Abstract
Calcifying fibrous tumor (CFT) is a rare, benign mesenchymal tumor usually affecting children and young adults, and it shows a predilection for the soft tissue and the abdominal cavity. Intrinsic visceral CFT is extremely rare and we present herein the case of a 59-year-old man with an asymptomatic gastric lesion, incidentally detected 1 month before this presentation. Thus, gastric endoscopy revealed a polypoid submucosal mass in the fundus, covered by an erythematous mucosa. The polypoid mass was a 3.9 × 2.7 cm-sized well-defined tumor located in the proper muscle, with extension to the subserosa. The tumor showed characteristic hypocellular sclerosis with coarse collagen, mononuclear inflammatory infiltrates, sparse fibroblastic spindle cells and occasional, psammomatous or dystrophic calcifications. Immunohistochemically, the spindle cells were negative for CD117, CD34, platelet-derived growth factor receptor-alpha, S100, smooth muscle actin, desmin and anaplastic lymphoma kinase.
Keywords: Stomach, Gastrointestinal stromal tumors, Calcifying fibrous tumor
INTRODUCTION
Calcifying fibrous tumor (CFT) is a rare, benign fibrous lesion, usually affecting children and young adults, and originally described as childhood fibrous tumor with psammoma bodies [1]. CFT is composed of hyalinized fibrous tissue with interspersed bland fibroblastic spindled cells, scattered psammomatous, and/or dystrophic calcifications, and variably prominent mononuclear inflammatory infiltrate. CFTs show a predilection for the soft tissue and the abdominal cavity. However, CFT of the stomach is very rare [2-5]. Herein, we present a rare case of gastric CFT, with a review of the literature and differential diagnosis.
CASE REPORT
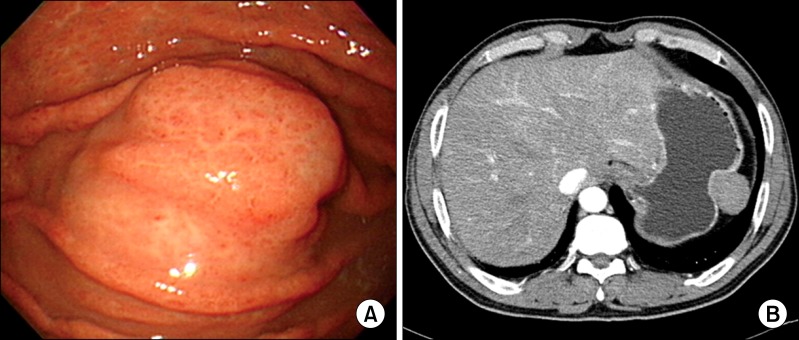
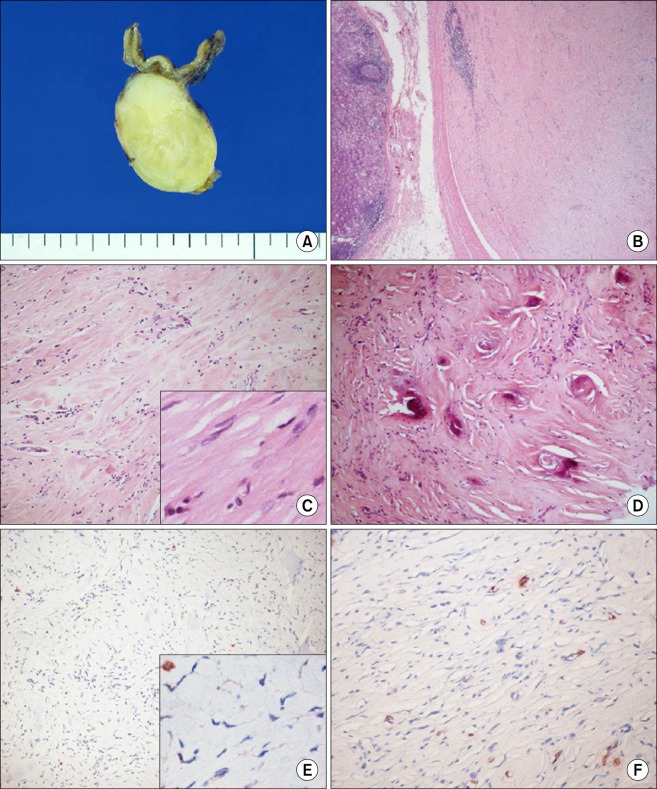
A 59-year-old man was admitted to Chonbuk National University Hospital for the evaluation of an asymptomatic gastric lesion that had been detected incidentally 1 month earlier. Physical examination of the abdomen was unremarkable and laboratory findings, including tumor markers, were found within normal limit. The patient underwent an endoscopic evaluation, which revealed a polypoid submucosal mass, with erythematous mucosa, at the greater curvature of the gastric fundus (Fig. 1A). Contrast-enhanced computed tomography scan revealed a well-circumscribed, non-enhanced, and homogeneous round mass, at the great curvature of the gastric fundus (Fig. 1B). Surgery was determined as the best treatment option, and the patient underwent laparoscopic wedge resection of the gastric fundus. Macroscopically, the mass was covered by intact mucosa. Cut-sections revealed that it was a relatively well-defined tumor, measuring 3.9 × 2.7 cm, and located in the proper muscle, with extension to the subserosa. Sections showed a homogeneous yellow to white, firm surface (Fig. 2A). Microscopically, the tumor was well-circumscribed, but not encapsulated, and was surrounded by a peripheral cuff of lymphoid aggregates (Fig. 2B). The tumor showed characteristic hypocellular sclerosis, with wavy, vaguely storiform coarse collagen, and either scattered or patchy mononuclear inflammatory infiltrates, composed of plasma cells, lymphocytes and mast cells. Small nodular lymphoid aggregates were common, and usually located at the periphery of the tumor. Sparse spindle cells were dispersed among thick collagen bundles. These cells had ovoid, vesicular nuclei with fine chromatin, and did not show any cellular atypia or mitotic activity (Fig. 2C). Occasional psammomatous and dystrophic calcifications were found (Fig. 2D). Some of the calcifications revealed minute lumina, suggesting calcified vascular channels. Immunohistochemically, the spindle cells were negative for CD117 (Fig. 2E), CD34, platelet-derived growth factor receptor-alpha, S100, smooth muscle actin (SMA) (Fig. 2F), desmin and anaplastic lymphoma kinase (ALK). However, there were scattered CD117-positive mast cells and immunoglobulin G4-positive plasma cells. Two months after the operation, the patient remained in good condition, with no signs or symptoms of tumor recurrence.
Fig. 1.
(A) Endoscopic evaluation reveals the polypoid submucosal mass in the greater curvature of the gastric fundus with mild erythematous mucosa. (B) Contrast-enhanced computed tomography scan reveals the well-circumscribed, non-enhanced, homogeneous, round mass at the great curvature of the gastric fundus.
Fig. 2.
(A) Cut-section reveals the well-defined tumor measuring 3.9 × 2.7 cm covered by intact mucosa. The tumor is located in the proper muscle with extension to the subserosa and shows a homogeneous yellow to white, firm surface. (B) The tumor is well-circumscribed, but not encapsulated, and located in the proper muscle (H&E, ×20). (C) The tumor shows characteristic hypocellular sclerosis, with wavy collagen and scattered mononuclear inflammatory infiltrates (H&E, ×100). Note the sparse spindle cells dispersed among thick collagen bundles and some plasma cells (inset, H&E, ×400). (D) Occasional psammomatous and dystrophic calcifications are found (H&E, ×100). (E) Spindle cells show no immunoreactivity for CD117 (CD117, ×100). Note the immunoreactive mast cells, as the internal positive control (inset, CD117, ×400). (F) Spindle cells show no immunoreactivity for smooth muscle actin (SMA, ×200). Note the immunoreactive vascular walls, as the internal positive control.
DISCUSSION
CFTs are rare, benign lesions consisting of well circumscribed, un-encapsulated, paucicellular, hyalinized fibrosclerotic tissue, with a variable inflammatory infiltrate, consisting of lymphocytes and plasma cells. Lymphoid aggregates may be present. Calcifications, both psammomatous and dystrophic, are scattered throughout the tumor. CFTs show a predilection for the soft tissue and the abdominal cavity, especially subserosal location involving either the pleura or the visceral peritoneum [1]. However, intra-abdominal CFT occurring as an intrinsic, visceral lesion is rare. In addition, it has been shown that gastric CFTs are different from their soft tissue counterpart in their smaller tumor size, higher mean age at presentation and no tendency for local recurrence. These findings suggest different pathogenic pathways between gastric CFTs and their soft tissue counterpart, regardless of morphologic similarity.
CFT is thought to represent a true neoplasm, with a tendency for nondestructive local recurrence, rather than a reactive process resulting from abnormal tissue healing.
In the literature, gastric CFTs have been documented no predominance of both sex in occurrence, with a mean age of 52.5 years [2-5]. Mean age was slightly higher for women than for men (i.e., 55.6 years vs. 49.5 years). Tumor mean size was 1.9 cm. Eight tumors were originated in the body of the stomach, 2 in the lesser curvature, 1 in the antrum, 1 in the antrum junction, and 2 in unspecified gastric sites. None of the 9 patients with mean follow-up of 51 months developed recurrence. The clinical features of the present case are somewhat different from the previous cases, in that the age of the patient was older (59 years) than the reported mean age of male patients. In addition, the tumor size was larger (3.9 cm) than the reported mean size and located in the greater curvature of the fundus.
The differential diagnosis of gastric CFT includes gastrointestinal stromal tumor (GIST), inflammatory fibroid polyp (IFP), schwannoma, sclerosing leiomyoma, inflammatory myofibroblastic tumor (IMT) and plexiform fibromyxoma. Gastric GISTs are frequently hyalinized, with dystrophic calcifications in approximately 50% of cases [6]. However, psammomatous calcifications and lymphoplasmacytic infiltrates are not features of regressing GIST [6]. In addition, in sclerosing GIST, immunoreactivity for CD117 and CD34 is helpful in differentiation. Unlike gastric CFTs, gastric IFPs are located mostly in the antrum, are submucosally-based lesions with eosinophil-dominant inflammatory cells, higher cellularity and onion-skin pattern of the stromal cell arrangement [7]. Gastric schwannoma may reveal the residual schwannoma tissue and a strong immunoreactivity for S100 [8]. Sclerosing leiomyoma may also reveal residual smooth muscle tumor cells, which are immunoreactive for both SMA and desmin. IMTs reveal infiltrative growth of either fibroblasts or myofibroblasts, which are immunoreactive for both SMA and ALK [9]. Plexiform fibromyxomas are uniformly larger (>5 cm) tumors of the antrum, with prominent capillary network, fibromyxoid stroma, high cellularity and a common expression of SMA [10].
In general, gastric CFTs are under-recognized because they are unfamiliar with the surgeons and pathologists and because their small size, bland looking histology and benign course attract less attention from the clinicians. Awareness of the characteristic morphology of gastric CFTs will help to distinguish them from other gastric sclerosing stromal tumors.
Footnotes
No potential conflict of interest relevant to this article was reported.
References
- 1.Rosenthal NS, Abdul-Karim FW. Childhood fibrous tumor with psammoma bodies: clinicopathologic features in two cases. Arch Pathol Lab Med. 1988;112:798–800. [PubMed] [Google Scholar]
- 2.Puccio F, Solazzo M, Marciano P, Benzi F. Laparoscopic resection of calcifying fibrous pseudotumor of the gastric wall: a unique case report. Surg Endosc. 2001;15:1227. doi: 10.1007/s00464-001-4202-1. [DOI] [PubMed] [Google Scholar]
- 3.Elpek GO, Kupesiz GY, Ogus M. Incidental calcifying fibrous tumor of the stomach presenting as a polyp. Pathol Int. 2006;56:227–231. doi: 10.1111/j.1440-1827.2006.01951.x. [DOI] [PubMed] [Google Scholar]
- 4.Attila T, Chen D, Gardiner GW, Ptak TW, Marcon NE. Gastric calcifying fibrous tumor. Can J Gastroenterol. 2006;20:487–489. doi: 10.1155/2006/378532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Agaimy A, Bihl MP, Tornillo L, Wunsch PH, Hartmann A, Michal M. Calcifying fibrous tumor of the stomach: clinicopathologic and molecular study of seven cases with literature review and reappraisal of histogenesis. Am J Surg Pathol. 2010;34:271–278. doi: 10.1097/PAS.0b013e3181ccb172. [DOI] [PubMed] [Google Scholar]
- 6.Agaimy A, Wunsch PH, Hofstaedter F, Blaszyk H, Rummele P, Gaumann A, et al. Minute gastric sclerosing stromal tumors (GIST tumorlets) are common in adults and frequently show c-KIT mutations. Am J Surg Pathol. 2007;31:113–120. doi: 10.1097/01.pas.0000213307.05811.f0. [DOI] [PubMed] [Google Scholar]
- 7.Kolodziejczyk P, Yao T, Tsuneyoshi M. Inflammatory fibroid polyp of the stomach: a special reference to an immunohistochemical profile of 42 cases. Am J Surg Pathol. 1993;17:1159–1168. doi: 10.1097/00000478-199311000-00009. [DOI] [PubMed] [Google Scholar]
- 8.Daimaru Y, Kido H, Hashimoto H, Enjoji M. Benign schwannoma of the gastrointestinal tract: a clinicopathologic and immunohistochemical study. Hum Pathol. 1988;19:257–264. doi: 10.1016/s0046-8177(88)80518-5. [DOI] [PubMed] [Google Scholar]
- 9.Shi H, Wei L, Sun L, Guo A. Primary gastric inflammatory myofibroblastic tumor: a clinicopathologic and immunohistochemical study of 5 cases. Pathol Res Pract. 2010;206:287–291. doi: 10.1016/j.prp.2009.09.002. [DOI] [PubMed] [Google Scholar]
- 10.Miettinen M, Makhlouf HR, Sobin LH, Lasota J. Plexiform fibromyxoma: a distinctive benign gastric antral neoplasm not to be confused with a myxoid GIST. Am J Surg Pathol. 2009;33:1624–1632. doi: 10.1097/PAS.0b013e3181ae666a. [DOI] [PubMed] [Google Scholar]