Abstract
The role of CB2 in the central nervous system, particularly in neurons, has generated much controversy. Fueling the controversy are imperfect tools, which have made conclusive identification of CB2-expressing neurons problematic. Imprecise localization of CB2 has made it difficult to determine its function in neurons. Here we avoid the localization controversy and directly address the question if CB2 can modulate neurotransmission. CB2 was expressed in excitatory hippocampal autaptic neurons obtained from CB1 null mice. Whole-cell patch clamp recordings were made from these neurons to determine the effects of CB2 on short-term synaptic plasticity. CB2 expression restored depolarization induced suppression of excitation to these neurons, which was lost following genetic ablation of CB1. The endocannabinoid 2-arachidonylglycerol (2-AG) mimicked the effects of depolarization in CB2 expressing neurons. Interestingly, ongoing basal production of 2-AG resulted in constitutive activation of CB2, causing a tonic inhibition of neurotransmission that was relieved by the CB2 antagonist AM630 or the diacylglycerol lipase inhibitor RHC80267. Through immunocytochemistry and analysis of spontaneous EPSCs, paired pulse ratios and coefficients of variation we determined that CB2 exerts its function at a presynaptic site of action, likely through inhibition of voltage gated calcium channels. Therefore CB2 expressed in neurons effectively mimics the actions of CB1. Thus, neuronal CB2 is well suited to integrate into conventional neuronal endocannabinoid signaling processes, with its specific role determined by its unique and highly inducible expression profile.
Keywords: CB2, cannabinoid receptor, autaptic neurons, DSE, endocannabinoid2
1. Introduction
The CB2 cannabinoid receptor has been nicknamed the “peripheral cannabinoid receptor.” This title followed from several studies that failed to find it in the brain, as compared to the CNS-abundant CB1 cannabinoid receptor (Atwood and Mackie, 2010). Subsequent studies found evidence of CB2 in the brain, however, which cells express CB2 remains unsettled. There is general agreement that microglia, the resident immune cells of the brain, express CB2 (Ashton et al., 2007; Walter et al., 2003). Other reports suggest CB2 is present in neurons of both the peripheral and central nervous system. These reports range from expression under only specific conditions (e.g.Wotherspoon et al., 2005) to widespread expression throughout the brain (e.g. Gong et al., 2006). Ascertaining the true location of CB2 in the nervous system is complicated by the inducibility of CB2, the lack of sufficiently specific antibodies, and imperfect pharmacology (Atwood and Mackie, 2010). These complications make it difficult to determine the function of neuronal CB2. Specifically, it is not known for certain if CB2 receptors in neurons are capable of modulating synaptic transmission. Morgan and colleagues reported finding miniature action potential dependent inhibitory currents in the medial entorhinal cortex that were sensitive to CB2-selective ligands, but did not identify the anatomical localization of the CB2 receptor involved (Morgan et al., 2009). Very recently it has been reported that CB2 ligands activate a calcium-dependent chloride current in rodent layer II/III prefrontal cortex pyramidal neurons, decreasing spontaneous firing (den Boon et al., 2012). Using a behavioral model of cocaine self-administration, Xi et al. demonstrated that CB2 ligands when given systemically, intranasally or intra-accumbens reduced cocaine self administration and this effect was absent in CB2 null mice (Xi et al., 2011). This study was also limited by the lack of knowledge of the anatomical localization of CB2. As CB2 is located on microglia and microglia are capable of modulating synaptic plasticity (Ben Achour and Pascual, 2010), it remains to be determined if the results of these studies are due to neuronal or glial CB2. Many other studies of CB2 in the CNS are impacted by similar considerations.
CB1 receptors are G protein coupled receptors (GPCRs) that are abundantly expressed in the CNS. Endocannabinoids are retrograde signaling molecules that activate presynaptic CB1 receptors to inhibit neurotransmission, often through inhibition of voltage gated calcium channels (VGCCs). Strong depolarization of a post-synaptic neuron increases endocannabinoid production. At excitatory synapses this coupling between post-synaptic endocannabinoid production and presynaptic inhibition is known as depolarization suppression of excitation (DSE). DSE is a form of short-term synaptic plasticity that suppresses neurotransmission from seconds to minutes (Kano et al., 2009).
CB2 signals through many of the same effectors as CB1. Early pharmacological comparisons of these receptors found that CB2 coupled poorly to VGCCs (Felder et al., 1995; Ross et al., 2001). However, this poor coupling appears to be an example of functional selectivity as we recently found that CB2 inhibits VGCCs, in a fashion strongly dependent on the CB2 ligand used (Atwood et al., 2012). We hypothesized that if expressed in neurons, CB2 could also inhibit neurotransmission via inhibition of VGCCs. Autaptic neuronal cultures offer us a powerful means to test this hypothesis. Autaptic neurons are a well described, simple preparation useful for studying synaptic function (Bekkers et al., 1991). They have a complete complement of cannabinoid signaling proteins making them attractive for studying individual components of cannabinoid signaling (Straiker and Mackie, 2005). Furthermore, gene expression in these cultures is easily manipulated. Autaptic hippocampal neurons obtained from CB1 null mice lack DSE and are insensitive to cannabinoids (Straiker and Mackie 2005). We expressed CB2 in CB1 null neurons to determine if CB2 restored cannabinoid sensitivity and if it acted in a similar manner as CB1 to modulate neurotransmission. This approach allowed us to circumvent the issue of whether or not CB2 is expressed in neurons and directly ask the question: when CB2 is present in neurons, what might be its function?
2. Materials & Methods
2.1 Materials
Drugs and reagents were purchased from Tocris Cookson (Ellisville, MO, USA), Cayman Chemical (Ann Arbor, MI, USA) or Sigma-Aldrich (St Louis, MO, USA). Constructs were made such that the receptors had an N-terminal HA epitope tag for immunostaining and a pre-prolactin signaling sequence (pplss) to enhance protein expression and trafficking. pplss-HA-rCB1-pcDNA3.0, pplss-HA-CB2-pcDNA3.0, pplss-HA-CB2-CAG, and mCherry-CAG, were all constructed, amplified and purified using NEB buffers and restriction enzymes (New England BioLabs, Ipswich, MA) and Qiagen plasmid DNA purification kits (Valencia, CA) according to the manufacturer's instructions. Sequencing was performed to verify each construct's sequence (Indiana University Molecular Biology Institute). Primers for sequencing and cloning were purchased from Operon (Huntsville, AL).
2.2 Animals, Cell culture and transfection
All animal care and experimental procedures used in this study were approved by the Institutional Animal Care and Use Committee of Indiana University and conform to the Guidelines of the National Institutes of Health on the Care and Use of Animals. All efforts were made to reduce the number of animals used and to minimize their suffering during procedures. Heterozygote (CB1+/−) mice to establish the CB1 knock-out colony were generously provided by Dr Catherine Ledent (University of Brussels, Belgium; (Reibaud et al., 1999)). Mouse (CD1 strain) hippocampal neurons isolated from the CA1–CA3 region were cultured on microislands as previously described (Furshpan et al., 1976; Bekkers and Stevens, 1991). Neurons were obtained from animals (at postnatal day 0–2, killed via rapid decapitation without anesthesia) and plated onto a feeder layer of hippocampal astrocytes that had been laid down previously (Levison and McCarthy, 1991). Cultures were grown in high-glucose (20 mM) minimum essential media containing 10% horse serum, without mitotic inhibitors and used for recordings after 8 days in culture and for no more than 3 h after removal from culture medium (Straiker and Mackie, 2005). All electrophysiological experiments were performed exclusively on excitatory neurons. Neuronal transfection was done using calcium phosphate transfection reagents (Clontech, Mountain View, CA) according to the manufacturer's instructions. Neurons were co-transfected with plasmids encoding mCherry and a cannabinoid receptor 18 to 24 hours prior to recordings. All drugs were tested on cells from at least two different preparations.
2.3 Electrophysiology
When a single neuron is grown on a small island of permissive substrate, it forms synapses – or `autapses' – onto itself. All experiments were performed on isolated autaptic neurons. Whole-cell, voltage-clamp recordings from autaptic neurons were carried out at room temperature using an Axopatch 200B amplifier (Axon Instruments, Burlingame, CA, USA). The extracellular solution contained (mM) NaCl 119, KCl 5, CaCl2 2, MgCl2 1, glucose 30 and HEPES 20. Continuous flow of solution through the bath chamber (2 mL·min-1) ensured rapid drug application and clearance. Drugs were typically prepared as a stock then diluted into extracellular solution at their final concentration and used on the same day. For low calcium recordings the CaCl2 concentration was lowered to 0.2 mM. Recording pipettes of 1.8–4 ΩM were filled with solution containing (mM): potassium gluconate 121.5, KCl 17.5, NaCl 9, MgCl2 1, HEPES 10, EGTA 0.2, MgATP 2 and LiGTP 0.5. Access resistance was monitored and only cells with a stable access resistance were included for data analysis. Electrophysiological recordings were performed and analyzed according to the methods reported in (Atwood et al., 2010). To examine inwardly rectifying currents at hyperpolarized potentials, neurons were depolarized to −50 mV then hyperpolarized to −130 mV. Peak currents at −130 mV were measured before and during drug application. Current voltage plots to examine peak outward currents were obtained by stepping voltage in 20 mV increments from −100 mV to +40 mV.
2.4 Immunocytochemistry
Coverslips with cultured autaptic neurons (grown 8–15 days) were fixed and washed in PBS (Straiker et al., 2009a). Cells were incubated with a neuron blocking solution (5% milk in 0.1M PBS + 0.1% Triton-X) for 30 min at room temperature to reduce non-specific staining. Neurons were next incubated with a mouse monoclonal antibody against the axonal marker 2H3 (Developmental Studies Hybridoma Bank, University of Iowa, 1:300) or the dendritic marker MAP2 (Millipore, Billerica MA, 1:1000) overnight at 4 °C and then washed six times with 0.1 M PBS. Ant ibodies against the HA tag (1:500, Covance) were also used. Cells were next incubated with Alexa633 or Alexa488 secondary antibodies (Invitrogen, Grand Island, NY, 1:500) for 1.5 hours at room temperature. Finally cover slips were washed, dried and mounted. Images were acquired with a Leica TCS SP5 confocal microscope (Leica Microsystems, Wetzlar, Germany) using Leica LAS AF software and a 63× oil objective. Images were processed using ImageJ (available at http://rsbweb.nih.gov/ij/) and/or Photoshop (Adobe Inc., San Jose, CA). Images were modified only in terms of brightness and contrast.
2.5 Data analysis
Data are reported as mean ± SEM (except EC50, IC50 and t1/2 data, which are reported as mean ± 95% CI). Relative EPSC charge data are presented as proportions (relative to baseline). Non-linear regression was used to fit the concentration response curves. Treatment effects on evoked and spontaneous EPSCs, paired pulse ratios data and coefficients of variation were evaluated using Student's t-tests and one-way ANOVA with Bonferroni multiple comparison tests where indicated. Statistical significance is indicated as follows: ***p < 0.001, **p < 0.01, and *p < 0.05. All graphs and statistical analyses were generated using GraphPad Prism 4.0 software (Hearne Scientific Software, Chicago, IL, USA).
3. Results
3.1 CB2 expression in CB1 null autaptic neurons restores DSE
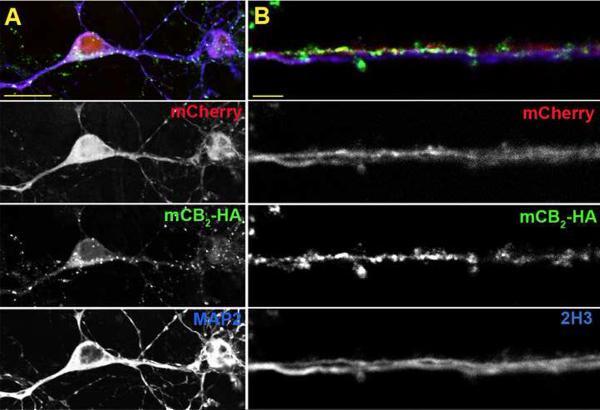
Wild type autaptic hippocampal neurons display DSE whereas CB1 null neurons do not. We have recently reported that transfecting a CB1 null neuron with CB1 restores the wild type phenotype (Straiker et al., 2011). In determining what effect CB2 expression in these CB1 null autaptic neurons would have on neurotransmission, we initially investigated whether transfection of these neurons with CB2 would result in expression and trafficking of CB2. These neurons were also co-transfected with a plasmid encoding mCherry as a fluorescent marker of transfected cells. The presence of CB2 in transfected CB1 null neurons was determined with immunocytochemistry using antibodies directed against the HA epitope on our CB2 construct. There was substantial overlap between CB2 immunoreactivity and the mCherry signal, suggesting that CB2 is not only expressed, but is also trafficked throughout the neuron (Fig. 1). To confirm this observation, we compared the localization of CB2 with immunostaining of specific neuronal compartments using antibodies that label MAP2, a marker of the somatodendritic compartment (Kosik and Finch, 1987), and 2H3, a marker of axon neurofilaments (Dodd et al., 1988). We detected CB2 in the axons of transfected neurons as determined by colocalization of the CB2 signal with 2H3 staining (Fig. 1A). We also detected CB2 in the soma and some neuronal processes that were MAP2 positive, suggesting that CB2 was also present in at least some dendrites (Fig. 1B). In processes that were positive for CB2, we found that the CB2 signal did not appear to be uniformly distributed (Fig. 1), but was often found in a clustered pattern. We found that mCherry fluorescent cells also expressed detectable CB2 in >90% of cells, allowing us to positively identify CB2 cells from which to record.
Fig. 1. CB2 is trafficked to both the axonal and somatodendritic compartments of transfected CB1 null neurons.
A) Micrograph shows composite (top) and component panels (below) for axons from a mCB2-HA/mCherry transfected neuron. mCherry shows the full outline of the transfected processes, HA staining identifies the mCB2 HA tag, and 2H3 labels axons. Arrows indicate examples of overlap between the HA and 2H3 immunoreactivity. B) A neuron transfected with HA-mCB2 and mCherry but stained for HA and the dendritic marker MAP2. Arrows indicate examples of overlap between the HA and MAP2 immunoreactivity. Scale bars: 5 μm and 20 μm for panels A and B, respectively.
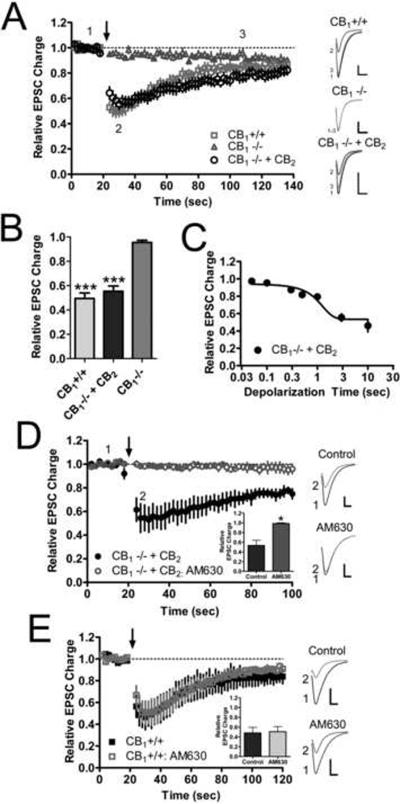
Having demonstrated that these autaptic neurons were able to express and traffic CB2, we next sought to ascertain if CB2 could modulate neurotransmission. We first looked at whether CB2 could mediate DSE, similar to CB1's function in wild type neurons and CB1 transfected CB1 null autaptic neurons. Consistent with our previous findings, depolarization resulted in robust DSE in wild type neurons, but had no effect on neurotransmission in CB1 null neurons (Fig. 2A). Interestingly, when CB2 was transfected into CB1 null neurons, DSE was restored. In wild type neurons 3 seconds of depolarization suppressed neurotransmission to 0.50 ± 0.044 of control which was significantly greater than that achieved in CB1 null neurons (Fig. 2B: 0.96 ± 0.018 of control, p<0.001). The magnitude of DSE elicited in CB2 neurons (Fig. 2B: 0.55 ± 0.043 of control) was not significantly different from wild type neurons but was much greater than CB1 null neurons (p<0.001). Despite having similar magnitudes of DSE, the wild type and CB2 transfected neurons recovered at different rates from the effects of depolarization (Fig. 2A). Wild type cells recovered from DSE with a t1/2 of 21 (17 to 29) seconds while CB2 cells had a t1/2 of 45 (24 to 270) seconds, which was significantly slower (p=0.010). Just as with wild type and CB1 transfected CB1 null neurons, increasing durations of depolarization resulted in increased suppression of neurotransmission in CB2 expressing CB1 null neurons (Fig. 2C). The maximal amount of suppression achieved in CB2 neurons was 0.53 ± 0.027 of control and this was also not significantly different than wild type (0.48 ± 0.068 of control, p>0.05, data not shown). The half maximal effective duration of depolarization (duration of depolarization necessary to elicit half maximal inhibition) for CB2 neurons was 1.6 (0.75 to 3.8) seconds. This is nearly identical to the half maximal effective duration of depolarization in wild type neurons (1.6 seconds) as previously reported (Straiker et al., 2011). To additionally ensure that the effects observed in CB2 transfected neurons were indeed due to CB2 activation, 1 μM AM630 (a CB2- preferring antagonist) was applied following establishment of a baseline DSE time course (Fig. 2D). AM630 completely blocked the expression of DSE in CB2-expressing cells that had previously demonstrated robust DSE (pre-AM630: 0.53 ± 0.11 of control; with-AM630: 0.99 ± 0.016 of control, p=0.028). AM630 had no effect on DSE in wild type neurons (Fig. 2E; pre-AM630: 0.49 ± 0.12 of control; with-AM630: 0.51 ±0.11 of control, p>0.05).
Fig. 2. CB2 expression restores DSE to CB1 null neurons.
(A) Time course of EPSCs recorded before and after a depolarizing step to 0 mV from a holding potential of −70 mV (arrow at 20 sec) in wild type (CB1+/+, n=6), CB1 null (CB1−/−, n=6) and CB2 transfected CB1 null neurons (CB1−/− + CB2, n=12). Scale bars = 1 nA, 10 ms. (B) Summary of the maximal inhibition of neurotransmission achieved by 3 seconds of depolarization in each class of neuron. Data analyzed using one way ANOVA with Bonferroni multiple comparison test. (C) Plot of the effect of increasing lengths of depolarization from 50 ms up to 10 s on EPSC magnitude for CB2 transfected CB1 null neurons (n=3–11 for each time point). (D) 1 μM AM630 prevents DSE following a 3 depolarizing stimulus in CB2 transfected CB1 null neurons that previously exhibited DSE, suggesting the rescue of DSE is due to the expression of functional CB2 receptors (n=4). Data analyzed using paired Student's t-test. (E) 1 μM AM630 did not prevent DSE following a 3 depolarizing stimulus in wild type neurons that previously exhibited DSE (n=3). Scale bars in (D) and (E) = 1 nA, 5 ms. Data in (D) and (E) analyzed using paired Student's t-test. *: p<0.05, ***: p<0.001.
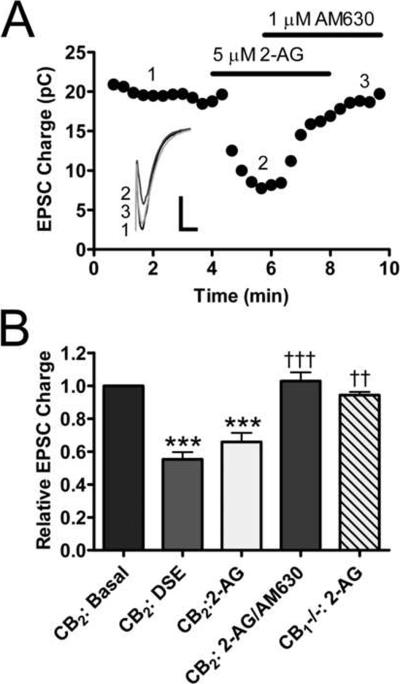
2-AG is the endocannabinoid that most likely mediates DSE in wild type autaptic neurons (Straiker and Mackie, 2005). To determine whether 2-AG could mimic the effects of depolarization that we observed in our CB2-transfected cells, we bath applied 5 μM 2-AG to these neurons and evaluated EPSC magnitude. As seen in the example in Fig. 3A, 2-AG reduced the magnitude of EPSCs and this reduction was reversed by 1 μM AM630. 2-AG significantly decreased the size of EPSCs (Fig. 3B; 0.66 ± 0.055 of control; p<0.001 vs. basal) and this suppression was not significantly different from that observed with depolarization for 3 seconds (0.55 ± 0.043 of control; p > 0.05 vs. 2-AG treatment). 1 μM AM630 significantly reversed the effects produced by 2-AG treatment (1.029 ± 0.053 of control; p < 0.001 vs. 2-AG, p > 0.05 vs. basal). The effects of AM630 alone are discussed in greater detail below. 2-AG had no significant effect on untransfected CB1 null neurons (0.94 ± 0.019 of control; p > 0.05). These data suggest that when expressed in neurons, CB2 has a similar function as CB1 in that it mediates DSE. As with CB1-mediated DSE, this is likely due to CB2 activation by 2-AG released following depolarization.
Fig. 3. 5 μM 2-AG mimics the effects of depolarization in CB2 neurons.
(A) Representative time course showing that treatment with 5 μM 2-AG suppresses neurotransmission and the inhibition can be reversed/blocked by 1 μM AM630. Inset shows individual traces for indicated time points. Scale bar = 1 nA, 10 ms (B) 5 μM 2-AG suppresses EPSCs (n=9) to a similar extent as 3 s depolarizing stimulus (n=11). This suppression is blocked by 1 μM AM630 (n=5). 2-AG has no effect on neurotransmission in non-transfected CB1 null neurons (n=4). Data analyzed using oneway ANOVA with Bonferroni's multiple comparison test. ***: p<0.001 vs. basal. ††: p<0.01 vs. 2-AG, †††: p<0.001 vs. 2-AG.
3.2 CB2 inhibits neurotransmission by acting at a presynaptic site
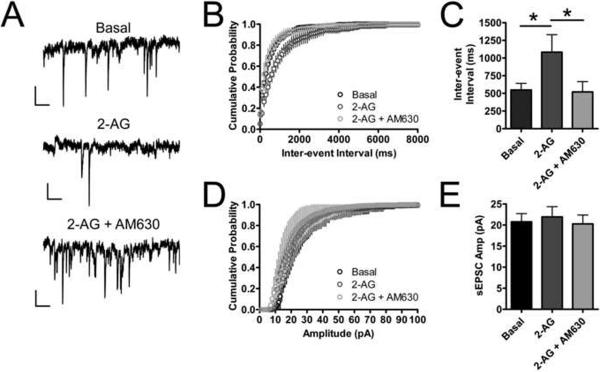
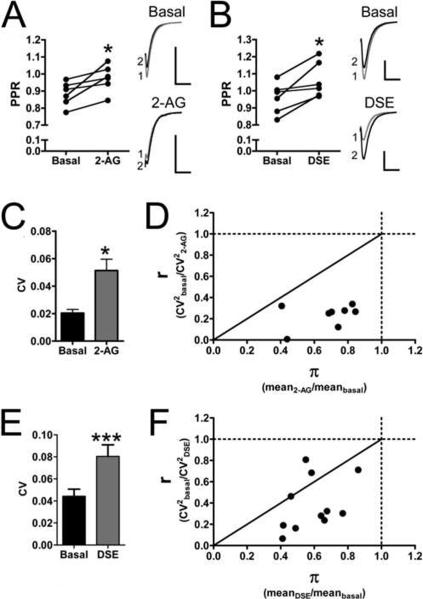
We hypothesized that since the effects of CB2 expression in these autaptic neurons mimicked that of the wild type CB1 expressing neurons, CB2 was likely acting through a similar mechanism as CB1. CB1 inhibits neurotransmission with a presynaptic site of action. The site of action of a receptor involved in modulation of neurotransmission in neuronal cultures can be determined by a combination of immunocytochemistry and measurements of paired-pulse ratios (PPR), spontaneous EPSCs (sEPSCs) and the coefficients of variation before and after agonist treatment or depolarization. Since our immunocytochemistry studies indicated that CB2 could be trafficked to both dendrites and axons, we turned to electrophysiological methods of determining the site of CB2's action. We first measured the frequency and amplitude of sEPSCs. Treating CB2 transfected neurons with 5 μM 2-AG increased the inter-event interval from 550.0 ± 95 ms to 1100.0 ± 250 ms (p<0.05) (Fig. 4A–C) without changing the mean sEPSC amplitude (basal = 17 ± 3.5 pA; 2-AG: 19 ± 3.5 pA, p>0.05) (Fig. 4D–E). This effect on sEPSC frequency could be reversed by 1 μM AM630, decreasing the inter-event interval to 520 ± 150 ms (p < 0.05 vs. 2-AG, p>0.05 vs. basal) (Fig. 4A–C). AM630 treatment also did not affect sEPSC amplitude (20 ± 3.3 pA, p>0.05 vs. basal and vs. 2-AG) (Fig. 4D–E). Decreasing sEPSC frequency, with no change in amplitude is consistent with a presynaptic site of action for CB2. We also measured PPRs before and after 2-AG treatment and depolarization. In CB2-transfected neurons, 5 μM 2-AG treatment increased PPR from 0.88 ± 0.029 to 0.98 ± 0.032 (p=0.021) (Fig. 5A). In addition, PPR increased following 3 seconds of depolarization (DSE) from 0.96 ± 0.037 to 1.063 ± 0.043 (p=0.016) (Fig. 5B). Increasing PPR with both depolarization and 2-AG application are also consistent with a presynaptic site of action for CB2-mediated inhibition of synaptic transmission. Finally, we calculated the coefficient of variation for each experiment in which we applied 2-AG or elicited DSE. 5 μM 2-AG increased the coefficient of variation from 0.020 ± 0.0027 to 0.051 ± 0.0083 (p=0.012) (Fig. 5C). Furthermore the comparison between the mean r value (0.23 ± 0.059) and the mean π value (0.68 ± 0.039) is also consistent with a presynaptic localization of synaptic depression (r < π < 1). Fig. 5D demonstrates this in that all 2-AG experiments produced r and π values that fell within the region of the graph typically believed to be associated with a presynaptic site of synaptic depression (Faber et al., 1991; Shen et al., 1999). Depolarization produced a similar effect. 3 seconds of depolarization increased the coefficient of variation from 0.044 ± 0.0064 to 0.080 ± 0.011 (Fig. 5E). The mean r value was 0.38 ±0.075 and the mean π value was 0.59 ± 0.044 (r < π < 1). Eight of the eleven values fell within the region of the graph associated with a presynaptic site of action (Fig. 5F). The other three fell within the region associated with a mixed presynaptic and postsynaptic site of action. Taken together, the evidence of axonal CB2 (Fig. 1A) and CB2 agonists affecting the frequency, but not the amplitude of sEPSCs (Fig. 4), increasing the PPRs and the coefficients of variation (Fig. 5) all suggest that CB2 is primarily exerting its inhibitory effect on neurotransmission by acting at a presynaptic locus, similar to what has been observed for CB1 in autaptic hippocampal neurons (Atwood et al., 2010a; Straiker and Mackie, 2005).
Fig. 4. CB2 activation decreases sEPSC frequency, but not amplitude suggesting a presynaptic site of action.
Spontaneous EPSCs (sEPSCs) were recorded from CB2 expressing neurons. Representative traces of sEPSCs recorded under (A) basal conditions, during 5 μM 2-AG treatment and during 5 μM 2-AG treatment with 1 μM AM630 present. Scale bars = 10 pA, 100 ms (B) Cumulative probability histogram of sEPSC frequency shows that the inter-event interval increases following 2-AG treatment and returns back to baseline when AM630 is applied. (C) Summary data for sEPSC frequency. (D) Cumulative probability histogram of sEPSC amplitude shows that 2-AG and AM630 do not alter the sEPSC amplitude. (E) Summary data for sEPSC amplitude. n=8 for all treatments. Data in (C) and (E) analyzed using one way ANOVA with Bonferroni's multiple comparison test. *: p<0.05 vs. 2-AG.
Fig. 5. CB2 activation increases the paired pulse ratio and coefficient of variation suggesting a presynaptic site of action.
(A) 5 μM 2-AG increases the paired pulse ratio (PPR) in CB1 null neurons expressing CB2 (n=6). (B) A 3 sec depolarization (DSE) also increases the PPR (n=6). The increases in PPR suggest a presynaptic site of action. (C) 5 μM 2-AG increases the coefficient of variation (CV) in CB1 null neurons expressing CB2. (D) Analysis of CV for 2-AG treatment demonstrates that r < π < 1 (n=8). (E) DSE also increases the CV (n=11). (F) Analysis of CV for DSE demonstrates that r < π < 1. The increases in CV as well as the measurements of r and π all point to a presynaptic site for CB2 activation resulting in synaptic depression. Data analyzed using paired Student's t-test. * p < 0.05 vs. basal. ***: p<0.001 vs. basal.
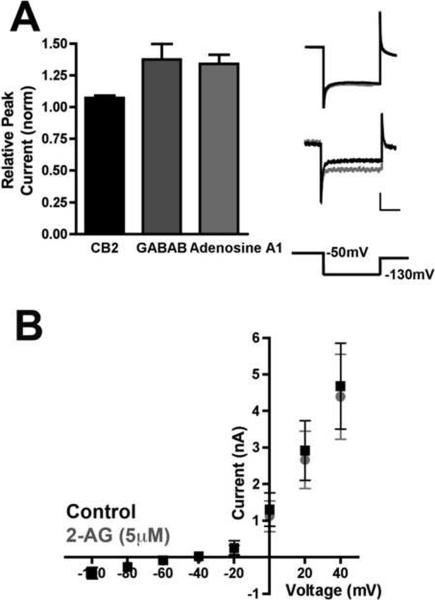
Despite the physiological evidence that CB2 acts presynaptically, we also found that CB2 was trafficked into the somatodendritic compartments of autaptic neurons (Fig. 1A). This raised the possibility that CB2 could have a post-synaptic function in addition to its presynaptic suppression of neurotransmission. We therefore tested whether 2-AG affected the electrophysiological properties of the CB2-expressing neurons. We determined that 5 μM 2-AG did not alter the holding current (basal: 73 ± 15 pA; 2-AG: 74 ± 16 pA; p>0.05) or the input resistance (basal: 9.5 ± 1.5 MΩ; 2-AG: 9.8 ± 1.7 MΩ; p>0.05). We have found that autaptic hippocampal neurons exhibit a robust G protein coupled inwardly rectifying potassium (GIRK) current in response to activation of GABAB and Adenosine A1 G protein coupled receptors. The GABAB receptor agonist baclofen and the adenosine A1 receptor agonist N6-cyclopentyladenosine each increased postsynaptic GIRK currents (25 μM baclofen: 1.38 ± 0.10, n=5; 200 nM N6-cyclopentyladenosine: 1.34 ± 0.07, n=4), as measured by changes in peak potassium currents following a voltage step to −130 mV after a brief depolarization to −50 mV. CB1 activation does not modulate these currents (data not shown). Activation of CB2 transfected into these neurons gave results similar to CB1 (5 μM 2AG: 1.07 ± 0.02; p<0.05 for Baclofen, N6-cyclopentyladenosine vs. 2-AG). (Fig. 6A). We also determined that 2-AG had no effect on voltage dependent peak outward currents (Fig. 6B). These data support our conclusion that CB2, despite being present post-synaptically, is primarily exerting a presynaptic effect.
Fig. 6. CB2 activation by 2-AG does not alter GIRK or outward currents.
A) 2-AG (5 μM) did not alter peak inwardly rectifying potassium currents observed while hyperpolarizing CB2-transfected neurons to −130mV after a brief depolarization to −50mV. These currents are reliably elicited by postsynaptic GABAB (25μM baclofen, n=5) and adenosine A1 (200 nM N6-cyclopentyladenosine, n=4) receptor activation in wild type neurons. Top trace: representative 2-AG treatment of CB2 expressing neuron. Bottom trace: representative trace for a baclofen treated neuron. Scale bars: 500pA, 50msec. B) Current-Voltage plot shows that peak outward currents in response to successive voltage steps are unaltered by application of 2-AG (5μM) in CB2-expressing neurons. p<0.05 for GABAB and Adenosine A1 vs. CB2 using one way ANOVA with Dunnett's posthoc test.
3.3 CB2 displays constitutive activity when expressed in neurons
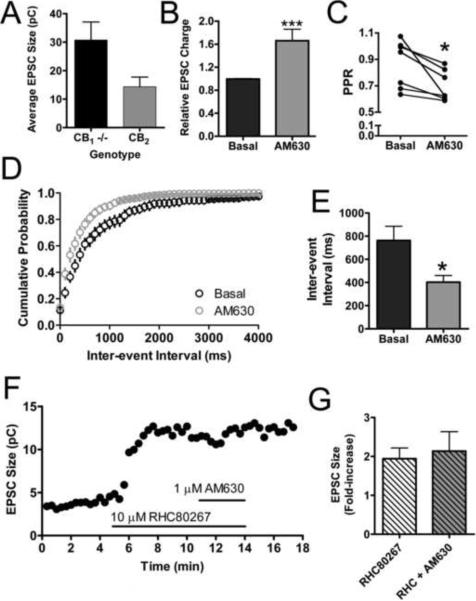
It has been reported that cells transfected with and expressing high levels of CB2, can display constitutive activity (Bouaboula et al., 1999). In our experiments with CB2-transfected neurons, we noticed signs that CB2 might also be displaying constitutive activity in this expression system. We noted that CB2 expressing cells qualitatively appeared to have smaller EPSCs than either wild type or untransfected CB1 null neurons. To quantify this observation we matched CB2 transfected and nontransfected neurons from the same cultures, on the same coverslips, and subjected to the same transfection procedures. When quantitatively assessed, our qualitative observation was confirmed (Fig. 7A). CB2 expressing cells had smaller EPSCs (14 ± 3.4 pC) than CB1 null neurons (31 ± 6.3 pC, p=0.052 vs. CB2). This implies that simply expressing CB2 in these neurons suppresses neurotransmission. To confirm that transfection itself did not alter the magnitude of EPSCs, we compared neurons that were transfected with CB2 and mCherry to neurons that were transfected with mCherry alone. We found that mCherry alone transfected neurons had an average EPSC size of 2.03 ± 0.40 nA, whereas CB2 plus mCherry transfected neurons in these experiments had an average EPSC size of 1.1 ± 0.18 nA (p=0.042 vs. mCherry alone), suggesting it is the over expression of CB2 that is leading to the smaller EPSC. To determine the mechanism of the decreased EPSCs, we performed additional electrophysiological experiments that would reveal whether the smaller EPSCs were due to ongoing CB2 activity. First we treated CB2 expressing neurons with the CB2 inverse agonist 1 μM AM630 alone (Fig. 7B). This increased the magnitude of EPSCs 1.7 -fold above basal levels (1.7 ± 0.20; p=0.013 vs. basal). 1 μM AM630 treatment also decreased the PPR in CB2 neurons from 0.87 ± 0.068 to 0.70 ± 0.044 (p=0.018)(Fig. 7C). As seen in Fig. 4A–C, AM630 slightly increased the frequency of sEPSCs above baseline following 2-AG treatment. When we applied 1 μM AM630 alone, we found that AM630 decreased the inter-event interval of sEPSCs from 760 ± 120 ms to 400 ± 58 ms (Fig. 7D–E) without affecting amplitudes (basal: 24 ± 3.2 pA; AM630: 23 ± 3.0 pA). Together these data support our hypothesis that CB2, when expressed in cultured autaptic neurons, exhibits ongoing activity. The source of this activity could either be persistent receptor activation intrinsic to the receptor itself (i.e., constitutive activity) or due to continuous release of endocannabinoids and subsequent activation of the receptor. To discriminate between these two possibilities we recorded EPSCs from CB2 transfected neurons and treated them with 10 μM RHC80267, a diacylglycerol lipase (DGL) inhibitor. DGL inhibition or genetic ablation decreases 2-AG production and disrupts endocannabinoid-mediated synaptic plasticity (Hashimotodani et al., 2008; Jung et al., 2011; Tanimura et al., 2010). If the ongoing activity of CB2 was due to continuous 2-AG production, we would expect RHC80267 treatment to increase EPSC magnitude. This was indeed the case. Fig. 7F and 7G shows that in a CB2 expressing neuron 10 μM RHC80267 increased the size of EPSCs. Furthermore it occluded the inverse agonist effect normally seen with AM630 treatment (Fig. 7B). Quantification of these observations revealed that RHC80267 substantially increases EPSC size (1.9 ± 0.28 fold increase over basal) and AM630 only produces a modest, statistically insignificant increase beyond this (2.1 ± 0.50, p = 0.72) (Fig. 7G). Thus it appears that at least in neuronal expression systems, ongoing 2-AG production engages CB2 receptors to produce a basal state of activation. This also suggests that CB2 in these neurons is sensitive to even basal levels of 2-AG production. This sensitivity is one possible explanation for the slow recovery from depolarization observed in Fig. 2A. It is important to note here that the effect of AM630 alone (Fig. 7B) varied somewhat from experiment to experiment (range: from 1.2 to 2.5 fold above basal) and was different from the 2-AG plus AM630 data in Fig. 3. We speculate that this could be due to differences in the level of CB2 expression or the levels of endogenous 2-AG production in each of these experiments.
Fig. 7. CB2 expressed in neurons displays constitutive activity.
(A) CB2 expressing CB1 null neurons have smaller EPSCs than untransfected CB1 null neurons from the same coverslip (n=5). (B) Treatment with 1 μM AM630 increases the sizes of EPSCs in CB2 expressing neurons (n=8). (C) AM630 decreases the PPR in CB2 neurons, suggesting a presynaptic site of action (n=7). (D) Cumulative probability histogram of sEPSC frequency shows that the inter-event interval decreases following 1 μM AM630 treatment (n=8). (E) Summary data for sEPSC frequency. (F) Representative time course of neuron treated with 10 μM RHC80267, a DGL inhibitor and 1 μM AM630. (G) Summary of data from neurons treated with RHC80267 (n=9 for RHC80267 alone and n=4 for RHC80267 + AM630). RHC80267 increases the relative size of EPSCs and occludes the AM630 increase seen in (B) suggesting CB2 constitutive activity is due to ongoing 2-AG synthesis. Data in (A) and (G) analyzed using unpaired Student's t-test. Data in (B),(C), and (E) analyzed using paired Student's t-test. *: p<0.05, ***: p<0.001.
3.4 CB2 inhibits voltage-gated calcium channels in autaptic neurons
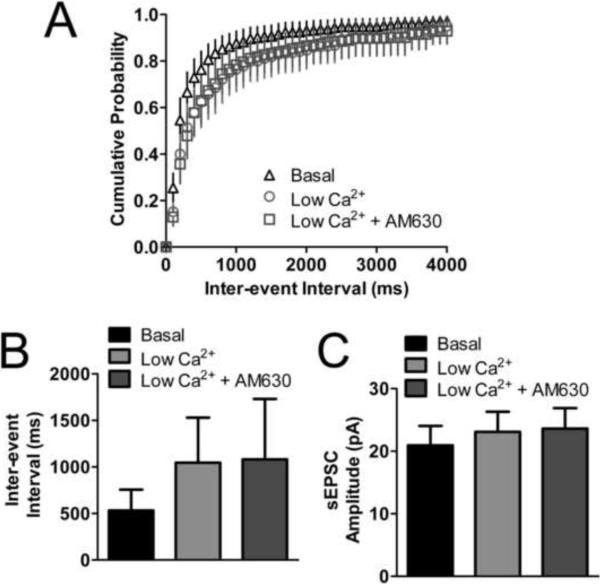
Based on our data presented thus far, we suspected that CB2 was completely compensating for the absence of CB1 in the CB1 null neurons. This leads to the possibility that CB2 was coupling to presynaptic calcium channels. We have recently reported that CB2 does indeed inhibit voltage gated calcium channels when expressed in AtT20 cells, albeit in a ligand dependent manner (Atwood et al., 2012). We sought to determine if CB2 was inhibiting calcium channels in our autaptic neuronal preparation. Since we couldn't directly measure synaptic calcium currents we used an indirect method of determining if CB2 was inhibiting calcium channels. We exchanged our normal extracellular solution for a low calcium external solution and determined if this would occlude the effects of cannabinoid ligands on sEPSC frequency. This low extracellular calcium solution increased the inter-event interval of sEPSCs recorded in these neurons from 530 ± 220 ms to 1000 ± 480 ms (p>0.05 vs. basal)(Fig. 8A,B), but did not alter the amplitudes (basal: 21 ± 3.1 pA; low calcium: 23 ± 3.2 pA; p>0.05)(Fig. 8C). Because this decreased frequency was now close to the limit of detection for this experiment we recognized that we would be unlikely to observe any further reduction in frequency with 2-AG treatment or depolarization. Instead we returned to our findings shown in Fig. 7 which demonstrated that 1 μM AM630 decreased the inter-event interval on its own in CB2 expressing neurons (Fig. 7D,E). We hypothesized that if CB2 were constitutively inhibiting neurotransmission through inhibition of voltage gated calcium channels, that the low calcium solution would occlude the inverse agonist action of AM630. Consistent with this hypothesis, 1 μM AM630 did not affect the inter-event intervals of sEPSCs in the presence of low calcium (1100 ± 650 ms, p>0.05 vs. low calcium)(Fig. 8A,B). It also had no effect on sEPSC amplitude (24 ± 3.3 pA; p>0.05 vs. low calcium)(Fig. 8C). These data suggest that, consistent with our previous work in AtT20 cells (Atwood et al., 2012), CB2 inhibits neuronal voltage gated calcium channels. It is likely that this is the mechanism whereby CB2 exerts its inhibitory effect on neurotransmission.
Fig. 8. CB2 inhibits neurotransmission through inhibition of voltage gated calcium channels.
(A,B) In CB2 expressing CB1 null neurons, exchanging the external medium with medium containing 0.2 mM CaCl2 (low Ca2+) increases the inter-event interval of sEPSCs (n=9). The low Ca2+ medium occludes the inverse agonist effect of AM630 observed in Fig. 7. (C) Exchanging the medium with low Ca2+ does not alter sEPSC amplitude. AM630 also has no further effect on sEPSC amplitude (n=9). Data in (B) and (C) analyzed using one-way ANOVA with Bonferroni's multiple comparison test.
4. Discussion
Despite originally being thought of as the “peripheral” cannabinoid receptor, considerable functional and anatomical evidence suggests that CB2 is expressed in the nervous system—certainly in activated microglia and possibly in some neurons. However, there is quite a bit of disagreement between studies and these disagreements most likely result from imperfect tools, non-selective ligands (Lauckner et al., 2008), functional selectivity of ligands (Atwood et al., 2012), improper controls, and even the inducibility of CB2 expression under very specific conditions (Pietr et al., 2009; Wotherspoon et al., 2005; Zhang et al., 2003). Due to all of these discrepancies and the imperfect tools at our disposal, it has been quite difficult to ascertain the function of CB2 in neurons, if any. Many studies rely on indirect pharmacological evidence of CB2's presence in neurons. For example, a recent report found that CB2 selective ligands reduce inhibitory transmission in the entorhinal cortex, but did not identify the cellular location of the CB2 receptors involved (Morgan et al., 2009). The pharmacological effects reported may indeed be due to CB2 being present in neurons, but alternatively, CB2 receptors may also be found on brain microglia (Ashton et al., 2007; Walter et al., 2003) and microglia are capable of influencing synaptic plasticity (Ben Achour and Pascual, 2010). Others have looked at the role of CB2 in neurons, but from a behavioral standpoint. For example, mice that overexpress CB2 in the CNS have reduced neuropathic pain, are resistant to depression-like behaviors, and have decreased anxiety (García-Gutiérrez and Manzanares, 2011; García-Gutiérrez et al., 2010, Racz et al., 2008). Here we have addressed the role of CB2 specifically expressed in neurons and at the cellular level to determine its effects on synaptic transmission. We found that CB2 inhibits synaptic transmission via a presynaptic mechanism and participates in endocannabinoid-mediated synaptic plasticity.
4.1 CB2 mediates DSE in CB1−/− neurons likely via calcium channel inhibition
Previous work from our lab suggests that, contrary to earlier reports, CB2 is indeed able to inhibit VGCCs, but this ability is highly dependent on the identity of the CB2 ligand used (Atwood et al., 2012). In those studies we found that 2-AG was a ligand that efficaciously inhibited VGCCs through CB2. Since 2-AG, the endocannabinoid that mediates DSE in hippocampal autaptic cultures was able to activate CB2, resulting in VGCC inhibition in AtT20 cells, we hypothesized that if CB2 was expressed in a neuron it, too, would respond to 2-AG to inhibit VGCCs and reduce neurotransmitter release. We confirmed our hypothesis, demonstrating that CB2 not only restores DSE to CB1 null neurons, but that it does so in a manner very similar to wild type neurons. Specifically, CB2 expression restores sensitivity to 2-AG and suppresses neurotransmission by a presynaptic mechanism, presumably through inhibition of voltage-gated calcium channels.
4.2 CB2-mediated inhibition of neurotransmission displays some differences from wild type, CB1-mediated inhibition
Even though CB2 mediated DSE is similar to that mediated by CB1 in wild type cells, there were some differences. DSE in CB2 expressing neurons has a longer duration than in wild type neurons, likely due to CB2's greater sensitivity to 2-AG as indicated by significant activation of CB2 by basal levels of 2-AG production. To further support this hypothesis we have found that CB1 inverse agonist rimonabant does not increase the magnitude of EPSCs in wild type neurons (data not shown) contrasting with AM630's effects in CB2 transfected cells suggesting that CB1 is less sensitive to the 2-AG tone observed in Fig. 7. Another difference between wild type and CB2 expressing CB1 null neurons is the distribution of the receptor. In wild type neurons CB1 is predominately localized to axons (Straiker and Mackie, 2005). We found that when CB2 is transfected into CB1 null neurons, it is trafficked non-uniformly in the neuron to both axonal and somatodendritic neuronal compartments. The CB2-positive puncta seen in Fig. 1 are likely synapses to which CB2 is trafficked, but it is also apparent that since the CB2 signal is not ubiquitous throughout the axons, CB2 is not present at all synapses. This may account for the partial effect of CB2 activation on synaptic transmission. This lack of directed trafficking to either axons or dendrites may be a consequence of the heterologous expression of the receptor, as when CB1 is heterologously expressed in CB1 null neurons, it loses its axonal-targeting and is expressed in both axons and dendrites (data not shown). Despite being indiscriminately trafficked, CB2 nevertheless inhibits synaptic transmission at a presynaptic site. Evidence for this includes its effects on PPR, sEPSC frequency and the coefficient of variation as well as its lack of effect on sEPSC amplitude, holding current and input resistance as well as an absence of effect on GIRK currents and voltage-dependent outward currents. Our findings contrast with a recent study that found a CB2-mediated decrease in excitability in prefrontal cortex pyramidal cells (den Boon et al., 2012). A predominant presynaptic locus for CB2 activity is also supported by the ability of low extracellular Ca2+ to occlude the increase in sEPSC frequency seen following AM630 treatment. In addition, this occlusion by low calcium also suggests that CB2 is decreasing synaptic transmission via inhibition of VGCCs. It is possible that the occlusion seen in low extracellular calcium is due to a decrease in the production of endogenous 2-AG since 2-AG production is calcium dependent. However, based on our earlier work, 2-AG production in these autaptic neurons is likely due to calcium release from internal stores rather than extracellular sources (Straiker and Mackie, 2005). Therefore we conclude that low extracellular calcium occludes the CB2-mediated inhibition of voltage-gated calcium channels in axon terminals.
Altogether these data imply that CB2 is effectively taking the place of the absent CB1 receptor in these CB1 null neurons and restoring cannabinoid sensitivity to the axon terminals resulting in inhibition of VGCC and subsequent inhibition of neurotransmitter release. It is possible that CB2 could be activated postsynaptically to produce some other retrograde messenger (e.g. nitric oxide (Makara et al., 2007)). However, this seems unlikely, as our experiments with AtT20 cells establish that selected synthetic and endogenous cannabinoids can inhibit voltage gated calcium channels via activation of CB2 receptors as well as our experiments in neurons demonstrating Cd2+ occlusion of the effect of AM630.
4.3 Functional implications of CB2-mediated modulation of neurotransmission
These data challenge a commonly held belief in cannabinoid research. It has long been held that CB1 couples to voltage gated calcium channels and inwardly-rectifying potassium channels and that CB2 does not (Felder et al., 1995; Howlett et al., 2002). There are number of possibilities that may account for this major difference in results. First, as demonstrated in Fig. 7, CB2 possesses constitutive activity when overexpressed in neurons and AtT20 cells (Atwood et al, 2011) (it remains to be determined if this is true for endogenously expressed CB2). As also shown in Fig. 7 in neurons this is likely due to ongoing synthesis of an endocannabinoid (most likely 2-AG) that can activate CB2. If CB2 is expressed in cells that constitutively produce 2-AG, it follows that a basal level of inhibition of calcium channels will be present in non-drug treated conditions. As was the case in previous work (Felder et al., 1995), CB2 over expression can decrease the efficacy of other receptors to signal through the same pathways. Indeed in our AtT20 experiments we found that CB2 expression significantly reduced the effectiveness of oxotremorine-m, a muscarinic receptor agonist that inhibits VGCCs (Atwood et al., 2012). If the drug tested does not activate CB2 with high efficacy for calcium channel inhibition, any effect of the ligand would be lost in the background of the basal tone. Another possibility is the functional selectivity of CB2 ligands as we have previously reported. Based on our data in that report and the data presented here 2-AG has a demonstrated high efficacy at CB2 in inhibiting VGCCs. However, other studies that employ other cannabinoid ligands may arrive at different conclusions if those ligands do not effectively promote inhibition by CB2 of VGCCs.
While this study does not address the issue of whether CB2 is present in neurons, its significance lies in demonstrating that when CB2 is present in neurons, for example if it is induced, then it will modulate synaptic transmission. We have demonstrated that CB2 can essentially mimic the signaling role of CB1 in its ability to modulate neurotransmission. If CB2 is expressed in neurons and signals in a nearly identical manner as CB1 one may speculate as to the physiological relevance of having CB1 and CB2 redundantly perform the same function in neurons. A hint comes from the marked inducibility of CB2. It could be that CB2 expression is quite low in most neurons under normal conditions. However, if the neuron is subjected to injury (Wotherspoon et al., 2005; Viscomi et al., 2009), having an inducible cannabinoid receptor to additionally control neurotransmission could confer physiological or protective benefits. The heightened sensitivity of CB2 to basal levels of 2-AG (Fig. 7) may confer additional protective benefits. Ongoing work aims to determine when, where and how CB2 is expressed in the CNS and whether that expression occurs in neurons. Our studies here provide an answer as to what function CB2 may have when it is expressed in neurons.
Acknowledgements
This work was supported by the National Institutes of Health (Grants DA011322, DA009158, DA021696, & RR025761); the Indiana METACyt Initiative of Indiana University, through a major grant from the Lilly Endowment, Inc.; and the Indiana University Light Microscopy Imaging Center. We would like to thank Natasha Murataeva for help with neuronal culture preparation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Abbreviations: 2-AG, 2-arachidonoylglycerol; CB1, cannabinoid receptor 1; CB2, cannabinoid receptor 2; CNS, central nervous system; DGL, diacylglycerol lipase; DSE, depolarization induced suppression of excitation; EPSC, excitatory post-synaptic current; GPCR, G protein coupled receptor; HA, hemagglutinin; PPR, paired pulse ratio; sEPSC, spontaneous excitatory post-synaptic current; VGCC, voltage gated calcium channel
References
- Ashton JC, Rahman RM, Nair SM, Sutherland BA, Glass M, Appleton I. Cerebral hypoxia-ischemia and middle cerebral artery occlusion induce expression of the cannabinoid CB2 receptor in the brain. Neurosci. Lett. 2007;412:114–117. doi: 10.1016/j.neulet.2006.10.053. [DOI] [PubMed] [Google Scholar]
- Atwood BK, Huffman J, Straiker A, Mackie K. JWH018, a common constituent of `Spice' herbal blends, is a potent and efficacious cannabinoid CB receptor agonist. Br. J. Pharmacol. 2010;160:585–593. doi: 10.1111/j.1476-5381.2009.00582.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atwood BK, Mackie K. CB2: a cannabinoid receptor with an identity crisis. Br. J. Pharmacol. 2010;160:467–479. doi: 10.1111/j.1476-5381.2010.00729.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atwood BK, Wager-Miller J, Haskins C, Straiker A, Mackie K. Functional selectivity in CB2 cannabinoid receptor signaling and regulation: implications for the therapeutic potential of CB2 ligands. Mol. Pharmacol. 2012;81:250–263. doi: 10.1124/mol.111.074013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bekkers JM, Stevens CF. Excitatory and inhibitory autaptic currents in isolated hippocampal neurons maintained in cell culture. Proc. Natl. Acad. Sci. U. S. A. 1991;88:7834–7838. doi: 10.1073/pnas.88.17.7834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben Achour S, Pascual O. Glia: the many ways to modulate synaptic plasticity. Neurochem. Int. 2010;57:440–445. doi: 10.1016/j.neuint.2010.02.013. [DOI] [PubMed] [Google Scholar]
- Bouaboula M, Dussossoy D, Casellas P. Regulation of peripheral cannabinoid receptor CB2 phosphorylation by the inverse agonist SR 144528. Implications for receptor biological responses. J. Biol. Chem. 1999;274:20397–20405. doi: 10.1074/jbc.274.29.20397. [DOI] [PubMed] [Google Scholar]
- den Boon FS, Chameau P, Schaafsma-Zhao Q, van Aken W, Bari M, Oddi S, Kruse CG, Maccarrone M, Wadman WJ, Werkman TR. Excitability of prefrontal cortex pyramidal neurons is modulated by intracellular type-2 cannabinoid receptors. Proc. Natl. Acad. Sci. USA. 2012;109:3535–3539. doi: 10.1073/pnas.1118167109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodd J, Morton SB, Karagogeos D, Yamamoto M, Jessell TM. Spatial regulation of axonal glycoprotein expression on subsets of embryonic spinal neurons. Neuron. 1988;1:105–116. doi: 10.1016/0896-6273(88)90194-8. [DOI] [PubMed] [Google Scholar]
- Felder CC, Joyce KE, Briley EM, Mansouri J, Mackie K, Blond O, Lai Y, Ma AL, Mitchell RL. Comparison of the pharmacology and signal transduction of the human cannabinoid CB1 and CB2 receptors. Mol. Pharmacol. 1995;48:443–450. [PubMed] [Google Scholar]
- Furshpan EJ, MacLeish PR, O'Lague PH, Potter DD. Chemical transmission between rat sympathetic neurons and cardiac myocytes developing in microcultures: evidence for cholinergic, adrenergic, and dual-function neurons. Proc. Natl. Acad. Sci. U. S. A. 1976;73:4225–4229. doi: 10.1073/pnas.73.11.4225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García-Gutiérrez MS, Pérez-Ortiz JM, Gutiérrez-Adán A, Manzanares J. Depression-resistant endophenotype in mice overexpressing cannabinoid CB(2) receptors. Br. J. Pharmacol. 2010;160:1773–1784. doi: 10.1111/j.1476-5381.2010.00819.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García-Gutiérrez MS, Manzanares J. Overexpression of CB2 cannabinoid receptors decreased vulnerability to anxiety and impaired anxiolytic action of alprazolam in mice. J. Psychopharmacol. 2011;25:111–120. doi: 10.1177/0269881110379507. [DOI] [PubMed] [Google Scholar]
- Gong JP, Onaivi ES, Ishiguro H, Liu QR, Tagliaferro PA, Brusco A, Uhl GR. Cannabinoid CB2 receptors: immunohistochemical localization in rat brain. Brain Res. 2006;1071:10–23. doi: 10.1016/j.brainres.2005.11.035. [DOI] [PubMed] [Google Scholar]
- Hashimotodani Y, Ohno-Shosaku T, Maejima T, Fukami K, Kano M. Pharmacological evidence for the involvement of diacylglycerol lipase in depolarization-induced endocanabinoid release. Neuropharmacology. 2008;54:58–67. doi: 10.1016/j.neuropharm.2007.06.002. [DOI] [PubMed] [Google Scholar]
- Howlett AC, Barth F, Bonner TI, Cabral G, Casellas P, Devane WA, Felder CC, Herkenham M, Mackie K, Martin BR, Mechoulam R, Pertwee RG. International Union of Pharmacology. XXVII. Classification of cannabinoid receptors. Pharmacol. Rev. 2002;54:161–202. doi: 10.1124/pr.54.2.161. [DOI] [PubMed] [Google Scholar]
- Jung KM, Astarita G, Thongkham D, Piomelli D. Diacylglycerol lipase-alpha and -beta control neurite outgrowth in neuro-2a cells through distinct molecular mechanisms. Mol. Pharmacol. 2011;80:60–67. doi: 10.1124/mol.110.070458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kano M, Ohno-Shosaku T, Hashimotodani Y, Uchigashima M, Watanabe M. Endocannabinoid-mediated control of synaptic transmission. Physiol. Rev. 2009;89:309–380. doi: 10.1152/physrev.00019.2008. [DOI] [PubMed] [Google Scholar]
- Kosik KS, Finch EA. MAP2 and tau segregate into dendritic and axonal domains after the elaboration of morphologically distinct neurites: an immunocytochemical study of cultured rat cerebrum. J. Neurosci. 1987;7:3142–3153. doi: 10.1523/JNEUROSCI.07-10-03142.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauckner JE, Jensen JB, Chen HY, Lu HC, Hille B, Mackie K. GPR55 is a cannabinoid receptor that increases intracellular calcium and inhibits M current. Proc. Natl. Acad. Sci. U. S. A. 2008;105:2699–2704. doi: 10.1073/pnas.0711278105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levison SW, McCarthy KD. Characterization and partial purification of AIM: a plasma protein that induces rat cerebral type 2 astroglia from bipotential glial progenitors. J. Neurochem. 1991;57:782–794. doi: 10.1111/j.1471-4159.1991.tb08220.x. [DOI] [PubMed] [Google Scholar]
- Makara JK, Katona I, Nyiri G, Nemeth B, Ledent C, Watanabe M, de Vente J, Freund TF, Hajos N. Involvement of nitric oxide in depolarization-induced suppression of inhibition in hippocampal pyramidal cells during activation of cholinergic receptors. J. Neurosci. 2007;27:10211–10222. doi: 10.1523/JNEUROSCI.2104-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan NH, Stanford IM, Woodhall GL. Functional CB2 type cannabinoid receptors at CNS synapses. Neuropharmacology. 2009;57:356–368. doi: 10.1016/j.neuropharm.2009.07.017. [DOI] [PubMed] [Google Scholar]
- Pietr M, Kozela E, Levy R, Rimmerman N, Lin YH, Stella N, Vogel Z, Juknat A. Differential changes in GPR55 during microglial cell activation. FEBS Lett. 2009;583:2071–2076. doi: 10.1016/j.febslet.2009.05.028. [DOI] [PubMed] [Google Scholar]
- Racz I, Nadal X, Alferink J, Baños JE, Rehnelt J, Martín M, Pintado B, Gutierrez-Adan A, Sanguino E, Manzanares J, Zimmer A, Maldonado R. Crucial role of CB(2) cannabinoid receptor in the regulation of central immune responses during neuropathic pain. J. Neurosci. 2008;28:12125–12135. doi: 10.1523/JNEUROSCI.3400-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reibaud M, Obinu MC, Ledent C, Parmentier M, Bohme GA, Imperato A. Enhancement of memory in cannabinoid CB1 receptor knock-out mice. Eur. J. Pharmacol. 1999;379:R1–2. doi: 10.1016/s0014-2999(99)00496-3. [DOI] [PubMed] [Google Scholar]
- Ross RA, Coutts AA, McFarlane SM, Anavi-Goffer S, Irving AJ, Pertwee RG, MacEwan DJ, Scott RH. Actions of cannabinoid receptor ligands on rat cultured sensory neurones: implications for antinociception. Neuropharmacology. 2001;40:221–232. doi: 10.1016/s0028-3908(00)00135-0. [DOI] [PubMed] [Google Scholar]
- Straiker A, Mackie K. Depolarization-induced suppression of excitation in murine autaptic hippocampal neurones. J. Physiol. 2005;569:501–517. doi: 10.1113/jphysiol.2005.091918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straiker A, Wager-Miller J, Mackie K. The CB(1) cannabinoid receptor C-terminus regulates receptor desensitization in autaptic hippocampal neurons. Br. J. Pharmacol. 2011 doi: 10.1111/j.1476-5381.2011.01743.x. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanimura A, Kawata S, Hashimoto K, Kano M. Not glutamate but endocannabinoids mediate retrograde suppression of cerebellar parallel fiber to Purkinje cell synaptic transmission in young adult rodents. Neuropharmacology. 2009;57:157–163. doi: 10.1016/j.neuropharm.2009.04.015. [DOI] [PubMed] [Google Scholar]
- Viscomi MT, Oddi S, Latini L, Pasquariello N, Florenzano F, Bernardi G, Molinari M, Maccarrone M. Selective CB2 receptor agonism protects central neurons from remote axotomy-induced apoptosis through the PI3K/Akt pathway. J. Neurosci. 2009;29:4564–4570. doi: 10.1523/JNEUROSCI.0786-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter L, Franklin A, Witting A, Wade C, Xie Y, Kunos G, Mackie K, Stella N. Nonpsychotropic cannabinoid receptors regulate microglial cell migration. J. Neurosci. 2003;23:1398–1405. doi: 10.1523/JNEUROSCI.23-04-01398.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wotherspoon G, Fox A, McIntyre P, Colley S, Bevan S, Winter J. Peripheral nerve injury induces cannabinoid receptor 2 protein expression in rat sensory neurons. Neuroscience. 2005;135:235–245. doi: 10.1016/j.neuroscience.2005.06.009. [DOI] [PubMed] [Google Scholar]
- Xi ZX, Peng XQ, Li X, Song R, Zhang HY, Liu QR, Yang HJ, Bi GH, Li J, Gardner EL. Brain cannabinoid CB2 receptors modulate cocaine's actions in mice. Nat. Neurosci. 2011;14:1160–1166. doi: 10.1038/nn.2874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Hoffert C, Vu HK, Groblewski T, Ahmad S, O'Donnell D. Induction of CB2 receptor expression in the rat spinal cord of neuropathic but not inflammatory chronic pain models. Eur. J. Neurosci. 2003;17:2750–2754. doi: 10.1046/j.1460-9568.2003.02704.x. [DOI] [PubMed] [Google Scholar]