Zimmerman JE, Naidoo N, Baldwin DA, Jensen ST, Churchill GA, Pack AI. Macromolecule biosynthesis: a key function of sleep. Physiol Genomics. 2007;31(3):441–457. doi: 10.1152/physiolgenomics.00275.2006. [DOI] [PubMed] [Google Scholar]
- Mackiewicz M, Naidoo N, Zimmerman JE, Pack AI. Molecular mechanisms of sleep and wakefulness. Ann N Y Acad Sci. 2008;1129:335–349. doi: 10.1196/annals.1417.030. [DOI] [PubMed] [Google Scholar]
- Meier-Ewert HK, Ridker PM, Rifai N, Regan MM, Price NJ, Dinges DF, Mullington JM. Effect of sleep loss on C-reactive protein, an inflammatory marker of cardiovascular risk. J Am Coll Cardiol. 2004;43(4):678–683. doi: 10.1016/j.jacc.2003.07.050. [DOI] [PubMed] [Google Scholar]
- Mullington JM, Haack M, Toth M, Serrador JM, Meier-Ewert HK. Cardiovascular, inflammatory, and metabolic consequences of sleep deprivation. Prog Cardiovasc Dis. 2009;51(4):294–302. doi: 10.1016/j.pcad.2008.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Must A, Parisi SM. Sedentary behavior and sleep: paradoxical effects in association with childhood obesity. Int J Obes (Lond) 2009;33(Suppl 1):S82–S86. doi: 10.1038/ijo.2009.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nedeltcheva AV, Kilkus JM, Imperial J, Kasza K, Schoeller DA, Penev PD. Sleep curtailment is accompanied by increased intake of calories from snacks. Am J Clin Nutr. 2009;89(1):126–133. doi: 10.3945/ajcn.2008.26574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okun ML, Coussons-Read M, Hall M. Disturbed sleep is associated with increased C-reactive protein in young women. Brain Behav Immun. 2009;23(3):351–354. doi: 10.1016/j.bbi.2008.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliver SJ, Costa RJ, Laing SJ, Bilzon JL, Walsh NP. One night of sleep deprivation decreases treadmill endurance performance. Eur J Appl Physiol. 2009;107:155–161. doi: 10.1007/s00421-009-1103-9. [DOI] [PubMed] [Google Scholar]
- Opp MR, Born J, Irwin MR. Sleep and the immune system. In: Ader R, editor. Psychoneuroimmunology. 4th edn. Elsevier; Burlington: 2007. pp. 579–618. [Google Scholar]
- Patel SR, Blackwell T, Redline S, Ancoli-Israel S, Cauley JA, Hillier TA, Lewis CE, Orwoll ES, Stefanick ML, Taylor BC, Yaffe K, Stone KL. The association between sleep duration and obesity in older adults. Int J Obes (Lond) 2008;32:1825–1834. doi: 10.1038/ijo.2008.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlis ML, Swinkels CM, Gehrman PR, Pigeon WR, Matteson-Rusby SE, Jungquist CR. The incidence and temporal patterning of insomnia: a pilot study. J Sleep Res. 2010;19:31–35. doi: 10.1111/j.1365-2869.2009.00768.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Punjabi NM. Do sleep disorders and associated treatments impact glucose metabolism? Drugs. 2009;69(Suppl 2):13–27. doi: 10.2165/11531150-000000000-00000. [DOI] [PubMed] [Google Scholar]
- Reynolds CF, 3rd, Serody L, Okun ML, Hall M, Houck PR, Patrick S, Maurer J, Bensasi S, Mazumdar S, Bell B, Nebes RD, Miller MD, Dew MA, Nofzinger EA. Protecting sleep, promoting health in later life: a randomized clinical trial. Psychosom Med. 2010;72(2):178–186. doi: 10.1097/PSY.0b013e3181c870a5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson LC, Wingo PA, Zack MM, Zahran HS, King JB. Health-related quality of life in cancer survivors between ages 20 and 64 years: population-based estimates from the Behavioral Risk Factor Surveillance System. Cancer. 2008;112(6):1380–1389. doi: 10.1002/cncr.23291. [DOI] [PubMed] [Google Scholar]
- Salihu HM, Bonnema SM, Alio AP. Obesity: what is an elderly population growing into? Maturitas. 2009;63:7–12. doi: 10.1016/j.maturitas.2009.02.010. [DOI] [PubMed] [Google Scholar]
- SAS Institute . Base SAS 9.2 procedures guide: statistical procedures. SAS Institute; Cary: 2008. [Google Scholar]
- Schultes B, Schmid S, Peters A, Born J, Fehm HL. Sleep loss and the development of diabetes: a review of current evidence. Exp Clin Endocrinol Diabetes. 2005;113(10):563–567. doi: 10.1055/s-2005-872944. [DOI] [PubMed] [Google Scholar]
- Schuster DP. Changes in physiology with increasing fat mass. Semin Pediatr Surg. 2009;18(3):126–135. doi: 10.1053/j.sempedsurg.2009.04.002. [DOI] [PubMed] [Google Scholar]
- Spiegel K, Leproult R, Van Cauter E. Impact of sleep debt on metabolic and endocrine function. Lancet. 1999;354(9188):1435–1439. doi: 10.1016/S0140-6736(99)01376-8. [DOI] [PubMed] [Google Scholar]
- Spiegel K, Leproult R, L'Hermite-Balériaux M, Copinschi G, Penev PD, Van Cauter E. Leptin levels are dependent on sleep duration: relationships with sympathovagal balance, carbohydrate regulation, cortisol, and thyrotropin. J Clin Endocrinol Metab. 2004a;89(11):5762–5771. doi: 10.1210/jc.2004-1003. [DOI] [PubMed] [Google Scholar]
- Spiegel K, Tasali E, Penev P, Van Cauter E. Brief communication: sleep curtailment in healthy young men is associated with decreased leptin levels, elevated ghrelin levels, and increased hunger and appetite. Ann Intern Med. 2004b;141(11):846–850. doi: 10.7326/0003-4819-141-11-200412070-00008. [DOI] [PubMed] [Google Scholar]
- Spiegel K, Knutson K, Leproult R, Tasali E, Van Cauter E. Sleep loss: a novel risk factor for insulin resistance and type 2 diabetes. J Appl Physiol. 2005;99(5):2008–2019. doi: 10.1152/japplphysiol.00660.2005. [DOI] [PubMed] [Google Scholar]
- Strine TW, Okoro CA, Chapman DP, Balluz LS, Ford ES, Ajani UA, Mokdad AH. Health-related quality of life and health risk behaviors among smokers. Am J Prev Med. 2005;28(2):182–187. doi: 10.1016/j.amepre.2004.10.002. [DOI] [PubMed] [Google Scholar]
- Tasali E, Leproult R, Spiegel K. Reduced sleep duration or quality: relationships with insulin resistance and type 2 diabetes. Prog Cardiovasc Dis. 2009;51(5):381–391. doi: 10.1016/j.pcad.2008.10.002. [DOI] [PubMed] [Google Scholar]
- Thomas A, Schüssler MN, Fischer JE, Terris DD. Employees' sleep duration and body mass index: potential confounders. Prev Med. 2009;48:467–470. doi: 10.1016/j.ypmed.2009.03.012. [DOI] [PubMed] [Google Scholar]
- Ronksley PE, Hemmelgarn B, Heitman SJ, Hanly PJ, Faris PD, Quan H, Tsai WH. Obstructive sleep apnea is associated with diabetes in sleepy subjects. Thorax. 2009;64:834–839. doi: 10.1136/thx.2009.115105. [DOI] [PubMed] [Google Scholar]
- Tuomilehto H, Peltonen M, Partinen M, Lavigne G, Eriksson JG, Herder C, Aunola S, Keinänen-Kiukaanniemi S, Ilanne-Parikka P, Uusitupa M, Tuomilehto J, Lindström J. Sleep Duration, Lifestyle Intervention and Incidence of Type 2 Diabetes in Impaired Glucose Tolerance. The Finnish Diabetes Prevention Study. Diabetes Care. 2009;32:1965–1971. doi: 10.2337/dc08-1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueno LM, Drager LF, Rodrigues AC, Rondon MU, Braga AM, Mathias W, Jr, Krieger EM, Barretto AC, Middlekauff HR, Lorenzi-Filho G, Negrão CE. Effects of exercise training in patients with chronic heart failure and sleep apnea. Sleep. 2009;32(5):637–647. doi: 10.1093/sleep/32.5.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Cauter E, Holmback U, Knutson K, Leproult R, Miller A, Nedeltcheva A, Pannain S, Penev P, Tasali E, Spiegel K. Impact of sleep and sleep loss on neuroendocrine and metabolic function. Horm Res. 2007;67(Suppl 1):2–9. doi: 10.1159/000097543. [DOI] [PubMed] [Google Scholar]
- Van Cauter E, Spiegel K, Tasali E, Leproult R. Metabolic consequences of sleep and sleep loss. Sleep Med. 2008;9(Suppl 1):S23–S28. doi: 10.1016/S1389-9457(08)70013-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe M, Kikuchi H, Tanaka K, Takahashi M. Association of short sleep duration with weight gain and obesity at 1-year follow-up: a large-scale prospective study. Sleep. 2010;33(2):161–167. doi: 10.1093/sleep/33.2.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams CJ, Hu FB, Patel SR, Mantzoros CS. Sleep duration and snoring in relation to biomarkers of cardiovascular disease risk among women with type 2 diabetes. Diabetes Care. 2007;30(5):1233–1240. doi: 10.2337/dc06-2107. [DOI] [PubMed] [Google Scholar]
- Youngstedt SD, Kline CE. Epidemiology of exercise and sleep. Sleep Biol Rhythms. 2006;4:215–221. doi: 10.1111/j.1479-8425.2006.00235.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yue HJ, Bardwell W, Ancoli-Israel S, Loredo JS, Dimsdale JE. Arousal frequency is associated with increased fatigue in obstructive sleep apnea. Sleep Breath. 2009;13:331–339. doi: 10.1007/s11325-009-0252-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zizi F, Jean-Louis G, Brown CD, Ogedegbe G, Boutin-Foster C, McFarlane SI. Sleep duration and the risk of diabetes mellitus: epidemiologic evidence and pathophysiologic insights. Curr Diab Rep. 2010;10(1):43–47. doi: 10.1007/s11892-009-0082-x. [DOI] [PMC free article] [PubMed] [Google Scholar]


