Abstract
Purpose
Local recurrence, due to residual tumor, may occur after endoscopic resection for early gastric cancer. The aims of this study are to evaluate the predictive factors for local recurrence, and suggest an appropriate follow-up biopsy strategy.
Materials and Methods
We retrospectively reviewed 396 early gastric cancers from 372 consecutive patients, who underwent endoscopic resection between January 2002 and April 2008. Cumulative recurrence rates were determined by the Kaplan-Meier method, and Cox proportional hazard analysis was used to determine the risk factors for local recurrence.
Results
Local recurrence at the endoscopic resection site was found in 17 cases, among the total 396 lesions, during a median follow-up period of 48 months. The 5-year cumulative local recurrence rate was 4.8%. Multivariate analyses determined that tumor involvement at the lateral resection margin [hazard ratio: 35.9; P<0.001], uncheckable lateral resection margin [hazard ratio: 16.8; P<0.001], uncheckable or involved deep resection margin [hazard ratio: 3.76; P=0.047], and piecemeal resection [hazard ratio: 3.95; P=0.007] were associated with local recurrence. If a lesion was positive for any of these risk factors, the 5-year cumulative recurrence rate was 27.0%, while local recurrence was not found in any lesion that lacked these risk factors. Most episodes of recurrence were found during the first or second follow-up endoscopic biopsy at the ulcer scar.
Conclusions
Routine follow-up biopsies at the endoscopic resection site might be unnecessary in cases where an early gastric cancer lesion was endoscopically resected en bloc with tumor-free lateral and deep margins.
Keywords: Gastric cancer, Endoscopic resection, Recurrence, Risk factors, Biopsy
Introduction
Endoscopic resection (ER) is a treatment option for early gastric cancer (EGC) with minimal risk of lymph-node metastases that it is less invasive than surgical resection and offers a better quality of life.(1) Endoscopic mucosal resection (EMR) is associated with a high risk of local recurrence (range: 2~35%)(1-6) due to the piecemeal resection of large lesions.(7,8) Despite endoscopic submucosal dissection (ESD) having high en bloc and curative resection rates,(3,9,10) local recurrence has been found to occur after ESD as well (range: 1~5%).(8-12)
To detect local recurrence after ER, follow-up endoscopies were performed two to four times during the first year and annually thereafter, and biopsy specimens were routinely taken from the post-ER ulcer scar during each endoscopy irrespective of curability.(4,9,13) However, a recent study reported that use of follow-up endoscopy for the surveillance of local recurrences could be modified according to curability because there were no episodes of recurrence in patients with a complete resection, whereas local recurrence occurred in about 15% of patients with an incomplete resection.(11) There have been few reports about the proper strategy in terms of follow-up biopsies for the surveillance of local recurrence after ER. This is important because routinely obtaining biopsy specimens from the ER ulcer scar during each follow-up endoscopy increases medical costs and may increase patient anxiety.
In this study, we aimed to evaluate individual risk factors associated with local recurrence and determine the appropriate follow-up strategy for surveillance against recurrence after endoscopic treatment for EGC based on these risk factors.
Materials and Methods
1. Patients
Between January 2002 and April 2008, ERs were performed to treat 536 EGCs in 500 consecutive patients at the National Cancer Center, Goyang, Korea. Patients were followed-up to examine for recurrence until April 2011.
The criteria for ER were: histologically confirmed well- or moderately-differentiated adenocarcinoma with an endoscopic diagnosis of mucosal cancer, a lesion with diameter <3 cm, and no ulcerative findings. The following cases were excluded from risk factor analysis: cases without follow-up endoscopic examination or surgical resection; cases with argon plasma coagulation immediately after ER to eradicate possible residual cancer; cases with less than 6 months of follow-up; and cases with surgical resection immediately after ER.
Clinicopathological features and clinical outcomes of ER were obtained from a prospectively collected database. Patients provided informed consent before the ER procedure. The Institutional Review Board of the National Cancer Center approved this retrospective study (NCCNCS-09-269).
2. ER technique
ER was performed by ESD or EMR, either by a cap-fitted endoscope and suction method (EMR-C) or a circumferential mucosal incision and snaring method (EMR-P). Patients were sedated with midazolam (2.5~5.0 mg) and meperidine (25~50 mg) administered intravenously. EMR-C was performed with a single or two-channel endoscope (GIF-Q240 or GIF-2T240; Olympus Co. Ltd, Tokyo, Japan), transparent hoods (MH-594 or MAJ-665; Olympus Co. Ltd), and a crescent-shaped snare (SD-7P-1; Olympus Co. Ltd) as previously described.(14) The EMR-P was performed with a two-channel endoscope (GIF-2T240) as previously reported. (15) After making a circumferential mucosal incision with a needle papillotome (MTW Endoscopy, Wesel, Germany), the lesion was resected by direct snaring with an oval-shaped device (SD-16L-1; Olympus Co. Ltd). ESD was performed with a single-channel endoscope (GIF-H260; Olympus Co. Ltd) as previously described.(16) After making a circumferential incision, the submucosal layer was dissected with an ESD-knife (MTW Endoscopy) and/or a fixed flexible snare (Kachu Technology, Seoul, Korea).
3. Histological evaluation
The location of EGC was classified into upper, middle, and lower thirds of the stomach which were defined by subdividing both lesser and greater curvatures into 3 equal lengths.(17) Retrieved ER specimens were fixed in 10% formalin. Specimens were sectioned in 2-mm intervals and evaluated pathologically according to the World Health Organization classification of gastric cancer.(18) Well- to moderately-differentiated adenocarcinoma and papillary adenocarcinoma were defined as differentiated adenocarcinoma, while poorly-differentiated adenocarcinoma, signet ring cell carcinoma, and mucinous adenocarcinoma were defined as undifferentiated adenocarcinoma. Gastric cancers were defined histologically as either intramucosal carcinoma (category 5.1) or submucosal carcinoma or beyond (category 5.2) based on the Vienna classification. (19)
4. Definition of resection margin, en bloc resection, and complete resection
Resection margins were considered "free" if the tumor-free lateral margin was at least 2 mm and the tumor-free deep margin was at least 0.1 mm. If a tumor invaded the margin, the resection margin classified as "involved". We defined a resection margin as "uncheckable" when one of the following occurred:
- resection margin was impossible to evaluate due to burn injury at the margin
- tumor-free margin was less than 2 mm in a lateral resection margin or less than 0.1 mm in a deep resection margin
- lack of a complete reconstruction in a piecemeal resection.
A resection was determined to be an en bloc resection if the tumor was resected in a single piece endoscopically as opposed to piecemeal resection. A resection was determined to be a complete resection if a lesion was resected in an en bloc pattern or a complete reconstruction was possible with tumor-free lateral and deep resection margins.
5. Complications during the procedure
A bleeding complication was defined as profuse bleeding during the procedure that interfered with ER procedure and was controlled by hemostatic clips (HX-600-090L or HX-610-090L; Olympus Co. Ltd). Perforation was defined as a gross defect managed with endoscopic clipping. Delayed bleeding that occurred after ER procedure completion and micro-perforations diagnosed by the presence of free air on chest radiography after the procedure were not considered in the analysis because these might not affect the main outcome.
6. Follow-up and surgery
Patients who had an incomplete resection or a lesion out of expanded indication were recommended to receive surgical treatment.(20) Tumors with undifferentiated histology or lymphovascular invasion were also indications for further surgery. Subtotal or total gastrectomy with D2 lymph node dissection was performed in surgical cases. Patients with complete resections and patients with incomplete resections who declined additional surgery were examined endoscopically 3, 6, and 12 months after ER and annually thereafter. To evaluate local recurrence, two to four biopsy specimens were routinely obtained from the ER ulcer scar during each examination with standard fenestrated open-cup forceps (FB-25K-1; Olympus Co. Ltd) or ellipsoid fenestrated cup forceps with needle (FB-24K-1; Olympus Co. Ltd). Local recurrence was defined as the cancer detected at the ER ulcer scar in the follow-up biopsy regardless of period from ER.
7. Statistical analysis
Continuous variables are presented as mean±standard deviation. Statistical analysis was performed by Fisher's exact test or chi-square test for categorical variables and the Student t test for continuous variables. Cumulative probabilities of local recurrence in relation to risk factors were determined by the Kaplan-Meier method, and the results were compared by the log-rank test. Predictive factors for local recurrence were assessed by Cox proportional hazard analysis. Hazard ratios with 95% confidence intervals were calculated to estimate risk of local recurrence. Clinical and pathological factors associated with identified risk factors were evaluated by logistic regression analysis. Variables found to be statistically significant factors by univariate analysis were included in the multivariate analysis. Multivariate analysis with a forward-selection procedure was used. Two-sided P-values<0.05 were considered significant.
Results
1. Clinical course after ER
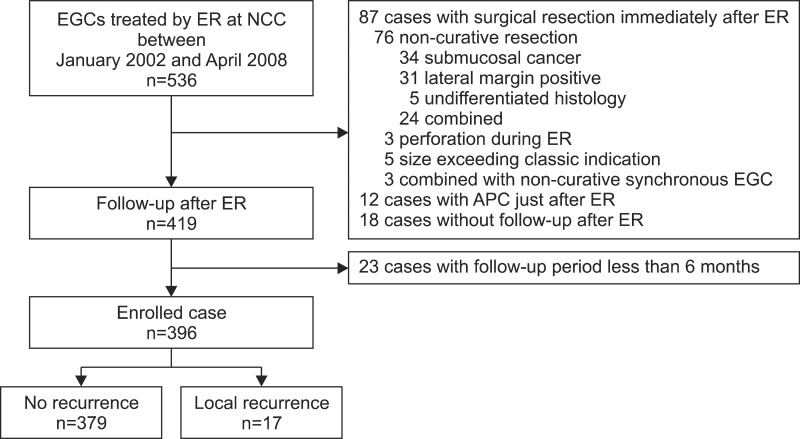
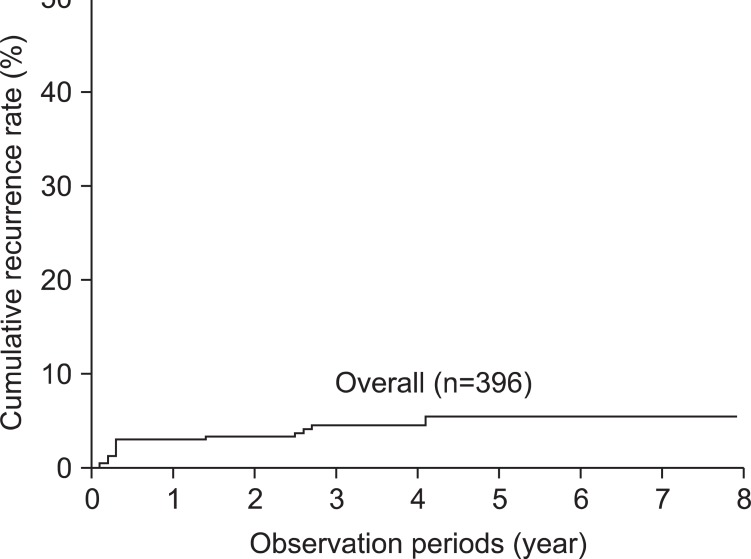
In 500 consecutive patients treated with ER, 140 of 536 EGC tumors were excluded from the analysis (excluded cases are detailed in Fig. 1). Thus, a total of 396 lesions in 372 patients were included in this study. En bloc resection and complete resection rates were 92.9% (368/396) and 83.6% (331/396), respectively. Among the 396 cases followed-up on with endoscopic examination, an episode of local recurrence occurred in 17 cases (Fig. 1). The 5-years cumulative recurrence rate was 4.8%. Recurrence was found in 12 of the 17 cases of local recurrence (71%) within 12 months, while local recurrence was detected in the other five cases (29%) after 12 months (range: 17~49 months; Fig. 2).
Fig. 1.
Flow chart. EGC = early gastric cancer; ER = endoscopic resection; NCC = National Cancer Center; APC = argon plasma coagulation.
Fig. 2.
Overall cumulative recurrence rate of the follow-up group after endoscopic resection for early gastric cancer.
2. Factors associated with local recurrence
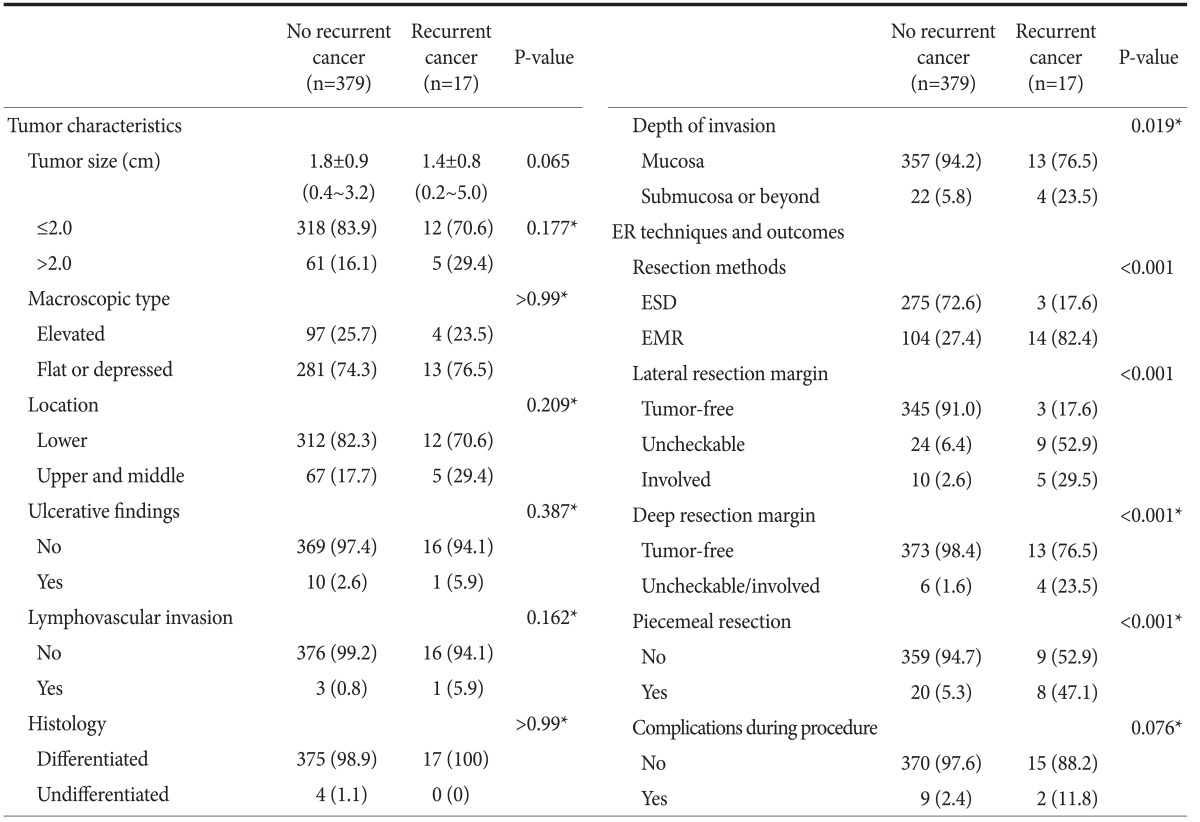
The mean age of the patients without recurrent cancer (n=355) was 61.7±9.9 years (range, 35~83 years), and the male to female ratio was 3.13 (269/86). On the other hand, the mean age of the patients with recurrent cancer (n=17) was 64.0±9.1 years (range, 47~85 years), and the male to female ratio was 3.25 (13/4). No significant difference was found between two groups in terms of age, gender (P=0.328, P>0.99, respectively). Tumor characteristics and the clinical outcomes of ER are summarized in Table 1. The relative frequency of submucosal invasion in the recurrent cancer group was significantly higher than that in the group without recurrent cancer (P=0.019). Of the parameters measured relating to the clinical outcomes of ER, the recurrent cancer group was significantly more likely to have had an EMR performed, uncheckable/involved lateral and deep resection margins, and piecemeal resection performed than the group without recurrent cancer (P<0.001).
Table 1.
Clinicopathological features of EGCs and clinical outcomes of ER according to the presence/absence of recurrent cancer
Values are presented as mean¡¾standard deviation (range) or n (%). EGC = early gastric cancer; ER = endoscopic resection; ESD = endoscopic submucosal dissection; EMR = endoscopic mucosal resection. *P-values were calculated with Fisher's exact test.
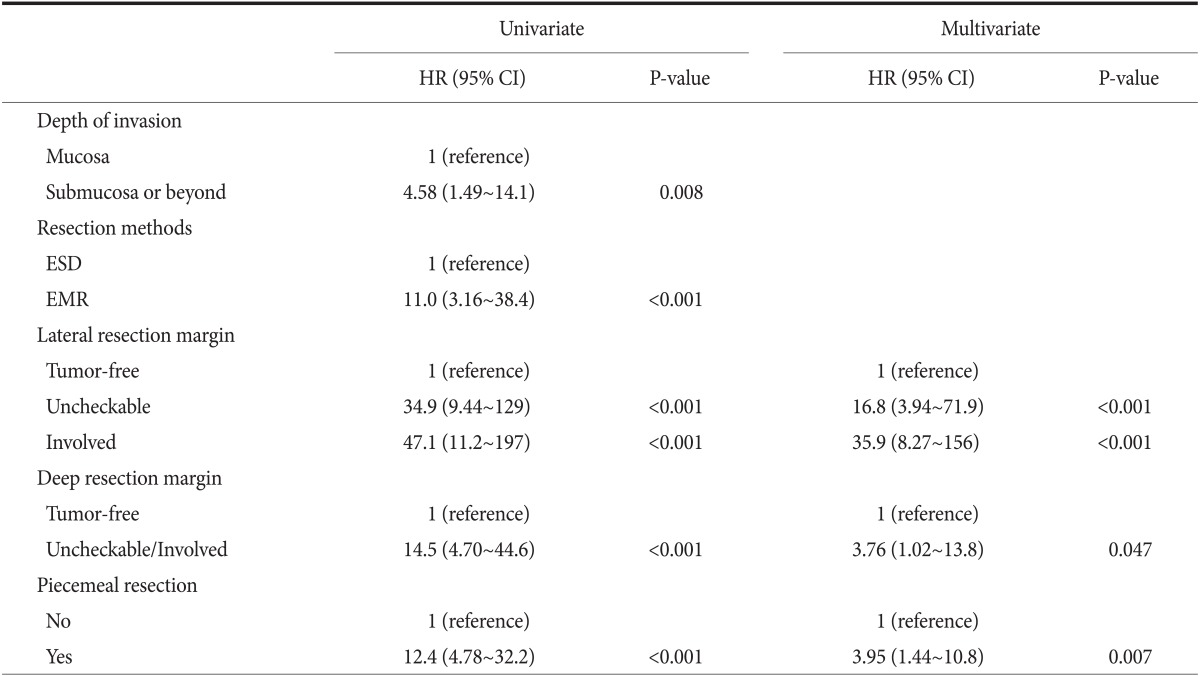
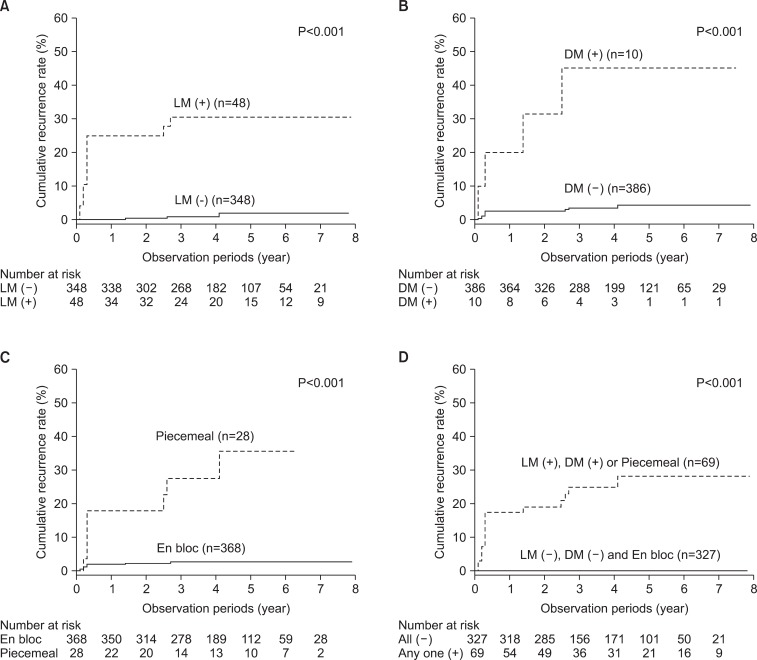
Risk factors for local recurrence were assessed by Cox proportional hazard analysis (Table 2). Multivariate analyses determined that an involved or uncheckable lateral resection margin, an uncheckable/involved deep resection margin, and a piecemeal resection were significantly associated with local recurrence. The 5-year cumulative recurrence rates in tumors with involved or uncheckable lateral resection margins, with uncheckable or involved deep resection margins, and that were resected in a piecemeal fashion were 30.2%, 45.1%, and 33.1%, respectively (Fig. 3A~C). These recurrence rates were significantly higher than the those for tumors that lacked the corresponding risk factor (no involved or uncheckable lateral resection margins: 1.3%, no uncheckable or involved deep resection margins: 3.8%, and no piecemeal resection: 2.5%; all P<0.001). There were no episodes of local recurrence in cases without any of the above risk factors, while the 5-years cumulative recurrence rate was 27.0% if a case had any of the risk factors (P<0.001; Fig. 3D).
Table 2.
Risk factors associated with local recurrence
HR = hazard ratio; CI = confidence interval; ESD = endoscopic submucosal dissection; EMR = endoscopic mucosal resection.
Fig. 3.
Kaplan-Meier plots in follow-up patients (n=396). Local recurrence rates according to the following risk factors. (A) Lateral resection margin status, (B) deep resection margin status, (C) piecemeal resection, (D) one or more risk factors. Log-rank test was used to evaluate the significance. LM (-) = free lateral resection margin; LM (+) = involved or uncheckable lateral resection margin; DM (-) = free deep resection margin; DM (+) = involved or uncheckable deep resection margin.
3. Factors indirectly associated with local recurrence
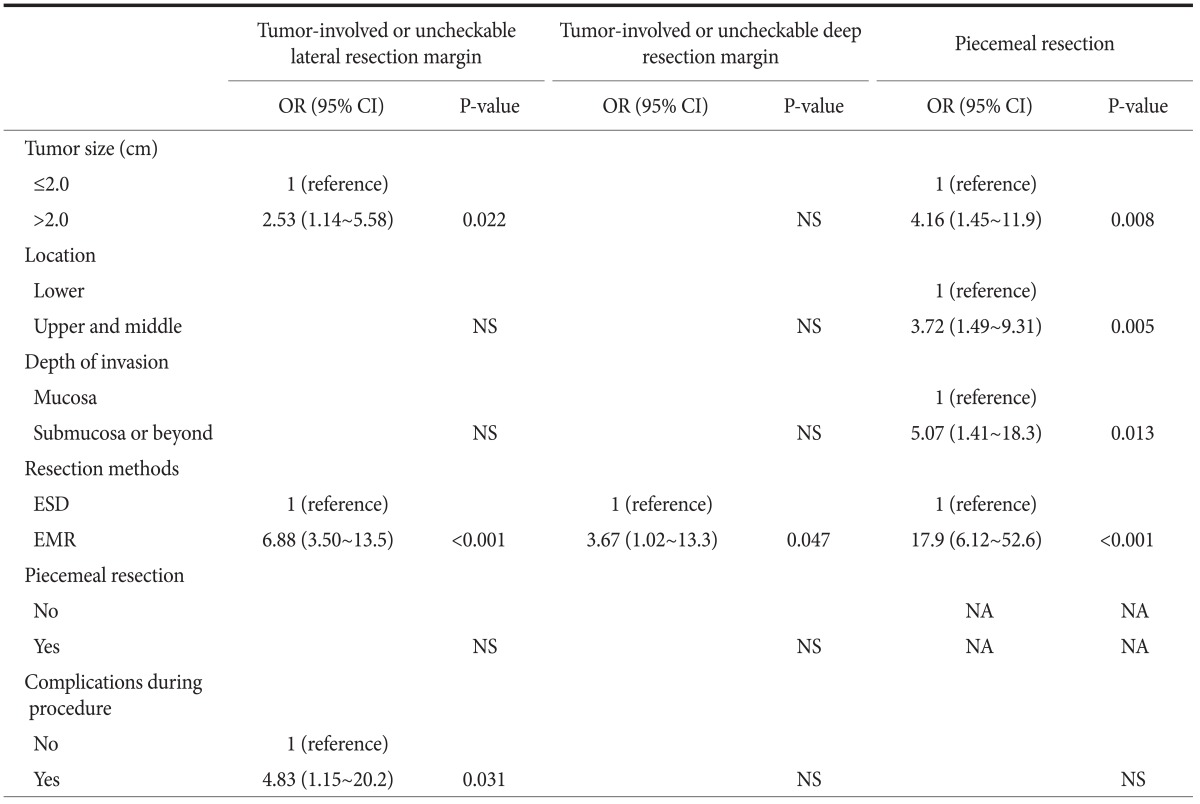
We evaluated the factors associated with the confirmed risk factors for local recurrence (Table 3). Multivariate analyses showed that tumors larger than 2 cm in diameter, the EMR method, and complications during the procedure were significantly associated with an involved or uncheckable lateral resection margin, while EMR was significantly associated with an involved or uncheckable deep resection margin. Tumors larger than 2 cm in diameter, tumors in the upper and middle part of the stomach, invasion into the submucosa, and EMR were all significantly associated with piecemeal resection.
Table 3.
Multivariate analyses of factors for tumor-involved or uncheckable lateral resection margins, tumor-involved or uncheckable deep resection margins, and piecemeal resection
OR = odds ratio; CI = confidence interval; ESD = endoscopic submucosal dissection; EMR = endoscopic mucosal resection; NS = not significant in multivariate logistic-regression models with the use of a forward-selection procedure; NA = not applicable.
4. Endoscopic findings of local recurrence and the risk factors for local recurrence
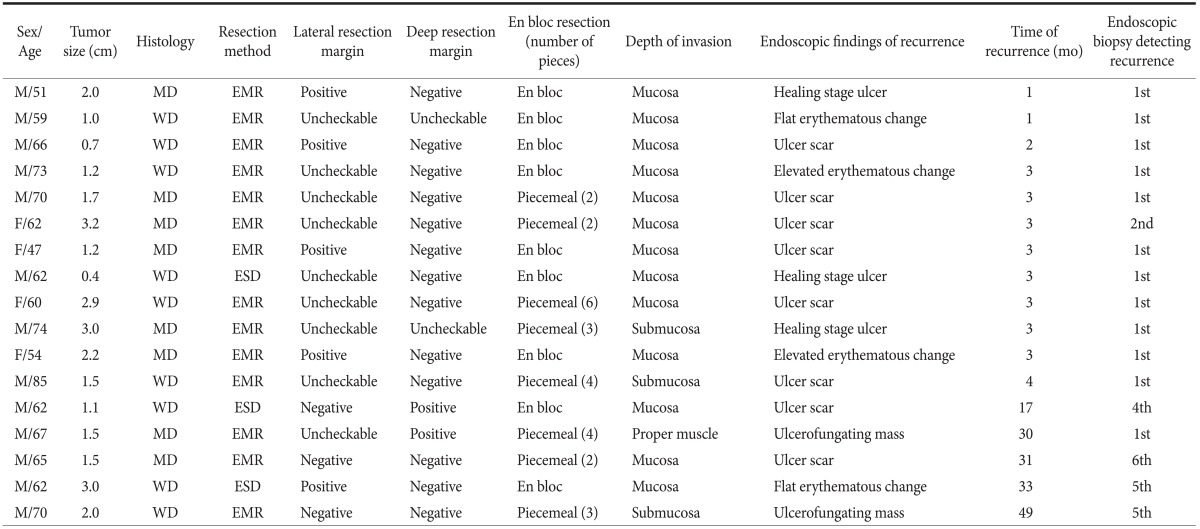
Table 4 shows the endoscopic findings and risk factors for local recurrence present in cases with an episode of local recurrence. All cases with an episode of recurrence had one or more risk factors for local recurrence (involved or uncheckable lateral resection margin, uncheckable or involved deep resection margin, and/or piecemeal resection).
Table 4.
Endoscopic findings and risk factors for local recurrence
M = male; F = female; MD = moderately differentiated adenocarcinoma; WD = well differentiated adenocarcinoma; EMR = endoscopic mucosal resection; ESD = endoscopic submucosal dissection.
Twelve episodes (71%) of local recurrence were found within 4 months after ER. In these cases, local recurrence consisted of endoscopic findings of ulcer scars, healing stage ulcers, or subtle erythematous changes. Recurrences were detected by the first (11 cases) or second (1 case) endoscopic follow-up biopsy at the site of the ulcer scar. Five cases (29%) were detected after 12 months. Recurrence led to endoscopic findings of ulcer scars and/or subtle erythematous changes in three cases, and these episodes of recurrence were detected by the fourth to sixth endoscopic follow-up biopsy. Endoscopic examination showed an ulcerofungating mass in the remaining two cases. One patient had a tumor that was resected in a piecemeal fashion and had an uncheckable lateral resection margin and involved deep resection margin. This patient refused additional surgery, and was lost to follow-up immediately after ER and was found to have an ulcerofungating mass upon returning 30 months later. The other patient had a tumor with lymphovascular invasion resected in a piecemeal fashion but refused additional surgery. This patient was followed for 12 months without any evidence of local recurrence (four consecutive endoscopic biopsies at the ulcer scar only showed chronic gastritis). The patient was then lost to follow-up for 37 months and upon returning, an ulcerofungating mass at the ER site was found via endoscopy.
Discussion
In this study, we evaluated risk factors for local recurrence of EGC at the ER site to help suggest an adequate follow-up biopsy strategy. Our data showed that involved or uncheckable lateral resection margin, uncheckable/involved deep resection margin, and piecemeal resection were significantly associated with local recurrence of EGC after ER. Since local recurrence due to residual tumor was not found without any of these risk factors, routine follow-up biopsies may not be necessary for cases in which the tumor is completely resected in an en bloc pattern.
Local recurrence is a main problem during follow-up after ER for EGC. Several studies showed that incomplete resection was most significantly associated with local recurrence.(5,9-12) The definition of incomplete resection, however, varies among studies and often includes several clinicopathological parameters of EGC and outcomes of ER, including lateral and deep resection margins, en bloc resection, depth of invasion, and differentiation.(5,9-12,21) Thus, we considered each of these parameters as individual risk factors rather than collectively considering them as complete or incomplete resection. In many studies, the cancer detected in follow-up endoscopy was regarded as residual cancer or local recurrence according to the timing of detection.(13,22,23) However, both residual and recurrent cancers are macro- or microscopically remaining cancer cells from original cancer tissue after ER. Moreover, small residual cancer at the resection site would have resulted in local recurrence long after ER (>12 months), if it had not been detected properly at earlier follow-up biopsies. Thus, we regarded all the cancers detected in the follow-up endoscopy after ER as local recurrence instead of dividing into residual cancer and local recurrence.
In our study, piecemeal resection was associated with local recurrence. A study including only EMR-P cases reported that local recurrence rate for lesions resected en bloc (4.1%) was significantly lower than that for lesions resected in multiple fragments (17%).(5) Moreover, when a lesion was resected in more than three pieces, the recurrence rate rose to above 20%.(24) These results support our data showing that piecemeal resection was a significant risk factor for local recurrence. In contrast, in studies including only ESD cases, local recurrence was strongly associated with incomplete resection but not with piecemeal resection.(10,11) However, since en bloc resection rates were much higher in patients who underwent ESD(2,3,8,25) than in patients who underwent EMR, this lack of significant association between local recurrence and piecemeal resection may be due to the studies not having a sufficient number of piecemeal resection cases.
Previous reports that evaluated factors in the local recurrence of EGC usually included either EMR(5) or ESD cases.(9-12) However, we included both EMR and ESD cases in analyses and regarded resection method as a parameter when analyzing predictive factors for local recurrence. Multivariate analysis showed that the resection method itself was not associated with local recurrence despite showing significance in univariate analysis. EMR, including EMR-C and EMR-P, was significantly associated with involved or uncheckable lateral resection margins, involved or uncheckable deep resection margins, and piecemeal resection, all of which were independent risk factors for local recurrence. Thus, the parameter of ER method lost significance in multivariate analysis.
In our study, no local recurrence occurred in cases without any of the identified risk factors. Several studies reported that local recurrence did not occur in lesions that underwent a complete, en bloc resection.(9-11) However, other studies reported that there were still episodes of local recurrence after complete resection.(5,12,21,26) Pathologists cannot always accurately evaluate margin status after ER because of burn injury and mechanical damage. It is possible that in studies reporting local recurrence after complete resection, an incompletely resected tumor was considered completely resected due to misinterpretation of the margin of the lesion.(12,26) Moreover, minute pathological findings such as lymphatic invasion can be overlooked.(27) Thus, careful histological evaluation is important in assessing curability and risk of local recurrence.
In many studies, routine follow-up endoscopies were performed several times during the first year and annually thereafter, and routine biopsy samples were obtained from the scar during each endoscopy to examine for local recurrence irrespective of curability. (4,7,9,11,13) Our data suggest that scheduling follow-up endoscopic examinations and biopsies should be individualized based on risk factors for local recurrence. Routine biopsies at the ulcer scar for surveillance for local recurrence may not be necessary after a complete, en bloc resection that lacked margin involvement due to low possibility of local recurrence. Further, this low possibility of recurrence may not justify the time and cost of performing multiple biopsies. Patients without any risk factors for local recurrence may require an annual endoscopy only for surveillance for metachronous gastric cancer.(28)
Endoscopic findings of local recurrence in our study were mostly just ulcer scars, healing stage ulcers, and subtle erythematous changes. Only two cases (12%), with 30- and 37-month losses to follow-up, showed an ulcerofungating mass on endoscopic examination. A study reported that the macroscopic types of recurrent gastric cancer after ER were EGC type I (1/15, 7%), IIa (9/15, 60%), and IIc (5/15, 33%).(29) However, in another report, the gross morphology of local recurrent gastric cancer was described as an ulcer scar in half of the cases (30/64, 47%).(30) Considering the latter report and our data, a significant portion of local recurrence may be overlooked if biopsy specimens are not obtained from the ER ulcer scar. Therefore, in cases with risk factors for local recurrence, routine biopsies should be obtained for histological evaluation at the ER scar even when the ER scar shows no abnormal features.
In our study, most episodes of local recurrence (12/17, 71%) were found within 1 year after ER. Previous studies also reported that most episodes of local recurrence were detected during the first year after ER.(10,11,26) Thus, patients with one or more risk factors for local recurrence might require frequent follow-up endoscopies and routine biopsies at the ER ulcer site for surveillance for local recurrence during the first year after ER. However, some studies reported episodes of local recurrence that occur after the first year post-ER.(9,21,31) In our study, some episodes of local recurrences were detected after 1 year (range: 17~49 months), and a recurrence among them was detected at the 6th follow-up biopsy. Thus, surveillance endoscopies along with routine biopsies at the ER scar in patients with risk factors for local recurrence should be continued past 1 year post-ER.
Our study has several limitations. This study was a retrospective, single-center analysis and had a limited number of cases with local recurrence of EGC. Thus, a prospective multicenter study of a large number of ER cases is necessary to confirm our results. Moreover, many patients underwent further surgical treatment because submucosal invasion and lateral margin involvement was found during the pathological evaluation of resected specimens. Exclusion of these patients from the analysis could have led to significant underestimation of the risk of local recurrence. In this study, deep resection margin was considered "free" if the tumor-free deep margin was at least 0.1 mm. However, it is somewhat dangerous to define the tumor-free deep margin as ≥0.1 mm considering the effects of mechanical damage during ER. Japanese concept of histological classification (differentiated vs. undifferentiated) was used in this study. However, care should be taken to interpret our results because this concept of histological classification is different from that of World Health Organization classification of gastric cancer.(18)
In summary, our study showed that local recurrence of gastric cancer after ER was not found in cases that lacked involvement of resection margins and in which piecemeal resection of the tumor was not performed. Thus, the schedule for follow-up endoscopies and biopsies should be individualized according to risk factors for local recurrence. If a lesion was resected en bloc with tumor-free margins, routine biopsies at the ER scar might not be necessary. This approach may reduce medical cost and patient anxiety that accompanies avoidable routine follow-up biopsies. However, surveillance endoscopy is still needed in these patients due to the risk of metachronous developments of gastric cancer.
Acknowledgments
This work was supported by a grant from the National Cancer Center, Korea (1210230).
References
- 1.Ono H, Kondo H, Gotoda T, Shirao K, Yamaguchi H, Saito D, et al. Endoscopic mucosal resection for treatment of early gastric cancer. Gut. 2001;48:225–229. doi: 10.1136/gut.48.2.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Oda I, Saito D, Tada M, Iishi H, Tanabe S, Oyama T, et al. A multicenter retrospective study of endoscopic resection for early gastric cancer. Gastric Cancer. 2006;9:262–270. doi: 10.1007/s10120-006-0389-0. [DOI] [PubMed] [Google Scholar]
- 3.Oka S, Tanaka S, Kaneko I, Mouri R, Hirata M, Kawamura T, et al. Advantage of endoscopic submucosal dissection compared with EMR for early gastric cancer. Gastrointest Endosc. 2006;64:877–883. doi: 10.1016/j.gie.2006.03.932. [DOI] [PubMed] [Google Scholar]
- 4.Tanabe S, Koizumi W, Mitomi H, Nakai H, Murakami S, Nagaba S, et al. Clinical outcome of endoscopic aspiration mucosectomy for early stage gastric cancer. Gastrointest Endosc. 2002;56:708–713. doi: 10.1067/mge.2002.129085. [DOI] [PubMed] [Google Scholar]
- 5.Miyamoto S, Muto M, Hamamoto Y, Boku N, Ohtsu A, Baba S, et al. A new technique for endoscopic mucosal resection with an insulated-tip electrosurgical knife improves the completeness of resection of intramucosal gastric neoplasms. Gastrointest Endosc. 2002;55:576–581. doi: 10.1067/mge.2002.122579. [DOI] [PubMed] [Google Scholar]
- 6.Manner H, Rabenstein T, May A, Pech O, Gossner L, Werk D, et al. Long-term results of endoscopic resection in early gastric cancer: the Western experience. Am J Gastroenterol. 2009;104:566–573. doi: 10.1038/ajg.2008.151. [DOI] [PubMed] [Google Scholar]
- 7.Noda M, Kodama T, Atsumi M, Nakajima M, Sawai N, Kashima K, et al. Possibilities and limitations of endoscopic resection for early gastric cancer. Endoscopy. 1997;29:361–365. doi: 10.1055/s-2007-1004216. [DOI] [PubMed] [Google Scholar]
- 8.Watanabe K, Ogata S, Kawazoe S, Watanabe K, Koyama T, Kajiwara T, et al. Clinical outcomes of EMR for gastric tumors: historical pilot evaluation between endoscopic submucosal dissection and conventional mucosal resection. Gastrointest Endosc. 2006;63:776–782. doi: 10.1016/j.gie.2005.08.049. [DOI] [PubMed] [Google Scholar]
- 9.Isomoto H, Shikuwa S, Yamaguchi N, Fukuda E, Ikeda K, Nishiyama H, et al. Endoscopic submucosal dissection for early gastric cancer: a large-scale feasibility study. Gut. 2009;58:331–336. doi: 10.1136/gut.2008.165381. [DOI] [PubMed] [Google Scholar]
- 10.Goto O, Fujishiro M, Kodashima S, Ono S, Omata M. Outcomes of endoscopic submucosal dissection for early gastric cancer with special reference to validation for curability criteria. Endoscopy. 2009;41:118–122. doi: 10.1055/s-0028-1119452. [DOI] [PubMed] [Google Scholar]
- 11.Takenaka R, Kawahara Y, Okada H, Hori K, Inoue M, Kawano S, et al. Risk factors associated with local recurrence of early gastric cancers after endoscopic submucosal dissection. Gastrointest Endosc. 2008;68:887–894. doi: 10.1016/j.gie.2008.03.1089. [DOI] [PubMed] [Google Scholar]
- 12.Jang JS, Choi SR, Qureshi W, Kim MC, Kim SJ, Jeung JS, et al. Long-term outcomes of endoscopic submucosal dissection in gastric neoplastic lesions at a single institution in South Korea. Scand J Gastroenterol. 2009;44:1315–1322. doi: 10.3109/00365520903254304. [DOI] [PubMed] [Google Scholar]
- 13.Miyata M, Yokoyama Y, Okoyama N, Joh T, Seno K, Sasaki M, et al. What are the appropriate indications for endoscopic mucosal resection for early gastric cancer? Analysis of 256 endoscopically resected lesions. Endoscopy. 2000;32:773–778. doi: 10.1055/s-2000-7712. [DOI] [PubMed] [Google Scholar]
- 14.Tada M, Inoue H, Yabata E, Okabe S, Endo M. Colonic mucosal resection using a transparent cap-fitted endoscope. Gastrointest Endosc. 1996;44:63–65. doi: 10.1016/s0016-5107(96)70231-6. [DOI] [PubMed] [Google Scholar]
- 15.Choi IJ, Kim CG, Chang HJ, Kim SG, Kook MC, Bae JM. The learning curve for EMR with circumferential mucosal incision in treating intramucosal gastric neoplasm. Gastrointest Endosc. 2005;62:860–865. doi: 10.1016/j.gie.2005.04.033. [DOI] [PubMed] [Google Scholar]
- 16.Lee JY, Choi IJ, Cho SJ, Kim CG, Kook MC, Lee JH, et al. Endoscopic submucosal dissection for metachronous tumor in the remnant stomach after distal gastrectomy. Surg Endosc. 2010;24:1360–1366. doi: 10.1007/s00464-009-0779-6. [DOI] [PubMed] [Google Scholar]
- 17.Japanese Gastric Cancer Association. Japanese Classification of Gastric Carcinoma - 2nd English Edition - Gastric Cancer. 1998;1:10–24. doi: 10.1007/s101209800016. [DOI] [PubMed] [Google Scholar]
- 18.Hamilton SR, Aaltonen LA, editors. World Health Organization classification of tumours. World Health Organization classification of tumours. Pathology and genetics of tumours of the digestive system. Lyon: IARC Press; 2000. [Google Scholar]
- 19.Schlemper RJ, Riddell RH, Kato Y, Borchard F, Cooper HS, Dawsey SM, et al. The Vienna classification of gastrointestinal epithelial neoplasia. Gut. 2000;47:251–255. doi: 10.1136/gut.47.2.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Soetikno R, Kaltenbach T, Yeh R, Gotoda T. Endoscopic mucosal resection for early cancers of the upper gastrointestinal tract. J Clin Oncol. 2005;23:4490–4498. doi: 10.1200/JCO.2005.19.935. [DOI] [PubMed] [Google Scholar]
- 21.Kim JJ, Lee JH, Jung HY, Lee GH, Cho JY, Ryu CB, et al. EMR for early gastric cancer in Korea: a multicenter retrospective study. Gastrointest Endosc. 2007;66:693–700. doi: 10.1016/j.gie.2007.04.013. [DOI] [PubMed] [Google Scholar]
- 22.Dinis-Ribeiro M, Pimentel-Nunes P, Afonso M, Costa N, Lopes C, Moreira-Dias L. A European case series of endoscopic submucosal dissection for gastric superficial lesions. Gastrointest Endosc. 2009;69:350–355. doi: 10.1016/j.gie.2008.08.035. [DOI] [PubMed] [Google Scholar]
- 23.Lee H, Yun WK, Min BH, Lee JH, Rhee PL, Kim KM, et al. A feasibility study on the expanded indication for endoscopic submucosal dissection of early gastric cancer. Surg Endosc. 2011;25:1985–1993. doi: 10.1007/s00464-010-1499-7. [DOI] [PubMed] [Google Scholar]
- 24.Ono H. Early gastric cancer: diagnosis, pathology, treatment techniques and treatment outcomes. Eur J Gastroenterol Hepatol. 2006;18:863–866. doi: 10.1097/00042737-200608000-00009. [DOI] [PubMed] [Google Scholar]
- 25.Nakamoto S, Sakai Y, Kasanuki J, Kondo F, Ooka Y, Kato K, et al. Indications for the use of endoscopic mucosal resection for early gastric cancer in Japan: a comparative study with endoscopic submucosal dissection. Endoscopy. 2009;41:746–750. doi: 10.1055/s-0029-1215010. [DOI] [PubMed] [Google Scholar]
- 26.Park JC, Lee SK, Seo JH, Kim YJ, Chung H, Shin SK, et al. Predictive factors for local recurrence after endoscopic resection for early gastric cancer: long-term clinical outcome in a single-center experience. Surg Endosc. 2010;24:2842–2849. doi: 10.1007/s00464-010-1060-8. [DOI] [PubMed] [Google Scholar]
- 27.Uedo N, Iishi H, Tatsuta M, Ishihara R, Higashino K, Takeuchi Y, et al. Longterm outcomes after endoscopic mucosal resection for early gastric cancer. Gastric Cancer. 2006;9:88–92. doi: 10.1007/s10120-005-0357-0. [DOI] [PubMed] [Google Scholar]
- 28.Nakajima T, Oda I, Gotoda T, Hamanaka H, Eguchi T, Yokoi C, et al. Metachronous gastric cancers after endoscopic resection: how effective is annual endoscopic surveillance. Gastric Cancer. 2006;9:93–98. doi: 10.1007/s10120-006-0372-9. [DOI] [PubMed] [Google Scholar]
- 29.Oka S, Tanaka S, Kaneko I, Mouri R, Hirata M, Kanao H, et al. Endoscopic submucosal dissection for residual/local recurrence of early gastric cancer after endoscopic mucosal resection. Endoscopy. 2006;38:996–1000. doi: 10.1055/s-2006-944780. [DOI] [PubMed] [Google Scholar]
- 30.Yokoi C, Gotoda T, Hamanaka H, Oda I. Endoscopic submucosal dissection allows curative resection of locally recurrent early gastric cancer after prior endoscopic mucosal resection. Gastrointest Endosc. 2006;64:212–218. doi: 10.1016/j.gie.2005.10.038. [DOI] [PubMed] [Google Scholar]
- 31.Sohn YJ, Jang JS, Choi SR, Kwon HC, Jung GJ, Kim MC, et al. Early detection of recurrence after endoscopic treatment for early gastric cancer. Scand J Gastroenterol. 2009;44:1109–1114. doi: 10.1080/00365520903121701. [DOI] [PubMed] [Google Scholar]