Abstract
Glomus tumors known as paragangliomas are neoplasms arising from the neural crest. They are named according to the place they originate from. Tumors originating from the carotid body at the carotid bifurcation are called Carotid Body Tumors (CBT). Surgical intervention is planned according to the Shamblin classification. 17 patients were operated after being diagnosed with CBT in our clinic between February 2007 and June 2010. 12 (70.5%) of the patients were male, and 5 (29.4%) of the patients were female. The average age was 42 (ages ranging between 32 and 47). Nine of the patients were diagnosed and treated with Shamblin type I tumor, seven of the patients with type II and one patient with type III. Only one patient had bilateral carotid tumor. In all patients with Shamblin type I and II, blunt dissection of the tumor was conducted smoothly by means of thermal cautery in the subadventitial plane. The patient with Shamblin type III had tumor invasion in the carotid artery and adjacent tissues were in an adherent state. Therefore mass resection was carried out by resecting 2 cm of the distal portion of the common carotid artery and 3 cm of the proximal portion of the internal carotid artery. 6 mm of synthetic polytetrafluoroethylene graft was interpositioned between the common carotid artery and the internal carotid artery. External carotid artery was anastomosed to this graft in an end-to-end fashion. The patient developed vocal cord paralysis postoperatively on the lesion side. The patient who underwent bilateral tumor excision developed Baroreflex Failure Syndrome. In the two patients thrombus developed in the internal carotid artery in the early postoperative period. These patients underwent thrombectomy and developed hemiplegia on the lesion side. One of them died on the seventh post-operative day while in follow-up in the intensive care unit. Surgical resection is the recommended treatment for carotid body tumors. Shamblin I and II type tumors’ dimensions and pathological characteristics allow dissection. However Shamblin III tumors may require carotid artery resection and reconstruction due to tissue invasion. The possibility of post-operative cranial nerve paralysis and arterial thrombosis should be taken into account.
Keywords: Carotid body tumor, Carotid artery, Reconstruction, Shamblin classification
Introduction
Glomus tumors known as paragangliomas are neoplasms originating from the neural crest. They are named according to the place they originate from. Tumors caused by carotid bodies at the carotid bifurcation are called Carotid Body Tumors (CBT).
CBT is the most commonly seen head and neck paraganglioma type. They are asymptomatic, slow-growing and generally benign. Early surgical treatment is advisable as soos as diagnosis is established since they may invade or compress adjacent vascular and neural tissues.
Surgical intervention is planned according to the Shamblin classification of the tumor. Surgical treatment involves resection of the mass and maintaining the continuity of arterial structure if needed. In this study, patients who were operated on with the diagnosis of CBT are reported on along with a literature review.
Materials and Methods
17 patients were operated after being diagnosed with CBT in our clinic between February 2007 and June 2010. 12(70.5%) of these patients were male and 5 (29.4%) were female. The age of the patients ranged between 32 and 47, and the average age was 42. Nine of the patients were diagnosed and treated with Shamblin type I tumor, seven of the patients with type II and 1 patient with type III. Only one patient had bilateral carotid tumor.
The main symptom patients exhibited was a slowly growing mass on the neck. Other symptoms included local tremor and ache in the mass site, flushing and pins and needles sensation. The patients did not have any neurological sequelae. The common finding physical examinations revealed was a pulsatile, hard and rubbery mass located below the mandibular angle which was palpable on the carotid arterial trace, mobile on the horizontal plane and immobile on the vertical plane. The minimum and maximum size of the tumors were 2 × 3 cm and 5 × 6 cm, respectively.
To confirm the diagnosis; Doppler ultrasound (USG), computed tomography (CT), digital substraction angiography (DSA) and magnetic resonance angiography (MRA) were performed (Figs. 1 and 2). According to the examinations carotid body tumors were determined to be type 3 in one patient, type 2 in seven patients and type 1 in nine patients.
Fig. 1.
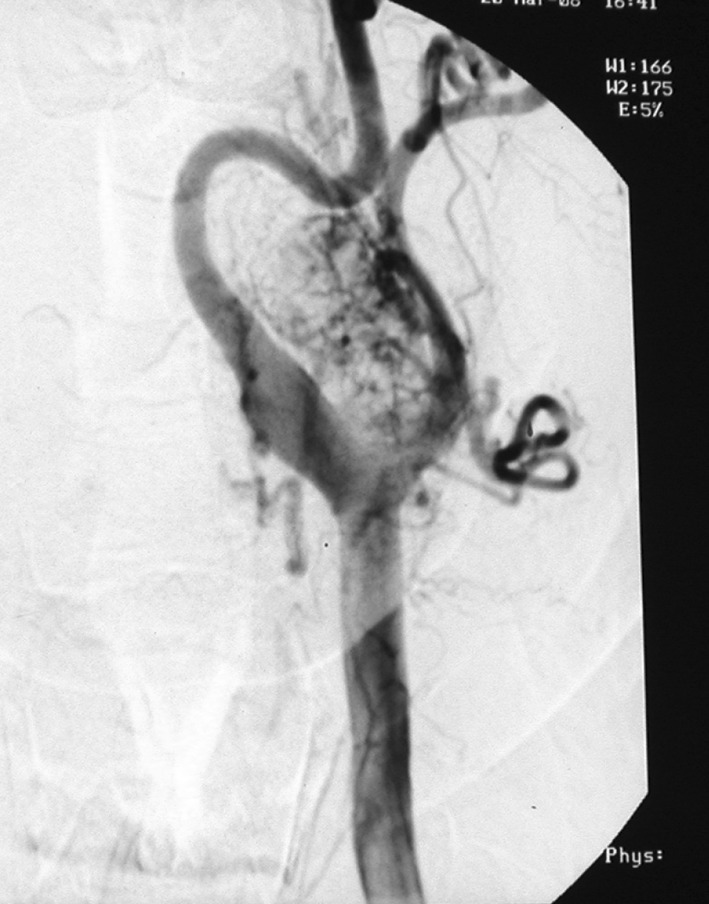
Digital substraction angiography (DSA) image of carotid body tumor
Fig. 2.
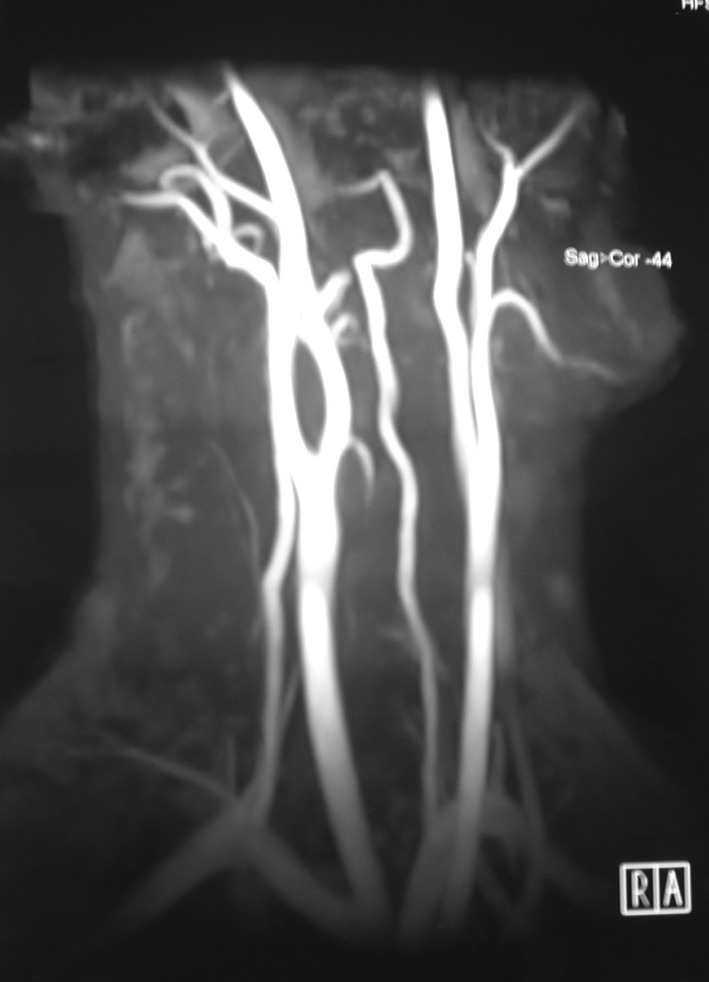
Magnetic resonance angiography (MRA) image of carotid body tumor
Patients were operated under general anaesthesia. Common carotid artery and branches were explored. Vagus and hypoglossal nerves were dissected and seperated from the surgical plane in order to prevent damage. Before the resection of the tumour common carotid artery, internal and external carotid veins were blocked with tape for the control of blood flow. In Shamblin I and II tumors, dissection was attempted from the artery bifurcation and extended onto the superior margin of the tumor. The tumor was dissected completely from the common carotid artery and its branches in the subadventitial plane. Blood flow was not stopped in these patients during the operation since the arterial structure was maintained as it was. The Carotid artery and its branches were clamped before the surgical resection in the Shamblin Type III case. The common carotid artery, the internal and external carotid artery were excised together with the mass, leaving 0.5 cm of healthy tissue as border. The spinal accesory, vagus and hypoglossal nerves were identified and preserved. After the excision of the mass, number 6 polytetrafluoroethylene graft of proper length was implanted between the common carotid artery and internal carotid artery. The external carotid artery was anastomosed to a shunt at the superior level. Blood flow was easy and smooth and no shunt leakage was observed.
Results
A female patient with Shamblin II and a male patient with Shamblin III developed post-operative vocal cord paralysis on the lesion side. Six months of follow-up found no remission in vocal cord paralysis.
None of the patients had pathologies pertaining to hypoglossal and facial nerves.
In the two of the male patients with a type II tumor had an early post-operative loss of consciousness and developed hemiplegia on the same side as the lesion. A Cranial magnetic resonance (MR) of these patients found infarction. Also Doppler USG showed thrombus in the internal carotid artery. These patients underwent thrombectomy without delay and was taken into the intensive care unit for follow-up. One of the them died on the fifth post-operative day.
All masses were confirmed pathologically as carotid body tumors and no trace of malignancy was found. Patients underwent contro once in 6 months in the first postoperative year and once a year in the following years. No evidence of local or distant metastasis was seen at the follow-up checks.
Discussion
The carotid body has autonomic control over the respiratory and cardiovascular system. Paraganglionic cells constituting this body sense pH, pO2 and pCO2 changes in the blood [1]. While the size of the body may vary, the mean size is 5 × 3 × 1.5 mm. Weight for adults range from 1 mg to 47.4 mg, with a mean of 12.1 mg [2].
CBT has a higher occurrence rate in females and bilatarelity is seen in 10% of the cases [3]. 7–9% of the cases have a family history [4]. Most of the lesions are in benign form; only 3–12.5% of the cases are malignant. Malignancy is identified by regional or distant metastasis of these tumors. Local lymph nodes are the most common locations for metastasis [5]. Spontaneus remission of these tumors has not been reported. Surgical treatment involves resection of the mass maintaining the continuity of the arterial structure if needed.
Carotid body tumors are rarely seen, they are asymptomatic, slow-growing and generally benign tumors. Early surgical treatment is advisable as soon as diagnosis is established since they may invade or compress adjacent vascular and neural tissues. A diffuse mass, growing slowly at hyoid bone level on the sternocleidomastoid muscle should be suggestive of this pathology. Usually the mass can be moved laterally but not cephalocaudally since it adheres to the carotid.This is called Fontaine sign [6]. Also sounds and murmurs can be heard through auscultation. Tumor’s progressive growth may cause symptoms like dysphagia, odinophagia and hoarseness because of the anatomical neighbourhood of the 10 and 12 cranial nerves and the carotid vessels [7].
Branchial cyst, parotid gland tumor, carotid artery aneurysm, lateral aberrant thyroid gland, malignant lymphoma, neurofibroma, tuberculosis lymphadenitis and metastatic carcinoma should be taken into consideration when differential diagnosis concerning carotid body tumors is to be established [8]. We did not perform biopsy on patients as a diagnostic method because of its contraindications for carotid body tumors [9].
Ultrasonography, CT, MR, MRA and DSA are requested of patients with suspected CBT for diagnosis. CT is useful in demonstrating the diffusion of the tumor to adjacent structures. MR imaging reveals the involvement between jugular vein, carotid artery and the tumor. Angiography is indispensable, because the hypervascular mass occupying the carotid bifurcation supports the diagnosis and provides preoperative data about the arterial blood support [10]. Recently, MRA has been preferred over MR because it both gives MR image and provides data on blood flow [11]. DSA is the gold standard for the diagnosis of Carotid body tumors [12]. Martinelli et al. demonstrated in their study that preoperative colour coded ultrasound (CCU) revealed the carotid body tumors (though four were not palpable) with 100% sensitivity. CCU allows an early and noninvasive detection of the tumor. The combined use of CCU and somatostatin receptor scintigraphy with both planar and single photon emission tomography provides effective data to identify the tumors and to evaluate their extent and carotid artery infiltration [13].
Shamblin et al. classified the carotid body tumors according to carotid artery involvement [14]. In Type I the carotid artery is minimally involved with the tumor. These kinds of tumors are easily resected. In Type II, the tumor partially encases the carotid arterial structures. These types of tumors are resectable, though difficulty in resection may be experienced. Type III is the most invasive of arterial structures. Surgical dissection of this tumor is not possible because it surrounds the arterial structures completely [15]. Careful subadventitial resection should be chosen as a treatment for Shamblin type I and II carotid body tumors. Treatment of Shamblin type III tumors requires the resection of the external and/or internal carotid artery. If the internal carotid artery is encased by the tumor or has been damaged during resection, reconstruction is essential. Arterial continuity should be maintained through graft interposition in type III cases [16]. The patient with Shamblin type III had tumor invasion in the carotid artery and adjacent tissues were in an adherent state. Therefore mass resection could be carried out by resecting 2 cm of the distal portion of the common carotid artery and 3 cm of the proximal portion of the internal carotid artery. 6 mm of polytetrafluoroethylene interposition was performed between the common carotid artery and internal carotid artery using synthetic graft. The external carotid artery was anastomosed to this graft in an end-to-end fashion. The patient did not have any cerebrovacular sequelae but developed vocal cord paralysis on the same side with the lesion. A follow-up of 1 year showed no remission in paralysis. Dan Ma et al. performed resection of the external carotid artery on five cases and resection of both external and internal carotid arteries in three cases in overall 18 CBT cases [12].
For highly vascular carotid body tumors larger than five centimeters, preoperative embolization (with polyvinyl alcohol and gelfoam) is recommended [17]. A study carried out by Koç et al. reported a 95% reduction in tumor vascularity enabled by the embolization of ascending pharyngeal artery feeding the tumor [10]. However some publications report that embolization could cause embolic ischemia. Though we observed high vascularity in our cases, we did not apply preoperative embolization due to possible complications.
Postsurgical mortality rate is reported to be 5–13% [18, 19], postoperative cranial nerve paralysis rate 32–44% [20] and postoperative cerebrovascular insufficency rate 8–20% [18, 19]. Mortality is observed in one case (8.3%) and two cases (16.6%) developed vocal cord paralysis.
Radiotherapy is indicated to be useful in the treatment of carotid body tumors in recent studies [21]. For this reason radiotherapy is recommended for regional lymph node metastasis of carotid body tumors which are large in size, recurrent and malign.
Conclusion
Carotid body tumors are slow-growing and mostly asymptomatic masses. USG, CT, MRA and DSA play important roles in diagnosis. As the tumor grows, compressing surrounding neurovascular tissues; symptoms like hoarseness, dysphagia and syncope may arise. Also, possible metastases and progressive local invasion may lead to an inoperable situation. Surgical treatment should be preferred for patients who are under the age of 50 and expected to have a long lifespan. Surrounding neurovascular tissues should be identified carefully and not be damaged during surgical resection. Radiotherapy should also be remembered as a treatment option for cases with recurring tumors and metastasis.
References
- 1.Baysal BE, Myers EN. Etiopathogenesis and clinical presentation of carotid body tumors. Microsc Res Tech. 2002;59:256–261. doi: 10.1002/jemt.10200. [DOI] [PubMed] [Google Scholar]
- 2.Netterville JL, Reilly KM, Robertson D, Reiber ME, Armstrong WB, Childs P. Carotid body tumors: a review of 30 patients with 46 tumors. Laryngoscope. 1995;105:115–126. doi: 10.1288/00005537-199502000-00002. [DOI] [PubMed] [Google Scholar]
- 3.Ridge BA, Brewster DC, Darling RC, Cambria RP, LaMuraglia GM, Abbott WM. Familial carotid body tumors: incidence and implications. Ann Vasc Surg. 1993;7:190–194. doi: 10.1007/BF02001015. [DOI] [PubMed] [Google Scholar]
- 4.Pacheco-Ojeda L. Malignant carotid body tumors: report of three cases. Ann Otol Rhinol Laryngol. 2001;110:36–40. doi: 10.1177/000348940111000107. [DOI] [PubMed] [Google Scholar]
- 5.Rodríguez-Cuevas S, López-Garza J, Labastida-Almendaro S. Carotid body tumors in inhabitants of altitudes higher than 2000 meters above sea level. Head Neck. 1998;20:374–378. doi: 10.1002/(SICI)1097-0347(199808)20:5<374::AID-HED3>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 6.Sarı M, Serin GM, Özdemir N ve Ark (2006) Karotid Cisim Tümörlerinde Tedavi Yaklaşımlarımız. KBB-Forum 5(3):113–117
- 7.Mey AG, Jansen JC, Baalen JM. Management of carotid body tumors. Otolaryngol Clin North Am. 2001;34:907–924. doi: 10.1016/S0030-6665(05)70354-6. [DOI] [PubMed] [Google Scholar]
- 8.Matticari S, Credi G, Pratesi C, Bertini D. Diagnosis and surgical treatment of the carotid body tumors. J Cardiovasc Surg. 1995;36:233–239. [PubMed] [Google Scholar]
- 9.Plukker JT, Brongers EP, Vermey A, Krikke A, Dungen JJ. Outcome of surgical treatment for carotid bodyparaganglioma. Br J Surg. 2001;88:1382–1386. doi: 10.1046/j.0007-1323.2001.01878.x. [DOI] [PubMed] [Google Scholar]
- 10.Koç A, Bilgili AM, Altıntaş O, Han T. Bir Olgu Nedeniyle Glomus Karotikum Tümörleri ve Cerrahi Tedavi Öncesi Embolizasyon. Türk Otolarengoloji Arşivi. 2004;42(2):120–123. [Google Scholar]
- 11.Biliciler N, Yazıcıoğlu E, Mirata D, Üstündağ E. Glomus caroticum. Kulak Burun Bogaz Ihtis Derg. 1992;2:336–339. [Google Scholar]
- 12.Ma Dan, Liu Min, Yang Hua, Ma Xiaogan, Zhang Chaojun. Diagnosis and surgical treatment of carotid body tumor: a report of 18 cases. J Cardiovasc Dis Res. 2010;1(3):122–124. doi: 10.4103/0975-3583.70905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Martinelli O, Irace L, Massa R, Savelli S, Giannoni F, Gattuso R, Gosetti B, Benedetti-Valentini F, Izzo L. Carıtid body tumors: radioguided surgical approach. J Exp Clin Cancer Res. 2009;28:148. doi: 10.1186/1756-9966-28-148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shamblin WR, Remine WH, Sheps SG, et al. Carotid body tumor (chemodectoma): clinicopathologic analysis of 90 cases. Am J Surg. 1971;122:732–739. doi: 10.1016/0002-9610(71)90436-3. [DOI] [PubMed] [Google Scholar]
- 15.Kunt A, Bulut F, Demir CS (2003) Karotis cisim tümörleri. Türk Göğüs Kalp Damar Cer Derg 11:198–200
- 16.Erentuğ V, Bozbuğa NU, Sareyyüpoğlu B, Mansuroğlu D, Antal A, Kırali K ve ark (2004) Karotis cisim tümörlerinde cerrahi yaklaşımlar. Türk Göğüs Kalp Damar Cer Derg 12:277–279
- 17.Hallett JW, Jr, Nora JD, Hollier LH, Cherry KJ, Jr, Pairolero PC. Trends in neurovascular complications of surgical management for carotid body and cervical paragangliomas: a 50-year experience with 153 tumors. J Vasc Surg. 1988;7:284–291. [PubMed] [Google Scholar]
- 18.Hirsch JH, Killien C, Troupin RH. Bilateral carotid body tumours and cyanotic heart disease. AJR Am J Roentgenol. 1980;134:1073–1075. doi: 10.2214/ajr.134.5.1073. [DOI] [PubMed] [Google Scholar]
- 19.Keating JF, Miller GA, Keaveny TV. Carotid body tumours: report of six cases and a review of management. J R Coll Surg Edinb. 1990;35:172–174. [PubMed] [Google Scholar]
- 20.Persky MS, Setton A, Yasunari N, Hartman J, Frank D, Berenstein A. Combined endovascular and surgical treatment of head and neck parangliomas: a team approach. Head Neck. 2002;24:423–431. doi: 10.1002/hed.10068. [DOI] [PubMed] [Google Scholar]
- 21.Gerosa M, Visca A, Rizzo P, Foroni R, Nicolato A, Bricolo A. Glomus jugulare tumors: the option of gamma knife radiosurgery. Neurosurgery. 2006;59:561–569. doi: 10.1227/01.NEU.0000228682.92552.CA. [DOI] [PubMed] [Google Scholar]


