Abstract
Biomaterials that modulate innate and adaptive immune responses are receiving increasing interest as adjuvants for eliciting protective immunity against a variety of diseases. Previous results have indicated that self-assembling β-sheet peptides, when fused with short peptide epitopes, can act as effective adjuvants and elicit robust and long-lived antibody responses. Here we investigated the mechanism of immunogenicity and the quality of antibody responses raised by a peptide epitope from P. falciparum circumsporozoite (CS) protein, (NANP)3,conjugated to the self-assembling peptide domain Q11. The mechanism of adjuvant action was investigated in knockout mice with impaired MyD88, NALP3, TLR-2, or TLR-5 function, and the quality of antibodies raised against (NANP)3-Q11 was assessed using a transgenic sporozoite neutralizing (TSN) assay for malaria infection. (NANP)3-Q11 self-assembled into nanofibers, and antibody responses lasted up to 40 weeks in C57BL/6 mice. The antibody responses were T cell- and MyD88-dependent. Sera from mice primed with either irradiated sporozoites or a synthetic peptide, (T1BT*)4-P3C, and boosted with (NANP)3-Q11 showed significant increases in antibody titers and significant inhibition of sporozoite infection in TSN assays. In addition, two different epitopes could be self-assembled together without compromising the strength or duration of the antibody responses raised against either of them, making these materials promising platforms for self-adjuvanting multi-antigenic immunotherapies.
INTRODUCTION
Vaccines based on peptide and protein subunits that focus the host’s immune response on epitopes known to play a role in protective immunity are attractive owing to their compositional definition and their advantageous safety profiles [1-3]. However, the immunogenicity of subunit vaccines depends heavily on adjuvants, many of which currently suffer from imprecise chemical definition, instability, local toxicity, or an inability to confer optimal protection [4, 5]. In recent years, the presentation of peptides and small molecule antigens on the surface of macromolecular assemblies has emerged as a powerful strategy for eliciting immune responses without adjuvants [6-13]. Antigenic formulations composed of peptide epitopes coupled to lipopeptides [10-12, 14], coiled-coil oligomerization domains [8, 9], polymers [15], and virus-like particles [7, 13, 16] have demonstrated excellent adjuvanting capability and induced robust antibody and cellular responses. We recently reported that a self-assembling β-sheet fibrillar peptide, Q11 (Ac-QQKFQFQFEQQ-Am), can act as an immune adjuvant when fused to a peptide antigen [6]. Peptide ligands, epitopes, or small chemical moieties that are appended to the N-terminus of Q11 can be displayed on the surface of the nanofibers, retaining their biofunctionality [17-19]. Fusion peptides containing Q11 and the antigenic peptide OVA323-339 (OVA323-339-Q11), raised robust long-lived, anti-OVA antibody responses in mice, which were comparable to OVA323-339 administered in complete Freund’s adjuvant (CFA) [6, 20]. In contrast, Q11 by itself was non-immunogenic, even when delivered in CFA. The antibody response to OVA323-339-Q11 was found to be dependent on CD4+ T cells, and disrupting fibril formation via targeted mutations in the core of Q11 also led to loss of antibody responses [20]. Another self-assembling peptide KFE8 (Ac-FKFEFKFE-Am) was also shown to have an immunological profile similar to Q11 when conjugated to OVA323-339 suggesting that self-assembling peptides, while being non-immunogenic themselves, can act as potential immune adjuvants for applications in vaccine development and immunotherapies [20].
To develop a better understanding of the immune responses associated with self-assembling peptides, we sought to investigate the mechanisms through which Q11 nanofibers activate the immune system and elicit robust antibody responses. It is now well known that most adjuvants act through the stimulation of the innate immune system, which further regulates the adaptive immune response [4, 21]. Antigen presenting cells like dendritic cells (DCs) express pattern recognition receptors (PRRs) that recognize molecular signatures, leading to their maturation and expression of co-stimulatory molecules along with antigen processing and presentation [22, 23]. The most studied PRRs are the toll-like receptors (TLRs), which are found on the surface of DCs and macrophages and in their intracellular compartments [24]. Due to their fibrillar morphology, which is similar to bacterial flagellin and curli, we hypothesized that Q11 nanofibers could activate the innate immune system through specific TLRs; conversely, due to their particulate nature similar to alum, they could activate alternative pathways [25-27]. Alum has been shown to act through the inflammasome pathway involving NOD-like receptors (NLRs) [27]. Also, previous work demonstrating the adjuvant activity of Q11 was limited to the model antigen OVA323-339. Therefore, to investigate the mechanism of adjuvant activity and quality of the antibody response, we chose the malaria peptide antigen (NANP)3 (NANPNANPNANP) derived from circumsporozoite (CS) protein of P. falciparum [28]. Antibodies recognizing the tandem repeat peptide, (NANP)n, have been identified as a major protective component in the sera of animals immunized with sporozoites and people living in malaria-endemic regions [29, 30]. Many potential malaria vaccines based on synthetic peptides [31], multiple antigenic peptides (MAPs) [32], polyoxime branched peptides [33], and virus-like particles (VLPs) [8, 34] have utilized (NANP)3 as a major protective epitope. By inhibiting sporozoite motility, anti-NANP antibodies block host hepatocyte invasion and prevent the blood-stage infection that causes clinical disease [35].
In this work, the malaria peptide epitope (NANP)3 was conjugated to Q11, and the fibrillization behavior and secondary structure were investigated. The longevity of antibody responses was investigated in C57BL/6 mice and the mechanism of adjuvant action was investigated using knockout mice lacking T cell receptors, toll-like receptors, or inflammasome complexes. Protection against infection with murine malaria transgenic sporozoites was investigated using a prime/boost regimen with (NANP)3-Q11, alone or in mice primed with irradiated sporozoites (Irr. Spz.) or the multi-epitope peptide derivative (T1BT*)4-P3C [33]. Inhibition of sporozoite infection was measured using TSN assays [36, 37]. Finally, to test the feasibility of raising antibody responses against multiple different antigens administered together on the same nanofibers, mice were immunized with co-assemblies of (NANP)3-Q11 and OVA323-339-Q11, and the antibody responses against both antigens were measured.
MATERIALS AND METHODS
Peptide Synthesis and Purification
Peptides Q11 (Ac-QQKFQFQFEQQ), (NANP)3-Q11 (NANPNANPNANP-SGSG-Q11), and OVA-Q11 (ISQAVHAAHAEINEAGR-SGSG-Q11), were synthesized on a CSBio136-XT peptide synthesizer using standard Fmoc chemistry. Peptides were purified using reverse-phase HPLC and water/acetonitrile gradients. The peptides were lyophilized and stored at −20 °C until further use. Peptide identity and purity (>90% for all peptides used in the study) were confirmed by MALDI-MS and HPLC, respectively. Endotoxin levels of all formulations were tested using a limulus amebocyte lysate (LAL) chromogenic end point assay (Lonza, USA) at the same volume and peptide concentration used for immunizations. Endotoxin levels in all immunization formulations were found to be less than 0.22 EU/mL within acceptable limits [38]. The polyoxime peptide (T1BT*)4-P3C, containing the minimal B epitope (NANP)3, T helper epitope (T1-DPNANPNVDPNANPNV), universal T epitope (T*-EYLNKIQNSLSTEWSPCSVT), and an endogenous adjuvant palmitoyl-S-glyceryl cysteine (P3C), was synthesized as described in [33] and [39].
Transmission Electron Microscopy
Stock solutions of 1 mM peptides were allowed to fibrillize in water overnight at 4 °C, diluted in PBS to 0.25 mM, and further incubated for 4 h at room temperature for OVA-Q11 or overnight at 4 °C for (NANP)3-Q11. Peptide solutions were then pipetted onto carbon-coated 200 mesh lacey grids (Electron Microscopy Sciences). For the co-assembled fibrils, OVA-Q11 and (NANP)3-Q11 peptides were mixed as dry powders, dissolved in deionized water, and diluted in PBS to produce working peptide concentration of 0.25 mM. After incubating overnight, peptide solutions containing the co-assembled fibers were applied to the grids. The grids were stained with 1% uranyl acetate for 2 minutes and imaged with a FEI Tecnai F30 transmission electron microscope (TEM).
Circular dichroism spectroscopy
An AVIV 215 circular dichroism spectropolarimeter was used with 0.1 cm path length quartz cells. Stock solutions were prepared by dissolving the peptides in ultrapure water (Millipore Milli-Q system) and diluting them to a working concentration of 1 mM. The wavelength range was 190-260 nm, the scanning speed was 0.5 nm/sec, and the bandwidth was 0.5 nm. Each spectrum is the average of three scans. Under the solution conditions described, adequate signal strength was observed at wavelengths up to 200 nm. The solvent background was subtracted and resultant CD signals were converted to mean residue ellipticity.
Animals and Immunizations
Peptides were dissolved in sterile water (8 mM stock), incubated overnight at 4 °C, and diluted in sterile PBS (2 mM working concentration) prior to immunizations. For (NANP)3-Q11, peptides were additionally incubated at 4 °C in PBS overnight to ensure complete fibril formation, as the kinetics of assembly for (NANP)3-Q11 was observed to be slower than for the previously investigated OVA-Q11 [6]. To prepare co-assembled fibers of OVA-Q11 and (NANP)3-Q11, the peptides were combined as dry powders, mixed thoroughly, and dissolved in sterile water. The peptides were allowed to incubate overnight at 4 °C and diluted to working concentrations with sterile PBS. Female C57BL/6 (B6), B6.129P2-Tcrbtm1MomTcrdtm1Mom/J (T cell receptor knockout), B6.129-Tlr2tm1Kir/J (TLR-2 knockout), B6.129P2(SJL)-MyD88tm1.1Defr/J (MyD88 knockout), and BALB/c were purchased from Jackson Labs. Breeder pairs of the TLR-5 knockout mice were the kind gift of Dr. A.T. Gewirtz, Emory University. Mice were immunized subcutaneously in the flank with two 50 μL injections containing 100 nmol of peptide each and boosted at 28 days with half the amount of the primary immunization. Blood was drawn via the submandibular maxillary vein, and sera were stored at −80 °C until analysis. In all animal work, institutional guidelines for the care and use of laboratory animals were strictly followed under a protocol approved by the University of Chicago’s and New York University’s Institutional Animal Care and Use Committees.
Enzyme-linked Immunosorbent Assay (ELISA)
ELISA plates (eBioscience) were coated with 20 μg/mL of peptide in PBS overnight at 4 °C and blocked with 200 μL of 1% BSA in PBST (0.5% Tween-20 in PBS) for 1 h. Serum was applied (1:100, 100 μL/well) for 1 h at room temperature followed by peroxidase-conjugated goat anti-mouse IgG (H+L) (Jackson Immuno Research) (1:5000 in 1 % BSA-PBST, 100 μL/well). Plates were developed using TMB substrate (100 μL/well, eBioscience) and absorbance values were read at 450 nm. Absorbance values of PBS (no antigen) coated wells were subtracted to account for background. The plates were washed between each step with PBST. Serum ELISA techniques for mice primed with Irr. Spz. or (T1BT*)4-P3C peptide and boosted with (NANP)3-Q11 have been described in detail elsewhere [40]. ELISAs for the knockout mice and corresponding wild type mice were conducted at the following days and serum dilutions post-immunization: MyD88 KO mice (day 35, 1:100), TLR-2 KO mice (day 35, 1:100), TLR-5 KO mice (day 43, 1:160), and NALP3 KO mice (day 10, 1:100).
Boosting pre-infected mice with (NANP)3-Q11
PfPb, the recombinant P. berghei rodent malaria parasite bearing P. falciparum CS protein repeats, was generated as described previously [41]. Mice (n=8) were primed with 2 doses of irradiated PfPb sporozoites through 15-20 mosquito bites per mouse 14 days apart, and pre-boost sera were collected up to 93 days after infection. A second cohort of mice was immunized with a single dose of the synthetic vaccine construct, (T1BT*)4-P3C, as previously described [39], and pre-boost sera was collected for up to 107 days after immunization. Antibody titers were equivalent at 93 days and 107 days after priming with irradiated sporozoites and (T1BT*)4-P3C peptide respectively. Half the mice in each cohort (n=4) were then boosted with 100 nmol (NANP)3-Q11, and the other half did not receive boosting. Geometric mean antibody titers against sporozoites or (T1B) repeat peptide in the sera were subsequently evaluated by ELISA.
Transgenic Sporozoite Neutralizing Assay (TSNA)
The detailed methods for the transgenic sporozoite neutralization assay have been described elsewhere [37]. Briefly, 2×104 PfPb sporozoites were incubated with antisera from immunized mice or control mice at 1:5 dilution for 45 min. The sporozoites were then added to wells containing HepG2 hepatoma cell cultures. 48 h post infection, cells were harvested, and parasites were measured by quantitative real-time PCR, using primers for parasite 18 S rRNA.
Statistical analysis
Data represent the mean and standard deviation within a group of mice. Statistical analysis was performed by Student’s t-test or ANOVA with Tukey or Bonferroni post-hoc comparisons, with p-values < 0.05 considered significant.
RESULTS
(NANP)3-Q11 self-assembled into nanofibers and adopted a β-sheet structure
By TEM, it was observed that (NANP)3-Q11 self-assembled into fibrous structures similar to Q11 and other Q11 derivatives. Fibril formation was detected as early as 30 min after the addition of PBS (Fig 1a); however, thicker fibrils were observed after overnight incubation (Fig 1b). Secondary structure analysis of (NANP)3-Q11 indicated a predominant β-sheet character similar to Q11 (Fig 1c). In previous studies, similar fibril morphology and secondary structure were observed when cell-binding ligands, small chemical moieties, or antigenic peptides were conjugated to Q11 [6, 17-19]. Collectively, these data indicated that like these previous peptides, (NANP)3-Q11 could also self-assemble into β-sheet rich nanofibers. For many previously studied Q11-based peptides including those containing ovalbumin epitopes [6] and cell binding ligands of various charge and hydrophobicity [17], the ligand or epitope domain was found to be enriched on the surface of the peptide fibrils using TEM and immunogold labeling. Although immunogold labeling of the malaria epitope was not performed for (NANP)3-Q11 in the present study, the morphological similarities between (NANP)3-Q11 fibrils and previous Q11 fibrils make it likely that the (NANP)3 epitope domains were also enriched on the nanofibers’ surfaces.
Figure 1.
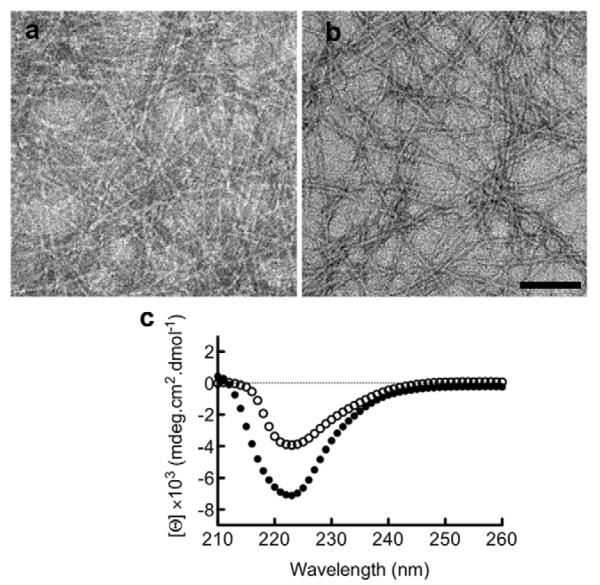
(NANP)3-Q11 fibril formation and structural analysis. TEM images of (NANP)3-Q11 nanofibers after incubation in PBS for 30 min (a) and overnight (b). Scale bar = 100 nm. Secondary structure of (NANP)3-Q11 (open circles) and Q11 (solid circles) showing beta sheet structure.
Antibody responses to (NANP)3-Q11 were long-lived and T cell-dependent
In B6 mice, unadjuvanted self-assembled (NANP)3-Q11 raised strong antibody responses that were detectable for at least 40 weeks, the duration of the study (Fig. 2a). Average titers peaked at the twelfth week, but a significant booster response was not observed. Mice immunized with equivalent amounts of Q11 lacking the (NANP)3 epitope failed to elicit any antibodies, even upon boosting (Fig. 2b), indicating that the epitope was critical for generating antibody responses. In BALB/c mice, (NANP)3-Q11 did not raise any significant antibody responses (Fig 2c). The fact that (NANP)3-Q11 raised strong, persistent responses in B6 mice (H-2b haplotype) but not in BALB/c (H-2d) suggested to us that proper restriction and presentation within MHC molecules was critical, and that the antibody responses were likely to be T cell mediated. Therefore we investigated the immunogenicity of (NANP)3-Q11 in T cell receptor knockout mice (B6.129P2-Tcrbtm1MomTcrdtm1Mom/J, on a B6 background), chosen for their specific and durable lack of functional T cells. These mice did not raise antibody responses against (NANP)3-Q11, whereas they were fully capable of generating antibody responses against NP-Ficoll, a known T-independent antigen (Figure 3). This result demonstrated that T cells are required for antibody production against self-assembled (NANP)3-Q11 materials, which could aid in producing stronger recall responses and immunological memory.
Figure 2.
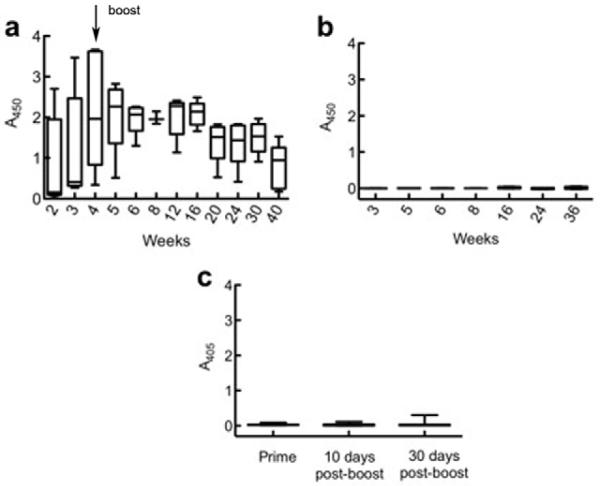
Antibody responses to (NANP)3-Q11 were durable in B6 mice (a). In contrast, Q11 did not elicit any antibody responses (b). Antibody responses were not observed in BALB/c mice (c).
Figure 3.
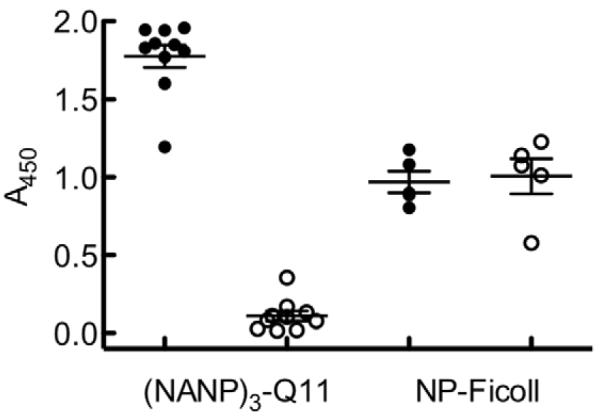
Antibody responses to (NANP)3-Q11 were T cell-dependent. T cell receptor knockout mice (open circles) did not produce antibodies against (NANP)3-Q11, but did respond to a T-independent antigen, NP-Ficoll. B6 mice (solid circles) responded to both antigens.
Antibody responses against (NANP)3-Q11 were MyD88-dependent
The mechanism of action of self-assembled Q11-based adjuvants has not previously been fully outlined, so we employed additional knockout mouse models to ascertain the requirement for several signaling components that have been shown to be critical for the adjuvanticity of other systems. Toll-like receptors (TLRs) play a critical role in the mechanism of action of many adjuvants; bacterial flagellin and curli proteins signal through TLR-5 and TLR-2 respectively [25, 26]. Alum has been shown to exert its adjuvant activity through the inflammasome pathway, specifically NALP3 [27]. Given the fibrillar and proteinaceous nature of Q11 nanofibers and their morphological similarity to flagellin and curli, we investigated antibody responses in TLR-5 and TLR-2 knockout mice. Given their nanoparticulate nature, which is in some respects similar to the particulate nature of alum, we also investigated them in NALP3 knockout mice. In MyD88-knockout mice, antibody responses to (NANP)3-Q11 were abolished (Fig 4a) but they remained in NALP3-knockout mice (Fig. 4b). With respect to specific TLRs, mice lacking functional TLR-2 were capable of generating antibody responses against (NANP)3-Q11 (Fig. 4c), as were those lacking functional TLR-5 (Fig. 4d). Collectively, these data indicated that the adjuvant activity of Q11, at least in the context of (NANP)3-Q11, is dependent on T cells and MyD88, but not NALP3. Although we have not yet exhaustively determined if any TLR signaling is important, it is clear that TLR-2 and TLR-5 are not required.
Figure 4.
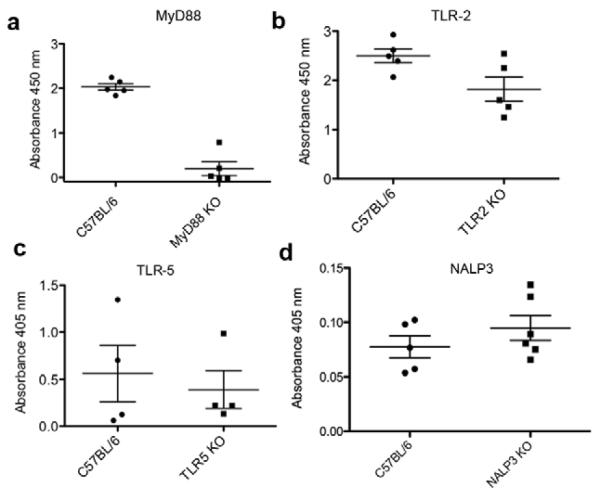
Anti-(NANP)3-Q11 responses in knockout mouse models, measured by ELISA. Antibody production was abolished in MyD88-knockout mice (a), but not in knockout mice lacking functional TLR-2 (b), TLR-5 (c), or NALP3 (d).
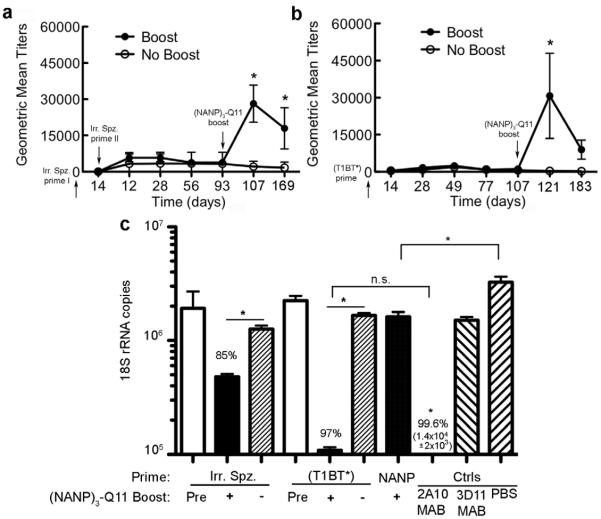
Boosting pre-infected mice with (NANP)3-Q11 protected against sporozoite infection
The quality of the antibody responses raised by (NANP)3-Q11 was assessed by evaluating antisera in transgenic sporozoite neutralizing (TSN) assays. Using this assay, the ability of antisera to inhibit sporozoite infection of human HepG2 hepatoma cells was measured [37]. Groups included antisera raised with primary immunizations of irradiated PfPb sporozoites (delivered via mosquito bites) or of (T1BT*)4-P3C peptide and booster immunizations of (NANP)3-Q11; and prime/boost regimens of (NANP)3-Q11 only. Mice primed with PfPb sporozoites and boosted with (NANP)3-Q11 had significantly higher levels of antibodies against the falciparum CS repeat peptide (T1B)4 compared to mice that did not receive the boost (Fig 5a). Antibody levels also remained significantly higher for up to 76 days after the boost. Similarly, mice primed with (T1BT*)4-P3C peptide and boosted with (NANP)3-Q11 showed significant antibody responses compared to mice that did not receive a boost (Fig 5b). TSN assays showed that sporozoite infection was reduced by 85% when incubated with immune sera from mice that were primed with irradiated sporozoites and boosted with (NANP)3-Q11 (Fig 5c). Similarly, 97% inhibition was observed in mice primed with (T1BT*)4-P3C peptide and boosted with (NANP)3-Q11, a level of protection that was statistically similar to that achieved using the positive control monoclonal antibody 2A10, directed against P. falciparum CS repeats (99.6%). This antibody is a standard for inhibition studies, and 90% inhibition is usually considered significant and commensurate with in vivo protection [37]. As a negative control, no significant inhibition was observed in sporozoites incubated with the P. berghei-directed monoclonal antibody 3D11. Sera from mice immunized with (NANP)3-Q11 alone did not show clinically relevant levels of protection. Taken together, these data suggest that boosting with antigen-Q11 nanofibers might be advantageous in situations where strong recall responses are required against previously encountered antigens.
Figure 5.
Recovery of antibody titers in mice boosted with (NANP)3-Q11, after priming with irradiated sporozoites (a) or (T1BT*)4-P3C peptide (b). Closed circles represent boosted mice, open circles indicate mice that did not receive a boost, and the error bars indicate SEM. TSN assay showing sporozoite neutralizing activity in HepG2 cells after incubation with antisera from immune mice (c). Immune sera from mice immunized with PBS and the monoclonal antibodies 2A10 or 3D11 were used as controls. Mice primed with irradiated sporozoites or (T1BT*)4-P3C are labeled Irr. Spz. and (T1BT*) respectively. Mice that received (NANP)3-Q11 only are labeled NANP. Pre, +, and - denote antibody titers pre-boost, post-boost with (NANP)3-Q11, or no-boost within each group. The levels of parasites in HepG2 cells were measured using qPCR. * p<0.05 by ANOVA.
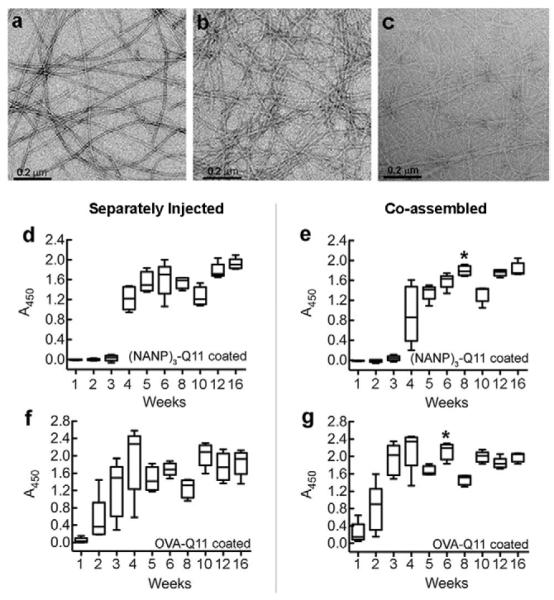
Antibody responses to two different epitopes can be maintained upon their co-assembly
Transmission electron microscopy indicated that OVA-Q11, (NANP)3-Q11, and co-assembled OVA-Q11:(NANP)3-Q11 (1:1) self-assembled into nanofibers having similar morphologies (Figure 6a-c) [6], although mixed fibrils stained to a lesser extent with uranyl acetate. To investigate if antibodies could be raised simultaneously against multiple antigens co-assembled together, mice were immunized with the co-assembled fibrils, and their responses were compared to those produced in mice receiving separate immunizations of the two different peptides. The total peptide concentration was kept the same in both groups. Injecting the peptides either separately or as co-assembled fibrils resulted in similar antibody responses for up to 16 wks (Fig 6d-g). This suggested that the presence of one peptide did not adversely affect the antibody response raised by the other when the two were co-fibrillized together, opening the possibility of designing multi-epitope fibers using the Q11 system.
Figure 6.
Fibril formation was not disrupted when peptides bearing two different epitopes were mixed together. TEM images of OVA-Q11 fibrils (a), (NANP)3-Q11 fibrils (b), and co-assembled fibrils of OVA-Q11 and (NANP)3-Q11 (c). Antibody levels in mice raised against (NANP)3-Q11 (d, e) and OVA-Q11 (f, g) after immunization with individual epitopes (d, f) or co-assembled epitopes (e, g) respectively. * p<0.05 by t-test between groups at corresponding time points.
DISCUSSION
Ideally, an adjuvant is an immune stimulant that amplifies or modifies immune responses to co-delivered antigens but does not elicit any specific responses of its own [4, 21]. Many adjuvants currently in use or under investigation are chemically heterogeneous mixtures of plant- or pathogen-derived molecules or formulations of mineral salts [4]. Until recently, in the USA, aluminum-based formulations were the only clinically approved adjuvants, and their mechanism of action is still not completely understood [42, 43]. Although powerful adjuvants like CFA elicit strong immune responses, they are generally associated with deleterious side effects such as toxicity and autoimmunity, precluding human use [4, 44]. In addition to requiring adjuvants, many subunit vaccines also require multiple injections to achieve long-lived antibody responses, presenting logistical and economical challenges, especially in developing countries [4]. The present results indicate that in mice, self-assembled (NANP)3-Q11 can elicit durable antibody responses without the need for multiple boosts or supplemental adjuvants. Following a single boost at 4 weeks, antibodies were detectable in the sera of mice for as long as 40 weeks. We did not observe detectable antibody responses to unmodified Q11, even when Q11 was administered in CFA, [6] indicating that Q11 was non-immunogenic by itself, which is a desirable feature in an adjuvant. In addition, no overt inflammation or nodule formation was observed at the injection sites of any Q11-based peptides, but the tissue response, clearance, and clinical safety of these materials remains to be systematically and completely investigated.
In previous work, the mechanism of immunological activity for Q11-based materials has not been fully determined [6, 20], so we investigated several mechanistic aspects involving adaptive and innate immunity. Our initial observation was that antibody production against (NANP)3-Q11 was haplotype dependent. C57BL6 mice of the H-2b haplotype generated robust antibody responses, while BALB/c mice (H-2d) failed to raise significant responses (Fig 2c). (NANP)3 has been reported to be a poor immunogen in BALB/c mice, and conjugation to Q11 did not alter this property [45]. The requirement of T cells, suspected from the haplotype dependence, was confirmed when T cell receptor knockout B6 mice failed to raise antibodies against (NANP)3-Q11 even after a boost (Fig 3). This T cell dependence may be advantageous for conferring long-lasting immunological memory [47]. It is also known that most adjuvants enhance humoral and cellular responses by engaging components of the innate immune system [4]. Recently, many natural and synthetic biomaterials have been investigated for their ability to activate the immune system and as vaccine adjuvants against a variety of diseases [22, 48, 49]. It has recently been shown that some polymeric materials such as poly(lactide-co-glycolide) (PLGA) and alginate are recognized as pathogen-associated molecular patterns (PAMPs) by innate immune cells such as macrophages and dendritic cells (DCs) [48, 50-52]. (NANP)3-Q11 immunogenicity was abolished in MyD88-knockout mice, but not in mice with defective TLR-5, TLR-2, or NALP3 (Figure 4), so it appears that these materials signal through MyD88, but it is not clear whether they engage a specific type of TLR or NLR. In sum, it is possible that the nanofibers act directly on certain innate immune receptors or indirectly mediate the release of activators of innate immunity, which subsequently act through the MyD88 pathway. At this time, however, it is not known which cell types require MyD88 to respond to the fibrils, as the experiments reported here have been performed in mice lacking any MyD88 at all. This question could be resolved in models having MyD88 deficiencies in only a limited set of cell types. It is also possible that the nanofibers could be providing an antigen depot for long-term activation of immune cells. However, at this time it is not clear if such a depot exists at either the immunization site or in the draining lymph nodes, or whether the long-term antibody production is instead the result of long-lived plasma cells.
Early studies demonstrated that immunizing rodents and humans with irradiated sporozoites results in sterile immunity, considered to be a gold standard for malaria vaccine development [53-55]. Immunization with (NANP)3-Q11 alone did not generate high levels of neutralizing antibodies, although the inhibition of infection was significant compared to mice that received only PBS. This may be due to lack of appropriate T helper epitopes, which are present in sporozoites and are included in the (T1BT*)4-P3C peptide, suggesting future embodiments of the approach that contain T helper epitopes [32, 40, 56]. Although antibodies raised against (NANP)3-Q11 by itself were not sufficient to confer protection, the high levels of protective antibodies raised in sporozoite-primed mice boosted with (NANP)3-Q11 suggest the possibility that these materials could be useful for boosting responses to previously encountered antigens.
Although long-term antibody production against protein and peptide antigens has been reported with other synthetic particulate adjuvants such as PLGA microparticles [57, 58], some practical advantages may be offered by self-assembled peptide adjuvants. Polymeric microparticles require emulsification techniques and organic solvents that may affect antigen stability [59, 60], and the process can also result in antigen loss into the aqueous phase that may be difficult to control [59]. In contrast, previous work has demonstrated that Q11-based materials can be engineered so that they undergo minimal compositional drift. That is, each ligand- or epitope-bearing Q11 peptide can be assembled predictably, so that specific ratios of ligands or epitopes can be maintained [8, 10]. In the present work, we observed that OVA-Q11 and (NANP)3-Q11 could be co-assembled without diminishing the immunogenicity of either epitope. This modularity may prove useful in the design of multi-antigen vaccines with tightly controlled amounts of more than one epitope. Traditionally, peptide epitopes are employed within vaccines by conjugating them to carrier proteins and delivering them with an additional adjuvanting compound or mixture of compounds. In the system reported here, co-assembly of multiple epitope-bearing Q11 peptides into a single formulation may be a comparatively straightforward route for producing multi-antigen materials able to raise strong antibody responses alone, without requiring a conjugation step or co-delivery with an adjuvant.
CONCLUSIONS
Immunizing with self-assembled peptides containing Q11 and the minimal epitope (NANP)3 produced vaccines capable of raising long-lived antibody responses without the requirement for frequent boosts. Unadjuvanted antibody responses were durable, lasting at least 40 weeks, and these responses were dependent on T cells and MyD88. Mice primed with irradiated PfPb sporozoites or (T1BT*)4-P3C and boosted with (NANP)3-Q11 showed significant boosts of antibody titers, and antisera from these mice conferred significant protection against sporozoite infection in HepG2 human hepatoma cell cultures. Two different epitope-bearing peptides could be co-assembled without diminishment of the antibody responses to either epitope, suggesting the feasibility of developing multi-epitope vaccines.
ACKNOWLDGEMENTS
This research was supported in part by the National Institutes of Health (NIBIB 1R01EB009701 (JHC), NIAID 1R21AI094444 (JHC and ASC), and NIAID R01AI083655 (EHN), and by the Chicago Biomedical Consortium (CBC), with support from the Searle Funds at the Chicago Community Trust. We thank Rita Altszuler for technical assistance with malaria assays. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH or the CBC. We thank Philip Jung, Ye Tian, and Joshua Gasiorowski for assistance with the TEM, which was performed at the University of Chicago Biophysics Core facility.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Purcell AW, McCluskey J, Rossjohn J. More than one reason to rethink the use of peptides in vaccine design. Nat Rev Drug Discov. 2007;6:404–14. doi: 10.1038/nrd2224. [DOI] [PubMed] [Google Scholar]
- 2.Black M, Trent A, Tirrell M, Olive C. Advances in the design and delivery of peptide subunit vaccines with a focus on toll-like receptor agonists. Expert Rev Vaccines. 2010;9:157–73. doi: 10.1586/erv.09.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Corradin G, Kajava AV, Verdini A. Long synthetic peptides for the production of vaccines and drugs: a technological platform coming of age. Sci Transl Med. 2010;2:50rv3. doi: 10.1126/scitranslmed.3001387. [DOI] [PubMed] [Google Scholar]
- 4.Coffman RL, Sher A, Seder RA. Vaccine adjuvants: putting innate immunity to work. Immunity. 2010;33:492–503. doi: 10.1016/j.immuni.2010.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.O’Hagan DT, De Gregorio E. The path to a successful vaccine adjuvant-the long and winding road. Drug Discov Today. 2009;14:541–51. doi: 10.1016/j.drudis.2009.02.009. [DOI] [PubMed] [Google Scholar]
- 6.Rudra JS, Tian YF, Jung JP, Collier JH. A self-assembling peptide acting as an immune adjuvant. Proc Natl Acad Sci U S A. 2010;107:622–27. doi: 10.1073/pnas.0912124107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kaltgrad E, Sen Gupta S, Punna S, Huang CY, Chang A, Wong CH, et al. Anti-carbohydrate antibodies elicited by polyvalent display on a viral scaffold. Chembiochem. 2007;8:1455–62. doi: 10.1002/cbic.200700225. [DOI] [PubMed] [Google Scholar]
- 8.Kaba SA, Brando C, Guo Q, Mittelholzer C, Raman S, Tropel D, et al. A nonadjuvanted polypeptide nanoparticle vaccine confers long-lasting protection against rodent malaria. J Immunol. 2009;183:7268–77. doi: 10.4049/jimmunol.0901957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schroeder U, Graff A, Buchmeier S, Rigler P, Silvan U, Tropel D, et al. Peptide nanoparticles serve as a powerful platform for the immunogenic display of poorly antigenic actin determinants. J Mol Biol. 2009;386:1368–81. doi: 10.1016/j.jmb.2008.11.023. [DOI] [PubMed] [Google Scholar]
- 10.Boato F, Thomas RM, Ghasparian A, Freund-Renard A, Moehle K, Robinson JA. Synthetic virus-like particles from self-assembling coiled-coil lipopeptides and their use in antigen display to the immune system. Angew Chem Int Ed. 2007;46:9015–18. doi: 10.1002/anie.200702805. [DOI] [PubMed] [Google Scholar]
- 11.Ghasparian A, Riedel T, Koomullil J, Moehle K, Gorba C, Svergun DI, et al. Engineered synthetic virus-like particles and their use in vaccine delivery. Chembiochem. 2011;12:100–9. doi: 10.1002/cbic.201000536. [DOI] [PubMed] [Google Scholar]
- 12.Riedel T, Ghasparian A, Moehle K, Rusert P, Trkola A, Robinson JA. Synthetic virus-like particles and conformationally constrained peptidomimetics in vaccine design. Chembiochem. 2011;12:2829–36. doi: 10.1002/cbic.201100586. [DOI] [PubMed] [Google Scholar]
- 13.Braun M, Jandus C, Maurer P, Hammann-Haenni A, Schwarz K, Bachmann MF, et al. Virus-like particles induce robust human T-helper cell responses. Eur J Immunol. 2012;42:330–40. doi: 10.1002/eji.201142064. [DOI] [PubMed] [Google Scholar]
- 14.Toth I, Simerska P, Fujita Y. Recent advances in design and synthesis of self-adjuvanting lipopeptide vaccines. Int J Pept Res Ther. 2008;14:333–40. [Google Scholar]
- 15.Puffer EB, Pontrello JK, Hollenbeck JJ, Kink JA, Kiessling LL. Activating B cell signaling with defined multivalent ligands. ACS Chem Biol. 2007;2:252–62. doi: 10.1021/cb600489g. [DOI] [PubMed] [Google Scholar]
- 16.Chackerian B, Durfee MR, Schiller JT. Virus-like display of a neo-self antigen reverses B cell anergy in a B cell receptor transgenic mouse model. J Immunol. 2008;180:5816–25. doi: 10.4049/jimmunol.180.9.5816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jung JP, Nagaraj AK, Fox EK, Rudra JS, Devgun JM, Collier JH. Co-assembling peptides as defined matrices for endothelial cells. Biomaterials. 2009;30:2400–10. doi: 10.1016/j.biomaterials.2009.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jung JP, Jones JL, Cronier SA, Collier JH. Modulating the mechanical properties of self-assembled peptide hydrogels via native chemical ligation. Biomaterials. 2008;29:2143–51. doi: 10.1016/j.biomaterials.2008.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jung JP, Moyano JV, Collier JH. Multifactorial optimization of endothelial cell growth using modular synthetic extracellular matrices. Integr Biol. 2011;3:185–96. doi: 10.1039/c0ib00112k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rudra JS, Sun T, Bird KC, Daniels MD, Gasiorowski JZ, Chong AS, et al. Modulating adaptive immune responses to peptide self-assemblies. ACS Nano. 2012;6:1557–64. doi: 10.1021/nn204530r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mbow ML, De Gregorio E, Valiante NM, Rappuoli R. New adjuvants for human vaccines. Curr Opin Immunol. 2010;22:411–16. doi: 10.1016/j.coi.2010.04.004. [DOI] [PubMed] [Google Scholar]
- 22.Hubbell JA, Thomas SN, Swartz MA. Materials engineering for immunomodulation. Nature. 2009;462:449–460. doi: 10.1038/nature08604. [DOI] [PubMed] [Google Scholar]
- 23.Kim J, Mooney DJ. In vivo modulation of dendritic cells by engineered materials: towards new cancer vaccines. Nano Today. 2011;6:466–77. doi: 10.1016/j.nantod.2011.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pulendran B. Modulating vaccine responses with dendritic cells and Toll-like receptors. Immunol Rev. 2004;199:227–50. doi: 10.1111/j.0105-2896.2004.00144.x. [DOI] [PubMed] [Google Scholar]
- 25.Salazar-Gonzalez RM, McSorley SJ. Salmonella flagellin, a microbial target of the innate and adaptive immune system. Immunol Lett. 2005;101:117–22. doi: 10.1016/j.imlet.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 26.Tukel C, Nishimori JH, Wilson RP, Winter MG, Keestra AM, van Putten JP, et al. Toll-like receptors 1 and 2 cooperatively mediate immune responses to curli, a common amyloid from enterobacterial biofilms. Cell Microbiol. 2010;12:1495–505. doi: 10.1111/j.1462-5822.2010.01485.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Eisenbarth SC, Colegio OR, O’Connor W, Sutterwala FS, Flavell RA. Crucial role for the Nalp3 inflammasome in the immunostimulatory properties of aluminium adjuvants. Nature. 2008;453:1122–6. doi: 10.1038/nature06939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nardin EH. The past decade in malaria synthetic peptide vaccine clinical trials. Hum Vaccin. 2010;6:27–38. doi: 10.4161/hv.6.1.9601. [DOI] [PubMed] [Google Scholar]
- 29.Kumar KA, Sano G, Boscardin S, Nussenzweig RS, Nussenzweig MC, Zavala F, et al. The circumsporozoite protein is an immunodominant protective antigen in irradiated sporozoites. Nature. 2006;444:937–40. doi: 10.1038/nature05361. [DOI] [PubMed] [Google Scholar]
- 30.Nussenzweig V, Nussenzweig RS. Rationale for the development of an engineered sporozoite malaria vaccine. Adv Immunol. 1989;45:283–334. doi: 10.1016/s0065-2776(08)60695-1. [DOI] [PubMed] [Google Scholar]
- 31.Lopez JA, Weilenman C, Audran R, Roggero MA, Bonelo A, Tiercy JM, et al. A synthetic malaria vaccine elicits a potent CD8(+) and CD4(+) T lymphocyte immune response in humans. Implications for vaccination strategies Eur J Immunol. 2001;31:1989–98. doi: 10.1002/1521-4141(200107)31:7<1989::aid-immu1989>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 32.Nardin EH, Oliveira GA, Calvo-Calle JM, Castro ZR, Nussenzweig RS, Schmeckpeper B, et al. Synthetic malaria peptide vaccine elicits high levels of antibodies in vaccinees of defined HLA genotypes. J Infect Dis. 2000;182:1486–96. doi: 10.1086/315871. [DOI] [PubMed] [Google Scholar]
- 33.Nardin EH, Calvo-Calle JM, Oliveira GA, Nussenzweig RS, Schneider M, Tiercy JM, et al. A totally synthetic polyoxime malaria vaccine containing Plasmodium falciparum B cell and universal T cell epitopes elicits immune responses in volunteers of diverse HLA types. J Immunol. 2001;166:481–89. doi: 10.4049/jimmunol.166.1.481. [DOI] [PubMed] [Google Scholar]
- 34.Alonso PL, Sacarlal J, Aponte JJ, Leach A, Macete E, Milman J, et al. Efficacy of the RTS,S/AS02A vaccine against Plasmodium falciparum infection and disease in young african children: randomised controlled trial. Lancet. 2004;364:1411–20. doi: 10.1016/S0140-6736(04)17223-1. [DOI] [PubMed] [Google Scholar]
- 35.Hollingdale MR, Nardin EH, Tharavanij S, Schwartz AL, Nussenzweig RS. Inhibition of entry of Plasmodium-falciparum and Plasmodium-vivax sporozoites into cultured cells - an in vitro assay of protective antibodies. J Immunol. 1984;132:909–13. [PubMed] [Google Scholar]
- 36.Kumar KA, Oliveira GA, Edelman R, Nardin EH, Nussenzweig V. Quantitative Plasmodium sporozoite neutralization assay (TSNA) J Immunol Methods. 2004;292:157–164. doi: 10.1016/j.jim.2004.06.017. [DOI] [PubMed] [Google Scholar]
- 37.Othoro C, Johnston D, Lee R, Soverow J, Bystryn JC, Nardin EH. Enhanced immunogenicity of plasmodium falciparum peptide vaccines using a topical adjuvant containing a potent synthetic toll-like receptor 7 agonist, imiquimod. Infect Immun. 2009;77:739–48. doi: 10.1128/IAI.00974-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Malyala P, Singh M. Endotoxin limits in formulations for preclinical research. J Pharm Sci. 2008;97:2043–46. doi: 10.1002/jps.21152. [DOI] [PubMed] [Google Scholar]
- 39.Nardin EH, Calvo-Calle JM, Oliveira GA, Clavijo P, Nussenzweig R, Simon R, et al. Plasmodium falciparum polyoximes: highly immunogenic synthetic vaccines constructed by chemoselective ligation of repeat B-cell epitopes and a universal T-cell epitope of CS protein. Vaccine. 1998;16:590–600. doi: 10.1016/s0264-410x(97)00238-7. [DOI] [PubMed] [Google Scholar]
- 40.Oliveira GA, Clavijo P, Nussenzweig RS, Nardin EH. Immunogenicity of an alum-adsorbed synthetic multiple-antigen peptide-based on B-Cell and T-Cell epitopes of the plasmodium falciparum CS protein - possible vaccine application. Vaccine. 1994;12:1012–17. doi: 10.1016/0264-410x(94)90337-9. [DOI] [PubMed] [Google Scholar]
- 41.Persson C, Oliveira GA, Sultan AA, Bhanot P, Nussenzweig V, Nardin EH. Cutting edge: A new tool to evaluate human pre-erythrocytic malaria vaccines: Rodent parasites bearing a hybrid Plasmodium falciparum circumsporozoite protein. J Immunol. 2002;169:6681–85. doi: 10.4049/jimmunol.169.12.6681. [DOI] [PubMed] [Google Scholar]
- 42.Lambrecht BN, Kool M, Willart MAM, Hammad H. Mechanism of action of clinically approved adjuvants. Curr Opin Immunol. 2009;21:23–29. doi: 10.1016/j.coi.2009.01.004. [DOI] [PubMed] [Google Scholar]
- 43.Marrack P, McKee AS, Munks MW. Towards an understanding of the adjuvant action of aluminium. Nat Rev Immunol. 2009;9:287–93. doi: 10.1038/nri2510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mellinkoff SM, Pearson CM, Wood FD, Eshelmano, Dixon W. Studies of polyarthritis induced in rats by injection of mycobacterial adjuvant. VI. Effects of dietary alterations. Am J Clin Nutr. 1962;10:398–402. doi: 10.1093/ajcn/10.5.398. [DOI] [PubMed] [Google Scholar]
- 45.Good MF, Berzofsky JA, Maloy WL, Hayashi Y, Fujii N, Hockmeyer WT, et al. Genetic control of the immune response in mice to a plasmodium falciparum sporozoite vaccine. Widespread nonresponsiveness to a single malaria T epitope in highly repetitive vaccine. J Exp Med. 1986;164:665–660. doi: 10.1084/jem.164.2.655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Del Giudice G, Cooper JA, Merino J, Verdini AS, Pessi A, Togna AR, et al. The antibody response in mice to carrier-free synthetic polymers of Plasmodium falciparum circumsporozoite repetitive epitope is I-Ab-restricted: possible implications for malaria vaccines. J Immunol. 1986;137:2952–2955. [PubMed] [Google Scholar]
- 47.Ahlers JD, Belyakov IM. Molecular pathways regulating CD4(+) T cell differentiation, anergy and memory with implications for vaccines. Trends Mol Med. 2010;16:478–91. doi: 10.1016/j.molmed.2010.07.007. [DOI] [PubMed] [Google Scholar]
- 48.Jones KS. Biomaterials as vaccine adjuvants. Biotechnol Prog. 2008;24:807–14. doi: 10.1002/btpr.10. [DOI] [PubMed] [Google Scholar]
- 49.Demento SL, Siefert AL, Bandyopadhyay A, Sharp FA, Fahmy TM. Pathogen-associated molecular patterns on biomaterials: a paradigm for engineering new vaccines. Trends Biotechnol. 2011;29:294–306. doi: 10.1016/j.tibtech.2011.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kou PM, Babensee JE. Macrophage and dendritic cell phenotypic diversity in the context of biomaterials. J Biomed Mater Res A. 2011;96:239–60. doi: 10.1002/jbm.a.32971. [DOI] [PubMed] [Google Scholar]
- 51.Yoshida M, Mata J, Babensee JE. Effect of poly(lactic-co-glycolic acid) contact on maturation of murine bone marrow-derived dendritic cells. J Biomed Mater Res A. 2007;80A:7–12. doi: 10.1002/jbm.a.30832. [DOI] [PubMed] [Google Scholar]
- 52.Yang D, Jones KS. Effect of alginate on innate immune activation of macrophages. J Biomed Mater Res A. 2009;90:411–8. doi: 10.1002/jbm.a.32096. [DOI] [PubMed] [Google Scholar]
- 53.Nussenzweig RS, Vanderberg J, Most H, Orton C. Protective immunity produced by injection of x-irradiated sporozoites of plasmodium berghei. Nature. 1967;216:160–2. doi: 10.1038/216160a0. [DOI] [PubMed] [Google Scholar]
- 54.Herrington D, Davis J, Nardin EH, Beier M, Cortese J, Eddy H, et al. Successful immunization of humans with irradiated malaria sporozoites - humoral and cellular responses of the protected individuals. Am J Trop Med Hyg. 1991;45:539–47. doi: 10.4269/ajtmh.1991.45.539. [DOI] [PubMed] [Google Scholar]
- 55.Hoffman SL, Goh LML, Luke TC, Schneider I, Le TP, Doolan DL, et al. Protection of humans against malaria by immunization with radiation-attenuated Plasmodium falciparum sporozoites. J Infect Dis. 2002;185:1155–64. doi: 10.1086/339409. [DOI] [PubMed] [Google Scholar]
- 56.Moreno CA, Rodriguez R, Oliveira GA, Ferreira V, Nussenzweig RS, Castro ZRM, et al. Preclinical evaluation of a synthetic Plasmodium falciparum MAP malaria vaccine in aotus monkeys and mice. Vaccine. 1999;18:89–99. doi: 10.1016/s0264-410x(99)00184-x. [DOI] [PubMed] [Google Scholar]
- 57.Matzelle MM, Babensee JE. Humoral immune responses to model antigen co-delivered with biomaterials used in tissue engineering. Biomaterials. 2004;25:295–304. doi: 10.1016/s0142-9612(03)00531-3. [DOI] [PubMed] [Google Scholar]
- 58.Carcaboso AM, Hernandez RM, Igartua M, Rosas JE, Patarroyo ME, Pedraz JL. Enhancing immunogenicity and reducing dose of microparticulated synthetic vaccines: Single intradermal administration. Pharm Res. 2004;21:121–26. doi: 10.1023/b:pham.0000012159.20895.5b. [DOI] [PubMed] [Google Scholar]
- 59.De Koker S, Lambrecht BN, Willart MA, van Kooyk Y, Grooten J, Vervaet C, et al. Designing polymeric particles for antigen delivery. Chem Soc Rev. 2011;40:320–39. doi: 10.1039/b914943k. [DOI] [PubMed] [Google Scholar]
- 60.Schwendeman SP, Costantino HR, Gupta RK, Siber GR, Klibanov AM, Langer R. Stabilization of tetanus and diphtheria toxoids against moisture-induced aggregation. Proc Natl Acad Sci U S A. 1995;92:11234–38. doi: 10.1073/pnas.92.24.11234. [DOI] [PMC free article] [PubMed] [Google Scholar]