Abstract
Purpose
We developed a standardized critical pathway for gastric cancer surgery and then determined the increase of application, which resulted in an improvement in terms of measurable indices, such as hospital stay and cost.
Materials and Methods
A critical pathway was revised and used widely from the 2nd quarter of 2009. We collected clinical data, such as length of stay and complication rate, as clinical indices of quality prospectively. The total cost paid at the patient's discharge, as well as the daily hospital income, were calculated and compared by each quarter from January 2008 to December 2009.
Results
The application rate of critical pathway was 11.8% and 87.8% in 2008 and 2009, respectively. There were no perioperative deaths. There was no difference in the complication rates between 2008 and 2009 (P=0.45). However, the mean length of stay was significantly different between the 2 years (P<0.05). Although the total cost was not different, the daily hospital income was significantly higher in the latter year (P<0.05).
Conclusions
An increase in the application of critical pathway for gastrectomy resulted in significant decreases in length of stay and increases in the daily hospital income without a compromise on the clinical indices.
Keywords: Critical pathway, Gastrectomy, Clinical index, Length of stay, Cost
Introduction
Gastric cancer is the most common cancer in Korea, accounting for more than 25,000 new cases per year, and the second most common cause of cancer-related deaths. Although the incidence has decreased gradually and the proportion of early-stage cases has increased to 40~50%,(1) gastric cancer presents a notable burden to health payers in Korea.
The critical pathway (CP) is a standardized measure that is planned on a time-based schedule for patient management. The use of CP has been proposed as a way of improving health care processes, outcomes, and costs.(2,3) In the surgical setting, each step of treatment can be standardized using CP, which can prevent unnecessary or ineffective procedures. Therefore, we can expect a reduction in inappropriate care, an increased clinical efficiency, better control of health care spending, and a higher level of patient satisfaction.
For these reasons, there has been an increasing worldwide interest over the last 2 decades in CP development and implementation.(4-7).
Despite the growing utilization of these pathways in medicine, the effect of a CP for gastrectomy has not been fully investigated. In this study, we developed a "CP of Operable Gastric Cancer" and examined the effect of increased application rate of this CP for gastrectomy in terms of cost and length of hospital stay in patients with gastric cancer.
Materials and Methods
1. Development and revision
We developed a standardized CP for gastric cancer patients in 2004.(8) Briefly, our CP was developed by a committee, "We Dream Team," which included gastric surgeons, nurses, nutritionists, administrative officials, and members of the clinical support services. After an extensive review of the literature to identify the best demonstrated practices, a pre-operative work-up process and post-operative care plan were determined. The CP was implemented for those patients who were expected to undergo curative resection and had no severe co-morbidities. Patients with following factors were excluded from CP. 1) patients with cancer-related complications such as perforation or bleeding, 2) patients with concomittent other malignancy, 3) patients with severe comorbidities which is not appropriate to general anesthesia, 4) patients with incurable factors, 5) patients without consents.
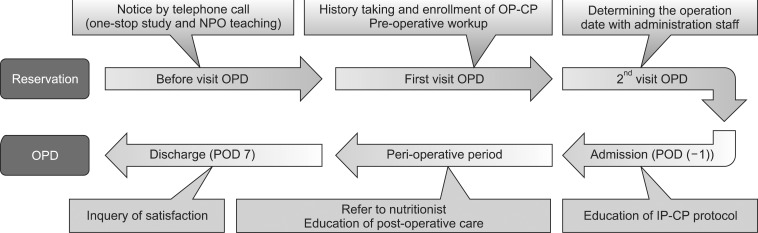
Then we evaluated the practical impact of the CP on the clinical outcome, as well as with regard to the economical benefits. The positive aspects of the CP were analyzed and reported. However, there were some practical barriers to the implementation of the CP in the clinical system. The CP has not been applied to the clinical care program as we had expected; therefore, the mean CP application rate before 2008 was less than 10%. In 2009, with the opening of a new hospital, our institution recruited specialized nurses, known as clinical nurse specialists (CNSs), to raise the application rate of CP. The CNS is deeply involved in every step of the CP (Fig. 1). At the same time, the house staff tried to set up a CP protocol on a daily basis at the patients' initial visits to our out-patient clinic. With the effort to minimize the evaluation period pre-operatively and train our in-patient care nursing staff who routinely worked with and educated the patients, the CP application rate has increased to 87.8%.
Fig. 1.
Roles of the clinical nurse specialist in each step of critical pathway. NPO = not per oral; OP-CP = outpatient critical pathway; IP-CP = inpatient critical pathway.
2. Elements of the CP
In 2009, as part of our revision, we separated the CP into 2 parts: the out-patient CP (OPCP) and the in-patient CP (IPCP). All patients with histologically confirmed, resectable, gastric adenocarcinoma were eligible. From a total of 452 patients who underwent curative resection of stomach, 397 patients (87.8%) were included in this CP in 2009. These patients were divided as OPCP (n=290) and IPCP (n=107).
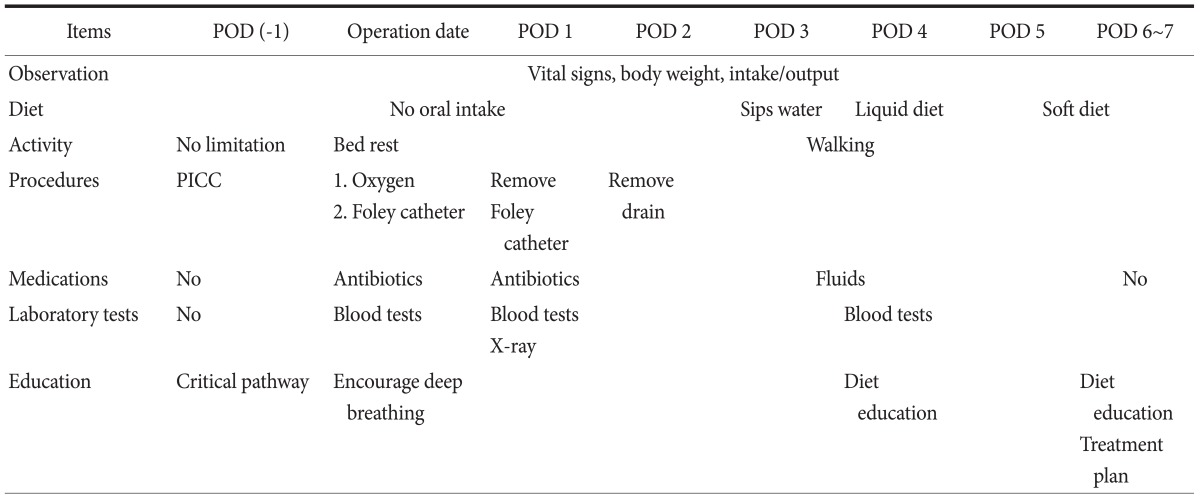
If the patient had no comorbidity or any minor illness, he/she was enrolled in the OPCP group, underwent all pre-operative workups, and was admitted 1 day before operation. The workup evaluation consisted of the patient's history, physical examination, laboratory tests including tumor markers (carcinoembrionic antigen and CA 19-9), chest radiography, endoscopy, 3-dimensional computed tomography (CT), and bone scintigraphy. If the patient had a major illness, severe malnutrition, or severe anemia, the pre-operative workup and build-up proceeded simultaneously after admission. These patients were enrolled in the IPCP group, which started at 1 day before operation. The routine process from 1 day before operation was the same in both CPs. No nasogastric tube was used and no bowel preparation was performed before surgery. We made a pathway brochure, which explained the anticipated sequence of events in lay terms for the patients and their family. This brochure was broken into the following categories: diet, activity, procedures, medication, laboratory tests, and education (Table 1). The patients started taking small sips of water on post-operative day 3 and solid meals on post-operative day 5. On post-operative day 4 and the day of discharge (post-operative day, POD #6), the patients were educated about adequate nutrition. They also received a detailed instruction sheet upon discharge that outlined their recommended eating habits, level of activity, expected follow-up schedule, and post-operative life-style modifications.
Table 1.
Pathway brochure* for the patient
POD = post-operative day; PICC = peripherally inserted central line. *Originally this brochure was written in Korean.
3. Patients
Between 2008 and 2009, a total of 745 patients underwent operation for gastric cancer. Six hundred ninety-four (93%) of these patients-those who underwent gastrectomy-were reviewed in this study. To analyze the clinical impact of the actual CP application, we directly compared the clinical indices and hospital charges between years 2008 and 2009. The clinicopathological characteristics and post-operative outcomes, including peri-operative morbidity and length of stay (LOS), were collected from our database. The LOS and cost were analyzed according to the type of resection and each quarter to see the periodic differences. Data such as the total cost paid at discharge and daily hospital income were provided by the Health Insurance Review and Assessment Service of Korea. This study was approved by the Institutional Review Board (IRB KC12RISI0378).
4. Statistical analysis
To compare the continuous variables between the groups, the Mann-Whitney U test was used. The dichotomous variables were compared using the chi-square test or, when appropriate, Fisher's exact test. The results are expressed as mean (standard deviation) unless otherwise stated. A P-value of less than 0.05 was considered to indicate statistical significance.
Results
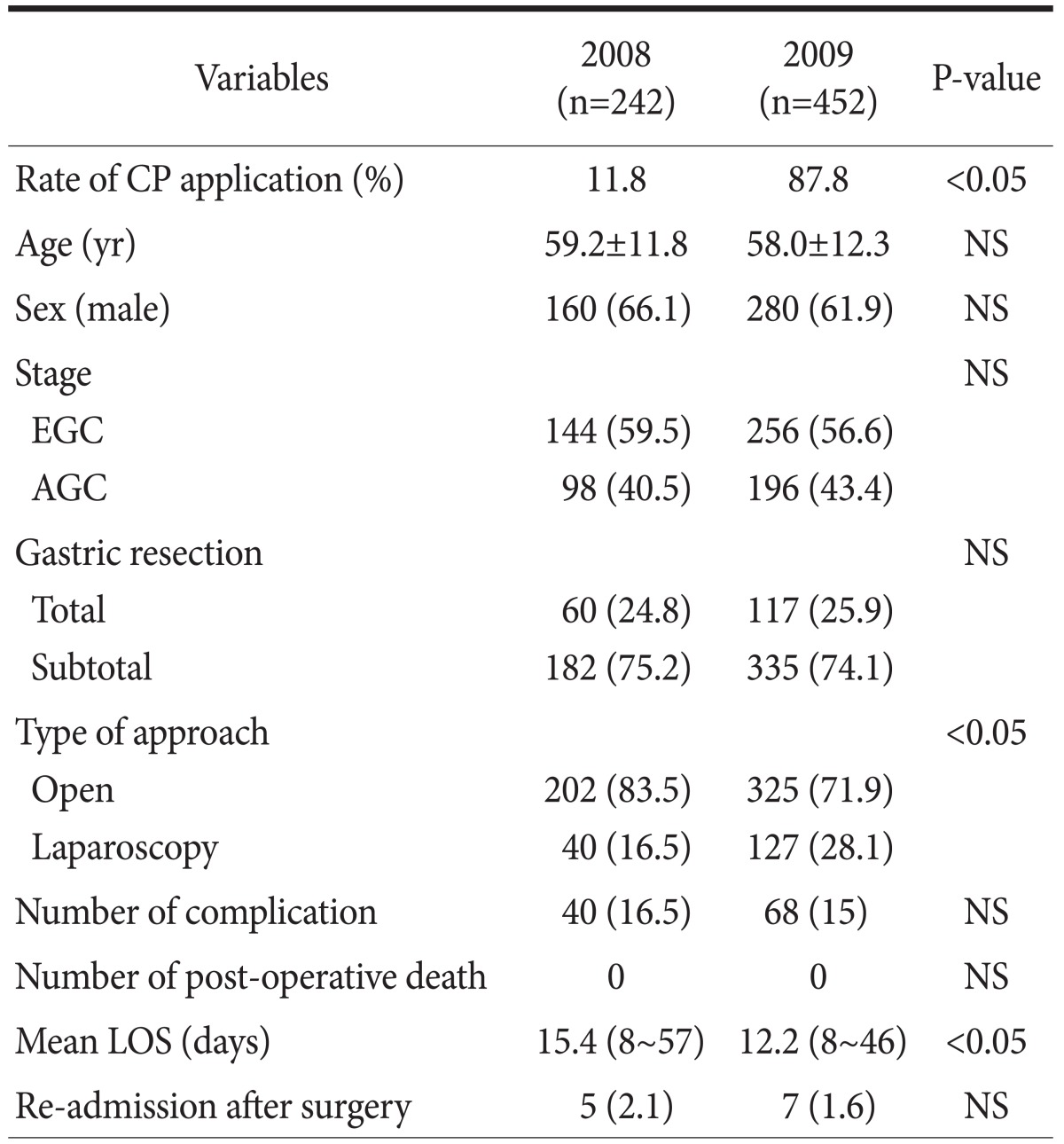
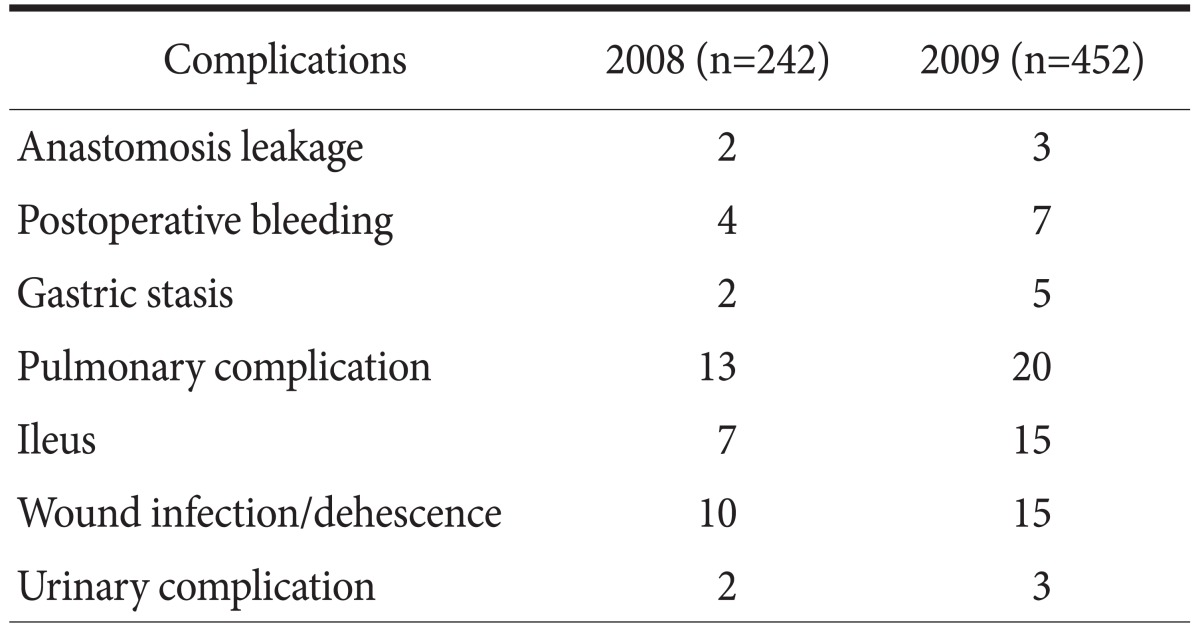
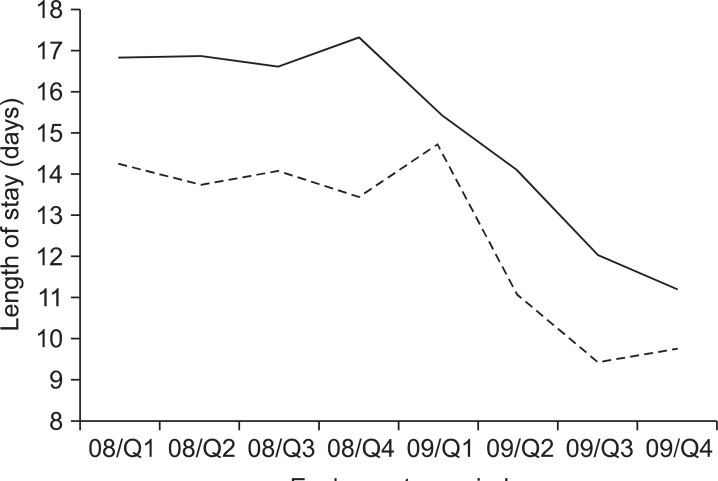
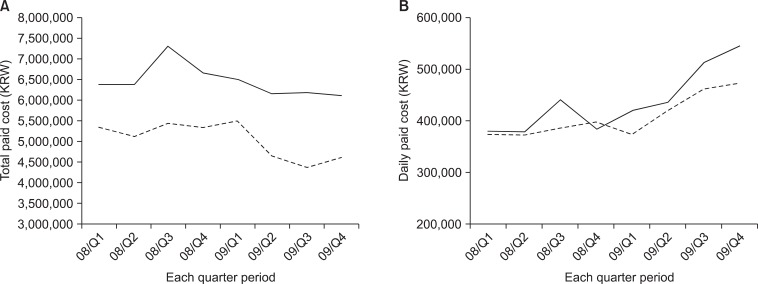
The clinicopathological characteristics are shown in Table 2. Gastrectomy was performed in 242 and 452 patients in 2008 and 2009, respectively. The only different factor was the rate of laparoscopic surgery (16.5% vs. 28.1% in 2008 and 2009, respectively). The application rate of CP was 11.3% in 2008 and 87.8% in 2009, respectively. The overall drop rate of patients in 2009 was 14.6%. The reasons included the post-operative complication, patients' willingness not to back their home, or some cases of additional treatment during their stay. There was no peri-operative death in either year, and there was no significant difference in the complication rate between the 2 years (16.5 vs. 15.0%, P>0.05) (Table 3). However, the mean LOS was significantly different between the 2 years (15.4 vs. 12.2 days, P<0.05). The mean LOS did not change in each quarter 2008, but it gradually decreased in each quarter 2009 as application rate of CP increased (Fig. 2). There was also the difference of LOS between CP and non-CP patients (12.2 vs. 18.6 days, P<0.05). However, no difference was noted of post-operative hospital stays between CP and non-CP patients (8.5 vs. 9.9 days, P>0.05). Although the total cost was not different, the daily hospital income was significantly higher in the latter period (388,830 vs. 454,739 KRW, P<0.05). The daily hospital income also increased gradually in 2009 (Fig. 3).
Table 2.
Clinical characteristics of patients who underwent gastrectomy between 2008 and 2009
Values are presented as mean±standard deviation or number (%) or number (range). CP = critical pathway; EGC = early gastric cancer; AGC = advanced gastric cancer; LOS = length of stay; NS = not significant.
Table 3.
Operation-related complications
Fig. 2.
Mean length of stay in each quarter (Q) period. Solid line is for total gastrectomy and dotted line is for subtotal gastrectomy.
Fig. 3.
Total and daily paid cost in each quarter (Q) period. (A) Total cost, (B) daily hospital income. Solid line is for total gastrectomy and dotted line is for subtotal gastrectomy. One US dollar stands for 1,100 Korean Won (KRW) in February of 2011.
Discussion
It is possible to develop and apply CP to gastric cancer patients. The application of CP to daily clinical practices could also decrease the duration of hospital stay and increase the daily hospital income per practice. In addition, the maintenance of high qualities of CP would depend upon systemic support, such as the hiring of health professionals for CP and the setting-up of an institution-wide support system. Most importantly, mutual understanding and cooperation between the CP team is mandatory for a successful CP.
The pre-operative preparation process was the key barrier to applying CP to gastrectomy patients, whereas the post-operative hospital stays were not significantly different between the CP-applied and non-CP-applied patients. To minimize the total hospital stays, there was a need to reduce the pre-operative hospital stays. We performed pre-operative evaluation studies, such as duodenoscopy, CT, positron emission tomography, and blood panels, on clinic-based out-patients as a "One-stop" system. We also refrained from inserting the Levin tube pre-operatively and skipped the pre-operative bowel preparation process. These simplifications of pre-operative preparation set admission at 1 day before surgery. Clinical safety was established by our many years of clinical experience. This was supported by demonstration of the same complication rate and operation-related mortality between the non-CP era and CP eras. The standardized management protocol and application of minimally invasive surgery also resulted in increasing the quality of post-operative patient care. However, strictly speaking, the application of one-stop system in pre-operative evaluation studies, not the CP application, can change for the better LOS and cost.
The limitation of this study is that it did not directly compare the clinical outcomes of the CP and non-CP groups. Since the clinical characteristics of the CP and non-CP patients were totally different, the comparison of these 2 groups' clinical indices would lead to some misinterpretation of data. Instead, we compared the clinical indices and cost of all patients, including CP and non-CP patients, in 2008 and 2009 to see the clinical efficacy of CP application. As mentioned previously, the application rate of CP in 2008 was significantly lower than that in 2009. We reviewed the clinical impact of the increase of the CP application rate. The only clinical difference of patients in 2008 and 2009 was that the rate of laparoscopic gastrectomy increased in 2009. Laparoscopic surgery can decrease post-operative pain, which can affect post-operative hospital stay. And it also can increase the costs and this fact can affect the analysis. This would be a critical limitation of this study. However, the hospital stays of patients who underwent open gastrectomy and patients who had laparoscopic gastrectomy did not differ under the standardized CP program. Therefore, the operative procedure itself did not critically affect the duration of hospital stay in either patient group. Also, the costs for laparoscopic surgery were not significantly different between two time periods. Therefore, we believed that the comparison of this study would have a meaning despite of a limitation.
Since gastric cancer is the most common cancer in Korea, many gastric surgeons have developed experience with standard surgical procedures.(9) Many subspecialties and subdivision-based patient care for gastric cancer have also been developed. This environment has enhanced the quality of care for patients with gastric cancer and led to higher survival and lower post-operative complication rates than those of Western countries.(10) In particular, we previously reported a less than 1% operation-related mortality, which reflects the high quality of our surgical team and post-operative patient care program.(1,10) It was because of this that we developed a CP protocol for gastric cancer patients.
Our CP team had already developed a CP protocol for gastric cancer patients in 2004. This team comprised 3 surgeons with specialties in gastric cancer, a nursing specialist, a nutritional specialist, and an administrative team. This CP team was named as "We Dream Team". During the first year, CP was applied to 26 patients and the results were compared with those of 91 patients to whom the CP protocol was not applied. The hospital stay was shortened and daily hospital income per practice was increased. However, there was no difference in the quality of patient care, such as post-operative complications or mortality rate. Although it was proved that CP had many advantages for improving the quality of clinical practice, its actual application rate stagnated at less than 10%. This stagnation was caused by (1) the shortage of professional personnel for CP management, (2) the absence of an institutional support system, such as the setting up of an electronic chart system and financial support, (3) interdisciplinary conflicts, (4) the pressures placed on the house staff by the application of CP, and (5) the manner in which variations in personal general nutritional condition and combined morbidity prohibited the application of CP.
There is striking difference of the application rate of CP between 2008 and 2009. We believe that several reasons can explain this fact. First, with the opening of a new hospital, 2 CNSs for gastric cancer were employed. Second, we set up distinctive CP protocols for admitted patients and clinic-based out-patients. If there was the need for any pre-operative correction of patients' general health status or consultation for underlying co-morbidity, then the customized CP application would be used. Third, the electronic medical records system was also set up as part of the CP program. This institution-wide support and interdisciplinary cooperation improved CP application up to 90%.
This study demonstrates that it is possible to develop and apply CP to gastric cancer patients and CP can impact on hospital stay and costs, while patients' safety remains unaffected. But the most important thing to remind is that the maintenance of high qualities of CP would depend upon an institution-wide support system. Moreover, mutual understanding and co-operation between the CP team is mandatory for a successful CP.
Acknowledgments
The authors thank all participants of "We Dream team".
This study was partly supported by the Cancer Research Fund of the Catholic Comprehensive Cancer Institute in 2010.
References
- 1.Park CH, Song KY, Kim SN. Treatment results for gastric cancer surgery: 12 years' experience at a single institute in Korea. Eur J Surg Oncol. 2008;34:36–41. doi: 10.1016/j.ejso.2007.03.004. [DOI] [PubMed] [Google Scholar]
- 2.Melbert RB, Kimmins MH, Isler JT, Billingham RP, Lawton D, Salvadalena G, et al. Use of a critical pathway for colon resections. J Gastrointest Surg. 2002;6:745–752. doi: 10.1016/s1091-255x(02)00038-0. [DOI] [PubMed] [Google Scholar]
- 3.Coffey RJ, Richards JS, Remmert CS, LeRoy SS, Schoville RR, Baldwin PJ. An introduction to critical paths. Qual Manag Health Care. 2005;14:46–55. doi: 10.1097/00019514-200501000-00006. [DOI] [PubMed] [Google Scholar]
- 4.Pritts TA, Nussbaum MS, Flesch LV, Fegelman EJ, Parikh AA, Fischer JE. Implementation of a clinical pathway decreases length of stay and cost for bowel resection. Ann Surg. 1999;230:728–733. doi: 10.1097/00000658-199911000-00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Archer SB, Burnett RJ, Flesch LV, Hobler SC, Bower RH, Nussbaum MS, et al. Implementation of a clinical pathway decreases length of stay and hospital charges for patients undergoing total colectomy and ileal pouch/anal anastomosis. Surgery. 1997;122:699–703. doi: 10.1016/s0039-6060(97)90076-3. [DOI] [PubMed] [Google Scholar]
- 6.Porter GA, Pisters PW, Mansyur C, Bisanz A, Reyna K, Stanford P, et al. Cost and utilization impact of a clinical pathway for patients undergoing pancreaticoduodenectomy. Ann Surg Oncol. 2000;7:484–489. doi: 10.1007/s10434-000-0484-0. [DOI] [PubMed] [Google Scholar]
- 7.Delaney CP, Zutshi M, Senagore AJ, Remzi FH, Hammel J, Fazio VW. Prospective, randomized, controlled trial between a pathway of controlled rehabilitation with early ambulation and diet and traditional postoperative care after laparotomy and intestinal resection. Dis Colon Rectum. 2003;46:851–859. doi: 10.1007/s10350-004-6672-4. [DOI] [PubMed] [Google Scholar]
- 8.Song KY, Kim SN, Park CH. Critical pathway for operable gastric cancer. J Korean Gastric Cancer Assoc. 2005;5:95–100. [Google Scholar]
- 9.Hur H, Song KY, Park CH, Jeon HM. Follow-up strategy after curative resection of gastric cancer: a nationwide survey in Korea. Ann Surg Oncol. 2010;17:54–64. doi: 10.1245/s10434-009-0676-1. [DOI] [PubMed] [Google Scholar]
- 10.Strong VE, Song KY, Park CH, Jacks LM, Gonen M, Shah M, et al. Comparison of gastric cancer survival following R0 resection in the United States and Korea using an internationally validated nomogram. Ann Surg. 2010;251:640–646. doi: 10.1097/SLA.0b013e3181d3d29b. [DOI] [PubMed] [Google Scholar]