Abstract
The rise in blood pressure with age is a major risk factor for cardiovascular and renal disease, stroke and type-2 diabetes. Age-related increases in blood pressure have been observed in almost every population, except among hunter-gatherers, farmers and pastoralists. Here we test for age-related increases in blood pressure among Tsimane forager-farmers. We also test whether lifestyle changes associated with modernization lead to higher blood pressure and a greater rate of age-related increase in blood pressure. We measured blood pressure longitudinally on 2,296 adults age 20+ (n=6,517 observations over eight years). Prevalence of hypertension is 3.9% for women and 5.2% for men, although diagnosis of persistent hypertension based on multiple observations reduces prevalence to 2.9% for both sexes. Mixed-effects models reveal systolic, diastolic and pulse blood pressure increases of 2.86 (p < 0.001), 0.95 (p < 0.001) and 1.95 (p < 0.001) mm Hg per decade for women and 0.91 (p < 0.001), 0.93 (p < 0.001) and −0.02 (p = 0.93) for men, substantially lower than rates found elsewhere. Lifestyle factors, such as smoking and Spanish fluency have minimal effect on mean blood pressure and no effect on age-related increases in blood pressure. Rise in age-related blood pressure varies by distance to market and body mass index. Effects of modernization are therefore deemed minimal among Tsimane, in light of their lean physique, active lifestyle and protective diet.
Keywords: hypertension, Tsimane, blood pressure, modernization
INTRODUCTION
An age-related increase in blood pressure (BP) is viewed as a universal feature of human aging1–3. Among Westerners over age 40, systolic pressure (SBP) increases by about 7 mm Hg per decade4. Epidemiological surveys show a progressive increase in SBP with age, reaching an average of roughly 140 mm Hg by the eighth decade5. Diastolic pressure (DBP) also increases with age but at a lower rate than SBP; DBP may even fall at late ages6. Women show lower SBP and DBP than men up until the age of menopause, when women’s SBP surpasses that of men7. By age 70, over three-fourths of U.S. adults have hypertension.
Understanding the conditions affecting age-related BP increase is of obvious clinical importance. Higher BP is associated with cardiovascular and renal disease across diverse populations, even controlling for other factors5. Hypertension is the leading cause of cardiovascular mortality, and age-related BP increase is a high priority target for intervention8.
The only reported cases of no age-related BP increase come from studies of subsistence-level populations9–11. These studies, however, are problematic: they are cross-sectional, use small, sometimes biased samples, and often do not specify explicit measurement methods. Age estimates of older adults are also poor12. Due to epidemiologic and economic transition, cohort effects may also have muted age effects; younger adults may have higher BP than older adults did when they were younger.
Nonetheless, results from many studies suggest that “modernization” results in changes in diet, adiposity, activity, and psychosocial stress, leading to higher BP and greater age-related increases in BP13–15,. While available evidence shows that hypertension is more common among those with modern lifestyles, it is unclear whether these changes impact the rate of increase in BP. It is also unclear whether these changes impact everyone equally, or just high risk subpopulations. Heterogeneity in susceptibility and modernization could reveal further variability in longitudinal age trajectories of BP.
Here we assess the extent to which BP increases with age using longitudinal and cross-sectional data collected among Tsimane of the Bolivian Amazon. Tsimane are lowland forager-horticulturalists (pop. ~11,000) subsisting on plantains, rice, corn and manioc, fish and hunted game. Tsimane are currently undergoing epidemiological and technological transition16, although there was no electricity, running water, or waste management at time of study. Villages vary in their degree of healthcare access. Modernization takes several forms: visits to the town of San Borja (pop. ~24,000), wage labor with loggers or colonists, debt peonage with itinerant river merchants, and schooling. Schools now exist in over 75% of villages but many older adults have little or no schooling.
We first assess hypertension prevalence, and examine age-related changes in SBP, DBP and pulse pressure (PP), to test the general hypothesis that BP increase is a robust feature of human aging. We then test whether both BP and age-related increase in BP increase with modernization, operationalized by Spanish fluency, distance to town, smoking frequency and body mass index (BMI). We also assess whether an increase in BP with age occurs uniformly or is instead concentrated among a high-risk subpopulation.
METHODS
Study population
2,248 adults aged 20 to 90 (n=82 villages) participated in the Tsimane Health and Life History Project from July 2002 to December 2010. Adults were sampled anywhere from one to nine times during medical rounds, yielding a sample of 6,468 person-observations; 61% of adults were sampled at least twice, and 36% at least four times (Table S1). Sample size varied from 268to 1,186 individuals across nine medical rounds (Table S2).
BP and controls
SBP and DBP were measured on the right arm by trained Bolivian physicians with a Welch Allyn Tycos Aneroid 5090 sphygmomanometer and Littman stethoscope. Patients were seated or supine for at least 20 minutes before measurements. After 2008, all hypertensive readings were repeated after 30+ minutes to confirm preliminary diagnoses. No Tsimane has ever taken medication to control hypertension. We use the Joint National Committee on Prevention, Detection and Treatment of High Blood Pressure classification scheme to define BP categories: hypertensive (SBP ≥140 mm Hg or DBP ≥90 mm Hg), prehypertensive (120–139 SBP or 80–89 DBP) and normotensive (SBP <120 and DBP <80).
Height (cm) was measured by trained Tsimane research assistants with a portable Seca stadiometer. Weight (kg) and body fat percentage were measured using a Tanita BF-572 weigh scale.
Modernization
Village-level variance in distance to San Borja is substantial (mean±SD=41±23 km, min=6, max=82). Highest level of schooling and Spanish fluency were assessed during census updates and demographic interviews. Cumulative smoking was measured in cigarette pack years based on interviews of number of cigarettes smoked per week and age at which the interviewee started smoking. One pack year is equal to a pack of cigarettes smoked per day for one year. Given potential problems with recall bias, cumulative smoking experience was categorized into terciles: first (0.003–0.07 pack years), second (0.07–0.30), or third (>0.30).
Statistical Analysis
Cross-sectional analyses
We employed mixed and fixed effect models with linear and non-linear age parameters. Linear models were fit with the lm and lme procedures in R 2.13.1. Non-linear models employed generalized additive models (GAM)17,18. GAMs use a thin-point spline to fit non-linear age patterns while allowing for the simultaneous inclusion of parametric terms. GAMs were fit with gam in the mgcv package and gamm4 in the gamm4 package. Mixed models were used to control for both individual variation in age trajectories and correlated errors between repeated samples19.
Longitudinal rates of BP change
Longitudinal analyses included only individuals with ≥5 years between first and last observation (please see http://hyper.ahajournals.org). Repeat BP values were recoded as changes from the mean of a subject’s BP measures (ΔBP); times were coded as days before or after the subject’s median exam date. Linear models were fit to ΔBP including subject ID, a subject-by- time interaction term, season and pregnancy status as controls. Parameter values for ΔBP were obtained from the subject-by- time interaction terms.
Two stage mixed-model
To examine the effect of modernization on absolute BP levels and rates of BP change, we employ a two stage mixed model (Table 2, S3). In the first stage a standard mixed GAM was run with a non-parametric age term and individual variation in slope was modeled as a random effect. Individual slopes are obtained by adding the overall population slope for an individual’s age plus that individual’s random slope, both from the stage 1 model. These slopes were used as the dependent variable in model 2 to examine factors affecting rate of BP change.
Table 2.
Two-stage mixed models. Stage 1 models have a random slope and intercept for each individual in the study, with BP as the dependent variable. Stage 2 models use the individual random-effect slopes plus population main effect of age from Stage 1 as the dependent variable. Age was included as a non-linear thin-plate spline in both models. Only individuals with data for all variables and at least two observations were included (n=695 individuals, n=2,876 observations). For more details please see http://hyper.ahajournals.org.
| Parameter | Stage 1 | Stage 2 | ||||
|---|---|---|---|---|---|---|
| Main effects (mm Hg)
|
ΔBP (mm Hg/decade)
|
|||||
| SBP | DBP | PP | SBP | DBP | PP | |
| Constant | 94.5‡ | 57.6‡ | 36.3‡ | 2.0‡ | 0.9‡ | 0.6 |
| Sex (Male) | 2.7† | 1.4* | 1.1 | −2.7‡ | 0.03 | −3.0‡ |
| BMI | 0.6‡ | 0.4‡ | 0.3‡ | 0.02 | 0.01 | 0.03 |
| Years of Schooling | 0.1 | 0.1 | 0.01 | 0.01 | 0.01 | 0.01 |
| Distance to San Borja (per 10 km) | −0.3* | −0.1 | −0.2 | 0.03 | 0.01 | 0.08‡ |
| Pregnant | −2.8† | −3.3‡ | 0.4 | |||
| Smoking (tercile§) | ||||||
| 1st | −1.2 | −1.4* | 0.2 | −0.1 | −0.03 | −0.20 |
| 2nd | 1.1 | 0.5 | 1.1 | −0.01 | 0.10 | −0.10 |
| 3rd | 0.5 | −0.4 | 1.2 | −0.02 | 0.05 | −0.08 |
| Spanish fluency|| | ||||||
| Moderate | 1.1 | 0.5 | 0.5 | 0.05 | 0.07 | 0.01 |
| Fluent | −1.1 | 0.4 | −1.8* | 0.08 | 0.06 | 0.2 |
| Season¶ | ||||||
| Dry | 3.9‡ | 2.8‡ | 1.0* | |||
| Intermediate | −0.6 | 0.02 | −0.6 | |||
p≤0.05;
p≤0.01;
p≤0.001
Relative to no smoking
Relative to speaks no Spanish
Relative to wet season
Ethical concerns
Informed consent was obtained for all protocols at three levels: Tsimane government, community, and individual. After explanation of protocols by bilingual Spanish-Tsimane research assistants, consent forms were either signed by literate participants, or fingerprinted by non-literate participants. All protocols have been approved by the Institutional Review Boards at UNM and UCSB.
RESULTS
Sample characteristics
Average age was 38.0 and 39.3 years for women and men, respectively (Table 1). Women represent 52.6% of observations. In comparison to normotensives, hypertensive men and women are older, shorter, have more body fat, are less likely to be non-smokers, are less educated and are more likely to speak Spanish.
Table 1.
Sample means by BP status for Tsimane adults aged 20+. For each individual the median value of repeated measures on a given variable was used to calculate group means and determine hypertensive category.
| Variable | Women
|
Men
|
Sex by BP Group|| | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Blood Pressure Group | P-value§ | Blood Pressure Group | P-value§ | ||||||||||
| Normal (N) | Pre (P) | Hyper (H) | NvP | NvH | PvH | Normal (N) | Pre (P) | Hyper (H) | NvP | NvH | PvH | ||
| Age (years) | 38.0 | 56.0 | 66.5 | ‡ | ‡ | ‡ | 39.3 | 46.8 | 65.8 | ‡ | ‡ | ‡ | ‡ |
| Height (cm) | 150.8 | 150.8 | 148.3 | ns | † | † | 162.7 | 162.2 | 159.5 | ns | ‡ | † | ns |
| Weight (kg) | 53.6 | 56.3 | 52.5 | † | ns | * | 62.8 | 63.7 | 63.3 | † | ns | ns | ns |
| Body fat (%) | 25.9 | 28.5 | 28.2 | ‡ | † | ns | 17.5 | 18.9 | 21.1 | ‡ | ‡ | * | ns |
| BMI (kg/m2) | 23.6 | 24.7 | 23.8 | ‡ | ns | ns | 23.6 | 24.2 | 24.8 | ‡ | * | ns | ns |
| Years of schooling | 1.4 | 0.5 | 0.5 | ‡ | ns | ns | 2.8 | 2.5 | 1.0 | ns | ns | ns | ns |
| Distance to San | |||||||||||||
| Borja (km) | 37.7 | 35.0 | 35.9 | ns | ns | ns | 38.5 | 41.1 | 36.2 | ns | ns | ns | ns |
| SBP (mm Hg) | 103.8 | 122.2 | 146.5 | ‡ | ‡ | ‡ | 107.9 | 122.5 | 144.3 | ‡ | ‡ | ‡ | ‡ |
| DBP (mm Hg) | 64.4 | 73.9 | 80.0 | ‡ | ‡ | ‡ | 66.6 | 74.0 | 95.0 | ‡ | ‡ | ‡ | ‡ |
| PP (mm Hg) | 39.2 | 47.0 | 65.2 | ‡ | ‡ | ‡ | 41.6 | 47.3 | 54.7 | ‡ | ‡ | ns | ‡ |
| Smoking tercile (%) | |||||||||||||
| None | 78.9% | 69.3% | 58.8% | * | * | ns | 15.8% | 21.4% | 18.8% | ns | ns | ns | ns |
| 1st | 14.9% | 13.3% | 23.5% | 27.5% | 21.4% | 18.8% | |||||||
| 2nd | 3.9% | 16.0% | 11.8% | 27.5% | 23.3% | 31.3% | |||||||
| 3rd | 2.3% | 1.3% | 5.9% | 29.3% | 34.0% | 31.3% | |||||||
| Spanish fluency (%) | |||||||||||||
| None | 55.8% | 72.1% | 38.5% | * | ns | * | 24.6% | 31.4% | 16.7% | ns | ns | ns | ns |
| Moderate | 27.3% | 18.0% | 38.5% | 36.6% | 36.4% | 66.7% | |||||||
| Fluent | 16.9% | 9.8% | 23.1% | 38.8% | 32.2% | 16.7% | |||||||
| n (cases) | 942 | 158 | 44 | 838 | 288 | 51 | |||||||
p≤0.05;
p≤0.01;
p≤0.001.
P-values for comparisons are from a Mann-Whitney U or chi-square test.
P-values indicate the significance of a sex x blood pressure group interaction in a linear model with Gaussian link or a geneneralized linear model with ordinal response and logit link function. Models also included main effect terms for sex and hypertension group (not shown).
Abbreviations: NvP=normal vs. prehypertensive; NvH=normal vs. hypertensive; PvH=prehypertensive vs. hypertensive.
Mean BP and hypertension prevalence
In the largest medical round (Oct. 2008–2009), any observation of hypertension was followed with a confirmatory reading within a half hour. Mean BP for Tsimane men and women, respectively, is: 113, 108 mmHg (SBP), 70, 66 mmHg (DBP) and 43, 41 mmHg (PP). This cross-sectional analysis shows a notable increase in SBP and PP with age for women, and a very modest increase in SBP for men (Figure S1). Prevalence of hypertension is 3.9% for women and 5.2% for men (Figure S2). It is highest among women over age 70 (30.4%). Isolated systolic hypertension accounts for 49.3% of hypertensive cases, and isolated diastolic 22.3%. Pre-hypertension prevalence is 17.4% for women and 29.1% for men.
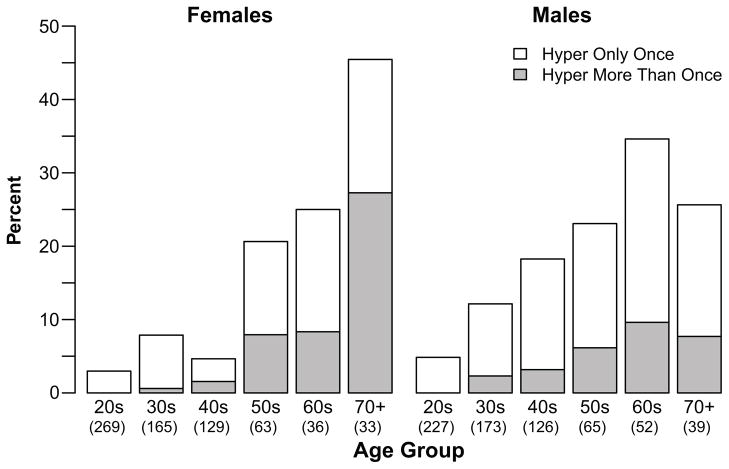
Prevalence of hypertension declines substantially if we require additional observations of elevated BP in other rounds. Among people sampled at least 3 times, only 38% were hypertensive more than once, and only 1% were hypertensive for all readings (Table S1). Even among those sampled 8 times, 50% of those with a hypertensive measurement were hypertensive only once. It is therefore likely that the true prevalence of hypertension may be as low as one-third the rates based on single measurements reported in Table S1 and preliminarily described in 20. Among those sampled multiple times, frequency of at least two instances of hypertension is low (Figure 1). Only 7.7% and 27.3% of men and women, respectively, in the highest risk age category (aged 70+), were hypertensive more than once, while an additional 18% of each sex were hypertensive only once. Overall, prevalence of repeat hypertension is 2.9% for both sexes.
Figure 1.
Prevalence of hypertension by age and sex among those sampled at least twice.
Rise in BP with age
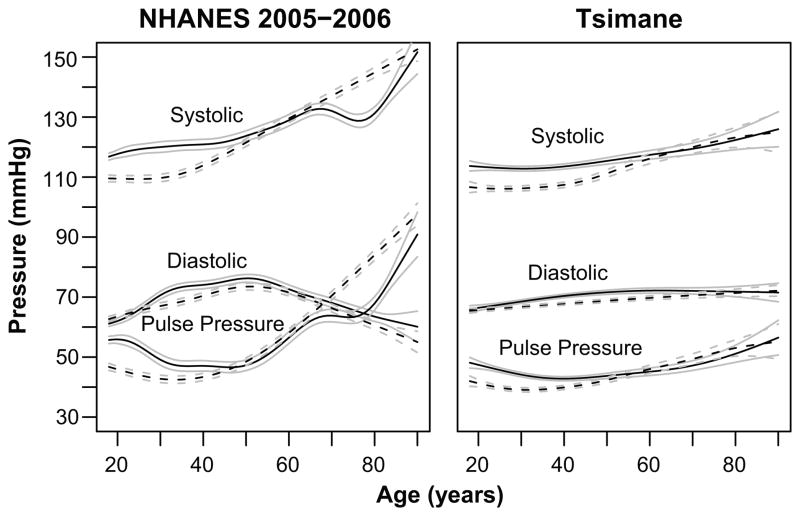
We estimated age trajectories of SBP, DBP, and PP for Tsimane and a U.S. comparison (NHANES 2005–2006) (Figure 2). Men’s SBP is much flatter across adulthood than women’s, whose SBP rises substantially around menopause. DBP increase with age is modest for women, whereas DBP decreases for men after age 60. This decrease in DBP is observed for both men and women in the U.S. PP increases for women after age 40 and less steeply for men after age 45. Despite these sex differences, Tsimane age profiles indicate substantially less change in BP with age than U.S. age profiles, even after controlling for BMI (Figure 2). However, both populations show similarities, including lower SBP for women than men at younger ages and increasing BP in women after menopause. Although blunted, Tsimane males also show an increase in DBP early in life and a decrease later in life. PP increases at later ages in both populations.
Figure 2.
BP by age and sex. Generalized additive models of SBP, DBP and PP for males (solid lines) and females (dashed lines), controlling for BMI and pregnancy status. Tsimane models are mixed models to control for repeated observations (n=5,528 observations, 1,749 individuals). NHANES models are based on a single time-point (n=7,359). Both models are illustrated at BMI=25.84, which is the midpoint between mean BMI for Tsimane (23.67) and NHANES (28.00). Gray lines are 95% confidence intervals for the mean.
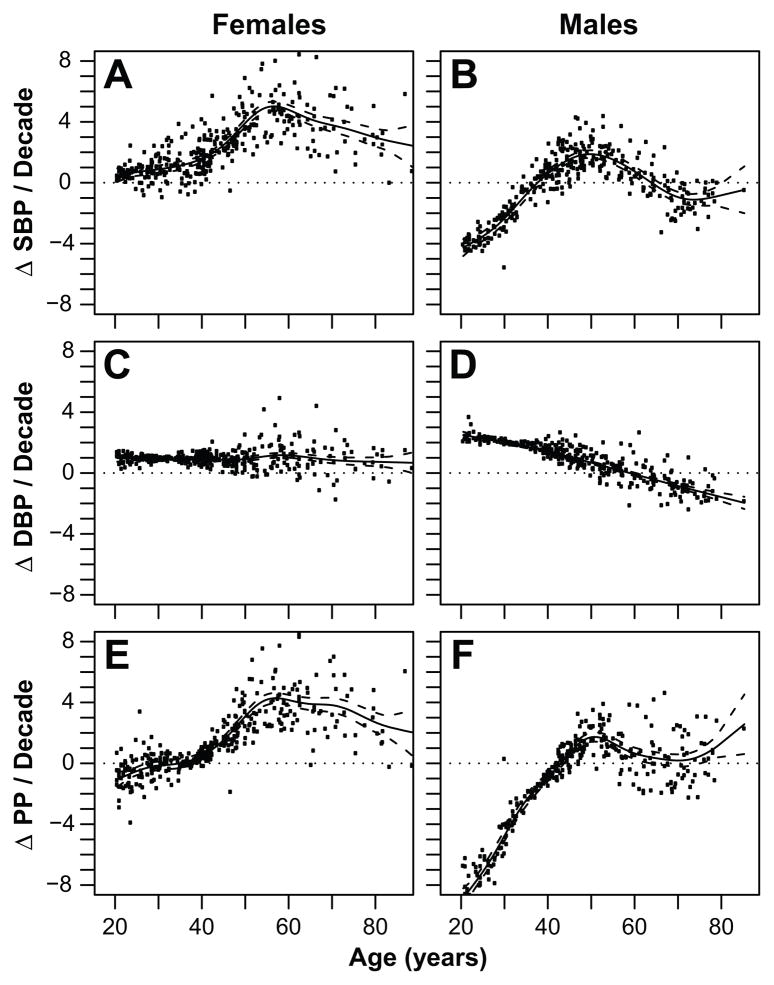
The two stage mixed modeling strategy tests for effects on both the intercept and rate of increase in BP for individuals (Table 2, S3). Stage 1 models main effects of predictors on BP, using random effects to control for repeated observations. Stage 2 assigns a slope to each individual consisting of the population slope for that age from Stage 1 plus the individual’s difference from the population mean obtained from the Stage 1 random effects model. These analyses include controls for sex, pregnancy status, season, BMI, Spanish fluency, years of schooling and distance to San Borja. Substantial variability exists among individuals in ΔBP (Figure 3). Overall, SBP increases throughout life for women. Average ΔSBP increases significantly among women aged 40–55, then declines gradually (Figure 3A). The net ΔSBP for men increases from a negative slope to a positive one by the mid-30’s, increases slightly for a few decades to a maximum of 2 mm Hg/decade, and then declines after age 50 (Figure 3B). ΔDBP is constant and positive at about 1 mm/decade for women, but declines continuously with age in men (Figures 3C–D). ΔPP shows a similar pattern as ΔSBP in women, given the lack of age-related change in ΔDBP. ΔPP changes little before age 40 given similar changes in ΔSBP and ΔDBP (Figure 3E). For men ΔPP increases from negative before age 40 to positive after age 40 and close to zero after age 60 (Figure 3F).
Figure 3.
Change in SBP (A, B), DBP (C, D) and PP (E, F) per decade by sex and age. Points are ΔBP versus mean observation age (Table 2, Step 1). Lines are spline fits and 95% confidence intervals for the slopes as a function of mean observation age, estimated with a generalized additive model (Table 2, Step 2).
Cross-sectional vs. Longitudinal Analysis
Although analyses above include repeated measures, they are cross-sectional because they estimate the overall population pattern for a given segment of time. An explicit longitudinal analysis looks at within individual changes. We estimated ΔBP for each individual with at least five years between first and last observation using linear regression models and controlling for season of measurement and pregnancy status. ΔBP varies somewhat among cross-sectional and longitudinal analyses, although less so when cross-sectional analyses are restricted to the same set of individuals with at least five observations (Table 3). Due to intra-individual lability of BP, standard errors of longitudinally estimated slopes are much higher than those estimated cross-sectionally, and in many cases slopes were not significantly different from zero.
Table 3.
Comparison of 10-year increase in BP as estimated from cross-sectional (CS) versus longitudinal (L) analyses. CS slopes were estimated on both the full sample and a subset with repeated observations at least five years apart, controlling for season, pregnancy, repeated measures and subject ID. For values from CS analyses, parameter estimates (β) are shown; for values from L analyses, mean parameters (Mβ) are shown. Significance is given for a one-sample t-test for results from L analyses and for the model parameter from CS analyses.
| Age | Men
|
Women
|
|||||
|---|---|---|---|---|---|---|---|
| CS All | CS ≥ 5 Yrs | L ≥ 5 Yrs | CS All | CS ≥ 5 Yrs | L ≥ 5 Yrs | ||
| β | β | Mβ | β | B | Mβ | ||
| 20–39 | ΔSBP | −0.33 | 3.65† | 2.09 | 1.83† | 2.59* | 1.82 |
| ΔDBP | 2.28‡ | 2.22* | −3.90 | 1.61‡ | 0.49 | −1.93 | |
| ΔPP | −2.71‡ | 1.32 | 5.99† | 0.11 | 1.89† | 3.75* | |
| 40–59 | ΔSBP | 2.03* | 0.85 | 0.69 | 4.36‡ | 5.62‡ | 2.12 |
| ΔDBP | 0.43 | −0.87 | −0.72 | 1.72† | 1.91* | −0.97 | |
| ΔPP | 1.75† | 1.67* | 1.41 | 2.83‡ | 3.66‡ | 3.09 | |
| 60+ | ΔSBP | −2.64 | −2.86 | −5.71 | 1.89 | 0.82 | 0.45 |
| ΔDBP | −4.08‡ | −4.50† | −7.26* | −0.96 | −2.03 | −5.07 | |
| ΔPP | 1.4 | 1.65 | 1.55 | 3.09* | 2.99 | 5.52 | |
| All | ΔSBP | 0.91‡ | 1.23† | 0.32 | 2.86‡ | 3.08‡ | 1.81 |
| Ages | ΔDBP | 0.93‡ | 0.23 | −2.99* | 0.95‡ | 0.72† | −1.86 |
| ΔPP | −0.02 | 0.97‡ | 3.31 | 1.95‡ | 2.38‡ | 3.67† | |
p≤0.05
p≤0.01
p≤0.001
Across ages, men had positive but moderate ΔSBPs, ranging from 0.32 in longitudinal to 1.23 mm/decade in the restricted cross-sectional analyses. Women had higher overall ΔSBP, ranging from 1.81 to 3.08 mm/decade. Men had little net increase in ΔDBP, with estimates ranging from −2.99 in longitudinal to 0.93 mm/decade in the cross-sectional analysis. Similarly, female ΔDBP ranged from −1.86 in longitudinal to 0.95 in cross-sectional analysis. PP increased the most in longitudinal analyses, 3.31 and 3.67 mm/decade, but this increase was modest in cross-sectional analysis, with −0.02 and 1.95 for men and women, respectively.
After segregating the sample by age, cross-sectional and longitudinal analyses show similarities, but with notable exceptions. Men age 20–39 had significantly decreasing ΔPP in the cross-sectional model including all Tsimane, but increasing ΔPP in the restricted sample. In all three models male ΔPP increased between ages 40 and 59, but only ΔSBP in the full cross-sectional model increased significantly above zero. Male ΔDBP declined significantly in individuals age 60+ in all analyses. Like men, women age 20–39 had increasing ΔPP in the restricted sample and no change in the full cross-sectional sample. ΔSBP increased in all three models, though not significantly in the longitudinal analysis. For women age 40–59, ΔSBP, ΔDBP and ΔPP increased in both cross-sectional analyses. Increases in ΔSBP and ΔPP in the longitudinal analysis were not statistically significant. Women aged 60+ showed increasing ΔSBP, declining ΔDBP, and increasing ΔPP, but only ΔPP changed significantly, and only in the full cross-sectional sample.
Variance in BP
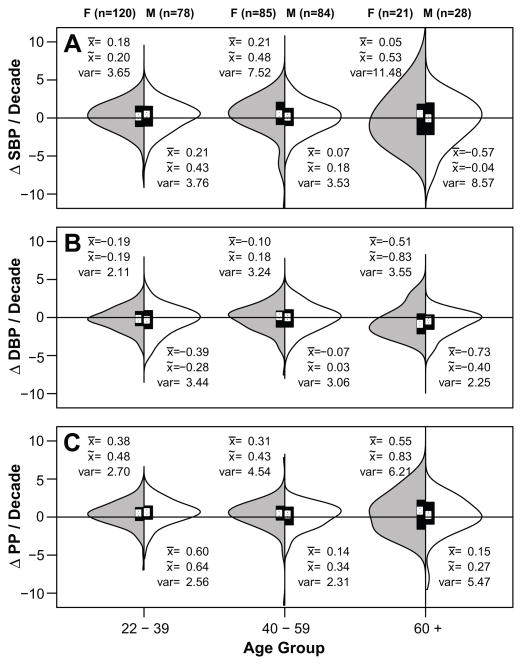
To test whether BP patterns were consistent for all individuals or appeared to affect subpopulations differentially, we examined differences in variance in BP and longitudinal slopes by sex, age, and population. Overall, variance in SBP, DBP and PP was higher in women than men and higher in Americans than Tsimane, particularly after age 40 (Figure 4, Table S4). Variance in both sexes and populations increased with age; both Tsimane and American women showed higher variance in BP with age. Examining longitudinal slopes, Tsimane women had higher variance over age 40, but variance did not increase significantly at age 60+ compared to ages 40–59 (Table S4). Tsimane men’s SBP variance increased after age 40 and men’s variance in slope also increased after age 60. Tsimane men’s variance in DBP did not change significantly with age, whereas Tsimane and American women’s DBP and PP variance increased with age (Figures 4, S4). Overall variance is greatest for SBP, and the greater variance with age among women is evident. By age 60, while mean and median slopes for women are positive for SBP and PP, a significant portion of women show slopes at or below zero.
Figure 4.
Distribution of individual ΔSBP (A), ΔDBP (B) and ΔPP (C) per decade by sex and age. Females are shown on the left (gray) and males on the right (white). Only individuals with two or more measures and at least five years between their earliest and latest BP measures were included. Boxplots show the first to third quartile range. Distributions are smoothed density plots. White circles indicate medians.
Effects of modernization
We examine effects of modernization on SBP, DBP and PP controlling for age, sex, season, and pregnancy status (Table 2: Stage 1). BMI is associated with higher SBP (β =0.61), DBP (β =0.39), and PP (β =0.25). BMI is not associated with significant differences in ΔBPs with age. Living farther from town is associated with lower SBP (β =−0.30 per 10 km), and a greater ΔPP (β =0.08 mm/10y per 10 km). Fluent Spanish speakers have lower PP than those with no Spanish fluency (β =−1.8 mmHg). Individuals in the lowest smoking tercile have lower DBP than non-smokers (β =−1.43), but other terciles do not differ from non-smokers. Smoking and Spanish fluency are not associated with significant ΔBPs, and schooling was not associated withsignificant changes in baseline BP or ΔBP.
DISCUSSION
Age-related increases in BP are modest among Tsimane compared to Westerners. BP changes little with age among Tsimane men, whereas a larger increase occurs among Tsimane women. Such increases are not uniform across the population. Longitudinal analyses reveal variability in age-related slopes, and variability increases with age, particularly among women. Overall, hypertension prevalence is low amongst Tsimane and point observations of hypertension are not sustained over time.
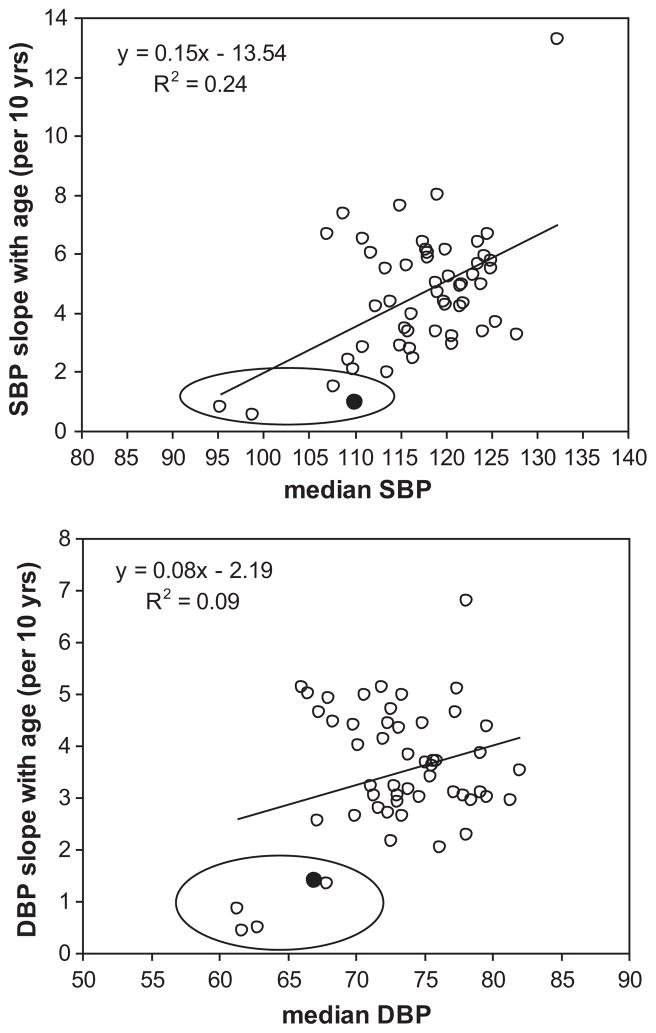
To place the Tsimane age-related increase in context, we compare Tsimane ΔSBP and ΔDBP with those from 52 populations from INTERSALT 21, a cross-sectional study of hypertension using standardized methodology among adults aged 20–59 (Figure 5). Tsimane slopes are derived from a mixed model with the same controls over the age range 20–59. Tsimane ΔSBP and ΔDBP are among the lowest, comparable with those from four other subsistence populations: the Xingu and Yanomamo of Brazil, Papua New Guinean highlanders and rural Kenyans. National populations show ΔSBPs that are 2–8 times higher, and ΔDBPs that are 2–4 times higher than Tsimane. Given their median level of adult SBP and DBP, Tsimane ΔBPs are smaller than that predicted by the regression lines (Figure 5). Overall, Tsimane BP and ΔBPs are small compared to other populations, even after controlling for BMI (Figure S3).
Figure 5.
Increase in (a) SBP and (b) DBP per decade. Cross-cultural sample includes 52 populations from the INTERSALT study (ages 20–59)21. Tsimane slope estimates are represented by black dots. Other populations inside ovals include the Brazilian Yanomamo and Xingu Amerindians, Papua New Guinea highlanders and Kenyans.
Despite the minimal age-related increases in BP, Tsimane BP age profiles share similarities with Western profiles. Women have lower BP than men at young ages, but beyond age 50, women’s BP equals men’s. Additionally, DBP declines at older ages across populations. Explanations for the late drop in DBP include “burned out” diastolic hypertension, reduced cardiac output, and increased large arterial stiffness6. “Burned out” hypertension seems unlikely given the DBP decrease in a population with minimal hypertension and longitudinal BP increase.
Effects of modernization are small and not consistent with the notion that greater exposure leads to poor health outcomes. While no indicator of modernization predicted a greater age-related increase in BP, BMI had the most substantial effect on BP level. Cohort increases in BMI have been linked to reduced physical activity, poor diet, and other changes associated with modernization22. Indeed, over 85% of hypertension diagnoses occur in overweight or obese individuals (BMI≥25 kg/m2) among Westerners 23. It might be expected, therefore, that behavioral changes associated with modernization should impact BP primarily through an indicator of obesity, i.e. BMI. BMI is almost universally positively and independently associated with morbidity and mortality from hypertension, cardiovascular and other chronic diseases and type II diabetes24. Greater body mass increases blood volume, viscosity, impairs pressure natriuresis and can lead to renal tubular sodium reabsorption25. Adipocytes also release angiotensinogen, a pre-cursor of angiotensin.
The effect of a unit change in BMI on BP is similar among Tsimane and Americans (β=0.39, 0,13, 0.26 for SBP, DBP, PP for NHANES; β=0.61, 0.39, 0.25 for Tsimane), but Tsimane BMI does not increase substantially throughout adulthood. Although obesity is rare among Tsimane (5.6% of women, 1.6% men age 20+), overweight is not uncommon: 27.8% of women and 21.9% of men. Heavy smokers and moderate Spanish speakers with greater schooling are more likely to be overweight or obese (Table S6). However, BMI was not greater in villages closer to town (Table S7), nor is overweight and obesity more prevalent (Table S6). Even if the average Tsimane were obese, Tsimane BP would not resemble U.S. patterns. Based on the model from Table 2, a Tsimane woman with U.S. average BMI at ages 40 and 70 would have SBP of 113 and 117, respectively, while an American woman with Tsimane average BMI at the same ages would have SBP of 116 and 122, respectively (Table S8).
Despite the significant relationship between BMI and BP among Tsimane, Tsimane display lower median SBP and DBP, and lower ΔSBP and ΔDBP, than expected based on comparative BMIs of 52 INTERSALT populations (Figure S3). Based on regressions using all INTERSALT populations, Tsimane ΔSBP and ΔDBP from ages 20–59 should be 339% and 134% greater, respectively, given their median BMI of 23.5. One possibility for the low BP given Tsimane BMI is that higher BMI amongst lean, active forager-horticulturalists reflects greater muscle rather than fat mass. However, this is not the case; BMI is highly correlated with body fat percentage in men and women across the BMI range (men: r=0.76, p<0.0001, women: r=0.55, p<0.0001, Figure S4). Body fat percentage per unit increase in BMI also appears similar among Tsimane and U.S. adults (1.5% from BMI of 20 to 35, Figure S4 for Tsimane women; ref26 for U.S. women).
Unlike patterns documented in the developed world23, Tsimane BMI reaches its peak by age 45 then declines by 1.0 kg/m2 by age 70 (Table S6), even though body fat percentage increases with age (men: r=0.27, p<0.0001; women: r=0.13, p<0.0001). So even though we find evidence that modernization may lead to higher BMI amongst Tsimane, only cumulative smoking increases with age, while schooling and Spanish fluency are greater among younger adults. The net effect is a decline in BMI at late ages, and only minimal age-related increase in BP.
Distance to town showed minimal effect on BP and a positive effect on PP rise with age. Similarly, indicators of modernization such as smoking, Spanish fluency and schooling showed no consistent negative effects on BP. This finding contrasts with many published patterns of “zero-slope” populations that underwent rapid modernization, where mean BP increased and also rose with age11. A meta-analysis of effects of modernization on BP shows universal positive effects with similar effect sizes worldwide (about 4 mm higher for SBP, 3 mm for DBP, on average)14. That study, however, did not examine modernization effects on rate of BP increase. Migration and initial contact (<3 years) in a modernized setting had the greatest positive impacts on BP, more than BMI or other variables. This high level of modernization is not representative of the Tsimane presently. Few Tsimane live in towns, and even those living in the most modernized villages still actively practice horticulture, fishing and hunting. Most Tsimane have not given up their traditional lifeways. Their diet remains rich in potassium, fiber, omega-3 fatty acids and low in saturated fat20. Perhaps the greatest differences across regions is in access to other market foods (e.g. sugar, salt, cooking oil), medical attention, and schools. A comparison of risk factors across regions does not show consistent high-risk in more acculturated regions (Table S7). For example, whereas women near town and the mission show highest Spanish fluency, literacy and schooling (Figure S5), women living downstream from San Borja show highest body fat and BMI, while women living in remote villages smoke more (Table S7; Figure S6). Despite increasing modernization, low hypertension prevalence and minimal age-related increase in BP among Tsimane is noteworthy given that Native Americans display higher susceptibility to hypertension; they show similar genetic profiles affecting salt avidity and cardiovascular reactivity as high-risk African populations, despite recent descent from cold-adapted north Asian populations15. This genetic propensity with rising obesity and changing diets is likely responsible for rising levels of CVD and metabolic disease among native North Americans. However, among North American Indians from the Strong Heart Study, BP increased substantially with age, but was minimally affected by obesity despite CVD being the leading cause of death27 (but see 28). North American Indians show similar rates of hypertension compared to other U.S. groups28. The non-trivial prevalence of pre-hypertension among Tsimane does suggest that imminent changes in cardiovascular risk-factors are likely if physical activity, diet or other hypertension-promoting conditions increase over time. Among “partially acculturated” island-dwelling Kuna, BP is also low and does not rise with age, whereas Kuna migrants to Panama City show relatively high prevalence of hypertension and rising BP with age29.
Finally, sex differences in Tsimane BP are striking. Most of the substantial rise in SBP and PP occurs in women, especially during the 40’s and 50’s (Figures S1, 2–4). We find greater variation in women’s BP and ΔBP with age (Figure 4, Table S4). Unlike the sex profiles of BP among Westerners, Tsimane women have higher rates of hypertension and are at greater risk of BP-related morbidity than men. Although age profiles of BMI do not vary markedly by sex, body fat increases at a higher rate among women (Figure S4: 17.2% vs. 12.2% per decade). BMI also has a 61% greater effect on SBP in women than men (β =1.16 vs. 0.72, Table S5). Postmenopausal increases in BP have been documented among Westerners and have been attributed to declines in estradiol production30. Estradiol influences vascular tone and structure, endothelial vasodilation, and has been attributed to inhibit vascular response to arterial injury31.
Strengths and limitations
To our knowledge, the Tsimane are the only subsistence foraging-horticultural population sampled longitudinally. Their active lifestyle, lack of BP medication and variable experience with modernization provided a unique opportunity to investigate BP change with age. Little bias is expected as 90+% of adults present were sampled per medical round. Few adults, however, were sampled at least five times, and the maximum time depth of the study was only eight years. While we include several measures of modernization, we did not consider its direct effects via individual-level measures of diet, physical activity, and other behavioral changes, although these are being collected in ongoing studies.
PERSPECTIVES
We found low levels of persistent hypertension and minimal age-related BP increase among Tsimane Amerindians compared to Westerners. Tsimane women are at greater risk of hypertension at late ages. Proximity to town affects SBP but not rate of BP increase in the predicted direction; BMI impacts BP level, but not BP slope, with age. Many aspects of traditional diet and activities are preserved even among more modern Tsimane, suggesting that they have not yet experienced severe changes that would otherwise promote greater hypertension and CVD. Pre-hypertension prevalence is moderate, suggesting that further changes in diet and behavior could place Tsimane at elevated risk.
Supplementary Material
NOVELTY AND SIGNIFICANCE.
1) What is new?
We provide the first systematic test of whether blood pressure increases with age in a subsistence population using longitudinal and cross-sectional data
We test whether modernization affects blood pressure and its rise with age
2) What is relevant?
Persistent hypertension is minimal (<3%) among adults age 40+, despite high levels of inflammation and variable experience with modernization
Hypertension and age-related increase in blood pressure is more prevalent among Tsimane women, unlike sex differences observed in Western countries
3) Summary
An increase in blood pressure with age is not a fundamental feature of human aging
Effects of age and modernization are minimal on blood pressure and its rise
The lean physique, active lifestyle and traditional diet may protect against hypertension in spite of increasing socioeconomic change
Acknowledgments
We thank Tsimane for their participation and collaboration, and Tsimane Health and Life History Project personnel.
SOURCES OF FUNDING
Research was supported by grants from the National Institutes of Health/National Institute on Aging (R01AG024119, R56AG024119) and National Science Foundation (BCS-0422690).
Footnotes
CONFLICT OF INTEREST
The authors declare no conflict of interest.
Contributor Information
Michael Gurven, Email: gurven@anth.ucsb.edu, Integrative Anthropological Sciences Program, University of California-Santa Barbara, Santa Barbara, CA 93106, Telephone: 805-893-2202; Fax: 805-893-8707.
Aaron D. Blackwell, Email: ablackwell@isber.ucsb.edu, Integrative Anthropological Sciences Program, University of California-Santa Barbara, Santa Barbara, CA 93106
Daniel Eid Rodriguez, Email: libremd@gmail.com, Universidad Mayor de San Simón, Departamento de Medicina, Cochabamba, Bolivia.
Jonathan Stieglitz, Email: j0nathan@unm.edu, Department of Anthropology, University of New Mexico, Albuquerque, NM 87131.
Hillard Kaplan, Email: hkaplan@unm.edu, Department of Anthropology, University of New Mexico, Albuquerque, NM 87131.
References
- 1.Finch C. The Biology of Human Longevity. San Diego, CA: Academic Press; 2007. [Google Scholar]
- 2.O’Rourke MF, Nichols WW. Aortic diameter, aortic stiffness, and wave reflection increase with age and isolated systolic hypertension. Hypertension. 2005;45:652–658. doi: 10.1161/01.HYP.0000153793.84859.b8. [DOI] [PubMed] [Google Scholar]
- 3.Baksi AJ, Treibel TA, Davies JE, Hadjiloizou N, Foale RA, Parker KH, Francis DP, Mayet J, Hughes AD. A meta-analysis of the mechanism of blood pressure change with aging. Journal of the American College of Cardiology. 2009;54:2087. doi: 10.1016/j.jacc.2009.06.049. [DOI] [PubMed] [Google Scholar]
- 4.Wolf-Maier K, Cooper RS, Banegas JR, Giampaoli S, Hense H-W, Joffres M, Kastarinen M, Poulter N, Primatesta P, Rodriguez-Artalejo F, Stegmayr B, Thamm M, Tuomilehto J, Vanuzzo D, Vescio F. Hypertension Prevalence and Blood Pressure Levels in 6 European Countries, Canada, and the United States. JAMA. 2003;289:2363–2369. doi: 10.1001/jama.289.18.2363. [DOI] [PubMed] [Google Scholar]
- 5.Whelton PK. Epidemiology of hypertension. Lancet. 1994;344:101. doi: 10.1016/s0140-6736(94)91285-8. [DOI] [PubMed] [Google Scholar]
- 6.Franklin SS, Gustin W, Wong ND, Larson MG, Weber MA, Kannel WB, Levy D. Hemodynamic patterns of age-related changes in blood pressure: the Framingham Heart Study. Circulation. 1997;96:308. doi: 10.1161/01.cir.96.1.308. [DOI] [PubMed] [Google Scholar]
- 7.Coylewright M, Reckelhoff JF, Ouyang P. Menopause and hypertension: an age-old debate. Hypertension. 2008;51:952–959. doi: 10.1161/HYPERTENSIONAHA.107.105742. [DOI] [PubMed] [Google Scholar]
- 8.Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, Jones DW, Materson BJ, Oparil S, Wright JT. Seventh report of the joint national committee on prevention, detection, evaluation, and treatment of high blood pressure. Hypertension. 2003;42:1206–1252. doi: 10.1161/01.HYP.0000107251.49515.c2. [DOI] [PubMed] [Google Scholar]
- 9.James GD, Baker PT. In: Hypertension, Pathophysiology, Diagnosis and Management. 2. Laragh JH, Brenner BM, editors. New York, NY: Raven Press; 1995. pp. 115–125. [Google Scholar]
- 10.Carvalho JJ, Baruzzi RG, Howard PF, Poulter N, Alpers MP, Franco LJ, Marcopito LF, Spooner VJ, Dyer AR, Elliot P. Blood pressure in four remote populations in the INTERSALT study. Hypertension. 1989;14:238–246. doi: 10.1161/01.hyp.14.3.238. [DOI] [PubMed] [Google Scholar]
- 11.Stevenson DR. Blood pressure and age in cross-cultural perspective. Human Biology. 1999;71:529. [PubMed] [Google Scholar]
- 12.Fleming-Moran M, Coimbra CEA., Jr Blood pressure studies among Amazonian native populations: a review from an epidemiological perspective. Social Science and Medicine. 1990;31:593–601. doi: 10.1016/0277-9536(90)90095-a. [DOI] [PubMed] [Google Scholar]
- 13.Waldron I, Nowotarski M, Freimer M, Henry JP, Post N, Witten C. Cross-cultural variation in blood pressure: A quantitative analysis of the relationships of blood pressure to cultural characteristics, salt consumption and body weight. Social Science & Medicine. 1982;16:419–430. doi: 10.1016/0277-9536(82)90050-8. [DOI] [PubMed] [Google Scholar]
- 14.Steffen PR, Smith TB, Larson M, Butler L. Acculturation to western society as a risk factor for high blood pressure: a meta-analytic review. Psychosomatic Medicine. 2006;68:386–397. doi: 10.1097/01.psy.0000221255.48190.32. [DOI] [PubMed] [Google Scholar]
- 15.Young JH, Chang Y-PC, Kim JD, Chretien J-P, Klag MJ, Levine MA, Ruff CB, Wang N-Y, Chakravarti A. Differential susceptibility to hypertension is due to selection during the Out-of-Africa expansion. PLoS Genetics. 2005;1:e82. doi: 10.1371/journal.pgen.0010082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gurven M, Kaplan H, Zelada Supa A. Mortality experience of Tsimane Amerindians: regional variation and temporal trends. American Journal of Human Biology. 2007;19:376–398. doi: 10.1002/ajhb.20600. [DOI] [PubMed] [Google Scholar]
- 17.Wood SN. Generalized additive models: an introduction with R. Vol. 66. CRC Press; 2006. [Google Scholar]
- 18.Hastie T, Tibshirani R. Generalized additive models. Statistical Science. 1986;1:297–310. doi: 10.1177/096228029500400302. [DOI] [PubMed] [Google Scholar]
- 19.Verbeke G, Molenberghs G. Linear Mixed Models for Longitudinal Data. New York: Springer Verlag; 2009. [Google Scholar]
- 20.Gurven M, Kaplan H, Winking J, Eid D, Vasunilashorn S, Kim J, Finch C, Crimmins E. Inflammation and infection do not promote arterial aging and cardiovascular disease among lean Tsimane forager-horticulturalists. PLoS One. 2009;4:e6590. doi: 10.1371/journal.pone.0006590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Intersalt CRG. Intersalt: an international study of electrolyte excretion and blood pressure. Results for 24 hour urinary sodium and potassium excretion. British Medical Journal. 1988;297:319–328. doi: 10.1136/bmj.297.6644.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Asia Pacific Cohort Studies C. Body mass index and cardiovascular disease in the Asia-Pacific Region: an overview of 33 cohorts involving 310,000 participants. International Journal of Epidemiology. 2004;33:751–758. doi: 10.1093/ije/dyh163. [DOI] [PubMed] [Google Scholar]
- 23.Haslam DW, James WPT. Obesity. The Lancet. 2005;366:1197–1209. doi: 10.1016/S0140-6736(05)67483-1. [DOI] [PubMed] [Google Scholar]
- 24.Pi-Sunyer FX. Medical hazards of obesity. Ann Intern Med. 1993;119:655–660. doi: 10.7326/0003-4819-119-7_part_2-199310011-00006. [DOI] [PubMed] [Google Scholar]
- 25.Hall JE. The kidney, hypertension and obesity. Hypertension. 2003;41:625–633. doi: 10.1161/01.HYP.0000052314.95497.78. [DOI] [PubMed] [Google Scholar]
- 26.Gallagher D, Heymsfield SB, Heo M, Jebb SA, Murgatroyd PR, Sakamoto Y. Healthy percentage body fat ranges: an approach for developing guidelines based on body mass index. The American Journal of Clinical Nutrition. 2000;72:694–701. doi: 10.1093/ajcn/72.3.694. [DOI] [PubMed] [Google Scholar]
- 27.Howard BV, Lee ET, Yeh JL, Go O, Fabsitz RR, Devereux RB, Welty TK. Hypertension in adult American Indians. Hypertension. 1996;28:256–264. doi: 10.1161/01.hyp.28.2.256. [DOI] [PubMed] [Google Scholar]
- 28.Wang W, Lee ET, Fabsitz RR, Devereux R, Best L, Welty TK, Howard BV. A longitudinal study of hypertension risk factors and their relation to cardiovascular disease: the Strong Heart Study. Hypertension. 2006;47:403–409. doi: 10.1161/01.HYP.0000200710.29498.80. [DOI] [PubMed] [Google Scholar]
- 29.Hollenberg NK, Martinez G, McCullough M, Meinking T, Passan D, Preston M, Rivera A, Taplin D, Vicaria-Clement M. Aging, acculturation, salt intake, and hypertension in the Kuna of Panama. Hypertension. 1997;29:171. doi: 10.1161/01.hyp.29.1.171. [DOI] [PubMed] [Google Scholar]
- 30.Dubey RK, Oparil S, Imthurn B, Jackson EK. Sex hormones and hypertension. Cardiovascular research. 2002;53:688–708. doi: 10.1016/s0008-6363(01)00527-2. [DOI] [PubMed] [Google Scholar]
- 31.Reckelhoff JF. Gender Differences in the Regulation of Blood Pressure. Hypertension. 2001;37:1199–1208. doi: 10.1161/01.hyp.37.5.1199. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.