Abstract
The purpose of this study was to determine the extent to which hindlimb muscles of mdx mice adapt to a voluntary endurance type of exercise. mdx and C57BL mice engaged in 8 weeks of wheel running or maintained normal cage activities. Beneficial adaptations that occurred in mdx mice included changes in muscle mass, fiber size, and fiber types based on myosin heavy chain (MHC) isoform expression. These adaptations occurred without increases in fiber central nuclei and embryonic MHC expression. An undesirable outcome, however, was that muscle mitochondrial enzyme activities did not improve with exercise in mdx mice as they did in C57BL mice. Cellular remodeling of dystrophic muscle following exercise has not been studied adequately. In this study we found that some, but not all, of the expected adaptations occurred in mdx mouse muscle. We must better understand these (non)adaptations in order to inform individuals with DMD about the benefits of exercise.
Keywords: Duchenne muscular dystrophy, endurance exercise, histochemistry, physical activity, skeletal muscle
Muscle weakness and fatigue are hallmarks of neuromuscular diseases. For example, individuals with neuromuscular diseases have reduced hand, quadriceps, and biceps muscle strength compared with healthy individuals of the same age,1 and boys with the specific neuromuscular disease of Duchenne muscular dystrophy (DMD) have reduced strength of elbow flexors10 and knee flexors and extensors.36 Skeletal muscle weakness is likewise exhibited by the mdx mouse, a mouse that is genetically similar to humans with DMD due to a mutation in the dystrophin gene.27 For example, soleus, extensor digitorum longus (EDL), and tibialis anterior muscles of mdx mice are 20 – 40% weaker than corresponding muscles from wild-type, C57BL mice.34,35,51 Muscle of mdx mice is also more fatigable relative to healthy mouse muscle,25,50 although fatigability in individuals with DMD is not as well substantiated.48
A common intervention for improving muscle weakness and fatigue is prescribed exercise. Endurance exercise promotes vascular and mitochondrial adaptations in skeletal muscle to enable more efficient oxygen delivery and adenosine triphosphate (ATP) production and utilization.17,52 These adaptations are well established in rodent hindlimb muscles following weeks of wheel running as demonstrated by increased capillarity and activities of mitochondrial enzymes involved in oxidative phosphorylation.11,28,56 Shifts in fiber types from the faster, more glycolytic type IIx and IIb fibers to the slower, more oxidative type IIa and I fibers occur in response to endurance exercise.2,44 In rodents, fiber size may also increase following wheel running.2 All of these exercise-induced adaptations are firmly documented in skeletal muscles of healthy rodents and humans, but the extent to which muscles of mdx mice and individuals with DMD adapt to exercise is undefined.
Some previous research has shown that a voluntary, endurance type of exercise can be beneficial to hindlimb muscles of the mdx mouse. Force-generating capacity of soleus muscles from mdx mice following 16 weeks or 1 year of voluntary wheel running was greater than that from muscles of mdx mice that did not run.26,61 Also, EDL muscles from mdx runners were more fatigue-resistant than in non-runners.26,61 Two similar studies showed that 4 weeks or 1 year of voluntary wheel running produced no effects on soleus or EDL muscle function of mdx mice.8,13 It is important to recognize that hindlimb muscle function of mdx mice was not affected detrimentally in any of the studies that utilized voluntary wheel running as the mode of exercise. This is in contrast to non-voluntary treadmill running, which is injurious to muscles of mdx mice,16,43 particularly when mice are forced to run downhill.7 To better understand the potential benefits of a voluntary endurance type of exercise to dystrophic muscle, we measured cellular adaptations that typically occur in response to endurance exercise. We hypothesized that the well-known endurance exercise–induced adaptations to mitochondria, capillaries, and fibers would occur in hindlimb muscles of mdx mice in response to voluntary wheel running.
METHODS
Animals and Study Design
The dystrophic mice used in this study were from our colony at the University of Minnesota. This colony was established from mdx:utrn+/− breeder pairs obtained from Virginia Polytechnic Institute and State University21 that descended from Washington University.19 The genotype of each offspring was determined by polymerase chain reaction (PCR) analysis of DNA isolated from a tail snip as described in detail previously.21 Mice that were dystrophin−/−– utrophin+/+ were the mdx mice used in the study, and age-matched, C57BL/10 mice served as controls. Mice that were dystrophin−/− – utrophin−/− (mdx:utrn−/−) were used in one preliminary study. Both male and female mice were used. All mice were given commercial rodent chow and water ad libitum and were housed on a 12-hour light– dark cycle with the dark cycle starting at 6:00 P.M. All protocols and animal care procedures were approved by the University of Minnesota Institutional Animal Care and Use Committee and complied with guidelines set by the American Physiological Society.
At 4 weeks of age (range 3.25–4.5 weeks), mice were randomly assigned to a sedentary (Sed) or a running (Run) group (C57BL Sed, n = 8; C57BL Run, n = 8; mdx Sed, n = 10; mdx Run, n = 10). Each Run mouse was individually housed in a standard mouse cage that contained running wheels.18 These wheels are considered low-resistance, or free-flying, because inertia was low (1.5 ± 0.4 g; n = 14 wheels).32 In a preliminary study, 8 mdx:utrn−/− mice aged 4 weeks were also housed individually in cages with wheels. Wheel-running distances were acquired in 24-hour intervals for 60 days, except for the mdx:utrn−/− mice, which ran for only 30 days. In addition, the number of 20-second active times were counted. That is, if the wheel rotated during a 20-second interval, then the mouse was considered active during that time. Counts were summed per hour such that the number of 20-second intervals out of a possible 180 was tabulated. These data on bouts of activity provide some indication if changes in total running distances (over time or between C57BL and mdx mice) were a result of differences in running engagement or running speed.
Sedentary mice of the same genotype and same gender were housed up to four per standard mouse cage for 60 days. At about day 50, subsets of mice from each Sed group (n = 6 each) were monitored for 24-hour cage activities using activity chambers (Med Associates, Inc., St. Albans, Vermont).59 The chambers contain three infrared arrays in the x-, y-, and z-axes, with two sets of beams in the x-direction, one being elevated above the other. Ambulation was measured by arrays located in the x- and y-axes, and vertical movement (i.e., jumping and hindlimb rearing) was measured by the second elevated x-array. Infrared sensors in the chamber registered an activity count each time one of the beams was disrupted such that movement was simultaneously measured in all three axes. Prior to measurement of activity, each mouse was familiarized with the activity-monitoring cage by spending 24 hours in a mock chamber. Mock chambers were identical to the real activity chamber but did not contain infrared sensors. Immediately after this familiarization period, mice were weighed and placed into an activity chamber for 24 hours. Physical activity measurements included distance traveled in the chamber (ambulation), rearing and jumping counts, time spent ambulating, time spent jumping, and total active time. Rearing time is not reported because it is not an independent variable; that is, the time spent rearing as reported by the activity monitor can also include time spent jumping and doing stereotypical activities. Therefore, total active time is reported instead. All data were acquired using Activity Monitor software, version 5 (Med Associates) on a PC.
At the end of the 60-day period, each C57BL and mdx mouse was weighed and anesthetized by an intraperitoneal injection of sodium pentobarbital (100 mg/kg body weight) with supplemental doses given as required to keep the mouse sedated and unresponsive during dissections. One soleus muscle and one EDL muscle were dissected and mounted in optimal cutting temperature (OCT) compound for histological analyses. The contralateral soleus and EDL muscles and both tibialis anterior muscles were next dissected, weighed, snap frozen in LN2, and stored at −80°C for subsequent biochemical analyses. Each mouse was euthanized by excision of the heart while still under pentobarbital anesthesia. The average age of the mice at the end of the study was 3.6 ± 0.7 months (mean ± SD).
Histological Assessments
Fourteen serial cross-sections (10 μm thick) from a subset of soleus and EDL muscles were cut on a microtome cryostat and mounted two sections per slide. The serial sections on the first and last slides were stained by hematoxylin–eosin to assess centrally nucleated fibers. Sections on adjacent slides were stained by periodic acid–Schiff reaction to evaluate capillarity.3 Sections on the three intermediary slides were used for determining fiber types based on myosin heavy chain (MHC) immunohistochemistry using MHC I antibody VP-M667 (Vector Laboratories, Burlingame, California) and antibodies to MHC 2A and 2B generated from hybridomas (ATCC, SC-71 and BF-F3, respectively).40,55 This sequential sectioning and staining was to quantify central nuclei and capillarity for each muscle fiber type, specifically types I, IIa, IIx, and IIb. If a fiber did not react with antibodies to MHC I, 2A, or 2B, it was classified as a type IIx fiber. Images were acquired on a microscope (Leica DM2000) with a digital camera (QImaging Micropublisher RTV 5.0). Fiber cross-sectional area was determined by measuring the circumference of each fiber using ImageJ software (National Institutes of Health, Bethesda, Maryland) at 100× magnification. Approximately 170 fibers from four or five soleus and EDL muscles were assessed (ranging from 168 to 172 fibers per soleus muscle and 145 to 203 fibers per EDL muscle). Mean data (fiber cross-sectional areas, number of capillaries around a fiber, and percent of fibers containing central nuclei) for each fiber type and a mean fiber type percentage were calculated for each muscle analyzed. These muscle means were then used for statistical analyses.
Additional 10-μm-thick sections from soleus muscles were stained for embryonic MHC (eMHC) to assess regenerating fibers. Slides were fixed in acetone for 10 minutes at 4°C. Anti-eMHC (Clone F1.652, Hybridoma Bank, Iowa State University, Ames, Iowa) was added to each slide at a 1:20 dilution in phosphate-buffered saline (PBS) and incubated for 90 minutes at 37°C. The mouse iso-IHC diaminobenzidene (DAB) kit (Innogenex; San Ramon, California) was used to visualize the antibody–antigen interactions. Six digital images at 400× magnification were acquired, and a single investigator blinded to the treatments counted the number of eMHC-positive cells per image.15 Data were normalized to area of the muscle examined and are presented as the number of eMHC-positive cells/mm2.
Mitochondrial Enzyme Assays
Mitochondrial enzyme activities of soleus, EDL, and tibialis anterior muscles were measured to determine whether there were differences in oxidative metabolism between Run and Sed mice and/or between genotypes. Each soleus and EDL muscle was homogenized using a glass tissue grinder in 80 μl of 33 mM phosphate buffer (pH 7.0) on ice. Tibialis anterior muscles were homogenized similarly in a volume of 1.0 ml. Cytochrome C oxidase (CCO) activity was determined in triplicate in homogenates by following the rate of oxidation of Cytochrome C at 550 nm and 25°C.46 The remaining homogenates were freezethawed three times, and citrate synthase (CS) activity was determined. This assay was performed in duplicate as described by Srere,57 except that the assays were done at 25°C, and only half as much acetyl-coenzyme A (acetyl-CoA) was used in each assay. The remaining tibialis anterior muscle homogenate was also assayed in triplicate for beta-hydroxy acyl-CoA dehydrogenase (β-HAD) activity.5
Statistical Analyses
Among the Run mice, two-way repeated-measures analyses of variance (ANOVAs) with Holm–Sidak post hoc tests were used to determine if genotype (C57BL vs mdx) affected wheel running over time. For the 24-hour distances, the repeated factor was time, and mean weekly running distances were used in this analysis. For the time-of-day running analysis, the repeated factor was hour. To access the 20-second run intervals, t-tests were used to compare data between mdx and C57BL mice for the three most active hours. Among the sedentary mice, t-tests were used to determine if genotype (C57BL vs. mdx) affected cage activities. Two-way ANOVAs were used to determine if genotype (C57BL vs. mdx) and/or activity level (Sed vs. Run) affected body or muscle masses, histological parameters, and mitochondrial enzyme activities. Chi-square tests were used to analyze the frequency distribution of fiber cross-sectional areas. Values are reported as mean (SE) or least-squares mean (SE) for comparisons among combinations of conditions.
RESULTS
Wheel Running
The mdx mice ran voluntarily on wheels, although they consistently ran ~25% less distance than the C57BL mice (P = 0.008; Fig. 1). The mean daily distances for the 60-day study period were 5.4 ± 0.5 and 7.5 ± 0.5 km per 24 hours for mdx and C57BL Run mice, respectively. There was also a main effect of time as mice gradually decreased their running throughout the 60-day period (P < 0.001; Fig. 1) with the effect not depending on genotype (interaction P = 0.714). Of the 10 mdx and 8 C57BL mice that wheel ran, 2 in each group were female, but running patterns were the same regardless of gender. The 4 mdx:utrn−/− mice voluntarily ran substantially less, particularly after about 2 weeks of running (Fig. 2). These mice were not assessed further.
FIGURE 1.
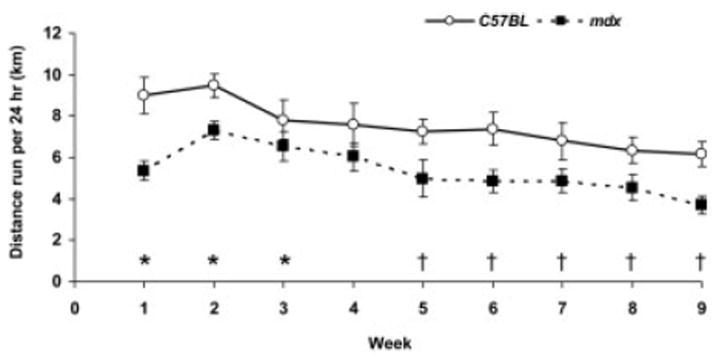
Distances run per 24 hours (mean, SE) shown as weekly averages per group. Post hoc analyses following the main effect of time show: *significant difference vs. week 9; and †significant difference vs. week 2. There was also a main effect of genotype that is not depicted on the graph.
FIGURE 2.
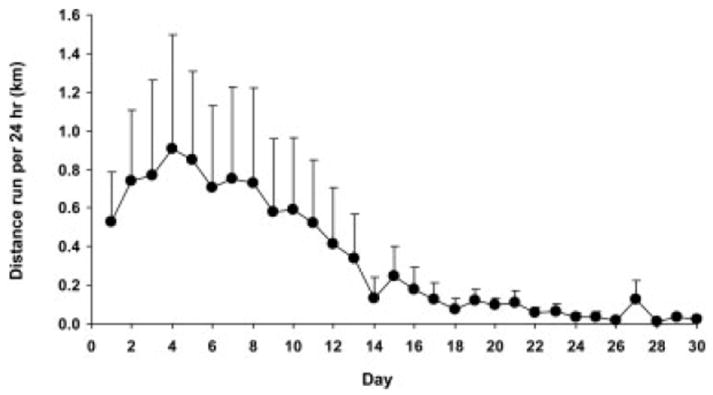
Distances run per 24 hours (mean, SE) by dystrophin/utrophin-deficient (mdx:utrn−/−) mice.
As expected, mice engaged in wheel running primarily during the dark hours, but there were statistically significant time-of-day differences between the groups (interaction, P < 0.001; Fig. 3). The mdx mice ran a greater percentage of their total daily running during the early dark hours, 6:00 –9:00 P.M., and less during the later dark hours, 10:00 P.M. to 4:00 A.M., relative to C57BL mice (P ≤ 0.021; Fig. 3). The most active running hours were the 8:00, 9:00, and 10:00 P.M. hours. The running activity during each of those three hours was analyzed for 20-second activity bouts. From 8:00 to 9:00 P.M., mdx and C57BL mice were wheel active in 121 and 125 of the total possible 180 20-second intervals, respectively (P = 0.189). From 9:00 to 10:00 P.M. and 10:00 to 11:00 P.M., mdx mice engaged in ~15% fewer 20-second intervals than did C57BL mice (117 and 107 vs. 135 and 129 intervals, respectively; P < 0.001). These data indicate that mdx mice ran less than C57BL mice because they got on the wheel less. We also compared the number of hourly 20-second activity bouts between the second and eighth weeks, that is, the peak and nadir of running. The average number of activity bouts was ~25% lower at week 8 compared with week 2 (P = 0.010) for both mdx and C57BL mice. This is an indication that, over the duration of the study, all mice had a drop in running distance due to a diminishing propensity to get on the wheel.
FIGURE 3.
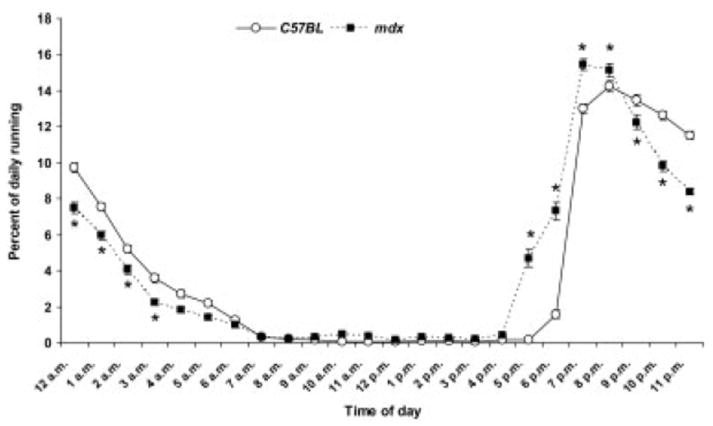
Percent of wheel running during each hour of the day (mean, SE). *Significant difference from C57BL at the corresponding hour.
Cage Activities
All of the physical activities that were monitored in the mice without running wheels (Sed mice) were less for mdx than C57BL mice (P ≤ 0.044; Fig. 4). The mdx mice ambulated about the cage, jumped, and reared ~40% less than the C57BL mice, and the times spent engaged in those activities were also ~40% less.
FIGURE 4.
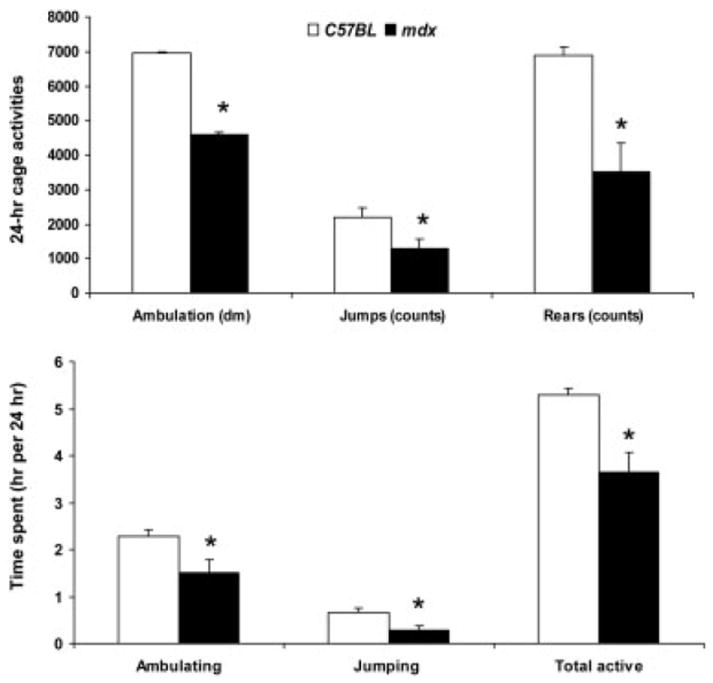
Cage activities (top) and time spent doing those activities (bottom) per 24 hours for mice without wheels (mean, SE). *Significantly different from C57BL.
Body and Muscle Masses
There was a significant interaction between genotype and activity level on body mass. Sixty days of wheel running resulted in a lower body mass for the C57BL Run mice compared with the C57BL Sed mice, but this effect was not observed in the mdx mice (Table 1). This interaction between genotype and activity level was not found for any of the muscle masses measured. The only significant main effect of wheel running activity was on normalized soleus muscle mass (Table 1). Run mice had soleus muscles that weighed 21% more than Sed mice when normalized to body mass. Genotype had a larger impact on muscle mass, as mdx mice had 20 –50% greater soleus, EDL, tibialis anterior, and heart masses compared with C57BL mice, independent of activity (Table 1). Even when normalized for the larger body mass of the mdx mice, soleus and tibialis anterior muscle masses were still ~30% greater in mdx mice than in C57BL mice.
Table 1.
Body, hindlimb muscle, and heart masses from C57BL and mdx mice that were sedentary or voluntarily ran on wheels for 60 days.
| C57BL Sed | C57BL Run | mdx Sed | mdx Run | P for genotype | P for activity | P for interaction | |
|---|---|---|---|---|---|---|---|
| Body (g) | 27.3 (1.2) | 23.8* (1.2) | 28.8 (1.1) | 30.7† (1.0) | – | – | 0.020 |
| Soleus muscle (mg) | 8.20 (0.99) | 9.11 (0.99) | 11.30 (0.93) | 14.20 (0.93) | <0.001 | 0.056 | 0.309 |
| Soleus:body (mg/g) | 0.300 (0.030) | 0.385 (0.030) | 0.394 (0.028) | 0.455 (0.028) | 0.008 | 0.017 | 0.685 |
| EDL muscle (mg) | 9.45 (0.91) | 9.01 (0.69) | 11.61 (0.61) | 11.86 (0.61) | 0.002 | 0.895 | 0.639 |
| EDL:body (mg/g) | 0.347 (0.037) | 0.379 (0.028) | 0.404 (0.025) | 0.386 (0.025) | 0.273 | 0.814 | 0.389 |
| Tibialis anterior muscle (mg) | 44.8 (3.1) | 39.8 (3.1) | 66.5 (2.9) | 65.7 (2.8) | <0.001 | 0.341 | 0.485 |
| Tibialis anterior: body (mg/g) | 1.64 (0.09) | 1.69 (0.09) | 2.29 (0.08) | 2.15 (0.08) | <0.001 | 0.574 | 0.298 |
| Heart (mg) | 118 (5.5) | 117 (5.5) | 134 (5.2) | 147 (5.2) | <0.001 | 0.259 | 0.215 |
| Heart:body (mg/g) | 4.34 (0.21) | 4.96 (0.21) | 4.70 (0.20) | 4.73 (0.20) | 0.771 | 0.122 | 0.161 |
Values expressed as mean (SE). Sed, sedentary; EDL, extensor digitorum longus muscle.
Significantly different vs. C57BL Sed mice.
Significantly different vs. C57BL Run mice.
Fiber Types
Soleus muscles from mdx and C57BL mice contained the same percentage of type I and IIa fibers (P ≥ 0.063), but mdx soleus muscles contained a greater percentage of IIx fibers than those of C57BL mice (P = 0.020; Fig. 5a). Soleus muscles from mdx mice also contained more fibers that co-expressed I and IIa MHC compared with those from C57BL mice (4.6 ± 0.6% vs. 1.1 ± 0.6%; P = 0.002). There were no effects of activity level on the percentages of the three fiber types or on fibers that expressed more than one MHC isoform in soleus muscles (P ≥ 0.071).
FIGURE 5.
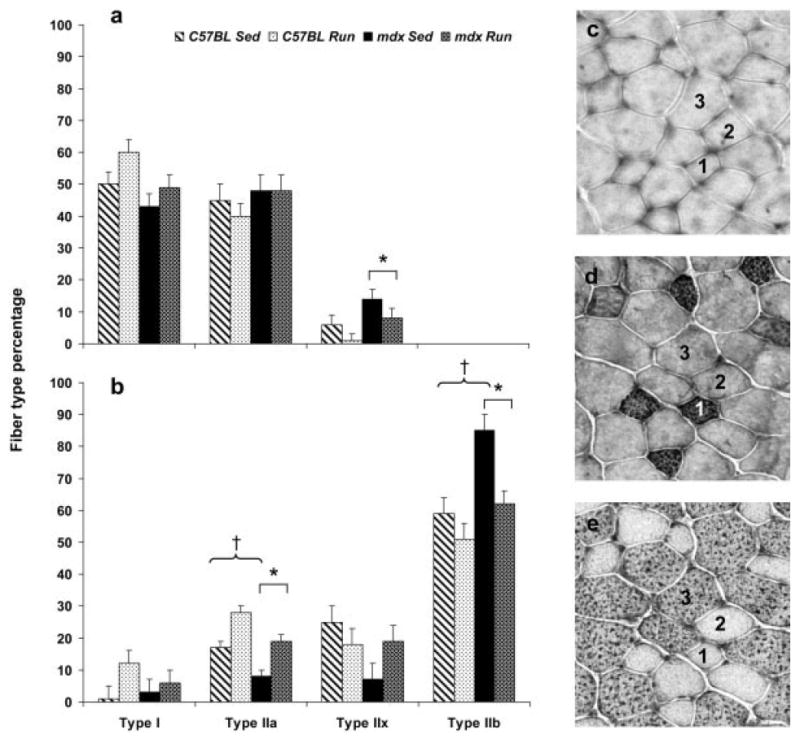
Percent of fibers that expressed type I, IIa, IIx, and IIb myosin heavy chain (MHC) in (a) soleus and (b) EDL muscles (mean, SE). *Main effect of genotype, that is, mdx is significantly different than C57BL regardless of activity; †main effect of activity, that is, Sed significantly different from Run regardless of genotype. (c) Representative cross-section of EDL muscle from a C57BL Run mouse stained with the MHC I antibody. (d) Serial section of the same EDL muscle stained with the MHC IIA antibody. (e) Serial section of the same EDL muscle stained with the MHC IIB antibody. Fiber 1 was classified as a type IIa fiber. Fiber 2 was classified as a type IIx fiber because it did not react with MHC I, IIA, or IIB antibodies. Fiber 3 was classified as a type IIb fiber.
In EDL muscles, there were no differences in the percentages of type I or IIx fibers between mdx and C57BL mice (P ≥ 0.116), but mdx EDL muscles contained fewer type IIa fibers and more type IIb fibers relative to C57BL EDL muscles (P = 0.002; Fig. 5b). The percentage of fibers in EDL muscles that coexpressed I and IIa MHC and fibers that coexpressed IIa and IIb MHC were <2% of all fibers and were not different between mdx and C57BL mice (P ≥ 0.092). Significant activity effects on fiber type percentages of EDL muscles were reflected in more IIa and fewer IIb fibers in EDL muscles from Run mice than from Sed mice (P ≤ 0.006; Fig. 5b). EDL muscles from mice that ran also exhibited more fibers that coexpressed types I and IIa MHC compared with Sed mice (1.9 ± 0.4% vs. 0.3 ± 0.4%; P = 0.025). There were no significant interactive effects of genotype with activity for any of these comparisons among soleus or EDL muscles (P ≥ 0.103).
Fiber Cross-Sectional Areas
The mean cross-sectional area of all fibers was 24% greater in soleus muscles from mdx mice relative to C57BL mice (1979 ± 104 μm2 vs. 1591 ± 99 μm2; P = 0.018). This is illustrated by the large distribution shift to the right for the mdx compared with the C57BL soleus muscle fiber cross-sectional areas (Fig. 6, top). The overall effect of genotype on fiber cross-sectional area was due to larger IIa fibers in the mdx soleus muscle relative to C57BL (1977 ± 89 μm2 vs. 1434 ± 85 μm2; P < 0.001), because type I fiber cross-sectional areas were not different between mdx and C57BL mice (1738 ± 128 μm2 vs. 1724 ± 121 μm2; P = 0.939). The greatest effect of wheel running was on large fibers, which we defined as those having a cross-sectional area of >3000 μm2. Chi-square tests revealed that running by C57BL mice resulted in more large fibers, whereas running by mdx mice resulted in fewer large fibers compared with respective Sed mice (P < 0.001; Fig. 6, top). Taken as a whole, the effect of running by the mdx mice was a leftward distribution shift in soleus muscle fiber cross-sectional areas back toward the C57BL profile.
FIGURE 6.
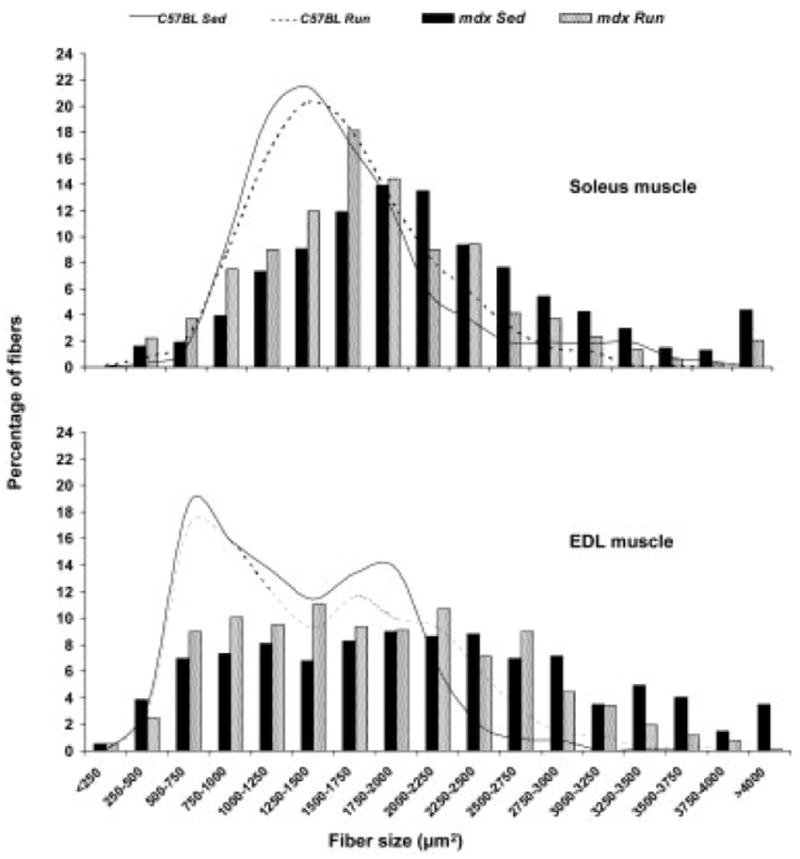
Fiber size frequency distributions of soleus (top) and EDL (bottom) muscles based on fiber cross-sectional areas. Note the overall rightward distribution shift of the mdx muscle fibers relative to the C57BL muscle fibers. In comparing mice that did not run (Sed) to those that did run (Run), among the C57BL mice there was a slight rightward shift, with running reflecting a modest overall fiber hypertrophy particularly in the EDL muscle. Among the mdx mice, the most notable finding was that the abnormally high percentages of large fibers (fibers >3000 μm2) decreased with running in both the soleus and EDL muscle and skewed the fiber size distribution back toward that of C57BL mice.
Fiber cross-sectional areas for EDL muscles were effected similarly by genotype and activity, except that the leftward distribution shift as a result of running by the mdx mice was even more pronounced than in soleus muscles (Fig. 6, bottom). Nearly 18% of the EDL muscle fibers from mdx Sed mice had cross-sectional areas >3000 μm2, whereas <8% of the fibers from mdx Run mice were that large (P < 0.001; Fig. 6, bottom). Irrespective of activity level, EDL muscle fibers from mdx mice had cross-sectional areas that were ~40% greater than those from C57BL mice (1892 ± 75 μm2 vs. 1349 ± 75 μm2; P < 0.001). This genotype effect was due to the most abundant type of fiber in the EDL muscle being larger in the mdx mice—that is, the type IIb fibers (P < 0.001). Type I, IIa, and IIx fibers had cross-sectional areas that were the same in EDL muscles in mdx and C57BL mice (P ≥ 0.158).
Muscle Fiber Capillarity
The number of capillaries in contact with each type I, IIa, and IIx fiber in soleus muscles was not affected by genotype or activity (P ≥ 0.133). The overall capillarity for C57BL and mdx soleus muscles was 4.37 ± 0.09 and 4.51 ± 0.10 capillaries per fiber (P = 0.331). The number of capillaries in contact with each type IIa, IIx, and IIb fiber in EDL muscles was not affected by genotype or activity either (P ≥ 0.117). The overall capillarity for C57BL and mdx EDL muscles was 3.07 ± 0.19 and 3.06 ± 0.19 capillaries per fiber (P = 0.107).
Because genotype affected fiber cross-sectional areas, particularly for fiber types that are highly abundant in each muscle, we tabulated the number of capillaries in contact with a fiber normalized by that fiber’s cross-sectional area. The mdx mice had significantly fewer capillaries per cross-sectional area of type IIa and IIx fibers from soleus muscles and type IIx and IIb fibers from EDL muscles compared with those from C57BL mice (P ≤ 0.036; Fig. 7). There were no effects of mouse activity on capillarity normalized to fiber size (P ≥ 0.271).
FIGURE 7.
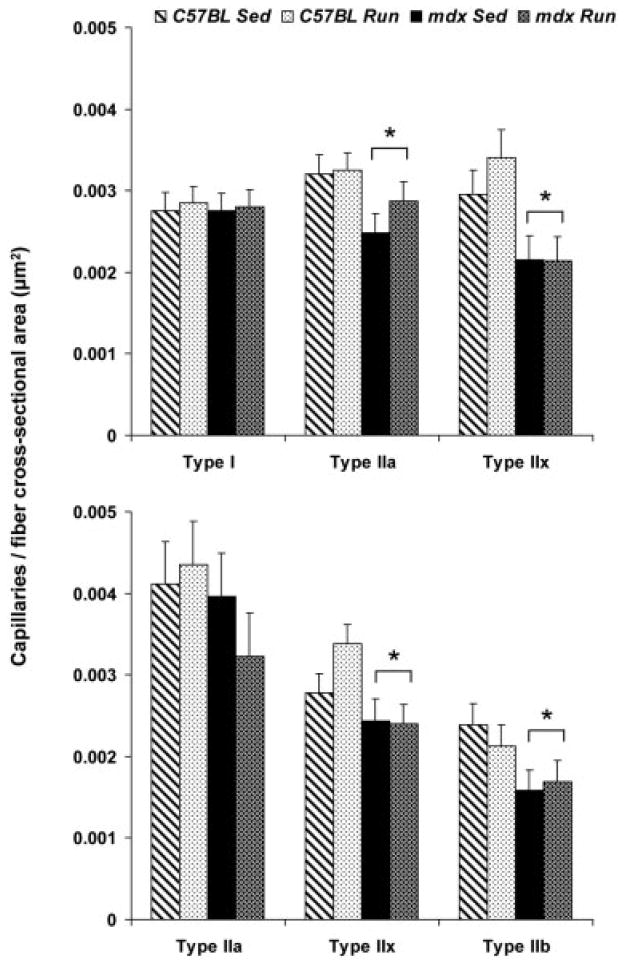
Number of capillaries in contact with type I, IIa, IIx, and IIb fibers normalized by each fiber’s cross-sectional area in soleus (top) and EDL (bottom) muscles (mean, SE). *Main effect of genotype, that is, mdx significantly different than C57BL regardless of activity. Capillarity was not evaluated in type I fibers of EDL muscles because there were so few of these fibers.
Fibers with Central Nuclei and Expressing eMHC
The majority of fibers in soleus and EDL muscles from mdx mice displayed central nuclei (70.0 ± 4.8% and 81.5 ± 2.2%, respectively), whereas fibers in those muscles from C57BL mice had substantially fewer central nuclei (9.0 ± 4.6% and 0.8 ± 2.3%, respectively). This genotype effect was observed for fiber types I, IIa, IIx, and IIb in soleus and EDL muscles (P ≤ 0.002; Fig. 8) and was independent of activity level (P ≥ 0.176). There were no statistically significant effects of wheel running on the appearance of central nuclei in either muscle, for any fiber type (P ≥ 0.150; Fig. 8).
FIGURE 8.
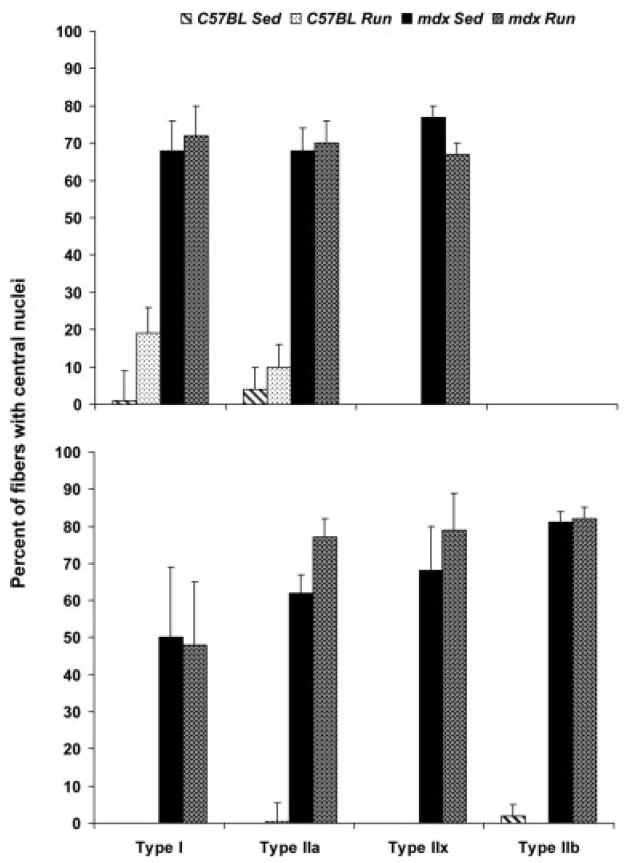
Percent of type I, IIa, IIx, and IIb fibers with central nuclei in soleus (top) and EDL (bottom) muscles (mean, SE). For each comparison there was a main effect of genotype but none of activity.
The expression eMHC by fibers of soleus muscles was affected by genotype (P < 0.001) but not by activity (P = 0.958; Fig. 9). Soleus muscles from mdx mice had ~60 fibers that were positive for eMHC per square millimeter of muscle, whereas those from C57BL mice had only ~6 fibers.
FIGURE 9.
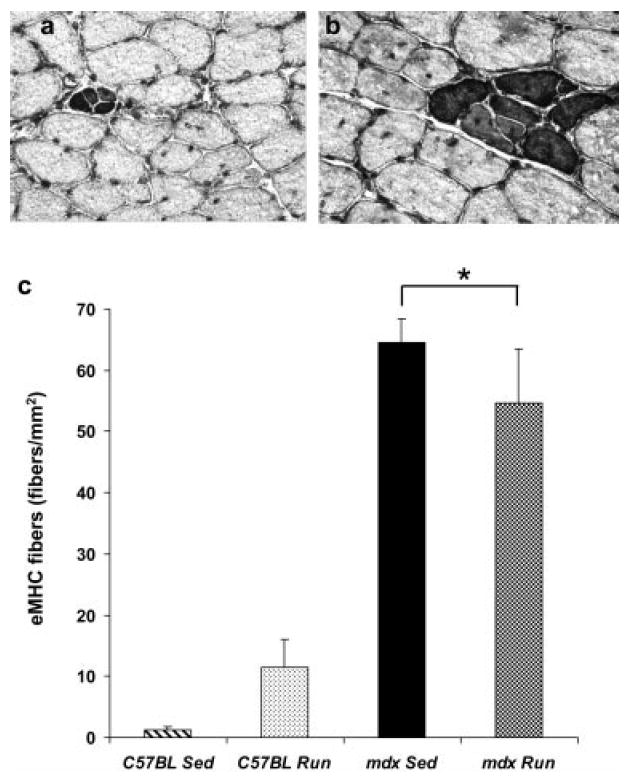
Fibers expressing embryonic myosin heavy chain (eMHC) in soleus muscle from the (a) C57BL Run mouse and (b) mdx Run mouse. (c) Number of eMHC-positive fibers per mm2 of soleus muscle (mean, SE). *Main effect of genotype, that is, mdx is significantly different than C57BL regardless of activity.
Mitochondrial Enzyme Activities
Soleus muscle oxidative capacity, as indicated by CCO and CS enzyme activities, was not affected by wheel running but was affected by genotype, with mdx soleus muscles having ~60% lower CCO activity (Table 2). CCO activity of EDL muscle increased with wheel running among the C57BL mice, but the adaptive response did not occur among mdx mice. CS activity of the EDL muscle was greater with wheel running, independent of genotype (Table 2). For the tibialis anterior muscle, CCO activity was less for mdx than C57BL mice, independent of activity level. CS and β-HAD enzyme activities in tibialis anterior muscles increased with wheel running among C57BL mice but the adaptive response did not occur among mdx mice. The result was that lower enzyme activities were found in tibialis anterior muscles from mdx Run mice than from C57BL Run mice (Table 2).
Table 2.
Mitochondrial enzymes activities of hindlimb muscles from C57BL and mdx mice that were sedentary and voluntarily ran on wheels for 60 days.
| C57BL Sed | C57BL Run | mdx Sed | mdx Run | P for genotype | P for activity | P for interaction | |
|---|---|---|---|---|---|---|---|
| Soleus CCO (μmol/min/g) | 22.2 (3.1) | 24.2 (3.5) | 14.7 (3.0) | 13.7 (3.3) | 0.008 | 0.869 | 0.646 |
| Soleus CS (μmol/min/g) | 55.2 (5.4) | 59.4 (5.7) | 49.7 (4.9) | 51.0 (5.4) | 0.202 | 0.611 | 0.797 |
| EDL CCO (μmol/min/g) | 15.1 (2.4) | 25.5* (2.5) | 15.2 (2.0) | 12.7† (2.2) | – | – | 0.009 |
| EDL CS (μmol/min/g) | 33.1 (3.9) | 39.0 (3.9) | 31.8 (3.1) | 42.7 (3.4) | 0.741 | 0.028 | 0.485 |
| Tibialis anterior CCO (μmol/min/g) | 44.6 (2.4) | 45.6 (2.7) | 38.1 (2.3) | 34.4 (2.4) | <0.001 | 0.581 | 0.353 |
| Tibialis anterior CS (μmol/min/g) | 43.7 (2.9) | 56.9* (3.2) | 38.4 (2.7) | 37.3† (2.9) | – | – | 0.019 |
| Tibialis anterior β-HAD(μmol/min/g) | 40.0 (2.5) | 54.4* (2.8) | 36.2 (2.4) | 40.2† (2.5) | – | – | 0.049 |
Values expressed as mean (SE) and activities expressed per gram of wet muscle weight. Sed, sedentary; CCO, Cytochrome C oxidase; CS, citrate synthase; β-HAD, beta-hydroxy acyl-CoA dehydrogenase; EDL, extensor digitorum longus muscle.
Significantly different vs. C57BL Sed mice.
Significantly different vs. C57BL Run mice.
DISCUSSION
The main finding of this study is that some, but not all, of the expected beneficial adaptations that typically occur in skeletal muscle as a result of endurance exercise training were found in muscles of mdx mice in response to voluntary wheel running (Table 3). This is a critical step in determining the efficacy of exercise for individuals with DMD. That is, even if appropriate exercise parameters (frequency, intensity, duration, and mode) that do not worsen the dystrophic condition are determined, the benefits of exercise to skeletal muscle will only be realized if favorable adaptations of that tissue can occur. It is encouraging that muscle lacking dystrophin can remodel in response to exercise, but equally as important are our results showing that chronic voluntary exercise by mdx mice did not worsen the pathology of the skeletal muscles investigated. Our findings that exercise was not deleterious to dystrophic muscle are in agreement with previous studies that used similar exercise protocols such as voluntary, low-resistance wheel running for 4 –52 weeks, with mdx mice aged 3– 4 weeks at the initiation of exercise,8,13,26,61 as well as 15 weeks of swimming exercise,25 and 8 weeks of low-intensity treadmill running.30 It is of interest that, in this latter report, a controlled, relatively slow speed of running on a treadmill induced beneficial long-term adaptations to mdx hindlimb muscles in contrast to other studies that used shorter-term, high-intensity treadmill running to induce injury to mdx mouse muscle (e.g., see refs. 16 and 43).
Table 3.
Summary of the muscle (mal)adaptations in response to 60 days of wheel running by C57BL and mdx mice.
| C57BL mice | mdx mice | mdx outcome | |
|---|---|---|---|
| Muscle mass (soleus/EDL and tibialis anterior) | ↑/↔ | ↑/↔ | Desirable |
| Percentage of large fibers (soleus/EDL) | ↓/↑ | ↓ | Desirable |
| Fiber type (soleus/EDL) | ↔/↑ IIa and ↓ IIb | ↔/↑ IIa and ↓ IIb | Desirable |
| Muscle capillarity | ↔ | ↔ | – |
| Muscle oxidative capacity | ↑ | ↔ | Undesirable |
| Fibers with central nuclei | ↔ | ↔ | Desirable |
| Fiber eMHC expression (soleus) | ↔ | ↔ | Desirable |
EDL, extensor digitorum longus; eMHC, embryonic myosin heavy chain; ↔, no change; ↑, increased; ↓, decreased; –, no outcome observed. If a muscle, soleus or EDL, is not stated, then response was the same in the two muscles.
Physical Activity and Exercise Behaviors of mdx Mice
An important finding was that mdx mice are relatively inactive in their cages under normal housing conditions. Whether this inactivity contributes to the overall mdx muscle pathology or if the inactivity is caused by the muscle pathology, is intriguing to consider. There have been reports that mdx mice have reduced motor activity at young ages.12,41 Contrary to the idea that physical activity is good for dystrophic muscle, there are some reports that reducing the activity of a dystrophic mouse has some benefits to muscle,38,39 and thus reduced physical activity by mdx mice could theoretically be a muscle-sparing strategy.
Despite the mdx mouse’s relative sedentary behavior in a typical cage, when such mice are provided the opportunity to be physically active, they do engage, albeit slightly less than C57BL mice, as previously described.8,13,26 There is one report of mdx mice voluntarily running on wheels in a much more intermittent pattern than control mice.23 In that study, the average continuous running time was 66 minutes for control mice and only 26 minutes for mdx mice, and the investigators concluded that this intermittent running pattern by mdx mice could be used as a phenotypic marker. We assessed the frequency of wheel running activity differently by totaling 20-second bouts per hour during the three most active hours and found that wheel activity by mdx mice was, at most, only 17% less than control mice. Therefore, our results counter the notion that there is a striking running abnormality in mdx mice. Overall, we consider the differences in total distance run per day, time-of-day running, and number of activity bouts between mdx and C57BL mice to be modest and not likely great enough to explain any differential outcomes of exercise training between mdx and control mice. A limitation to our study is that we do not know what the cage activities were for the mice that ran on wheels.
A second limitation of our study is the mouse model of DMD that was used, namely the mdx mouse, which has a mild phenotype. Thus, although mdx mice voluntarily ran and muscles adapted in response to the running, DMD patients may not engage and their muscles may not adapt so readily. With regard to participation, we specifically chose young mdx mice to best represent young DMD patients who would likely have the capacity and the eagerness to be physically active. We attempted to utilize a more severe model of DMD, the mdx:utrn−/− mouse, and we found that those mice voluntarily ran ~1 km/day, but only for about 1 week. The response during this first week was quite variable between the four mdx:utrn−/− mice we studied and, by the second week, all mice were running very little. We did not conduct any follow-up measurements on these mice because of the minimal distances they ran. Recently, however, we reported that running as little as 1.45 km/day can result in favorable adaptations in mouse soleus muscle,60 so some modified exercise protocol that could benefit muscles of these severely dystrophic mice is not out of the question. In addition, 129ReJ dy/dy mice, which also have a severe muscle pathology similar to DMD, showed some beneficial effects of voluntary wheel running, even though they ran only ~0.5 km/day.26
Typical, Beneficial Muscle Adaptations in Response to Exercise by Dystrophic Mice
Soleus muscles tended to hypertrophy in response to wheel running in both C57BL and mdx mice. When muscle mass was normalized to body mass, the Run mice had a statistically greater mass than the Sed mice. These adaptations by mdx mice have been reported previously.8,13,14,26 Soleus muscle mass relative to body mass is a critical adaptation, because the soleus, being a weight-bearing muscle, would be at a functional disadvantage if its mass did not change in proportion to body mass. The non-hypertrophic responses of the EDL and tibialis anterior muscles to running are in accordance with previous reports.2,8,26 The heart has been shown to hypertrophy in response to wheel running,2,31 but we did not observe this in either C57BL or mdx mice.
Wheel running resulted in an overall shift away from MHC IIb– expressing fibers toward more MHC IIa– expressing fibers in EDL muscles. This effect was particularly notable among the mdx mice, where the percentage of IIb fibers decreased from an abnormally high 86% in the Sed mdx mice to 63% in the Run mdx mice. Thus, after 60 days of wheel running, EDL muscles from mdx mice had the same percent of MHC IIb fibers as the C57BL mice (Fig. 5). This MHC isoform shift away from IIb toward IIa fibers has been demonstrated previously in C57BL2,24,44 and mdx26 mouse muscles in response to wheel running. The shift is considered a functional benefit because IIa fibers are normally associated with greater oxidative capacity while maintaining the high contractile velocity that the IIa MHC isoform confers to a fiber.
Atypical, Yet Beneficial Muscle Adaptation in Response to Exercise by Dystrophic Mice
Fibers of mdx muscles are classically characterized by a broader, more variable distribution of cross-sectional areas compared with fibers of control muscles. They have a particularly high frequency of large fibers, resulting in larger overall cross-sectional area in mdx fibers compared with controls.4 We found that wheel running caused a reduction in the number of abnormally large fibers in both the soleus and EDL muscles of mdx mice. This is a novel finding that we interpret to indicate that this exercise-induced shift in fiber cross-sectional area in mdx mice is beneficial, as the distribution becomes more similar to that of fibers from C57BL mice. Collectively, wheel running induced a relative hypertrophy of soleus muscle. The cellular adaptations of fiber MHC isoform expression and fiber cross-sectional area in soleus and EDL muscles of mdx mice are desirable (Table 3) and likely contribute to the functional improvements reported previously.26,61
Atypical or Nonadaptive Muscle Responses to Exercise by Dystrophic Mice
Mitochondrial oxidative capacity, as indicated by activities of enzymes of the Krebs cycle (CS), β-oxidation (β-HAD), and the electron transport chain (CCO), typically improves in mouse hindlimb muscles in response to wheel running.11,26 We found that this adaptation occurred in hindlimb muscles of C57BL mice, but it was not found in mdx mice. The C57BL Run mice had, on average, 25% greater mitochondrial enzyme activities compared with C57BL Sed mice (ranging from +9 to +69% improvements, respectively, among the seven enzyme–muscle combinations assayed; Table 2). In contrast, the average difference in enzyme activity between Run and Sed mdx mice was only 2% (ranging from −16% to +34%). This nonadaptive mitochondrial response is of particular interest in light of the normal adaptive MHC isoform response (shift from IIb toward IIa fibers) we found in mdx mouse muscle with running; typically, these two muscle adaptations occur in tandem. The nonadaptive mitochondrial enzyme response by mdx mouse muscles has only been noted for CS activity in soleus muscles following 8 weeks of wheel running.14 Although 12–28 days of low-frequency stimulation of mdx mouse EDL and tibialis anterior muscles was sufficient to elicit increases in CS and β-HAD activities in those muscles,49 low-intensity treadmill running was not a sufficient stimulus to increase mitochondrial enzyme activities.30 A more thorough analysis of mitochondrial enzymes and their adaptive potentials in dystrophic muscle is needed to adequately assess overall muscle adaptations to an endurance type of exercise.
A second notable finding with regard to mitochondrial enzyme activities is that, among the mice that did not engage in wheel running (i.e., the Sed mice), mitochondrial activities were 12% lower overall in muscles from mdx mice relative to those from C57BL mice. This is consistent with previous reports of low CS and β-HAD activities in EDL and tibialis anterior muscles49 and CCO activity in quadriceps muscle33 of mdx mice relative to controls. The reduced capacity for oxidative metabolism in muscles of mdx mice may reflect their physically inactive cage behaviors relative to C57BL mice. In other words, skeletal muscle of mdx mice may be considered untrained or deconditioned and hence have low oxidative capacities. It has also been proposed that the lack of dystrophin-associated proteins of the cytoskeleton in muscles of mdx mice causes a destabilization of enzyme complexes that results in metabolic deficiencies.9 Alternatively, the cytochrome contents of mitochondria isolated from skeletal muscles of mdx mice was found to be low and likely directly responsible for the low CCO enzyme activities.33 Those investigators suggested that mitochondrial impairments in mdx muscle are the result of calcium overload of fibers due to dystrophin deficiency. It follows that, regardless of the cause of mitochondrial alterations in mdx mice, beneficial mitochondrial adaptations in response to exercise would be attenuated if mitochondria are struggling to function under normal conditions in a dystrophin-deficient muscle.
The average number of capillaries in contact with a fiber was not different between muscles from mdx and C57BL mice, and the absolute values are similar to those published for rat muscles.45 However, because fibers in muscles of mdx mice were larger, capillarity expressed per fiber cross-sectional area diminished in dystrophic muscle. The inability of the mdx mouse to maintain capillaries per fiber cross-sectional area is important, because, for a given blood flow, perfusion and thus fiber pO2 would be lower. This would result in a reduced availability of oxygen to fibers and may contribute to functional ischemia. This has been reported in children with DMD53 and may also partially explain why vascular endothelial growth factor (VEGF), which induces angiogenesis, is useful for improving various treatments in muscular dystrophy.6,22,37 Previous studies of dystrophin-deficient muscle have shown normal capillary densities, but those data were expressed as capillaries per mm2 of muscle tissue, which does not take into account differences in fiber size and are therefore less meaningful in terms of supplying oxygen to individual muscle fibers.42,58
We did not observe an exercise-induced increase in capillarity in soleus or EDL muscles from either C57BL or mdx Run mice relative to Sed mice, indicating that our exercise stimulus was not sufficient to induce this adaptation. To our knowledge, capillary adaptations of dystrophic muscle in response to exercise have not been investigated previously. Capillary adaptations in response to ischemia and wounding are not worsened by the lack of dystrophin,58 and this suggests that mdx mouse muscle has the capacity for capillary adaptations to stressors such as exercise.
Indications that Voluntary Wheel Running Does Not Exacerbate the Pathology of Dystrophic Muscle
Fibers with central nuclei represent a muscle’s history of degeneration and regeneration. If voluntary wheel running were injurious to dystrophin-deficient muscle fibers, then an increase in the proportion of centrally nucleated fibers would be evident. This was not observed for mdx Run mice compared with mdx Sed mice in soleus or EDL muscle fibers, regardless of fiber type, corroborating findings from a similar report.30 It is of interest to note that there was no preferential expression of central nuclei with regard to fiber type, because all fiber types had about the same percentage of centrally nucleated fibers.
The expression of the eMHC isoform by a fiber indicates ongoing regeneration. We found that soleus muscles from mdx mice exhibited about 10 times more regenerating fibers than those from C57BL mice, which is typical for dystrophic muscle. Importantly, the occurrence of regenerating fibers in mdx muscle was not increased by wheel running. This is an indication that this type of exercise did not cause fiber injury and subsequent regeneration. A trend appeared for soleus muscle fibers of the C57BL mice to have more fibers with central nuclei and eMHC expression with wheel running, but it is known that a small amount of fiber injury occurs at the onset of voluntary wheel running and is considered a usual event in muscle adaptation to this type of exercise.29 This is an important point to consider, because seemingly unwanted physiological adaptations due to acute exercise by normally sedentary, cage-bound mice (whether dystrophic or not) are expected. An illustration of this concept was nicely depicted in a recent study by Kaczor and co-workers.30 They provided evidence that oxidative stress occurs in skeletal muscles of mdx mice, and it is likely heightened by acute exercise. Chronic, low-intensity exercise eventually attenuated the stress and resulted in lower lipid and protein oxidative damage. This concept of acute vs. chronic exercise is further exemplified by studies that have assessed acute (12– 48 hours) voluntary wheel running exercise by mdx mice. These studies showed increased inflammation and DNA damage47,54 and other studies utilizing high-intensity, short-term treadmill running exacerbated the dystrophic pathology.16,43 In addition to considering adaptations of acute vs. chronic exercise, the intensity of the exercise must also be considered. It appears that there is an exercise-intensity threshold for inducing beneficial vs. deleterious adaptations in dystrophic muscle, and we must better elucidate this intensity parameter.
In conclusion, cellular remodeling and the subsequent functional adaptations of dystrophic muscle to exercise have been inadequately studied. We hypothesized that a voluntary, endurance type of exercise training would improve mdx mouse muscle to the same extent that exercise benefited muscle from healthy, control mice. For the most part, this hypothesis was supported, but with the exception that mitochondrial adaptations did not occur in mdx mouse muscles (Table 3). This apparent abnormality should be investigated more thoroughly in future studies, as should additional avenues that have recently been recommended with regard to exercise in DMD.20 Because mdx mice and individuals with DMD are relatively inactive, the influence of physical inactivity on the dystrophic phenotype is also important to understand, as chronic inactivity contributes to secondary pathologies such as cardiovascular disease, diabetes, obesity, and osteoporosis. These are real clinical concerns for individuals with DMD, and they are worsened by prednisone treatment. Thus, remediation of skeletal muscle weakness and fatigue through exercise might not only improve the quality of life of individuals with DMD by enhancing muscle function but may also prevent secondary, unwanted consequences.
Acknowledgments
This study was supported by a Nash Avery Search for Hope Research Fund, University of Minnesota, and a grant from the Muscular Dystrophy Association (MDA4143). The authors thank Barry Prior for advice and discussions regarding capillary measurements and Robert Grange for critically evaluating the manuscript.
Abbreviations
- ANOVA
analysis of variance
- ATP
adenosine triphosphate
- β-HAD
beta-hydroxy acyl-CoA dehydrogenase
- CCO
cytochrome c oxidase
- CoA
coenzyme A
- CS
citrate synthase
- DAB
diaminobenzidine
- DMD
Duchenne muscular dystrophy
- EDL
extensor digitorum longus
- eMCH
embryonic myosin heavy chain
- MHC
myosin heavy chain
- PBS
phosphate-buffered saline
- Sed
sedentary
- VEGF
vascular endothelial growth factor
References
- 1.Aitkens SG, McCrory MA, Kilmer DD, Bernauer EM. Moderate resistance exercise program: its effect in slowly progressive neuromuscular disease. Arch Phys Med Rehabil. 1993;74:711–715. doi: 10.1016/0003-9993(93)90031-5. [DOI] [PubMed] [Google Scholar]
- 2.Allen DL, Harrison BC, Maass A, Bell ML, Byrnes WC, Leinwand LA. Cardiac and skeletal muscle adaptations to voluntary wheel running in the mouse. J Appl Physiol. 2001;90:1900–1908. doi: 10.1152/jappl.2001.90.5.1900. [DOI] [PubMed] [Google Scholar]
- 3.Andersen P. Capillary density in skeletal muscle of man. Acta Physiol Scand. 1975;95:203–205. doi: 10.1111/j.1748-1716.1975.tb10043.x. [DOI] [PubMed] [Google Scholar]
- 4.Anderson JE, Bressler BH, Ovalle WK. Functional regeneration in the hindlimb skeletal muscle of the mdx mouse. J Muscle Res Cell Motil. 1988;9:499–515. doi: 10.1007/BF01738755. [DOI] [PubMed] [Google Scholar]
- 5.Bass A, Brdiczka D, Eyer P, Hofer S, Pette D. Metabolic differentiation of distinct muscle types at the level of enzymatic organization. Eur J Biochem. 1969;10:198–206. doi: 10.1111/j.1432-1033.1969.tb00674.x. [DOI] [PubMed] [Google Scholar]
- 6.Bouchentouf M, Benabdallah BF, Bigey P, Yau TM, Scherman D, Tremblay JP. Vascular endothelial growth factor reduced hypoxia-induced death of human myoblasts and improved their engraftment in mouse muscles. Gene Ther. 2008;15:404–414. doi: 10.1038/sj.gt.3303059. [DOI] [PubMed] [Google Scholar]
- 7.Brussee V, Tardif F, Tremblay JP. Muscle fibers of mdx mice are more vulnerable to exercise than those of normal mice. Neuromuscul Disord. 1997;7:487–492. doi: 10.1016/s0960-8966(97)00115-6. [DOI] [PubMed] [Google Scholar]
- 8.Carter GT, Wineinger MA, Walsh SA, Horasek SJ, Abresch RT, Fowler WM., Jr Effect of voluntary wheel-running exercise on muscles of the mdx mouse. Neuromuscul Disord. 1995;5:323–332. doi: 10.1016/0960-8966(94)00063-f. [DOI] [PubMed] [Google Scholar]
- 9.Chinet AE, Even PC, Decrouy A. Dystrophin-dependent efficiency of metabolic pathways in mouse skeletal muscles. Experientia. 1994;50:602–605. doi: 10.1007/BF01921731. [DOI] [PubMed] [Google Scholar]
- 10.Cornu C, Goubel F, Fardeau M. Muscle and joint elastic properties during elbow flexion in Duchenne muscular dystrophy. J Physiol. 2001;533:605–616. doi: 10.1111/j.1469-7793.2001.0605a.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Davidson SR, Burnett M, Hoffman-Goetz L. Training effects in mice after long-term voluntary exercise. Med Sci Sports Exerc. 2006;38:250–255. doi: 10.1249/01.mss.0000183179.86594.4f. [DOI] [PubMed] [Google Scholar]
- 12.Dupont-Versteegden EE, Baldwin RA, McCarter RJ, Vonlanthen MG. Does muscular dystrophy affect metabolic rate? A study in mdx mice. J Neurol Sci. 1994;121:203–207. doi: 10.1016/0022-510x(94)90353-0. [DOI] [PubMed] [Google Scholar]
- 13.Dupont-Versteegden EE, McCarter RJ, Katz MS. Voluntary exercise decreases progression of muscular dystrophy in diaphragm of mdx mice. J Appl Physiol. 1994;77:1736–1741. doi: 10.1152/jappl.1994.77.4.1736. [DOI] [PubMed] [Google Scholar]
- 14.Dupont-Versteegden EE. Exercise and clenbuterol as strategies to decrease the progression of muscular dystrophy in mdx mice. J Appl Physiol. 1996;80:734–741. doi: 10.1152/jappl.1996.80.3.734. [DOI] [PubMed] [Google Scholar]
- 15.Fisher I, Abraham D, Bouri K, Hoffman EP, Muntoni F, Morgan J. Prednisolone-induced changes in dystrophic skeletal muscle. FASEB J. 2005;19:834–836. doi: 10.1096/fj.04-2511fje. [DOI] [PubMed] [Google Scholar]
- 16.Fraysse B, Liantonio A, Cetrone M, Burdi R, Pierno S, Frigeri A, et al. The alteration of calcium homeostasis in adult dystrophic mdx muscle fibers is worsened by a chronic exercise in vivo. Neurobiol Dis. 2004;17:144–154. doi: 10.1016/j.nbd.2004.06.002. [DOI] [PubMed] [Google Scholar]
- 17.Gollnick PD, Saltin B. Significance of skeletal muscle oxidative enzyme enhancement with endurance training. Clin Physiol. 1982;2:1–12. doi: 10.1111/j.1475-097x.1982.tb00001.x. [DOI] [PubMed] [Google Scholar]
- 18.Gorzek JF, Hendrickson KC, Forstner JP, Rixen JL, Moran AL, Lowe DA. Estradiol and tamoxifen reverse ovariectomy-induced physical inactivity in mice. Med Sci Sports Exerc. 2007;39:248–256. doi: 10.1249/01.mss.0000241649.15006.b8. [DOI] [PubMed] [Google Scholar]
- 19.Grady RM, Teng H, Nichol MC, Cunningham JC, Wilkinson RS, Sanes JR. Skeletal and cardiac myopathies in mice lacking utrophin and dystrophin: a model for Duchenne muscular dystrophy. Cell. 1997;90:729–738. doi: 10.1016/s0092-8674(00)80533-4. [DOI] [PubMed] [Google Scholar]
- 20.Grange RW, Call JA. Recommendations to define exercise prescription for Duchenne muscular dystrophy. Exerc Sport Sci Rev. 2007;35:12–17. doi: 10.1249/01.jes.0000240020.84630.9d. [DOI] [PubMed] [Google Scholar]
- 21.Grange RW, Gainer TG, Marschner KM, Talmadge RJ, Stull JT. Fast-twitch skeletal muscles of dystrophic mouse pups are resistant to injury from acute mechanical stress. Am J Physiol Cell Physiol. 2002;283:C1090–C1101. doi: 10.1152/ajpcell.00450.2001. [DOI] [PubMed] [Google Scholar]
- 22.Gregorevic P, Blankinship MJ, Allen JM, Crawford RW, Meuse L, Miller DG, et al. Systemic delivery of genes to striated muscles using adeno-associated viral vectors. Nat Med. 2004;10:828–834. doi: 10.1038/nm1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hara H, Nolan PM, Scott MO, Bucan M, Wakayama Y, Fisch-beck KH. Running endurance abnormality in mdx mice. Muscle Nerve. 2002;25:207–211. doi: 10.1002/mus.10023. [DOI] [PubMed] [Google Scholar]
- 24.Harrison BC, Bell ML, Allen DL, Byrnes WC, Leinwand LA. Skeletal muscle adaptations in response to voluntary wheel running in myosin heavy chain null mice. J Appl Physiol. 2002;92:313–322. doi: 10.1152/japplphysiol.00832.2001. [DOI] [PubMed] [Google Scholar]
- 25.Hayes A, Lynch GS, Williams DA. The effects of endurance exercise on dystrophic mdx mice. I. Contractile and histochemical properties of intact muscles. Proc R Soc Lond B Biol Sci. 1993;253:19–25. doi: 10.1098/rspb.1993.0077. [DOI] [PubMed] [Google Scholar]
- 26.Hayes A, Williams DA. Beneficial effects of voluntary wheel running on the properties of dystrophic mouse muscle. J Appl Physiol. 1996;80:670–679. doi: 10.1152/jappl.1996.80.2.670. [DOI] [PubMed] [Google Scholar]
- 27.Hoffman EP, Brown RH, Jr, Kunkel LM. Dystrophin: the protein product of the Duchenne muscular dystrophy locus. Cell. 1987;51:919–928. doi: 10.1016/0092-8674(87)90579-4. [DOI] [PubMed] [Google Scholar]
- 28.Houle-Leroy P, Garland T, Jr, Swallow JG, Guderley H. Effects of voluntary activity and genetic selection on muscle metabolic capacities in house mice Mus domesticus. J Appl Physiol. 2000;89:1608–1616. doi: 10.1152/jappl.2000.89.4.1608. [DOI] [PubMed] [Google Scholar]
- 29.Irintchev A, Wernig A. Muscle damage and repair in voluntarily running mice: strain and muscle differences. Cell Tissue Res. 1987;249:509–521. doi: 10.1007/BF00217322. [DOI] [PubMed] [Google Scholar]
- 30.Kaczor JJ, Hall JE, Payne E, Tarnopolsky MA. Low intensity training decreases markers of oxidative stress in skeletal muscle of mdx mice. Free Rad Biol Med. 2007;43:145–154. doi: 10.1016/j.freeradbiomed.2007.04.003. [DOI] [PubMed] [Google Scholar]
- 31.Konhilas JP, Maass AH, Luckey SW, Stauffer BL, Olson EN, Leinwand LA. Sex modifies exercise and cardiac adaptation in mice. Am J Physiol Heart Circ Physiol. 2004;287:H2768–H2776. doi: 10.1152/ajpheart.00292.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Konhilas JP, Widegren U, Allen DL, Paul AC, Cleary A, Leinwand LA. Loaded wheel running and muscle adaptation in the mouse. Am J Physiol Heart Circ Physiol. 2005;289:H455–H465. doi: 10.1152/ajpheart.00085.2005. [DOI] [PubMed] [Google Scholar]
- 33.Kuznetsov AV, Winkler K, Wiedemann FR, von Bossanyi P, Dietzmann K, Kunz WS. Impaired mitochondrial oxidative phosphorylation in skeletal muscle of the dystrophin-deficient mdx mouse. Mol Cell Biochem. 1998;183:87–96. doi: 10.1023/a:1006868130002. [DOI] [PubMed] [Google Scholar]
- 34.Louis M, Raymackers JM, Debaix H, Lebacq J, Francaux M. Effect of creatine supplementation on skeletal muscle of mdx mice. Muscle Nerve. 2004;29:687–692. doi: 10.1002/mus.20014. [DOI] [PubMed] [Google Scholar]
- 35.Lynch GS, Hinkle RT, Faulkner JA. Force and power output of diaphragm muscle strips from mdx and control mice after clenbuterol treatment. Neuromuscul Disord. 2001;11:192–196. doi: 10.1016/s0960-8966(00)00170-x. [DOI] [PubMed] [Google Scholar]
- 36.Marden FA, Connolly AM, Siegel MJ, Rubin DA. Compositional analysis of muscle in boys with Duchenne muscular dystrophy using MR imaging. Skeletal Radiol. 2005;34:140–148. doi: 10.1007/s00256-004-0825-3. [DOI] [PubMed] [Google Scholar]
- 37.Messina S, Mazzeo A, Bitto A, Aguennouz M, Migliorato A, De Pasquale MG, et al. VEGF overexpression via adeno-associated virus gene transfer promotes skeletal muscle regeneration and enhances muscle function in mdx mice. FASEB J. 2007;21:3737–3746. doi: 10.1096/fj.07-8459com. [DOI] [PubMed] [Google Scholar]
- 38.Mizuno Y. Prevention of myonecrosis in mdx mice: effect of immobilization by the local tetanus method. Brain Dev. 1992;14:319–322. doi: 10.1016/s0387-7604(12)80151-3. [DOI] [PubMed] [Google Scholar]
- 39.Mokhtarian A, Lefaucheur JP, Even PC, Sebille A. Hindlimb immobilization applied to 21-day-old mdx mice prevents the occurrence of muscle degeneration. J Appl Physiol. 1999;86:924–931. doi: 10.1152/jappl.1999.86.3.924. [DOI] [PubMed] [Google Scholar]
- 40.Moran AL, Warren GL, Lowe DA. Soleus and EDL muscle contractility across the lifespan of female C57BL/6 mice. Exp Gerontol. 2005;40:966–975. doi: 10.1016/j.exger.2005.09.005. [DOI] [PubMed] [Google Scholar]
- 41.Muntoni F, Mateddu A, Marchei F, Clerk A, Serra G. Muscular weakness in the mdx mouse. J Neurol Sci. 1993;120:71–77. doi: 10.1016/0022-510x(93)90027-v. [DOI] [PubMed] [Google Scholar]
- 42.Nguyen F, Guigand L, Goubault-Leroux I, Wyers M, Cherel Y. Microvessel density in muscles of dogs with golden retriever muscular dystrophy. Neuromuscul Disord. 2005;15:154–163. doi: 10.1016/j.nmd.2004.11.002. [DOI] [PubMed] [Google Scholar]
- 43.Okano T, Yoshida K, Nakamura A, Sasazawa F, Oide T, Takeda S, Ikeda S. Chronic exercise accelerates the degeneration–regeneration cycle and downregulates insulin-like growth factor-1 in muscle of mdx mice. Muscle Nerve. 2005;32:191–199. doi: 10.1002/mus.20351. [DOI] [PubMed] [Google Scholar]
- 44.Pellegrino MA, Brocca L, Dioguardi FS, Bottinelli R, D’Antona G. Effects of voluntary wheel running and amino acid supplementation on skeletal muscle of mice. Eur J Appl Physiol. 2005;93:655–664. doi: 10.1007/s00421-004-1237-8. [DOI] [PubMed] [Google Scholar]
- 45.Poole DC, Mathieu-Costello O. Relationship between fiber capillarization and mitochondrial volume density in control and trained rat soleus and plantaris muscles. Microcirculation. 1996;3:175–186. doi: 10.3109/10739689609148286. [DOI] [PubMed] [Google Scholar]
- 46.Prohaska JR, Wells WW. Copper deficiency in the developing rat brain: a possible model for Menkes’ steely-hair disease. J Neurochem. 1974;23:91–98. doi: 10.1111/j.1471-4159.1974.tb06920.x. [DOI] [PubMed] [Google Scholar]
- 47.Radley HG, Grounds MD. Cromolyn administration (to block mast cell degranulation) reduces necrosis of dystrophic muscle in mdx mice. Neurobiol Dis. 2006;23:387–397. doi: 10.1016/j.nbd.2006.03.016. [DOI] [PubMed] [Google Scholar]
- 48.Ratel S, Duche P, Williams CA. Muscle fatigue during high-intensity exercise in children. Sports Med. 2006;36:1031–1065. doi: 10.2165/00007256-200636120-00004. [DOI] [PubMed] [Google Scholar]
- 49.Reichmann H, Pette D, Vrbova G. Effects of low frequency electrical stimulation on enzyme and isozyme patterns of dystrophic mouse muscle. FEBS Lett. 1981;128:55–58. doi: 10.1016/0014-5793(81)81078-2. [DOI] [PubMed] [Google Scholar]
- 50.Rezvani M, Cafarelli E, Hood DA. Performance and excitability of mdx mouse muscle at 2, 5, and 13 wk of age. J Appl Physiol. 1995;78:961–967. doi: 10.1152/jappl.1995.78.3.961. [DOI] [PubMed] [Google Scholar]
- 51.Sacco P, Jones DA, Dick JR, Vrbova G. Contractile properties and susceptibility to exercise-induced damage of normal and mdx mouse tibialis anterior muscle. Clin Sci (Lond) 1992;82:227–236. doi: 10.1042/cs0820227. [DOI] [PubMed] [Google Scholar]
- 52.Saltin B, Gollnick PD. In: Skeletal muscle adaptability: significance for metabolism and performance. Peachy LH, Adrian RH, Greiger SR, editors. Baltimore, MD: Williams & Wilkins; 1983. pp. 555–631. [Google Scholar]
- 53.Sander M, Chavoshan B, Harris SA, Iannaccone ST, Stull JT, Thomas GD, Victor RG. Functional muscle ischemia in neuronal nitric oxide synthase–deficient skeletal muscle of children with Duchenne muscular dystrophy. Proc Natl Acad Sci USA. 2000;97:13818–13823. doi: 10.1073/pnas.250379497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sandri M, Carraro U, Podhorska-Okolov M, Rizzi C, Arslan P, Monti D, et al. Apoptosis, DNA damage and ubiquitin expression in normal and mdx muscle fibers after exercise. FEBS Lett. 1995;373:291–295. doi: 10.1016/0014-5793(95)00908-r. [DOI] [PubMed] [Google Scholar]
- 55.Schiaffino S, Gorza L, Sartore S, Saggin L, Ausoni S, Vianello M, et al. Three myosin heavy chain isoforms in type 2 skeletal muscle fibres. J Muscle Res Cell Motil. 1989;10:197–205. doi: 10.1007/BF01739810. [DOI] [PubMed] [Google Scholar]
- 56.Sexton WL. Vascular adaptations in rat hindlimb skeletal muscle after voluntary running-wheel exercise. J Appl Physiol. 1995;79:287–296. doi: 10.1152/jappl.1995.79.1.287. [DOI] [PubMed] [Google Scholar]
- 57.Srere PA. Citrate synthase. Meth Enzymol. 1969:3–9. [Google Scholar]
- 58.Straino S, Germani A, Di Carlo A, Porcelli D, De Mori R, Mangoni A, et al. Enhanced arteriogenesis and wound repair in dystrophin-deficient mdx mice. Circulation. 2004;110:3341–3348. doi: 10.1161/01.CIR.0000147776.50787.74. [DOI] [PubMed] [Google Scholar]
- 59.Teske JA, Levine AS, Kuskowski M, Levine JA, Kotz CM. Elevated hypothalamic orexin signaling, sensitivity to orexin A, and spontaneous physical activity in obesity-resistant rats. Am J Physiol Regul Integr Comp Physiol. 2006;291:R889–R899. doi: 10.1152/ajpregu.00536.2005. [DOI] [PubMed] [Google Scholar]
- 60.Warren GL, Moran AL, Hogan HA, Lin AS, Guldberg RE, Lowe DA. Voluntary run training but not estradiol deficiency alters the tibial bone–soleus muscle functional relationship in mice. Am J Physiol Regul Integr Comp Physiol. 2007;293:R2015–R2026. doi: 10.1152/ajpregu.00569.2007. [DOI] [PubMed] [Google Scholar]
- 61.Wineinger MA, Abresch RT, Walsh SA, Carter GT. Effects of aging and voluntary exercise on the function of dystrophic muscle from mdx mice. Am J Phys Med Rehabil. 1998;77:20–27. doi: 10.1097/00002060-199801000-00004. [DOI] [PubMed] [Google Scholar]


