Abstract
Objective
Contractures are a major clinical issue for patients with muscular dystrophies. However, it is unknown whether contractures are present in the widely used mdx mouse model of Duchenne muscular dystrophy. Therefore, the objectives of this study were to develop methods to measure muscle contractures in mice, to determine whether plantarflexion contractures are present in mdx mice, and to analyze the composition of the major muscles involved.
Design
Hindlimbs of eight wild type and six mdx mice were assessed every 2 wks during the course of a 12-wk study. Assessments included range of motion and in vivo torques about the ankle. At the end of the study, mice were euthanized, and muscles were analyzed for composition.
Results
The mdx mice had ~10 degrees less dorsiflexion, increased passive torque moving the ankle into dorsiflexion, and an increased passive-to-active torque ratio relative to wild type mice. Gastrocnemius muscle composition alterations included increased wet mass, decreased protein content, and increased collagen.
Conclusions
The results indicate that mdx mice have plantarflexion contractures similar to those seen in children with Duchenne muscular dystrophy. In future studies, these measures can be used to assess strategies to slow the progression of contractures that occur with muscular dystrophies.
Keywords: Duchenne Muscular Dystrophy, Strength, Range of Motion, Plantarflexors
Duchenne muscular dystrophy (DMD) is an X-linked neuromuscular disorder caused by an absence of the protein dystrophin due to mutation of the dystrophin gene.1 Lack of dystrophin on the plasma membrane of skeletal muscle fibers causes the hallmark feature of the disease, progressive muscle weakness. Muscle weakness results in compensatory postural adjustments during gait and stance and eventually wheel chair dependency.2 Congruent with muscle weakness, limb contractures emerge as a characteristic feature of DMD. Contracture is generally defined as a marked loss of range of motion (ROM) resulting from joint, muscle, or soft tissue limitation.3 Common contractures in DMD include those of the ankle plantarflexors, knee flexors, hip flexors, iliotibial bands, shoulder flexors and adductors, elbow flexors, wrist flexors, and finger flexors.4–6 The loss of function that children with DMD suffer because of contractures is significant. Upper limb contractures result in impairments in fine motor skills, grip strength, and performance of activities of daily living, whereas lower limb contractures impair balance during standing, gait, and comfortable positioning in bed.3,5,7,8 Physical therapy and exercise programs used in the management of contractures in DMD include passive ROM, daily standing or walking, positioning at rest, and splinting,3,9,10 although there is scant evidence for optimization of these modalities.
Alterations within dystrophin-deficient muscle include degeneration of muscle fibers, loss of muscle fibers, enlargement of muscle fibers, and infiltration of fibrotic and fatty tissue.3,11 Although much of the musculature undergoes atrophy with the progression of DMD, the gastrocnemius muscle undergoes notable enlargement. This enlargement is referred to as pseudohypertrophy, because although some fiber hypertrophy occurs in the early stage of disease in this muscle, a significant accumulation of fat and connective tissue also occurs.11 The gastrocnemius muscle is a primary muscle for plantarflexion and thus is principally involved in plantarflexion contractures in DMD. However, the cause and effect relationships among gastrocnemius muscle size, composition, strength, and plantarflexion contracture are not well understood in DMD.
The mdx mouse is the most frequently studied animal model of DMD. This mouse lacks the protein dystrophin as a result of a point mutation in the dystrophin gene.12 Similar to DMD, skeletal muscles of the mdx mouse display fiber loss, selective fiber atrophy and hypertrophy, inflammation, and progressive fibrosis.13 The mdx mouse has reduced strength, particularly when force is normalized to muscle cross-sectional area,14 increased thoracolumbar kyphosis,15 and engages in less volitional physical activity.16 However, an important feature of the DMD phenotype that has not been investigated in the mdx mouse is muscle contracture. A possible reason for this is that the phenotype of the mdx mouse is relatively mild compared with that of boys with DMD. For example, the mice live a near-normal life span17 and do not have obvious gait impairments. Nonetheless, if contractures are present in the mdx mouse because of lack of dystrophin, it could serve as a model to investigate anticontracture strategies such as exercise or pharmacological antifibrotic treatments, similar to how this mouse model is used to test other therapies for DMD.
Although it is unknown whether muscle contractures exist in the mdx mouse, contractures have been documented in another DMD animal model, the golden retriever muscular dystrophy (GRMD) dog. These dogs are severely affected by the disease, showing progressive muscle wasting, weakness, and contractures, as well as a significantly reduced life span.18,19 Contractures in GRMD dogs occur in the limbs, progress with age, and result in severe gait restrictions.19 Because of the limited availability of the dog model and frequent use of the mdx mouse model for studying DMD, it would be advantageous to know whether dystrophic mice exhibit muscle contractures. To date, no research has been conducted to determine whether contractures exist in the mdx mouse. Therefore, the objective of this study was to develop methods to measure muscle contractures in mice and then use that methodology to determine whether contractures are present in dystrophic mice. We hypothesized that plantarflexion contractures exist in mdx mice, as indicated by decreased dorsiflexion ROM, increased passive torque required to rotate the ankle into dorsiflexion, and decreased strength of the lower hindlimb muscles relative to those in healthy, control mice. We found evidence for plantarflexion contractures in mdx mice, which led to a second objective of this study, to characterize the contractures by analyzing the size and composition of the major muscles involved. We hypothesized that plantarflexion contractures would be associated with increased mass and collagen content and decreased protein content of the gastrocnemius muscle in the mdx mouse.
METHODS
Animals and Study Design
Wild type (WT) mice (C57BL/10ScSn) and mdx mice (C57BL/10ScSn-DMDmdx) were purchased from Jackson Laboratories (n = 8 each). All mice were male and were 6-wk old at the start of the study. Mice were housed four per cage, subjected to a 12:12-hr light-dark cycle, and provided rodent chow and water ad libitum. For the in vivo torque and ROM measurements, mice were anesthetized with a mixture of fentanyl citrate (10 mg/kg body weight), droperidol (0.2 mg/kg body weight), and diazepam (5 mg/kg body weight) given intraperitoneally. At the end of the study, each 19-wk-old WT and mdx mouse was euthanized with an intraperitoneal injection of sodium pentobarbital (200 mg/kg body weight). Muscles were dissected, weighed, frozen in liquid nitrogen, and stored at –80°C. All protocols and animal care procedures were approved by the Institutional Animal Care and Use Committee.
In Vivo Torque Measurements
The left hindlimb of each anesthetized mouse was prepared for torque measurements about the ankle as detailed previously.20 A mouse was positioned in the apparatus such that the femur was perpendicular to the tibia, and the tibia was perpendicular to the foot, with this ankle position being defined as 0 degree. The leg position was stabilized by clamping the knee and taping the foot to a foot plate, which was connected to the shaft of the servomotor. Passive torque measurements were then made as the servomotor rotated the ankle between different angles. The starting position for each passive torque measurement was 0 degree. The servomotor was programmed to randomly rotate the ankle to four different angles of dorsiflexion (5, 10, 15, and 20 degrees) and four different angles of plantarflexion (5, 10, 15, and 20 degrees) with respect to 0 degree. When the servo-motor positioned the ankle at a particular angle, the ankle was held in that position for 2 secs and then torque was measured. The servomotor returned the ankle to 0 degree and then after a 10-sec rest period, the procedure was repeated for the next angle. Five minutes after completion of the passive torque measurements, two sterilized platinum subdermal needle electrodes were inserted on either side of the left common peroneal nerve, and maximal active torque of the anterior crural muscles was measured as previously described.20 To elicit maximal torque, stimulator parameters were 0.5-msec square wave pulses at 300 Hz for 200 msec and 3–8 V (S48 and SIU5 Grass Technologies, West Warwick, RI). Passive and active torques were measured on each WT and mdx mouse at 6, 8, 10, 12, 14, 16, and 18 wks of age by the same investigator. The exception to this was that active torque was not determined at 12 and 16 wks of age to allow the skin to completely heal from the repeated impaling of this tissue by the electrodes.
ROM Measurements
Because procedures for measuring ROM about hindlimb joints of mice had not been established, we began our investigation by conducting intraand interrater reliability studies for potential ROM measurements of ankle dorsiflexion and extension of the hip using WT mice. These joint movements were initially chosen because posterior calf (plantarflexors) and anterior hip (hip flexors) are common sites for contracture development in boys with DMD. The ROM measurement that we settled on was dorsiflexion excursion about the ankle, and it was conducted as follows.
Immediately after the last torque measurement, the anesthetized mouse was repositioned on its right side on a backboard device that was designed to stabilize the spine, and a small mark was made over the lateral malleolus of the left hind-limb. Tape was placed around the mouse's lower rib cage and attached to the backboard to hold the mouse securely in place but not so tight as to compromise respiration. Tape was also used to hold the proximal segment of the tail in line with the spine. A digital camera was positioned on a tripod directly above the mouse. A heat lamp was also placed above the mouse to maintain surface temperature at 27°C–30°C. Next, an investigator moved the hip joint of the left hindlimb into 90 degrees of flexion while keeping the left knee fully extended. Then, with the hindlimb held in this position, the knee joint was palpitated, and a minutien pin was inserted into the skin precisely over the lateral aspect of the knee joint. Dorsiflexion excursion was then measured. To do this, the foot was moved from its resting position into dorsiflexion by manual force on the plantar surface of the foot until the end range was reached. For the purpose of this experiment, end range was defined as the point where a noticeable increase in resistance to ankle motion was detected by the investigator. The foot was held in this dorsiflexed position for 10 secs, during which time a digital image was acquired by a second investigator. Angles about the ankle were measured from the images using Image J software (Frederick, MD). With the lateral malleolus as the vertex, a line was extended parallel to the plantar surface of the lateral tarsal bones (calcaneus and cuboid) of the foot, and a second line was drawn from the lateral malleolus to the pin over the knee joint. This ROM measure was conducted by the same investigator for each mouse at the ages 6, 8, 10, 12, 14, 16, and 18 wks, and the investigator was blinded to the genotype of the mouse.
Dry Muscle Mass, Total Protein, and Collagen Contents
Tibialis anterior and gastrocnemius muscles from the left hindlimbs were lyophilized at –40°C and 133 × 10–3 mbar and weighed for dry mass. Dried muscles were then hydrolyzed in 6 M NaOH at 120°C for 20 mins. Total protein content of each muscle was determined by measuring the absorbance of the hydrolyzate at 280 nm (NanoDrop 1000 Spectrophotometer, Wilmington, DE).
Hydroxyproline content was also measured from the hydrolyzate to assess collagen content of the muscles.21 In brief, a stock solution of hydroxyproline (0.5 μg/μl) was used to create standards ranging from 0.5 to 14.0 μg. Fifty microliters of each hydrolyzate and each standard were mixed with 450 μl chloramine-T reagent and allowed to oxidize for 25 mins at room temperature. The chromophore was developed by adding 500 μl of Ehrlich's reagent and incubating in a water bath at 65°C for 20 mins. Samples and standards were brought to room temperature and read on a spectrophotometer (SpectraMax Plus 384, Molecular Devices; Sunnyvale, CA) at 550 nm. A linear regression of the hydroxyproline standards was used to determine the hydroxyproline content for each sample. Total collagen was calculated by dividing the total hydroxyproline content by 0.125, because hydroxyproline comprises ~12.5% of collagen.22
Statistics
Two mdx mice died during the study, one at 9 wks and the other at 11 wks of age. Data collected from these mice were not included in any analyses; therefore, statistical analyses were performed on data from eight WT and six mdx mice. Repeated measures two-way analysis of variances (mouse group × age), with age as the repeated measure, were used to analyze body mass, dorsiflexion excursion, passive torques, active torques, and the ratio of passive to active torque. When a main effect of age or an interaction was found to be significant, Tukey's post hoc tests were used. Data from measurements that were conducted only at the end of the study were analyzed using t tests. Statistical analyses were performed using SigmaStat version 3.5 (Systat Software; Point Richmond, CA) with an α level of 0.05. Values are reported as means (SE).
RESULTS
ROM About the Ankle
In preliminary trials, we found that inter- and intrarater ROM measurements about the hip were not reliable (intraclass correlation coefficient (ICC) ≤0.53). Therefore, hip flexor contractures were not considered for further study. Intrarater measurements for ankle ROM measures among four testers were reliable (ICC = 0.71–0.74). As such, ankle ROM was determined by a single investigator throughout the study, and this investigator was blinded to mouse group.
Measuring ROM is the most common method of monitoring contracture progression in boys with DMD, and a loss of dorsiflexion ROM is considered an indicator of gastrocnemius contracture severity. In mice, we found a significant interaction between group and age for dorsiflexion excursion (P = 0.013). Degrees of dorsiflexion were significantly less in mdx than in WT mice at six of seven ages (P ≤ 0.023; Fig. 1), with the mdx mice having ~10 degrees less dorsiflexion ROM. For both the mdx and WT mice, there was an 8- to 9-degree decrease in dorsiflexion excursion as the mice aged from 6 to 18 wks (Fig. 1).
FIGURE 1.
Dorsiflexion excursion was measured in WT and mdx mice from 6 to 18 wks of age. Values represent means. Error bars represent SE. There was an interaction between mouse group and age (P = 0.013). *Different from WT at corresponding age; #different from wk 6 within group; $different from wk 8 within group.
In Vivo Torque About the Ankle
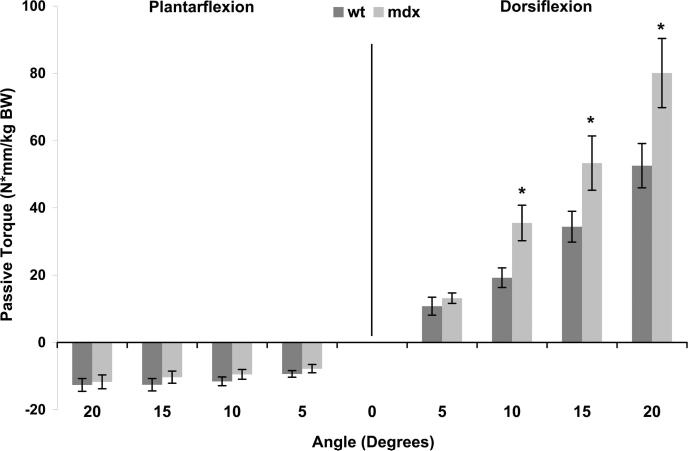
Passive torque represents the amount of force applied to the foot that is required to rotate the ankle when muscles of the lower leg are inactive. Higher torques when the ankle was passively moved into plantarflexed positions would indicate greater resistance from the dorsiflexor muscles. However, we found no differences between mdx and WT mice at the four plantarflexion positions at 6 wks of age (P ≥ 0.716; Fig. 2). Conversely, higher torques when the ankle was passively dorsiflexed would indicate greater resistance from the plantarflexor or calf muscles. The mdx mice displayed greater passive torques at 10, 15, and 20 degrees of dorsiflexion compared with WT mice (P ≤ 0.009; Fig. 2). Because the difference in means between the mdx and WT mice and the corresponding P values were the most significant for the 20-degree dorsiflexion position, we chose to focus on passive torque at this angle in the remainder of the results.
FIGURE 2.
Passive torque about the ankle was measured at 5-degree intervals from 20 degrees of plantarflexion to 20 degrees of dorsiflexion, with 0 degree defined as the foot being perpendicular to the tibia. Passive torque data shown are those that were measured at 6 wks of age in WT and mdx mice and are normalized to body weight. There was an interaction between mouse group and angle (P = 0.003). *Different from WT at corresponding angle.
Absolute passive torque at 20 degrees of dorsiflexion was nearly 50% higher in mdx than in WT mice, and there was also a main effect of age as passive torque increased over time (Table 1). Because from 12 wks of age and older, mdx mice weighed significantly more than WT mice (Table 1), we investigated passive torque normalized to body mass. A significant interaction between mouse group and age was found for normalized passive torque (P = 0.021), showing that as body mass changed with age, and passive torque remained greater in mdx relative to WT mice. In addition, passive torque at 18 wks of age normalized to wet mass or total protein content of the gastrocnemius muscle was still significantly higher in mdx than in WT mice (P ≤ 0.014). This is important because it indicates that more than just the large size of the mdx gastrocnemius muscle was causing the high-passive torque during dorsiflexion.
TABLE 1.
Body masses and torques of the dorsiflexor muscle group of WT and mdx mice from 6 to 18 wks of age
| Age, wks |
P
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 6 | 8 | 10 | 12 | 14 | 16 | 18 | Group | Age | Interaction | |
| Body mass, g | ||||||||||
| WT mice | 21.4 (0.4) | 24.2 (0.3) | 26.9 (0.5) | 28.5 (0.5) | 29.6 (0.5) | 30.2 (0.6) | 31.6 (0.6) | — | — | <0.001 |
| mdx mice | 21.2 (0.3) | 25.2 (0.9) | 28.6 (0.9) | 30.9 (1.2)a | 32.4 (0.9)a | 33.5 (1.1)a | 34.1 (0.9)a | |||
| Passive torque, N · mm | ||||||||||
| WT mice | 1.11 (0.12) | 1.26 (0.06) | 1.21 (0.08) | 1.63 (0.15) | 1.43 (0.08) | 1.05 (0.06) | 1.41 (0.11) | 0.006 | 0.014b | 0.407 |
| mdx mice | 1.67 (0.28) | 1.75 (0.16) | 1.90 (0.21) | 1.99 (0.22) | 2.14 (0.32) | 1.93 (0.30) | 1.83 (0.24) | |||
| Active torque, N · mm | ||||||||||
| WT mice | 1.91 (0.14) | 2.01 (0.14) | 2.09 (0.15) | ND | 2.22 (0.13) | ND | 2.23 (0.10) | 0.898 | <0.001c | 0.070 |
| mdx mice | 1.61 (0.13) | 1.89 (0.07) | 2.24 (0.11) | ND | 2.20 (0.24) | ND | 2.61 (0.22) | |||
Values are presented as means (SE). WT mice grew at every time point measured, except between wk 12 and wk 14, and between wk 14 and wk 16. Mdx mice grew at every time point measured, except between wk 14 and wk 16 and between wk 16 and wk 18.
Different from WT at corresponding age.
Wk 6 different from wk 12.
Wk 6 different from wks 10, 14, and 18 and wk 8 different from wk 18.
WT, wild type; ND, not determined.
Maximal active torque of the anterior lower leg muscles, which is predominated by the tibialis anterior muscle, is an indicator of dorsiflexor musculature strength. This was measured because progressive muscle weakness is a cardinal feature of DMD, and poor dorsiflexor strength can contribute to contracture formation of the plantarflexors. There was a main effect of age for absolute active torque of the dorsiflexors (Table 1), with torques increasing as the mice grew from 6 to 18 wks of age. However, when active torque was normalized to body mass to account for growth, there was no difference between mdx and WT mice and no change with age (P ≥ 0.087). Active torque of the dorsiflexors at 18 wks of age was also normalized to the mass of the tibialis anterior muscle to adjust for the large muscle masses of the mdx mice. Active torque normalized to tibialis anterior muscle mass was 30 ± 7% less in mdx than in WT mice (33.6 [3.3] vs. 48.1 [3.0] N·mm/g; P = 0.007), indicating reduced intrinsic force-generating capacity of the mdx mouse tibialis anterior muscle.
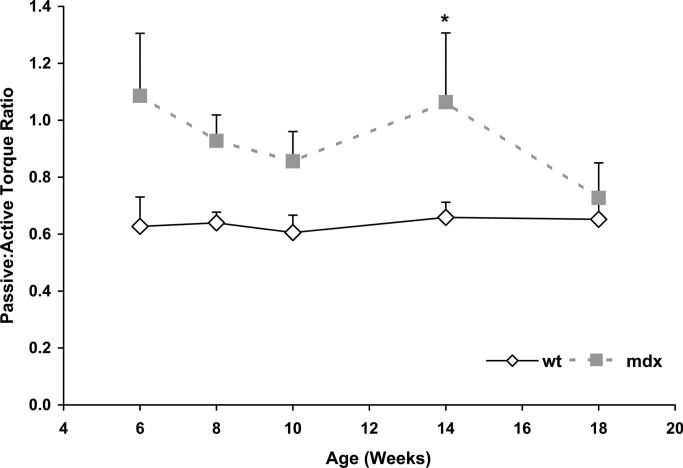
The ratio of passive to active torque at the ankle was calculated to probe the relationship between plantarflexor contracture and dorsiflexor weakness. This ratio represents the passive resistance to dorsiflexion by the calf relative to the force-generating capability of the anterior lower leg, namely the tibialis anterior muscle. The passive/active torque ratio was significantly greater for mdx than WT mice during the course of the study (P = 0.029; Fig. 3).
FIGURE 3.
Ratio of passive torque of the plantarflexors to the active torque of the dorsiflexors in WT and mdx mice from 6 to 18 wks of age. Values represent means. Error bars represent SE. *Main effect of mouse group (P = 0.029).
Composition of the Lower Leg Muscles Contributing to Ankle ROM and Torque
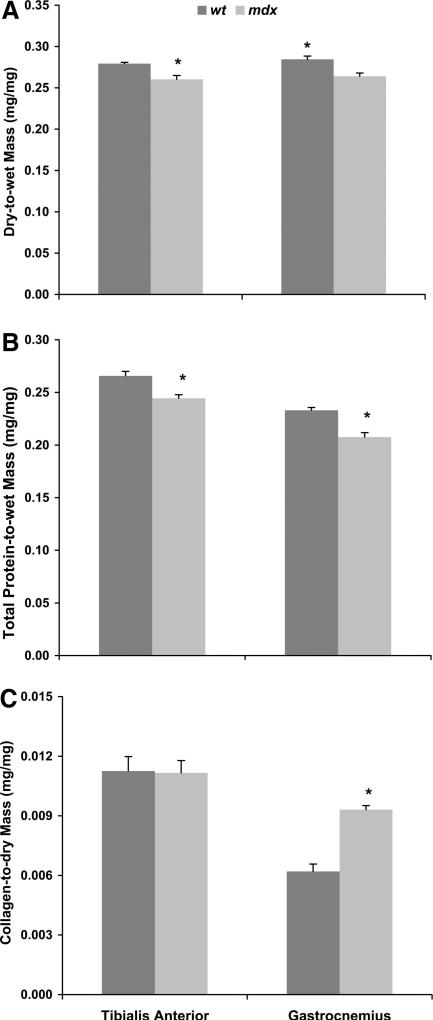
Dorsiflexor muscle masses of mdx mice were 45%–67% greater than those of WT mice (Table 2). When extensor digitorum longus and tibialis anterior muscle masses were normalized to body mass, these values were still 33% and 52% greater in mdx than in WT mice, respectively, indicating hypertrophy of the dorsiflexors. Dry mass and fluid and total protein contents of the tibialis anterior muscle were <50% greater in mdx than in WT mice (Table 2). However, when dry mass and total protein content were normalized to wet mass, muscles from mdx mice had ~8% less than those from WT mice (P ≤ 0.003; Fig. 4). Tibialis anterior muscles from mdx mice also had 52% more collagen than those from WT mice (Table 2). However, this greater collagen content was proportional to the greater amounts of dry mass and total protein content such that these muscles from mdx and WT were not different in collagen concentration (P ≥ 0.930; Fig. 4). Collectively, these data indicate that the tibialis anterior muscles of mdx mice undergo a substantial amount of hypertrophy and a slight disproportional accumulation of fluid but that collagen did not excessively accumulate.
TABLE 2.
Masses and contents of dorsiflexor muscles from WT and mdx mice at 18 wks of age
| WT Mice | mdx Mice | P | Percent mdx >WT | |
|---|---|---|---|---|
| Extensor digitorum longus muscle wet mass, mg | 11.14 (0.22) | 16.16 (0.51) | <0.001 | 45 |
| Tibialis anterior muscle | ||||
| Wet mass, mg | 47.30 (2.55) | 79.01 (4.31) | <0.001 | 67 |
| Dry mass, mg | 13.20 (0.69) | 20.51 (1.00) | <0.001 | 55 |
| Fluid, mg | 34.10 (1.89) | 58.50 (3.39) | <0.001 | 72 |
| Total protein, mg | 12.5 (0.6) | 19.3 (0.9) | <0.001 | 54 |
| Collagen, μg | 150 (14) | 229 (17) | 0.004 | 52 |
Values are presented as means (SE).
WT, wild type.
FIGURE 4.
Relative contents of dry mass (A), total protein (B), and collagen (C) in tibialis anterior and gastrocnemius muscles of WT and mdx mice at the end of the study when mice were 19 wks of age. *Different from WT.
The mdx mice had ~25% greater masses of the plantarflexor muscles compared with those of WT mice (Table 3). When the masses of these muscles were normalized to body mass, differences between mdx and WT mice became less, but mdx mice still had relatively larger gastrocnemius muscles by 15% (P = 0.024). Dry mass and total protein of the gastrocnemius muscle were only 15% and 11% greater in mdx than in WT mice, whereas the fluid content was 28% greater in mdx than in WT mice (Table 3). When dry and total protein contents were normalized to muscle mass, gastrocnemius muscles from mdx mice contained ~10% less of these proteins than WT mice (P ≤ 0.003; Fig. 4). These data indicate that the gastrocnemius muscle of mdx mice undergoes a modest amount of hypertrophy, and similar to the tibialis anterior muscle, there was a slight disproportional accumulation of fluid.
TABLE 3.
Masses and contents of plantarflexor muscles from WT and mdx mice at 18 wks of age
| WT Mice | mdx Mice | P | Percent mdx >WT | |
|---|---|---|---|---|
| Soleus muscle wet mass, mg | 9.38 (0.59) | 11.95 (0.68) | 0.014 | 27 |
| Plantaris muscle wet mass, mg | 18.60 (0.56) | 23.64 (2.41) | 0.037 | 27 |
| Gastrocnemius muscle | ||||
| Wet mass, mg | 165.5 (2.0) | 206.5 (8.0) | <0.001 | 25 |
| Dry mass, mg | 47.12 (0.92) | 54.37 (1.55) | 0.001 | 15 |
| Fluid, mg | 118.4 (1.5) | 152.1 (6.6) | <0.001 | 28 |
| Total protein, mg | 38.6 (0.3) | 42.7 (0.8) | <0.001 | 11 |
| Collagen, μg | 283 (19) | 505 (12) | <0.001 | 78 |
Values are presented as mean (SE).
WT, wild type.
Gastrocnemius muscles from mdx mice also had a significantly greater amount of collagen compared with WT mice (Table 3). In contrast to the tibialis anterior muscle, when collagen content of the gastrocnemius muscle was normalized to dry or total protein content of that muscle, this muscle still had ~50% more collagen compared with WT mice (P < 0.001; Fig. 4), i.e., there was an excess accumulation of collagen. The presence of excess collagen in the gastrocnemius muscle and its relationship to plantarflexion contractures are illustrated by the significant negative correlation between collagen content and dorsiflexion excursion (R = –0.600; P = 0.023).
DISCUSSION
The main finding of this study is that plantarflexion contractures are present in the mdx mouse. The primary characteristics of plantarflexion contracture include decreased dorsiflexion excursion and increased passive torque required to move the ankle into dorsiflexion. Furthermore, the plantarflexion contractures were accompanied by alterations in the compositions and sizes of the muscles surrounding the ankle. This study is a necessary precursor for research to assess the efficacy of therapies aimed at minimizing the severity of plan-tarflexion contractures in the mdx mouse.
The first objective of this study was to develop methods to measure muscle contractures in mice. Contractures in humans are typically assessed by ROM via goniometry, with a clinician determining the end range. Similarly, a study of contractures in GRMD dogs used a goniometer to measure the flexor surface tarsal joint angle at the ankle,19 but we found that goniometry was not possible with mice because of their small size. We developed alternative methods and found that dorsiflexion excursion was a reliable measure of ankle ROM. This measure was used to test the hypothesis that plantarflexion contractures are present in the mdx mouse, and our overall results support the hypothesis. For example, by the end of the study, ankles of the mdx mice had 22% lower dorsiflexion excursion than WT mice. Boys with DMD and dogs with GRMD also have ROM limitations about the ankle.8,19 Plantarflexion contractures in DMD commonly result in a loss of >30 degrees of ROM and can be as great as 70 degrees,6,23 whereas the mdx mice in our study had ~10 degrees of reduced dorsiflexion, indicating that the severity of the contracture is less in mice. This is consistent with the recognized shortcoming of the mdx mouse for modeling DMD, that is, the overall phenotype of the mdx mouse is mild relative to DMD.
Another indicator that we have determined to be important for demonstrating plantarflexor contracture in mdx mice is torque about the ankle. Passive torque while moving the ankle into dorsiflexion measures the resistance of the calf muscles to lengthening, and we hypothesized that this would be greater in mdx than in WT mice. We found that passive torques during dorsiflexion were nearly 50% higher in the mdx compared with WT mice throughout the 12-wk study. In preliminary studies, we have also measured passive torque about the ankle in mice that display a more severe phenotype of DMD. These mice are referred to as double-knockout mice because they lack both dystrophin and utrophin, and they do have an outward appearance of hindlimb contractures. We found even higher dorsiflexion passive torques in these mice relative to mdx mice (data not shown). These preliminary observations provide additional support for the concept that passive torque about the ankle is indicative of plantarflexor contracture in mice with muscular dystrophy.
We also hypothesized that weakness of the ankle musculature likely contributes to plantarflexor contracture. We found that maximal active torque of the dorsiflexors normalized to the mass of the tibialis anterior muscle was less in mdx than in WT mice, in line with a previous study.24 Therefore, the ratio of passive to active torque was consistently greater in mdx than in WT mice. The implication is that the dorsiflexor muscles of mdx mice are weak with respect to the passive torque of the contractured plantarflexor muscles, which is similar to the situation in boys with DMD and in GRMD-affected dogs.3,4,6 For example, it was demonstrated that tarsal joint contractures (analogous to plantarflexion contractures) correlated with weakness of the antagonist muscles in GRMD-affected dogs.18 This relationship between muscle weakness and contractures is critical to understand, because postural compensations for muscle weakness by children with DMD have been suggested to contribute to the early formation of plan-tarflexion contractures.3,4 Some studies have found a significant correlation between contractures and weakness of the opposing muscles,4,18 and another study determined that flexion contractures are rare when the antagonist extensor muscles have greater than or equal to antigravity strength.6
Another objective of this study was to characterize the nature of the plantarflexion contracture by analyzing the composition of the major muscles involved. Muscle (pseudo)hypertrophy was significant in the lower hindlimb muscles of the mdx mice, with the dorsiflexor muscles being ~50% larger than those of WT mice while the plantarflexor muscles were only ~25% larger. A signifi-cant finding was that both the tibialis anterior and gastrocnemius muscles of the mdx mice had a slight accumulation of fluid and reduced protein when normalized for muscle masses. These tissue alterations are comparable with the edema in muscles of patients with DMD, which are secondary to inflammation and muscle fiber necrosis.25 The most obvious tissue alteration of mdx muscle was collagen content. The gastrocnemius muscle of 19-wk old mdx mice contained 55% more collagen than that from age-matched WT mice, even when corrected for the increased wet or dry mass of the mdx muscle. Previously, it was shown that this deposition of collagen in the mdx gastrocnemius muscle increased progressively with age,26 and it parallels the ankle musculature in patients with DMD in whom fibers are replaced by fibrotic tissue and fat.3,11,27 Connective tissue is less extensible than muscle fibers; therefore, an increase in fibrosis contributes to greater resistance of the gastrocnemius muscle to lengthening. In contrast to the gastrocnemius muscle, the tibialis anterior muscle of mdx mice did not accumulate collagen, which likely explains why plantarflexion of the ankle was not affected.
A limitation of using a mouse model to study plantarflexion contracture is that the biomechanical factors that contribute to the development and progression of contractures in mice are likely very different than those in humans. A central dissimilarity to consider is the quadruped vs. biped nature of gait and stance. Moreover, mdx mice do not progress to a point that they are nonambulatory; therefore, prolonged positioning in sitting, which is known to accelerate the development of contractures with DMD,3,4 is not an issue in mice lacking dystrophin. These drawbacks of using mdx mice would make it difficult to investigate the effectiveness of certain rehabilitation modalities such as stretching or electrical stimulation on plantarflexion contracture and limit translation to the clinic. Despite these dissimilarities and limitations, there are commonalities that theoretically make the mdx mouse model acceptable for analyzing certain anticontracture strategies. For example, collagen accumulation in gastrocnemius muscle coupled with weak antagonist tibialis anterior muscle is a common feature related to plantarflexion contracture in both mdx mice and boys with DMD. Therefore, it would make sense to investigate the effectiveness of an antifibrotic agent, such as halofuginone, on contracture formation and progression in mdx mice. Halofuginone inhibits collagen formation in the heart and diaphragms of mdx mice and improves motor coordination and cardiac function of the mice.28 The agent's effectiveness on plantarflexion contracture is not known, but hypothetically it would enable mdx mice to have better dorsiflexion ROM, lower passive torque going into dorsiflexion, and lower ratios of passive to active torque about the ankle, relative to untreated mdx mice.
CONCLUSION
The collective results of this study show that mild plantarflexion contractures do exist in mdx mice. Characteristics of the mdx plantarflexion contracture include decreased dorsiflexion ROM, increased passive torque as the ankle is moved into dorsiflexion, and an increased ratio of passive to active torque about the ankle. These contractures seem to be related to increased collagen and fluid contents of the gastrocnemius muscle, indicative of muscle degeneration and replacement of muscle fibers with connective tissue. Overall, the disease process affects the muscles surrounding the ankle of the mdx mouse in a similar manner as in DMD; therefore, the mdx mouse model may be used to devise therapies and test exercise modalities to combat contractures that plague the muscular dystrophies.
ACKNOWLEDGMENTS
We thank Erin Foss, Kevin Gennrich, Leah Amundson, Kristin Herman, and Anthony Jaeger for their contributions in conceiving this project and assisting with the ROM measurements and Jared Bueche, Meghan Conrad, Hannah Dittner, Sheila Emms, Tonia Flategraff, and Sarah Fuller for assistance with the muscle analyses. We thank Marcia Margolis, PT, Jason Kelecic, DPT, and John Day, MD, PhD, for providing us with neuromuscular clinical experiences.
Supported by the Muscular Dystrophy Association grant MDA4143 (to D.A.L.) and NIH grants P30 (to the University of Minnesota Muscular Dystrophy Center) AR057220 and T32 AR07612 (for K.A.B. and J.A.C.). Presented in poster format at the American Physical Therapy Association, Combined Sections Meeting: “Plantarflexion contracture in Duchenne muscular dystrophy.” Ped Physical Ther. 21:88–136, 2009 and “Contracture properties of lower leg muscles due to Duchenne muscular dystrophy.” Ped Physical Ther. 21:88–136, 2009.
Footnotes
Disclosures: Financial disclosure statements have been obtained, and no conflicts of interest have been reported by the authors or by any individuals in control of the content of this article.
REFERENCES
- 1.Hoffman EP, Brown RH, Kunkel LM. Dystrophin: The protein product of the Duchenne muscular dystrophy locus. Cell. 1987;51:919–28. doi: 10.1016/0092-8674(87)90579-4. [DOI] [PubMed] [Google Scholar]
- 2.Sutherland DH, Olshen R, Cooper L, et al. The pathomechanics of gait in Duchenne muscular dystrophy. Dev Med Child Neurol. 1981;23:3–22. doi: 10.1111/j.1469-8749.1981.tb08442.x. [DOI] [PubMed] [Google Scholar]
- 3.McDonald CM. Limb contractures in progressive neuromuscular disease and the role of stretching, orthotics, and surgery. Phys Med Rehabil Clin N Am. 1998;9:187–211. [PubMed] [Google Scholar]
- 4.Brooke MH, Fenichel GM, Griggs RC, et al. Clinical investigation in Duchenne dystrophy: 2. Determination of the “power” of therapeutic trials based on the natural history. Muscle Nerve. 1983;6:91–103. doi: 10.1002/mus.880060204. [DOI] [PubMed] [Google Scholar]
- 5.Do T. Orthopedic management of the muscular dystrophies. Curr Opin Pediatr. 2002;14:50–3. doi: 10.1097/00008480-200202000-00009. [DOI] [PubMed] [Google Scholar]
- 6.McDonald CM, Abresch RT, Carter GT, Fowler WM, Johnson ER, Kilmer DD, Sigford BJ. Profiles of neuromuscular diseases. Duchenne muscular dystrophy. Am J Phys Med Rehabil. 1995;74:S70–92. doi: 10.1097/00002060-199509001-00003. [DOI] [PubMed] [Google Scholar]
- 7.Shimada T. Factors affecting appearance patterns of hip-flexion contractures and their effects on postural and gait abnormalities. Kobe J Med Sci. 1996;42:271–90. [PubMed] [Google Scholar]
- 8.Vignos PJ, Spencer GE, Archibald C. Management of progressive muscular dystrophy in childhood. JAMA. 1963;184:89–96. doi: 10.1001/jama.1963.03700150043007. [DOI] [PubMed] [Google Scholar]
- 9.Eagle M. Report on the muscular dystrophy campaign workshop: Exercise in neuromuscular diseases Newcastle, January 2002. Neuromuscul Disord 2002. 12:975–83. doi: 10.1016/s0960-8966(02)00136-0. [DOI] [PubMed] [Google Scholar]
- 10.Vignos PJ., Jr Physical models of rehabilitation in neuromuscular disease. Muscle Nerve. 1983;6:323–38. doi: 10.1002/mus.880060502. [DOI] [PubMed] [Google Scholar]
- 11.Cros D, Harnden P, Pellissier JF, et al. Muscle hypertrophy in Duchenne muscular dystrophy. A pathological and morphometric study. J Neurol. 1989;236:43–7. doi: 10.1007/BF00314217. [DOI] [PubMed] [Google Scholar]
- 12.Sicinski P, Geng Y, Ryder-Cook AS, et al. The molecular basis of muscular dystrophy in the mdx mouse: A point mutation. Science. 1989;244:1578–80. doi: 10.1126/science.2662404. [DOI] [PubMed] [Google Scholar]
- 13.Gosselin LE, Williams JE, Personius K, et al. A comparison of factors associated with collagen metabolism in different skeletal muscles from dystrophic (mdx) mice: Impact of pirfenidone. Muscle Nerve. 2007;35:208–16. doi: 10.1002/mus.20681. [DOI] [PubMed] [Google Scholar]
- 14.Lynch GS, Hinkle RT, Chamberlain JS, et al. Force and power output of fast and slow skeletal muscles from mdx mice 6–28 months old. J Physiol. 2001;535:591–600. doi: 10.1111/j.1469-7793.2001.00591.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Laws N, Hoey A. Progression of kyphosis in mdx mice. J Appl Physiol. 2004;97:1970–7. doi: 10.1152/japplphysiol.01357.2003. [DOI] [PubMed] [Google Scholar]
- 16.Landisch RM, Kosir AM, Nelson SA, et al. Adaptive and nonadaptive responses to voluntary wheel running by mdx mice. Muscle Nerve. 2008;38:1290–303. doi: 10.1002/mus.21141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chamberlain JS, Metzger J, Reyes M, et al. Dystrophin-deficient mdx mice display a reduced life span and are susceptible to spontaneous rhabdomyosarcoma. Faseb J. 2007;21:2195–204. doi: 10.1096/fj.06-7353com. [DOI] [PubMed] [Google Scholar]
- 18.Kornegay JN, Bogan DJ, Bogan JR, et al. Contraction force generated by tarsal joint flexion and extension in dogs with golden retriever muscular dystrophy. J Neurol Sci. 1999;166:115–21. doi: 10.1016/s0022-510x(99)00118-5. [DOI] [PubMed] [Google Scholar]
- 19.Kornegay JN, Sharp NJ, Schueler RO, et al. Tarsal joint contracture in dogs with golden retriever muscular dystrophy. Lab Anim Sci. 1994;44:331–3. [PubMed] [Google Scholar]
- 20.Baltgalvis KA, Call JA, Nikas JB, et al. The effects of prednisolone on skeletal muscle contractility in mdx mice. Muscle Nerve. 2009;40:443–54. doi: 10.1002/mus.21327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Reddy GK, Enwemeka CS. A simplified method for the analysis of hydroxyproline in biological tissues. Clin Biochem. 1996;29:225–9. doi: 10.1016/0009-9120(96)00003-6. [DOI] [PubMed] [Google Scholar]
- 22.Edwards CA, O'Brien WD. Modified assay for determination of hydroxyproline in a tissue hydrolyzate. Clin Chim Acta. 1980;104:161–7. doi: 10.1016/0009-8981(80)90192-8. [DOI] [PubMed] [Google Scholar]
- 23.Vignos PJ, Wagner MB, Karlinchak B, et al. Evaluation of a program for long-term treatment of Duchenne muscular dystrophy. Experience at the University Hospitals of Cleveland. J Bone Joint Surg Am. 1996;78:1844–52. doi: 10.2106/00004623-199612000-00007. [DOI] [PubMed] [Google Scholar]
- 24.Coulton GR, Curtin NA, Morgan JE, et al. The mdx mouse skeletal muscle myopathy: II. Contractile properties. Neuropathol Appl Neurobiol. 1988;14:299–314. doi: 10.1111/j.1365-2990.1988.tb00890.x. [DOI] [PubMed] [Google Scholar]
- 25.Marden FA, Connolly AM, Siegel MJ, et al. Compositional analysis of muscle in boys with Duchenne muscular dystrophy using MR imaging. Skeletal Radiol. 2005;34:140–8. doi: 10.1007/s00256-004-0825-3. [DOI] [PubMed] [Google Scholar]
- 26.Roig M, Roma J, Fargas A, et al. Longitudinal pathologic study of the gastrocnemius muscle group in mdx mice. Acta Neuropathol. 2004;107:27–34. doi: 10.1007/s00401-003-0773-3. [DOI] [PubMed] [Google Scholar]
- 27.Lovering RM, Porter NC, Bloch RJ. The muscular dystrophies: From genes to therapies. Phys Ther. 2005;85:1372–88. [PMC free article] [PubMed] [Google Scholar]
- 28.Turgeman T, Hagai Y, Huebner K, et al. Prevention of muscle fibrosis and improvement in muscle performance in the mdx mouse by halofuginone. Neuromuscul Disord. 2008;18:857–68. doi: 10.1016/j.nmd.2008.06.386. [DOI] [PubMed] [Google Scholar]