Abstract
Sera from 14 408 market sows from the Canadian domestic swine herd were tested for trichinellosis using an indirect-ELISA as a screening test and a competitive ELISA for confirmatory testing. Three sera (0.02%) gave low level reactions on the competitive ELISA. These reactions were considered nonspecific, and this designation was supported by data from previous and subsequent national surveys, in which serologic, trichinoscopic, and digestion test methodologies was used. This study provides additional evidence that Canada is free of trichinellosis in domestic swine.
Introduction
The use of enzyme-linked immunosorbent assays (ELISA) for the diagnosis of swine trichinellosis has been extensively studied (1,2,3,4,5,6,7). The World Organisation for Animal Health's Office International des Epizooties (OIE) has adopted this technique as a standardized diagnostic test (8). Accordingly, the Canadian Trichinellosis Control Program administered by the Science Division of the Canadian Food Inspection Agency (CFIA) uses this test to survey mature swine populations across Canada every 5 y in order to demonstrate freedom from this disease.
The prevalence of swine trichinellosis in Canada has been declining for several decades. Approximately 2% (n = 729) of tested pigs from Manitoba, Ontario, Quebec, and New Brunswick were found to be infected with the parasite in 1937 (9). In 1952, 0.4% (n = 1002) of pigs tested using the digestion technique were reported to have the parasite in Atlantic Canada (10). Prevalence in the same area over the period of 1968–1976 was estimated at 0.13% (n = 68 451) by the digestion technique (11). Since that time, only sporadic cases have been identified (12). National surveys have shown the Canadian national swine herd to be free of trichinellosis: 15 318 porcine sera were tested in 1985 (13), with 4 (0.026%) showing elevated titers (13). Pigs housed on the farms from which these animals originated were investigated, but no further seropositive animals were identified. Likewise, in 1990, more than 15 000 porcine sera were tested and 19 were found positive (unpublished observations). The results of the latest national serological survey for trichinellosis are presented here.
Materials and methods
Samples were collected from culled sows slaughtered at federally inspected abattoirs in all provinces and at provincially inspected abattoirs in Ontario, Manitoba, Alberta, and British Columbia over a period of 20 wk (November 1996 to April 1997). The number of sows sampled in each region of Canada reflected the proportion of swine relative to the national herd population. Sample collection from provincial abattoirs was necessary to maintain geographical representation, because Alberta, Manitoba, and Ontario exported most sows to the United States for slaughter, and in British Columbia, most sows were killed in provincial establishments. No set numbers of samples were assigned to any abattoir; rather, sampling continued until the goal for a specific region was met. Sows were surveyed because their prolonged presence in the herd over time would increase their risk of acquiring trichinellosis, as compared with the more transient market hogs. Therefore, freedom from disease in sows would be indicative of disease freedom in the general swine population.
Blood samples (10 mL) were allowed to clot over a 24-hour period; each was identified by ear-tag number, back-slap tattoo, and province of origin. Hemolyzed samples were discarded. Serum from each sample was collected and stored refrigerated, prior to shipping to the Animal Disease Research Institute (ADRI), Nepean, Ontario. A 400-μL aliquot from each sample was shipped to the Centre for Animal Parasitology (CAP) and stored at -20°C until tested for trichinellosis.
An indirect ELISA (i-ELISA) was used as a screening test to detect the presence of antibodies against Trichinella spp. Viable T. spiralis larvae, isolated from ground muscle tissue of infected laboratory rats by digestion in a 1% pepsin + 1% HCl solution at 37°C, were used as a source of excretory-secretory (ES) antigen. Larvae were cultured as described previously (3), with the exceptions that the culturing conditions were 20 h at 37°C (10% CO2 atmosphere) in Dulbecco's modified Eagle medium containing 10 mM HEPES and ampicillin (100 μg/mL). The retained volume after filtering was collected, diluted with phosphate-buffered saline (PBS) to a standard of 5 mg/mL total protein content, and stored frozen at -20°C.
Excretory-secretory antigen (100 μL of 5 μg/mL ES antigens in 50 mM carbonate buffer at pH 9.6) was used as the target for Trichinella-specific antibodies in an ELISA, similar to that described by Gamble et al (3). Briefly, coating of microtiter plates was performed overnight at 4°C. The wells were washed 3 times with 300-μL volumes of PBS containing 0.5% Tween-20. Sera were diluted 1/75 with PBS containing 0.05% Tween-20 and 10 mM ethylenediamine-tetraacetic acid (EDTA) and dispensed (100 μL) into each of 3 wells. Each microtiter plate contained serum from 2 known positives (strong and weak) and a pool of negative sera, known to be negative by enzyme digestion testing of muscle tissue from the source animals. Each control serum or serum pool was tested in triplicate. There were also 3 wells of a “No Serum” control per plate that contained only PBS-Tween-EDTA buffer. Plates were incubated at room temperature for 2 h, protected from dust by a plastic lid. The plates were washed 3 times. Appropriately diluted goat anti-swine IgG (heavy and light chain; KPL, Gaithersburg, Maryland, USA) conjugated with alkaline phosphatase (KPL) was added at 100 μL per well and incubated for 2 h at room temperature. The plates were again washed 3 times. The chromogenic reaction was initiated by the addition of prepared p-nitrophenyl phosphate substrate in diethanolamine buffer at 50 μL/well. To minimize plate-to-plate and day-to-day variation in test performance, color development was assessed by microplate spectrophotometer controlled by specific computer software (Monitor v1.2; Histotech Ltd., Saskatoon, Saskatchewan). The software monitored color development in designated wells containing the strong positive control serum. Optical density data measured at 405 nm was collected from the reader (Titertek, Huntsville, Alabama, USA) as soon as the mean of the strong positive controls wells (n = 3) met a predetermined color density [0.5 optical density (OD) units]. Values obtained in this ELISA that were 3 times higher than the mean of the triplicate pooled negative serum on that plate were considered significant and selected for further testing. Values below these standards were considered negative.
Competitive inhibition ELISA (c-ELISA) was performed, as previously described (7), on samples reacting above the negative threshold on i-ELISA. Greater than 50% color reduction was interpreted as positive.
Results
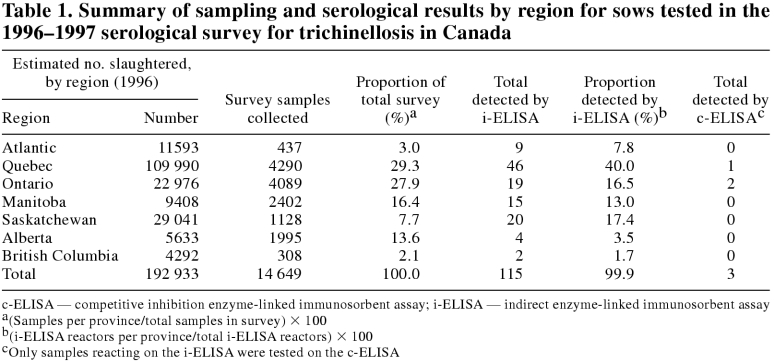
There were 14 649 samples collected during the swine serological survey. Table 1 shows the distribution of samples by province. Of these, 14 408 were tested for antibodies to T. spiralis and 241 were discarded, because they were unfit or provided insufficient serum.
Table 1.
By i-ELISA, 115 of the 14 408 (0.80%) samples tested gave reactions above the negative cut-off. The strong positive serum control (0.522, s = 0.050), was considered positive on every plate tested (n = 471) and had a coefficient of variation of 9.5%. Color development for the weak positive serum control (0.197, s = 0.046) fell between 3 and 4 times the mean of the triplicate pooled negative serum on that plate (4 × negative cut-off = 0.239, s = 0.076 and 3 × negative cut-off = 0.180, s = 0.057). Pooled negative sera used as a reference to determine cut-off value had a mean OD of 0.061, s = 0.019. The mean OD of all surveyed sera was 0.063, s = 0.025 (n = 14 408).
Three of the 115 samples that reacted on the i-ELISA showed competitive inhibition greater than 50% on the c-ELISA. The negative control defined maximal competitive antibody binding and was used as the standard for comparison of color development. Mean reduction in color generation compared with negative controls for all retested samples was 10.6%, s = 28.2%. The positive control inhibited color development by 69.5%, s = 9.7%. The reduction in color generation compared to negative controls for the 3 positive samples was 50.3%, 52.4%, and 54.1%.
Discussion
In accordance with the International Animal Health Code of the OIE (14), serological surveys are conducted on slaughter sow populations in Canada at 5-year intervals to assess the national swine herd for trichinellosis. The survey, which was begun in 1996 using an i-ELISA, sampled 14 408 animals, of which 115 (0.8%) showed reactions above the negative cut-off. The c-ELISA was chosen as the confirmatory serological test because of published data indicating that it had superior specificity (7). A confirmatory c-ELISA was done on the 115 samples detected by the i-ELISA; this failed to show strong serological evidence of trichinellosis in the national herd, as the 3 (0.02%) samples that it detected were marginally above the negative cut-off. Digestion tests on tissues from serologically reacting sows would have determined the validity of the results, but tissue collection was not possible under the conditions of the survey. However, the absence of trichinellosis in Canada was supported by surveillance data obtained previously (12) and has been further supported by subsequent surveys. Previous data included the 553 574 swine that were tested by trichinoscopy between 1980 and 1995, in which 3 positive swine were found in Nova Scotia, one each in 1990, 1993, and 1996, and the 15 328 hogs that were tested in the national swine serological survey in 1990 with 11 reactors, which were shown to be falsely positive by tissue digestion (12). In addition to the type of national serological survey described here, ongoing national surveillance by the CFIA includes a program in which 30 000 market swine are tested annually by laboratories using a quality controlled and validated digestion test method (15). In 1998, approximately 30 000 swine, randomly selected from the 14.7 million swine that were slaughtered, were tested by trichinoscopy or digestion techniques and found to be negative (16). A similar survey, in which approximately 30 000 market swine were tested by using digestion tests, was completed in 2000 and all samples were negative (unpublished observations, CFIA).
The last known case of trichinellosis in swine occurred in Atlantic Canada in 1996 (12). A single swine herd from Atlantic Canada was identified, based on a low level reaction on the i-ELISA alone, and a trace-back retrospective investigation performed. It was found that all sows from this herd were routinely slaughtered at a federally inspected abattoir, where they were tested for trichinellosis by using a trichinoscope and found to be negative. There was no evidence of trichinellosis on the premises of origin.
The regional differences in nonspecific reactor rates, as shown in Table 1, are probably related to management factors and sampling bias. This survey was designed to screen the culled sow population and, on a regional basis, did not control for over representation from individual producers or for regional differences in management that might alter exposure to other nematodes with cross reacting antigens. Future improvements in sample selection are possible, but true randomization, particularly in regions, is difficult, as sampling is dependent on voluntary submission of sows for slaughter.
Footnotes
Acknowledgments
The authors give thanks to Steven Loran (CAP) and Lori Renneberg (CAP) for their dedication to this project and expert technical assistance, to André Doré (CFIA) and Sarah Parker (CAP) for critical reading of the manuscript, and to David Gall (CFIA) for providing the geographic location data for the samples reacting on ELISA tests. CVJ
This work was supported by funding from Agriculture and Agri-Food Canada and the Canadian Food Inspection Agency. Greg D. Appleyard was supported by an Agriculture and Agri-Food Canada Fellowship in Biotechnology.
Address correspondence and reprint requests to Dr. Alvin Gajadhar.
This paper has been peer reviewed.
References
- 1.Van Knapen F, Franchimont JH, Ruitenberg EJ, et al. Comparison of four methods for early detection of experimental Trichinella spiralis infections in pigs. Vet Parasitol 1981;9:117–123. [DOI] [PubMed]
- 2.Murrell KD, Anderson WR, Schad GA, et al. Field evaluation of the enzyme-linked immunosorbent assay for swine trichinosis: efficacy of the excretory-secretory antigen. Am J Vet Res 1986;47: 1046–1049. [PubMed]
- 3.Gamble HR, Anderson WR, Graham CE, Murrell KD. Diagnosis of swine trichinosis by enzyme-linked immunosorbent assay (ELISA) using an excretory-secretory antigen. Vet Parasitol 1983;13:349–361. [DOI] [PubMed]
- 4.Gamble HR, Rapic D, Marinculic A, Murrell KD. Evaluation of excretory-secretory antigens for the serodiagnosis of swine trichinellosis. Vet Parasitol 1988;30:131–137. [DOI] [PubMed]
- 5.Smith HJ. Evaluation of the ELISA for the serological diagnosis of trichinosis in Canadian swine. Can J Vet Res 1987;51:194–197. [PMC free article] [PubMed]
- 6.Gamble HR. Detection of trichinellosis in pigs by artificial digestion and enzyme immunoassay. J Food Prot 1996;59:420–425. [DOI] [PubMed]
- 7.Gamble HR, Graham CE. Comparison of monoclonal antibody-based competitive and indirect enzyme-linked immunosorbent assays for the diagnosis of swine trichinosis. Vet Immunol Immunopathol 1984;6:379–386. [DOI] [PubMed]
- 8.OIE Standards Commission. Trichinellosis. In: Manual of Standards for Diagnostic Tests and Vaccines. Office International des Epizooties, 2000:322–327.
- 9.Cameron TW. Investigations on Trichinosis in Canada. I. A preliminary survey of the incidence of Trichinella spiralis in hogs in eastern Canada. Can J Res 1938;16:89–92.
- 10.Frank JF. A study of the incidence of trichinosis in swine in the maritime provinces. Can J Comp Med 1952;16:73–77. [PMC free article] [PubMed]
- 11.Smith HJ, Anzengruber A, DuPlessis DM. Current status of trichinosis in swine in the Atlantic provinces. Can Vet J 1976;17: 72–75. [PMC free article] [PubMed]
- 12.Gajadhar AA, Bisaillon JR, Appleyard GD. Status of Trichinella spiralis in domestic swine and wild boar in Canada. Can J Vet Res 1997;61:256–259. [PMC free article] [PubMed]
- 13.Smith HJ, Snowdon KE, Bishop LJ. Prevalence of trichinosis in Canadian swine determined serologically by enzyme-linked immunosorbent assay. Can J Vet Res 1988;52:392–393. [PMC free article] [PubMed]
- 14.Trichinellosis. In: International Animal Health Code. Paris: Office International des Epizooties, 1992:305–306.
- 15.Forbes L, Gajadhar A. A validated Trichinella digestion assay and an associated sampling and quality assurance system for use in testing pork and horse meat. J Food Prot 1999;62:1308–1313. [DOI] [PubMed]
- 16.Inch C, Doré A. The impact of Canada's animal health status on trade. Can Vet J 1999;40:435–439. [PMC free article] [PubMed]