Abstract
Purpose
Leptin plays an important role in the control of body weight and also has a growth-factor-like function in epithelial cells. Abnormal expression of leptin and leptin receptor may be associated with cancer development and progression. We evaluated the relationship among leptin and leptin receptors polymorphisms, body mass index (BMI), serum leptin concentrations, and clinicopathologic features with gastric cancer and determined whether they could be the risk factor of gastric cancer.
Methods
We measured the serum leptin concentrations of 48 Korean patients with gastric cancer and 48 age- and sex-matched controls. By polymerase chain reaction-restriction fragment length polymorphism, we investigated one leptin gene promoter G-2548A genotype and four leptin receptor gene polymorphisms at codons 223, 109, 343, and 656.
Results
There was no significant difference between the mean leptin concentrations of the patient and control groups, while BMI was significantly lower in gastric cancer cases (22.9 ± 3.6 vs. 24.5 ± 2.8 kg/m2, P = 0.021). There was significant association between the LEPR Lys109Arg genotype and gastric cancer risk, heterozygotes for GA genotype had been proved to increased the risk of gastric cancer, and its corresponding odds ratio was 2.926 (95% confidence interval, 1.248 to 6.861).
Conclusion
Our results suggested that LEPR gene Lys109Arg polymorphism is associated with gastric cancer in Korean patients.
Keywords: Leptin, Leptin receptors, Gastric cancer
INTRODUCTION
Leptin, the product of the ob gene, is secreted by adipocytes [1]. This adipocyte-derived cytokine plays an important role in the control of satiety and energy expenditure via interaction with leptin receptor (LEPR) in the hypothalamus [2]. Generally, plasma leptin levels reflect the size of fat stores [3]. Although initially thought to be exclusively expressed in and secreted by adipocytes, leptin expression has recently been detected in a number of additional tissues, including the placenta [4], pituitary cells [5], hepatic stellate cells [6], and gastric mucosa [7]. Leptin has been shown to act as a growth factor promoting the proliferation of cells, inhibiting the apoptosis of cells and promoting angiogenesis [8,9]. Although the mechanism underlying the association between obesity and cancer was not clearly demonstrated, recent studies have shown that obesity is associated with an increasing death rate of total cancer [10-13]. Many clinical studies have investigated the relationship between serum leptin levels and the risk of cancer [11-13]. Epidemiological evaluations have demonstrated that the number of patients with an increased body mass index (BMI) had a significant trend of increased death rates resulting from gastric cancer [10].
The leptin (LEP) gene is located at chromosome 7q31.3 and the LEPR gene is mapped to 1p31 and has one long isoform and three short isoforms. Several single nucleotide polymorphisms in the LEP and LEPR gene have been described. Among LEP polymorphisms, a common G-2548A leptin promoter variant, which results from a G to A substitution at nucleotide -2548 upstream of the ATG start site, has been shown to be associated with either variations in serum leptin levels or the degree of obesity. A mutation in the LEPR gene leads to abnormal mRNA splicing which gives rise to abnormal signal transduction and complete leptin resistance and obesity [14,15]. Gotoda et al. [16] identified four allelic variants associated with amino acid changes (Lys109Arg, Gln223Arg, Ser343Ser, and Lys656Asn). Enhanced expression of leptin and the leptin receptor was found in cases of human gastric cancer, and leptin stimulated the proliferation of gastric epithelial cells in vitro as measured by DNA synthesis [17,18]. Herein, a relationship between leptin and gastric cancer could be suggested. However, only a few studies have addressed the association between leptin and gastric cancer [17,19,20]; to date, few studies have addressed the association between polymorphisms within the LEP and LEPR gene and gastric cancer. The present study is a case-control study of gastric cancer among pre-treatment patients. We assessed the serum leptin levels and LEP and LEPR polymorphisms in Korean gastric cancer patients to clarify the role of leptin in relation to gastric cancer.
METHODS
Patients and specimens
Forty-eight Korean patients with gastric cancer (32 male and 16 female), diagnosed at the Department of Surgery, St. Vincent's Hospital, The Catholic University of Korea, from October 2005 to March 2007, were included in this study. The Institutional Review Board of St. Vincent's Hospital approved this study. All subjects signed a written informed consent form approved by the Institutional Review Board. None of the patients had ever received surgery, chemotherapy, or radiation therapy prior to the study. All patients had undergone a curative radical gastrectomy with standard lymph node dissection. According to the seventh International Union Against Cancer (American Joint Committee on Cancer/Union for International Cancer Control) tumor-node-metastasis staging system, patients were classified as stage I (n = 20), stage II (n = 16), and stage III (n = 12). Patients were categorized as Helicobacter pylori positive if positive in the rapid urease assay or histologic examination of gastric biopsy specimens for bacterial cells with characteristic morphology. After surgery, clinical follow-up data for all patients was collected at the outpatient clinic. The follow-up period ranged from 2.5 to 49.5 months, and median time was 37.7 months. Forty-eight controls were selected from the Health Screening Center of St. Vincent's Hospital. Controls were matched to cases by age and sex. Data in respect of demographic characteristics, underlying diseases, laboratories, anthropometric measurements, and lifestyle choices including alcohol consumption and cigarette smoking were obtained using interviewer-administered questionnaires. Subjects with underlying diseases or abnormal laboratory data were excluded from the study.
Measurement of BMI and serum leptin concentration
BMI was calculated as weight divided by height squared (kg/m2), and World Health Organization/International Association for the Study of Obesity/International Obesity Task Force (2000): Asian-Pacific standard was used to categorize BMI scores as follows: normal (18.5 to 23), overweight (23 to 24.9), obesity grade I (25 to 29.9), obesity grade II (≥30) [21]. Prior to surgery, 5 mL of blood was collected, centrifuged, and stored as serum at -20℃ until examined. Serum leptin concentration was measured in duplicate using the Human Leptin RIA Kit (Linco Research Inc., St. Charles, MO, USA).
Genotyping of the LEP and LEPR gene
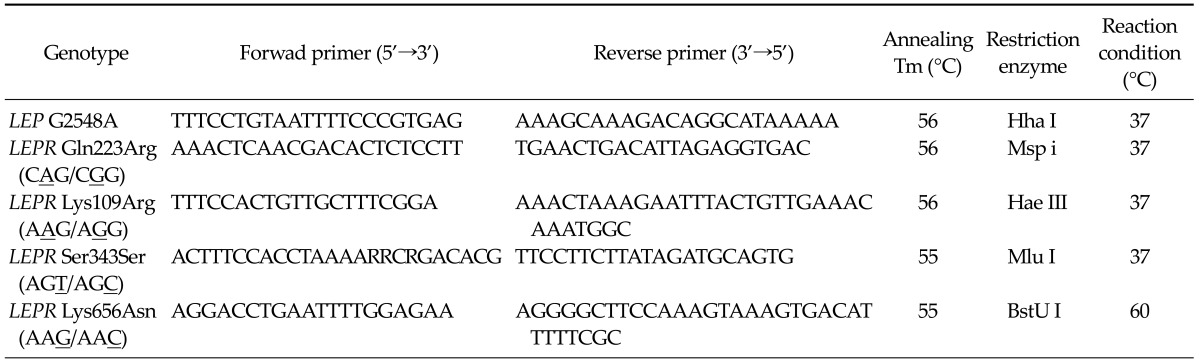
Genotyping of the LEP and LEPR gene was performed by direct sequencing. Genomic DNA was extracted from gastric mucosa using the AccuPrep Genomic DNA extraction kit (Bioneer Co., Daejeon, Korea). The LEP and LEPR gene polymorphisms were performed using the polymerase chain reaction-restriction fragment length polymorphism (PCR-RFLP) method. PCR reactions were set up using the i-Star Taq DNA polymerase (iNtRON Biotechnology Inc., Seongnam, Korea). In a total reaction volume of 20 µL, 2 µL 10 × buffer, 10 pmol each each primer, 2 µL genomic DNA, 2 µL genomic dNTP mixture were combined. Reactions were run on Bio-Rad My Cycler thermal cyclers (Bio-Rad Laboratories Inc., Hercules, CA, USA). 5 µL of the resulting PCR products were used for an overnight digest with the appropriate restriction enzyme (New England Biolabs, Beverly, MA, USA), and electrophoresis were performed in a 12% polyacrylamide gel electrophoresis gel (Table 1).
Table 1.
Sequences of primers for genotyping of human leptin (LEP) and leprin recetor (LEPR) gene variants
Statistical analysis
The data was statistically analyzed using SPSS ver. 11.5 (SPSS Inc., Chicago, IL, USA). The comparisons of the patient group and the control group for serum leptin levels and genotypes were performed with a non-parametric Mann-Whitney test. The relationship between genotypes and gastric cancer was also examined by odds ratios (ORs) with 95% confidence intervals (CIs). P-values less than 0.05 were considered statistically significant.
RESULTS
Relationships between serum letpin level and gastric cancer
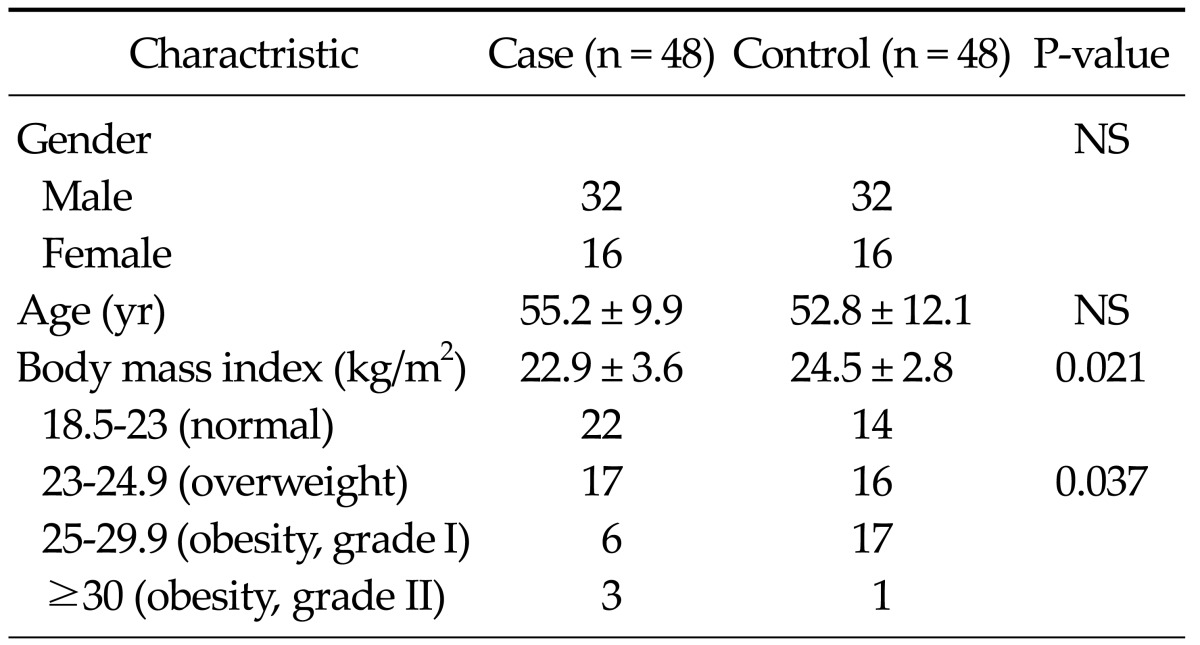
Table 2 shows the characteristics of 48 patients with gastric cancer and 48 healthy controls. There was no significant difference between the mean leptin concentrations of the patient and control groups (7.579 ± 8.536 vs. 7.793 ± 3.94 ng/mL, P = 0.139), while BMI was significantly lower in gastric cancer cases than the controls (22.9 ± 3.6 vs. 24.5 ± 2.8 kg/m2, P = 0.021). Cases with positive H. pylori on gastric mucosa showed significantly lower leptin levels than those with negative H. pylori (3.966 ± 3.404 vs. 9.573 ± 9.583 ng/mL, P = 0.01). There was no significant difference regarding leptin levels between early- and late-stage patients.
Table 2.
Charactristics of subjects
Values are presented as number or mean ± SD.
NS, not significant.
Relationships among genotypes of LEP and LEPR polymorphism, leptin level, and gastric cancer
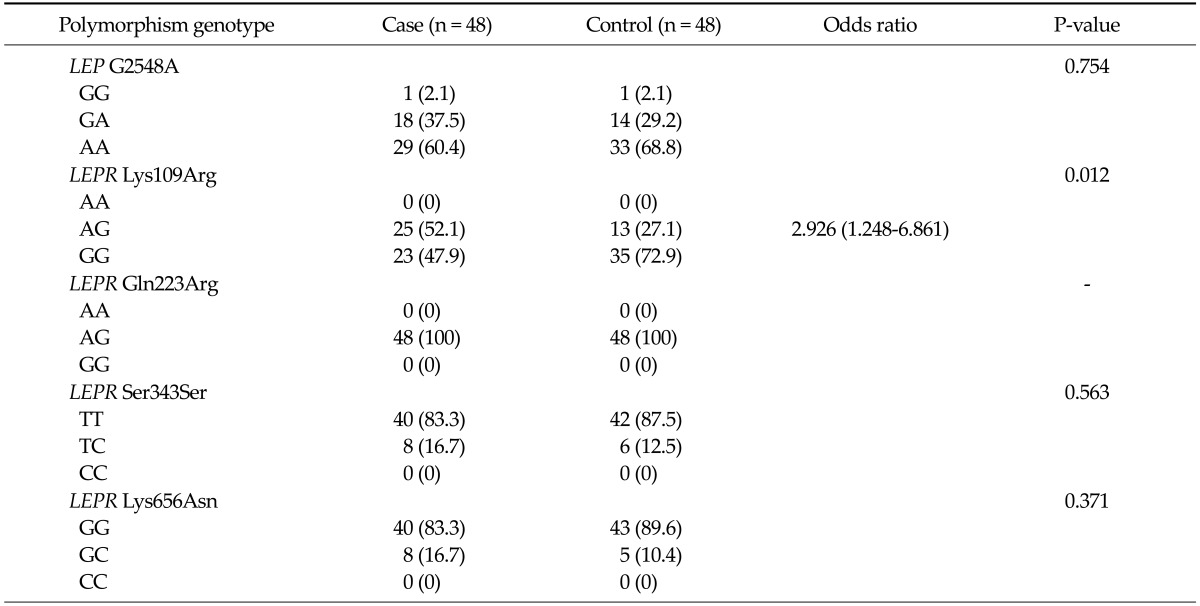
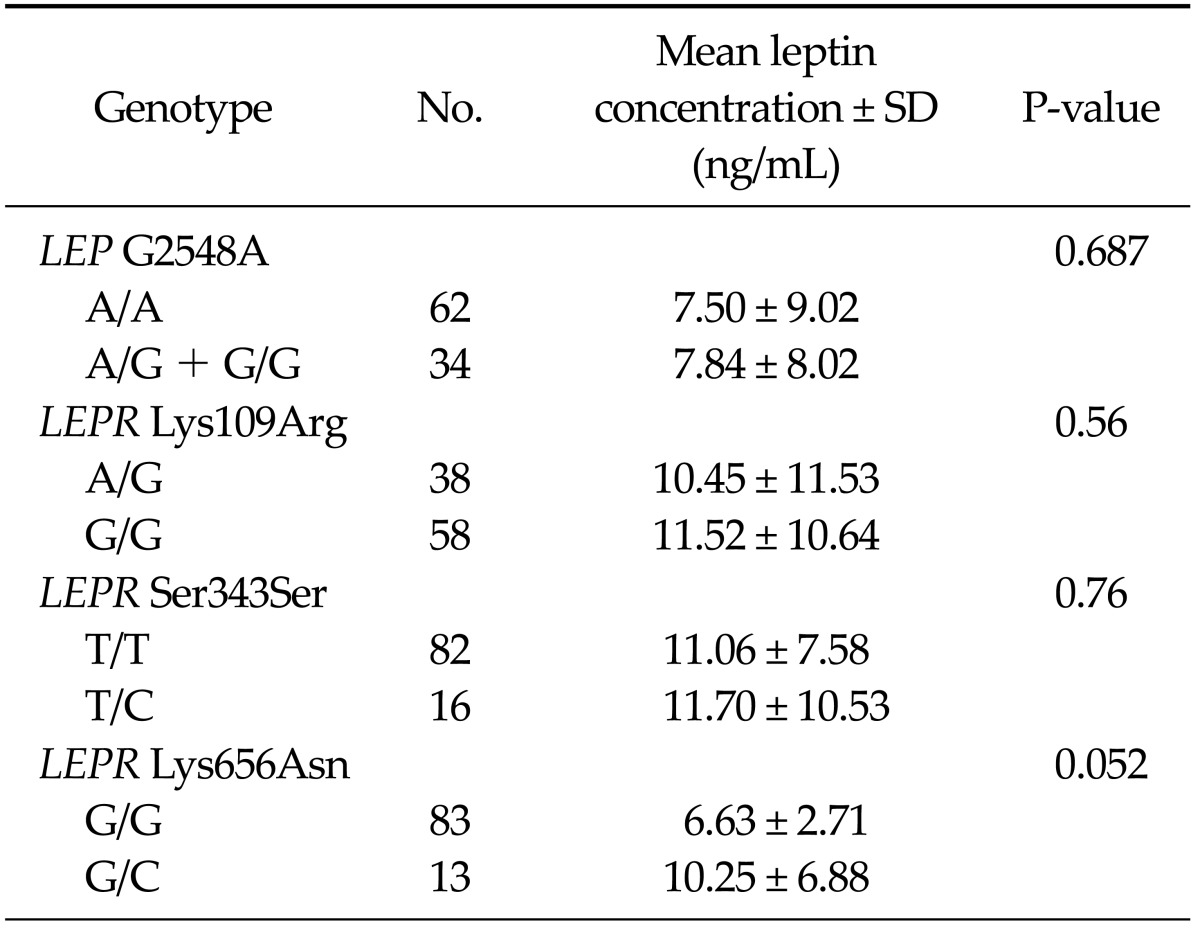
Table 3 shows the genotype frequencies for the one polymorphism of LEP genes and the four polymorphisms of LEPR genes in cases and controls, and their corresponding ORs. No significant difference in genotype and allele frequencies of the G2548A polymorphism was detected between cases and controls. The frequencies of the Lys109Arg and Gln223Arg wild-types were zero and those of Ser343Ser and Lys656Asn variant-types were zero. Comparing cases and controls, there was a significant association between Lys109Arg genotypes and risk of gastric cancer. Heterozygotes for AG genotype showed an increased risk of gastric cancer, and its corresponding OR was 2.926 (95% confidence interval [CI], 1.248 to 6.861). We analyzed the association between each genotypic variant and serum leptin concentration (Table 4). A trend was observed for cancer patients who were heterozygotic for Lys656Asn to have lower mean leptin concentrations compared to those with the wild-type (6.63 ± 2.71 vs. 10.25 ± 6.88 ng/mL, P = 0.052). Within the control group, there was no difference between genotypes and mean leptin concentrations.
Table 3.
Genotype frequencies of leptin (LEP) and leprin recetor (LEPR) gene polymorphisms in cases and controls and the association between genotypes and gastric cancer
Values are presented as number (%).
Table 4.
Mean leptin concentration according to leptin (LEP) and leprin recetor (LEPR) gene genotypes
DISCUSSION
In this study, the evaluation of the relationship of the LEPR Lys109Arg polymorphisms and gastric cancer risk showed that cases carrying LEPR 109Arg allele were at a modestly increased risk of gastric cancer (OR, 9.926; 95% CI, 1.248 to 6.861). Several studies have demonstrated that specific genotypes in the LEPR gene were associated with an increased risk for breast cancer. However, there are few previous studies that address the relationship between LEPR polymorphisms and gastric cancer risk. Our study is the first trial to analyze the LEP and LEPR polymorphisms in patients with gastric cancer. We selected cases that had not been treated in any manner that might affect leptin levels.
Circulating concentrations of leptin are highly correlated with body fat mass [22]. In our study, however, there was no significant difference observed regarding the mean serum leptin concentrations between the patient group and control group, while BMI was significantly lower in gastric cancer cases than the controls. Another mechanism suspected is that a sequence variation in gene coding leptin may affect the expression of leptin [23]. However, there have been conflicting reports about the potential effect of the G2548A LEP variant on leptin expression [23,24]. In the Tunisian population, no association was found between this LEP polymorphism and plasma leptin concentrations [23]. On the other hand, in men from a French cohort, the AA genotype was associated with increased plasma leptin levels [24]. Although not mentioned above, no association was found between LEP polymorphism and serum leptin concentrations. The issue regarding leptin level and gastric cancer requires debate and additional study with a larger number of cases to clarify this relationship.
Gastric leptin plays a role in serum leptin levels, regulating intestinal nutrient absorption, delaying gastric emptying, and signaling short-term satiety via vagal afferent nerves [25]. H. pylori induced inflammatory response affects many gastric cell types, including those responsible for leptin production [26]. Eradication of H. pylori has been reported to increase nutrition and weight, suggesting that H. pylori could play a role in the regulation of leptin expression [27]. In our study, cases with positive H. pylori on gastric mucosa showed lower concentrations of leptin than those with negative H. pylori. This result suggested that gastric H. pylori colonization reduces circulating levels of leptin by inhibiting their gastric production.
The frequencies of LEPR polymorphisms, except that of Ser343Ser, were different from those previously reported. In our study, the frequencies of Lys109Arg and Gln223Arg wild-types were zero, whereas in other studies, 53 to 97%, 23 to 66% of the subjects showed wild-type sequences at codons 109 and 223, respectively [16,28]. Most studies regarding LEPR polymorphisms were performed in Caucasian patients. The genotype frequencies for Lys109Arg and Gln223Arg in the Korean and Japanese population were similar to those in our study, although both studies were performed in breast cancer patients [29,30]. Therefore, further studies are needed to clarify racial differences in the genotype frequencies of LEPR polymorphisms between Asians and Caucasians.
In this study, there was no association between genotypes and serum leptin levels. A trend was observed for cancer patients who were hetorozygotic for Lys656Asn to have lower mean leptin concentrations compared to those with the wild-type. There have been a few previous studies that addressed a relationship between LEPR polymorphism and serum concentrations of leptin. Woo et al. [29] reported that being heterozygotic for Lys656Asn was significantly associated with lower leptin levels in premenopausal subjects and being homozygotic for Gln223 Arg and Lys109Arg was significantly associated with higher leptin levels in post-menopausal patients and in controls, respectively. Our result corresponds with the Woo et al. [29] report, although that report was performed in breast cancer patients.
Our study has several limitations. One limitation was the small size of screened population. Additional studies with a larger sample size would be helpful to demonstrate the relationship between LEPR polymorphisms and gastric cancer risk. Another was the paucity of epidemiological literature on the role of polymorphisms in the LEP and LEPR gene in gastric cancer susceptibility in most populations. The effect of genotypes such as LEPR Lys109Arg polymorphism on gastric cancer may vary from one population to the other as a result of marked differences in the distribution of the alleles in different populations. Further study is needed to clarify racial differences in the genotype frequencies of LEPR polymorphisms in different populations.
In conclusion, this study has demonstrated a modestly increased risk of gastric cancer in cases harboring the LEPR 109Arg allele of the LEPR Lys109Arg polymorphism of the leptin receptor gene. To the best of our knowledge, our study might be the first trial to provide information on the role of LEPR Lys109Arg polymorphism in gastric cancer risks in Koreans.
ACKNOWLEDGEMENTS
This work was supported by a research grant from St. Vincent's Hospital, The Catholic University of Korea.
Footnotes
No potential conflict of interest relevant to this article was reported.
References
- 1.Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM. Positional cloning of the mouse obese gene and its human homologue. Nature. 1994;372:425–432. doi: 10.1038/372425a0. [DOI] [PubMed] [Google Scholar]
- 2.Collins S, Kuhn CM, Petro AE, Swick AG, Chrunyk BA, Surwit RS. Role of leptin in fat regulation. Nature. 1996;380:677. doi: 10.1038/380677a0. [DOI] [PubMed] [Google Scholar]
- 3.Considine RV, Sinha MK, Heiman ML, Kriauciunas A, Stephens TW, Nyce MR, et al. Serum immunoreactive-leptin concentrations in normal-weight and obese humans. N Engl J Med. 1996;334:292–295. doi: 10.1056/NEJM199602013340503. [DOI] [PubMed] [Google Scholar]
- 4.Señarís R, Garcia-Caballero T, Casabiell X, Gallego R, Castro R, Considine RV, et al. Synthesis of leptin in human placenta. Endocrinology. 1997;138:4501–4504. doi: 10.1210/endo.138.10.5573. [DOI] [PubMed] [Google Scholar]
- 5.Jin L, Burguera BG, Couce ME, Scheithauer BW, Lamsan J, Eberhardt NL, et al. Leptin and leptin receptor expression in normal and neoplastic human pituitary: evidence of a regulatory role for leptin on pituitary cell proliferation. J Clin Endocrinol Metab. 1999;84:2903–2911. doi: 10.1210/jcem.84.8.5908. [DOI] [PubMed] [Google Scholar]
- 6.Briscoe CP, Hanif S, Arch JR, Tadayyon M. Leptin receptor long-form signalling in a human liver cell line. Cytokine. 2001;14:225–229. doi: 10.1006/cyto.2001.0871. [DOI] [PubMed] [Google Scholar]
- 7.Mix H, Widjaja A, Jandl O, Cornberg M, Kaul A, Goke M, et al. Expression of leptin and leptin receptor isoforms in the human stomach. Gut. 2000;47:481–486. doi: 10.1136/gut.47.4.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schneider R, Bornstein SR, Chrousos GP, Boxberger S, Ehninger G, Breidert M. Leptin mediates a proliferative response in human gastric mucosa cells with functional receptor. Horm Metab Res. 2001;33:1–6. doi: 10.1055/s-2001-12617. [DOI] [PubMed] [Google Scholar]
- 9.Sierra-Honigmann MR, Nath AK, Murakami C, Garcia-Cardena G, Papapetropoulos A, Sessa WC, et al. Biological action of leptin as an angiogenic factor. Science. 1998;281:1683–1686. doi: 10.1126/science.281.5383.1683. [DOI] [PubMed] [Google Scholar]
- 10.Calle EE, Rodriguez C, Walker-Thurmond K, Thun MJ. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. N Engl J Med. 2003;348:1625–1638. doi: 10.1056/NEJMoa021423. [DOI] [PubMed] [Google Scholar]
- 11.Stattin P, Lukanova A, Biessy C, Soderberg S, Palmqvist R, Kaaks R, et al. Obesity and colon cancer: does leptin provide a link? Int J Cancer. 2004;109:149–152. doi: 10.1002/ijc.11668. [DOI] [PubMed] [Google Scholar]
- 12.Stattin P, Soderberg S, Hallmans G, Bylund A, Kaaks R, Stenman UH, et al. Leptin is associated with increased prostate cancer risk: a nested case-referent study. J Clin Endocrinol Metab. 2001;86:1341–1345. doi: 10.1210/jcem.86.3.7328. [DOI] [PubMed] [Google Scholar]
- 13.Stephenson GD, Rose DP. Breast cancer and obesity: an update. Nutr Cancer. 2003;45:1–16. doi: 10.1207/S15327914NC4501_1. [DOI] [PubMed] [Google Scholar]
- 14.Lee GH, Proenca R, Montez JM, Carroll KM, Darvishzadeh JG, Lee JI, et al. Abnormal splicing of the leptin receptor in diabetic mice. Nature. 1996;379:632–635. doi: 10.1038/379632a0. [DOI] [PubMed] [Google Scholar]
- 15.Chen H, Charlat O, Tartaglia LA, Woolf EA, Weng X, Ellis SJ, et al. Evidence that the diabetes gene encodes the leptin receptor: identification of a mutation in the leptin receptor gene in db/db mice. Cell. 1996;84:491–495. doi: 10.1016/s0092-8674(00)81294-5. [DOI] [PubMed] [Google Scholar]
- 16.Gotoda T, Manning BS, Goldstone AP, Imrie H, Evans AL, Strosberg AD, et al. Leptin receptor gene variation and obesity: lack of association in a white British male population. Hum Mol Genet. 1997;6:869–876. doi: 10.1093/hmg/6.6.869. [DOI] [PubMed] [Google Scholar]
- 17.Zhao L, Shen ZX, Luo HS, Shen L. Possible involvement of leptin and leptin receptor in developing gastric adenocarcinoma. World J Gastroenterol. 2005;11:7666–7670. doi: 10.3748/wjg.v11.i48.7666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lewin MJ, Bado A. Gastric leptin. Microsc Res Tech. 2001;53:372–376. doi: 10.1002/jemt.1105. [DOI] [PubMed] [Google Scholar]
- 19.Ishikawa M, Kitayama J, Nagawa H. Expression pattern of leptin and leptin receptor (OB-R) in human gastric cancer. World J Gastroenterol. 2006;12:5517–5522. doi: 10.3748/wjg.v12.i34.5517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhao X, Huang K, Zhu Z, Chen S, Hu R. Correlation between expression of leptin and clinicopathological features and prognosis in patients with gastric cancer. J Gastroenterol Hepatol. 2007;22:1317–1321. doi: 10.1111/j.1440-1746.2007.04941.x. [DOI] [PubMed] [Google Scholar]
- 21.Low S, Chin MC, Ma S, Heng D, Deurenberg-Yap M. Rationale for redefining obesity in Asians. Ann Acad Med Singapore. 2009;38:66–69. [PubMed] [Google Scholar]
- 22.Maffei M, Halaas J, Ravussin E, Pratley RE, Lee GH, Zhang Y, et al. Leptin levels in human and rodent: measurement of plasma leptin and ob RNA in obese and weight-reduced subjects. Nat Med. 1995;1:1155–1161. doi: 10.1038/nm1195-1155. [DOI] [PubMed] [Google Scholar]
- 23.Ben Ali S, Kallel A, Ftouhi B, Sediri Y, Feki M, Slimane H, et al. Association of G-2548A LEP polymorphism with plasma leptin levels in Tunisian obese patients. Clin Biochem. 2009;42:584–588. doi: 10.1016/j.clinbiochem.2008.11.001. [DOI] [PubMed] [Google Scholar]
- 24.Mammes O, Betoulle D, Aubert R, Herbeth B, Siest G, Fumeron F. Association of the G-2548A polymorphism in the 5' region of the LEP gene with overweight. Ann Hum Genet. 2000;64(Pt 5):391–394. doi: 10.1017/s0003480000008277. [DOI] [PubMed] [Google Scholar]
- 25.Bado A, Levasseur S, Attoub S, Kermorgant S, Laigneau JP, Bortoluzzi MN, et al. The stomach is a source of leptin. Nature. 1998;394:790–793. doi: 10.1038/29547. [DOI] [PubMed] [Google Scholar]
- 26.Blaser MJ, Atherton JC. Helicobacter pylori persistence: biology and disease. J Clin Invest. 2004;113:321–333. doi: 10.1172/JCI20925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Azuma T, Suto H, Ito Y, Ohtani M, Dojo M, Kuriyama M, et al. Gastric leptin and Helicobacter pylori infection. Gut. 2001;49:324–329. doi: 10.1136/gut.49.3.324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chung WK, Power-Kehoe L, Chua M, Chu F, Aronne L, Huma Z, et al. Exonic and intronic sequence variation in the human leptin receptor gene (LEPR) Diabetes. 1997;46:1509–1511. doi: 10.2337/diab.46.9.1509. [DOI] [PubMed] [Google Scholar]
- 29.Woo HY, Park H, Ki CS, Park YL, Bae WG. Relationships among serum leptin, leptin receptor gene polymorphisms, and breast cancer in Korea. Cancer Lett. 2006;237:137–142. doi: 10.1016/j.canlet.2005.05.041. [DOI] [PubMed] [Google Scholar]
- 30.Ogawa T, Hirose H, Yamamoto Y, Nishikai K, Miyashita K, Nakamura H, et al. Relationships between serum soluble leptin receptor level and serum leptin and adiponectin levels, insulin resistance index, lipid profile, and leptin receptor gene polymorphisms in the Japanese population. Metabolism. 2004;53:879–885. doi: 10.1016/j.metabol.2004.02.009. [DOI] [PubMed] [Google Scholar]