Abstract
Gene delivery to bone is useful both as an experimental tool and as a potential therapeutic strategy. Among its advantages over protein delivery are the potential for directed, sustained and regulated expression of authentically processed, nascent proteins. Although no clinical trials have been initiated, there is a substantial pre-clinical literature documenting the successful transfer of genes to bone, and their intraosseous expression. Recombinant vectors derived from adenovirus, retrovirus and lentivirus, as well as non-viral vectors, have been used for this purpose. Both ex vivo and in vivo strategies, including gene-activated matrices, have been explored. Ex vivo delivery has often employed mesenchymal stem cells (MSCs), partly because of their ability to differentiate into osteoblasts. MSCs also have the potential to home to bone after systemic administration, which could serve as a useful way to deliver transgenes in a disseminated fashion for the treatment of diseases affecting the whole skeleton, such as osteoporosis or osteogenesis imperfecta. Local delivery of osteogenic transgenes, particularly those encoding bone morphogenetic proteins, has shown great promise in a number of applications where it is necessary to regenerate bone. These include healing large segmental defects in long bones and the cranium, as well as spinal fusion and treating avascular necrosis.
Keywords: Gene therapy, research translation, bone healing, osteogenesis imperfecta, animal models, facilitated endogenous repair
1. INTRODUCTION
Bone is subject to a number of systemic and local disorders that may be, to a greater or lesser degree, genetic or environmental in origin. Gene therapy is being investigated as a way to treat or cure several of these (Table 1).
TABLE 1.
Disorders affecting bone addressed by gene therapy approaches
| INDICATION | COMMENT | REPRESENTATIVE REFERENCES |
|---|---|---|
| Osteogenesis imperfecta | A monogenic, dominant negative, genetic disease. Some progress in silencing the mutant allele and restoring function. Lack of efficient cell homing to bone is a barrier to progress. | (69) |
| Osteoporosis | Some encouraging pre-clinical data in animal models. Transgenes delivered to sites where secreted factors will influence bone, or via MSCs. Gene therapy superseded by advances with more conventional therapies. | (24, 85–87) |
| Bone healing Fractures Osteoporotic fractures Non-unions Segmental defects Spine fusion Avascular necrosis |
Considerable body of encouraging pre- clinical data, especially in segmental defects and spine fusion. | (51, 81, 88, 89) |
| Tumors Osteosarcoma Ewing’s |
Research in this area is at an early stage. The translocations in Ewing’s provide targets for siRNA. | (90–92) |
| Osteolysis Cancer induced Aseptic loosening |
Transgene not delivered to bone, but to sites where secreted factors will influence bone formation/lysis or inhibit reaction to wear debris. There has been one clinical trial in aseptic loosening, targeting the pseudosynovium around the implant. | (93–96) |
Gene therapy is an obvious strategy for treating Mendelian diseases, including those that affect bone. Its use in treating complex genetic or non-genetic disorders is less evident. In such cases, the intent is not to compensate for a genetic defect, but to serve as a delivery system for therapeutic gene products, be these RNA or protein. The advantages of gene delivery over protein delivery are several (Table 2), including the flexibility to express the protein locally and focally, or in a disseminated fashion, as needed. Gene transfer is particularly useful for the delivery of intracellular proteins, which are otherwise difficult to target into cells. Unlike its recombinant equivalent, the protein delivered via gene transfer will be nascent and uncontaminated by a variable percentage of incorrectly folded, and possibly antigenic, molecules; moreover, proteins delivered via gene transfer will have undergone authentic post-translational modification. Additional advantages of gene delivery include the ability to express proteins for extended periods of time and to regulate the level of transgene expression, both quantitatively and temporally. Moreover, the use of tissue specific promoters opens additional possibilities for controlling the geography of gene expression. Depending on the application, there may also be advantages of cost, as a gene therapy may only need to be delivered once and in a relatively small amount.
TABLE 2.
Gene and protein therapy compared
| Gene Delivery | Protein Delivery |
|---|---|
| Use of viral vectors raises safety concerns | Safety concerns are psychologically lower |
| Sustained, local delivery is possible | Sustained, local delivery is difficult to achieve |
| Regulated gene expression, both temporally and quantitatively, is possible | Very difficult to achieve regulated delivery |
| Protein is nascent and authentically processed | Protein is usually stored and processing often inauthentic |
| Suitable for intra-cellular gene products (RNA and protein) | Difficult to deliver proteins to intra- cellular compartments |
| Therapy may only need to be delivered once | For many indications, therapy needs repeated delivery |
| Potentially less expensive, because one gene can give rise to large number of protein molecules | |
| Ultimately, the method of choice for Mendelian disorders |
In addition to its therapeutic potential, gene delivery is a valuable experimental tool for laboratory research into the biology of bone.
This article reviews strategies for gene transfer to bone and summarizes progress towards clinical application.
2. STRATEGIES FOR GENE TRANSFER
2.1 Vectors
Successful gene therapy requires vectors that deliver transgenes to the nuclei of target cells in an efficient manner that ensures adequate levels and duration of transgene expression. The therapeutic transgene product may be a protein or non-coding RNA. Vectors have been the subjects of numerous extensive reviews (e.g. (1–4)), so they will be dealt with only briefly.
Viruses are frequently used as the basis for vectors because they naturally transfer their genetic material very efficiently into the cells they infect. For gene therapy, the viral genome is modified to remove sequences that contribute to pathogenicity and, in most cases, viral replication without eliminating infectivity. Therapeutic genes, usually in the form of their cDNA equivalents, are cloned into the modified viral genome to produce a recombinant, viral vector. Their experimental use for gene delivery to bone includes vectors derived from oncoretroviruses (often referred to just as retroviruses), lentiviruses (also members of the retrovirus family), adenovirus, and adeno-associated virus (AAV). Each of these vectors has its own idiosyncratic properties (Table 3). Moreover, progressive modification of each virus has led to the production of different generations of viral vectors with altered properties, making generalizations difficult. Of most relevance to the present discussion is the distinction between first generation adenoviral vectors, which continue to express viral proteins at low levels in the cells they transduce, and later generation vectors, which do not (5). These viral proteins trigger cell-mediated immune responses that eliminate the cells that express them. Gene transfer using a virus is known as transduction.
TABLE 3.
Salient properties of the main viral vectors used in studies of gene transfer to bone
| Parent virus | Key properties of w.t. virus | Advantages | Disadvantages | Comment |
|---|---|---|---|---|
| Adenovirus | Double stranded DNA genome, ~ 35Kb | Straightforward production of recombinant vectors at high titers | Inflammatory and antigenic | Various generations with increasingly deleted genomes “Gutted” vectors have no viral coding sequences and large carrying capacity but are difficult to produce. |
| Non-enveloped | Transduces non-dividing cells | Tropism can be modified by altering coat proteins | ||
| Over 50 serotypes | Wide choice of serotypes | |||
| Genome episomal in infected cells | ||||
| Adeno-associated virus | Single-stranded DNA genome, 4.8 Kb | Perceived to be safe (w.t. virus causes no known disease) | Difficult to produce | W.t. virus cannot replicate without helper virus |
| Non-enveloped | Transduces non-dividing cells | Carrying capacity is insufficient for certain applications | W.t. virus integrates in a site-specific manner; recombinant virus remains as a stable, concatameric plasmid | |
| Growing number of serotypes identified | Thought to have low immunogenicity, but this is being re-evaluated | Transduction efficiency sometimes low | Limitations of single stranded genome now overcome by development of double copy (self complementing) DNA viruses | |
| Oncoretrovirus | RNA genome ~ 8–10 Kb | Straightforward production of recombinant vectors at moderate titers | Require host cell division | Usually used ex vivo |
| Enveloped | Pseudotyped vectors have wide host range | Risk of insertional mutagenesis | 2 genomes per virion, reverse transcribed into DNA | |
| Lentivirus | RNA genome ~ 8–10 Kb | Straightforward production of recombinant vectors at moderate titers | Risk of insertional mutagenesis, but non-integrating vectors being developed | 2 genomes per virion, reverse transcribed into DNA |
| Enveloped | Pseudotyped vectors have wide host range and are often very efficient |
w.t. = wild-type
Adapted from reference (97)
Because viral vectors can be expensive and complicated to make, and continue to raise safety concerns, interest in the use of non-viral vectors remains high (3, 4). These can be simple plasmids. Often the efficiency of gene transfer is improved by associating the DNA with a carrier such as a liposome or other polymer. Efficiency can also be improved by physical means such as electroporation or sonication. In general, non-viral vectors, although simpler, are far less efficient than viral vectors. Gene transfer using a non-viral vector is known as transfection.
The choice of vector depends upon a number of issues. In the first instance, there is a need to achieve the required level and duration of transgene expression. These parameters will vary, depending upon the nature of the disease or condition to be treated. A genetic disease is likely to require life-long expression of a transgene, while healing an osseous defect probably only requires transient expression of suitable osteogenic factors.
Long-term transgene expression is best achieved by the use of an integrating virus, such as retrovirus or lentivirus; certain non-viral constructs based upon transposons also integrate (6). Prolonged transgene expression can sometimes be obtained with non-integrating vectors if the host cells do not divide. As noted, cells transduced with first generation adenovirus vectors, unlike those transduced with retroviruses and AAV, continue to express antigenic viral proteins that trigger cell-mediated immune responses leading to elimination of the transduced cells. This need not be a disadvantage if long-term transgene expression is not necessary and success does not require persistence of the transduced cells. For certain indications it may be necessary to regulate the level and duration of transgene expression with more precision than is provided by the natural history of the virus and the cells it infects. In this case, it is possible to use a variety of native or engineered, inducible promoters that respond to exogenous or endogenous cues, such as a drug, inflammation or other local conditions (7–12). When clinical translation is intended, additional factors, such as safety, cost and intellectual property become very important (13).
AAV is widely perceived to be the safest of the commonly used viral vectors, as the wild-type virus causes no disease and cannot even replicate without a helper virus. The death in 2007 of a subject receiving intra-articular injections of AAV as part of an arthritis gene therapy trial was not attributed to AAV (14, 15). Clinical grade, recombinant AAV is, however, demanding to manufacture and it needs to be used at high multiplicities of infection; both of these factors increase costs. Retroviruses efficiently transduce dividing cells, but are known to cause insertional mutagenesis (16) and are unlikely to see clinical application in any but life-threatening diseases. Lentiviral vectors raise the same safety issues, but non-integrating lentiviral vectors have been developed and are very attractive, given the high transduction efficiency of lentiviruses and ability to transduce non-dividing cells (17) (Table 3).
Perceptions concerning the safety of adenoviral vectors have had to recover from the death of a subject in a gene therapy trial in 1999 (18). When delivered locally at moderate doses, there is no associated toxicity and when used in an ex vivo fashion, risk is reduced even more. The major issue surrounding the use of adenoviral vectors is their high immunogenicity, which may cause inflammation, curtail transgene expression and interfere with repeat dosing. Most humans have been infected naturally with adenovirus serotype 5, the one most commonly used for making vectors, and have circulating neutralizing antibodies that may interfere with even the first use of the vector (19). Using adenovirus in an ex vivo fashion reduces this problem (20), as does the use of an alternative serotype, such as adenovirus 35 (21). Use of first generation, serotype 5 adenovirus vectors is not constrained by issues of intellectual property, and their construction and manufacture are straightforward, thus reducing costs.
2.2 Modes of delivery
In general terms, the vector can be introduced into the patient directly (in vivo delivery) or indirectly, using cells that are first genetically modified outside the body and then injected, infused or implanted (ex vivo delivery). In the latter case it is usual to use autologous cells, although there is increasing interest in the use of allografted cells for certain purposes. These delivery modes can be used for systemic or local transgene delivery. Systemic delivery aims to disseminate and express the transgene widely in the skeleton and is particularly useful for applications such as osteoporosis or osteogenesis imperfecta, which affect the entire skeleton. Local delivery, in contrast, introduces and expresses the transgene in a limited, defined area such as a fracture or a tumor.
For many purposes with ex vivo delivery there would be advantages to using cells that can home to bone after systemic administration. If the intent is to promote osteogenesis, it may be a further advantage to use cells that can also differentiate into osteoblasts. A number of types of cells are being investigated in this regard, particularly so-called mesenchymal stem cells (MSCs). These are able to differentiate into osteoblasts, but they do not seem to home to bone very efficiently after intra-venous injection, as approximately 98% of the injected cells are lost to the liver and spleen (22). Homing to bone seems to require expression of CXCR4 and about 30% of murine MSCs express this molecule (23). Restoration of bone mass and mechanical strength has been achieved in a murine model of osteoporosis by the injection of transduced MSCs expressing CXCR4 and Cbfa-1 (24). Incorporation into bone is increased in areas of bone turnover (25) and in sites of injury (26). Homing to bone is also enhanced by expression of CD49d (27).
Niyibizi and colleagues have succeeded in grafting MSCs by suspending the cells in collagen and injecting them in an intra-medullary fashion (28). This appears to be a useful technique for the regional treatment of an individual bone. Because large numbers of autologous MSCs may be necessary for the treatment of osteogenesis imperfecta, Li et al. have suggested using induced pleuripotent stem (iPS) cells that are differentiated into MSCs prior to use (29). MSCs have the potential not only to serve as convenient, osteogenic cells with the possibility to home to bone, but also to secrete helpful trophic factors (30). Other types of circulating osteoprogenitor cells, reviewed recently by Pignolo and Kassem (31), may also be useful for systemic transgene delivery to bone, but remain to be evaluated.
Although systemic delivery holds promise for treating disseminated diseases of bone, most of the literature describes local gene delivery to accelerate the healing of fractures and large segmental defects.
3. LOCAL GENE DELIVERY TO BONE
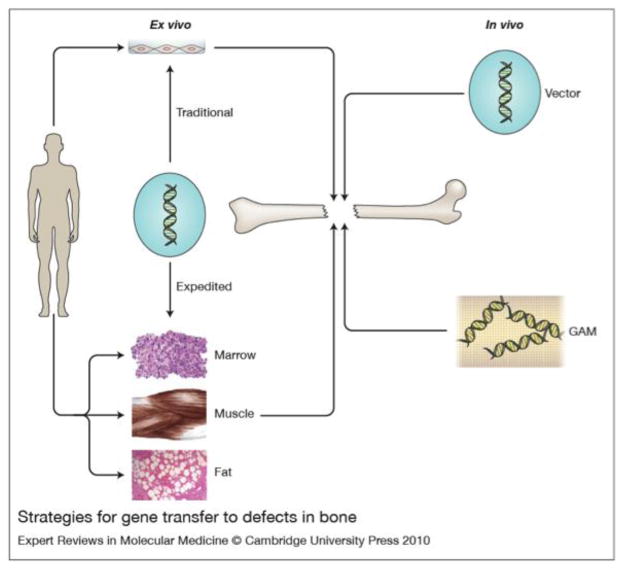
Based upon the principles described above, four different strategies have been described in the pre-clinical literature (figure 1). Two of these are ex vivo techniques and two of them are in vivo.
Figure 1. Strategies for gene transfer to bone.
There are two general strategies: in vivo (right hand side) and ex vivo (left hand side). For in vivo gene delivery, the vector is introduced directly into the site of the osseous lesion, either as a free suspension (top, right hand side) or incorporated into a gene activated matrix (GAM) (bottom, right hand side). For ex vivo delivery, vectors are not introduced directly into the defect. Instead they are used for the genetic modification of cells, which are subsequently implanted. Traditional ex vivo methods (top, left hand side) usually involve the establishment of cell cultures, which are genetically modified in vitro. The modified cells are then introduced into the lesion, often after seeding onto an appropriate scaffold. Expedited ex vivo methods (bottom, left hand side) avoid the need for cell culture by genetically modifying tissues such as marrow, muscle and fat, intraoperatively and inserting them into the defect during a single operative session.
From reference (81) with permission.
3.1. In vivo delivery
3.1.1. Gene Activated Matrices (GAMs)
Gene activated matrices (GAMs) consist of a matrix with associated vectors that are released into surrounding tissues after implantation (Table 4). They are usually designed to be stable and “off-the-shelf”.
TABLE 4.
Different types of GAMs and their use for bone formation in experimental animals
| Scaffold | Vector | Transgene | Application | Species | Ref |
|---|---|---|---|---|---|
| Allograft bone | AAV | BMP-2; caALK2; VEGF and RANKL | Femoral defect | Mouse | (38–40) |
| Demineralized bone | Adenovirus | Nell-1 | Spinal fusion | Rat | (98) |
| Fibronectin-apatite | Plasmid | BMP-2 | Subcutaneous | Rat | (99) |
| Silk | Adenovirus | BMP-2 | Cranial defect | SCID Mouse | (100) |
| Chitosan/collagen | Adenovirus | BMP-2 and VEGF | Dental implant | Dog | (101) |
| Triacrylate/amine- gelatin | Plasmid | BMP-2 | Cranial defect | Rat | (102) |
| Collagen/calcium phosphate | Plasmid | VEGF-165 | Intra-femoral | Mouse | (103) |
| Porous polymer | Adenovirus | RunX2 | Osteonecrosis | Rat | (104) |
| Chitosan/collagen | Adenovirus | BMP-7 | Mandible | Dog | (105) |
| PEG-block polycation | Plasmid | caALK6 and RunX2 | Cranial defect | Mouse | (106) |
| PLGA | Plasmid condensed with poly(ethylenimine) | BMP-4 | Cranial defect | Rat | (107) |
| Collagen | Plasmid/Calcium phosphate | BMP-2 | Tibial defect | Rat | (108) |
| Collagen | Plasmid | VEGF-165 | Radial defect | Rabbit | (109) |
| Collagen | Plasmid | PTH 1–34 | Metacarpal defect | Horse | (110) |
| Collagen | Plasmid | BMP-4 or PTH 1–34 | Femoral defect | Rat | (32) |
| Collagen | Plasmid | PTH 1–34 | Tibial defect | Dog | (33) |
PEG-block polycation: poly(ethyleneglycol) (PEG)-block-polycation (PEG-b-P[Asp-(DET)]): PEG-b-polyasparagine carrying the N-(2-aminoethyl)aminoethyl group (CH2)2NH(CH2)2NH2 as the side chain)
PLGA: poly(lactic-co-glycolic) acid
The first in vivo strategy to be described for bone used a GAM comprising a collagen sponge impregnated with plasmid DNA encoding parathyroid hormone (PTH) 1–34 or bone morphogenetic protein (BMP) - 4. It was designed to deliver DNA to infiltrating reparative cells when implanted into an osseous defect. By expressing the transgene, the infiltrating cells create an autocrine and paracrine osteogenic environment. Very promising results were demonstrated in segmental defect models in rats (32) and dogs (33). Improvements to this system use viral vectors instead of plasmid DNA, and a construct incorporating an adenovirus vector expressing platelet-derived growth factor (PDGF) has shown promise in peridontal lesions (34, 35). As the same construct has given encouraging results in human clinical trials for healing diabetic foot ulcers (36, 37), it is well positioned to enter clinical trials for bone healing.
Schwarz’s group has developed a different type of GAM. This comprises allograft bone coated with recombinant AAV expressing one or more genes associated with osteogenesis or bone remodeling. Compelling data have been reported using a murine, segmental defect model with AAV carrying cDNAs encoding BMP-2 (38), constitutively active Alk-2 (a BMP receptor) (39), or a combination of receptor activator of NF-κB ligand (RANKL) and vascular endothelial growth factor (VEGF) (40). When freeze-dried onto allograft bone, AAV is stable enough for “off-the-shelf” use.
Reference (41) provides a comprehensive, recent review of viral gene delivery from scaffolds.
3.1.2. Direct Injection
Direct injection provides an alternative way to introduce transgenes into osseous lesions. Success has been reported in experimental animals using several different genes delivered by recombinant adenoviral, retroviral or plasmid vectors (Table 5). Success seems to vary by the test species, at least when delivering BMP-2 with an adenovirus. Encouraging results have been reported with rabbits (42), rats (43) and horses (44), but not sheep (45). An immune response to both the adenovirus and the human BMP-2 by the sheep seemed to play a role in the lack of success in this species (45, 46). Efficacy in the horse is noteworthy, as successful treatment in large animal models is normally a requirement before clinical trials can start.
TABLE 5.
In vivo gene delivery to bone
| Transgene | Vector | Model | References |
|---|---|---|---|
| BMP-2 | Adenovirus | Rabbit femur segmental defect | (42) |
| BMP-2 | Adenovirus | Infected rabbit femur segmental defect | (49) |
| BMP-2 | Adenovirus | Rat femur segmental defect | (43, 111, 112) |
| BMP-2 | Adenovirus | Distraction osteogenesis–rat mandible | (113) |
| BMP-6 | Adenovirus | Rabbit ulna, horse metatarsal | (44, 114) |
| BMP-2, BMP-9 | Adenovirus | Rat mandible | (115) |
| BMP-4; COX-2; LMP-1 | Retrovirus | Rat femoral fracture | (116–118) |
| VEGF | Adenovirus | Rat femur drilling | (119) |
| VEGF | Adenovirus | Rabbit avascular necrosis | (120) |
| BMP-9; BMP-2 BMP-2 + BMP-7 |
Adenovirus | Rat spine fusion | (121, 122) |
| BMP-6 | Adenovirus | Rabbit spine fusion | (123) |
| BMP-7 | Plasmid | Rat spine fusion | (124) |
| BMP-2 | Adenovirus | Sheep tibial defect | (45, 46) |
| BMP-9 | Plasmid (electroporation) | Mouse radial non-union | (125) |
COX-2: cyclooxygenase-2
One safety concern with in vivo delivery is dissemination of the vector and adverse side effects in non-target sites. However, when using adenovirus in a rabbit segmental defect model little transgene was expressed outside of the osseous lesion (47). Indeed, most of the transgene was expressed in the muscle surrounding the defect. However, heterotopic or ectopic bone formation was not been observed in a rat model (43). It is interesting that, although direct adenoviral delivery of BMP-2 formed copious bone in femoral segmental defects in rats, intra-muscular injection of the same vector formed no bone in immunocompetent rats (43). The immune response is probably important in this dichotomy, because this vector formed far more bone after intramuscular injection into SCID mice than immunocompetent mice (48).
Less promising results were seen when the healing environment was more challenging, as in an infected, sclerotized, non-union in rabbits (49), and an atrophic non-union in rats (Kelly and Simpson, personal communication) although marker gene transfer to cells within an experimental, atrophic non-union in rabbits has been confirmed (50).
In vivo gene delivery to bone has recently been reviewed by Pelled et al (51).
3. 2. Ex vivo delivery
The direct introduction of vectors into the body always raises safety concerns and, as already discussed, the effectiveness of strongly antigenic viruses, such as adenovirus, can be reduced by the immune system. Ex vivo technologies help obviate these concerns as all genetic manipulations are performed outside the body.
3.2.1. Traditional Ex Vivo approaches
Traditional ex vivo strategies enable the use of expanded cultures of particular cell types, such as MSCs. Recombinant adenovirus (52) has been most commonly used in such studies, although retrovirus (53), lentivirus (54) and non-viral vectors (55) have also been evaluated (Table 6). Human and rat bone marrow MSCs have recently been reported to resist efficient transduction by AAV serotype 2 (56), although previous authors have not noted this problem (12, 57, 58). MSCs have been obtained most frequently from bone marrow, but similar cells derived from fat (59) and muscle (53) have also proved effective. When using transduced cells in this way it is usually necessary to attach the cells to a scaffold. Discussion of such scaffolds is beyond the scope of this paper; see Zippel et al (60) for recent review. The ex vivo delivery of genetically modified cells using a variety of different transgenes has given promising results in the healing of segmental defects in long bones, cranium and mandible, as well as in spinal fusion. In some cases, efficacy has been demonstrated in large animal models (Table 6).
TABLE 6.
Gene delivery to bone using traditional ex vivo methods
| Transgene | Vector | Model | References |
|---|---|---|---|
| BMP-2 | Adenovirus | Rodent long bone | (52, 59, 126) |
| IGF-1* | Plasmid* | (26) | |
| VEGF | Plasmid | (55) | |
| BMP-4 | Retrovirus | (53) | |
| BMP-2 | Lentivirus | (54, 66) | |
| FGF-2 | Plasmid | Rabbit radial defect | (127) |
| BMP-2, -7 | Retrovirus, Adenovirus | Rodent cranial defect | (128) (129) |
| BMP-4 + VEGF | Adenovirus | (130) | |
| Cbfa1 | Adenovirus | (131) | |
| Osterix | Retrovirus | (132) | |
| LMP-1 | Plasmid – liposomes | Rat spine fusion | (133) |
| BMP-7 | Adenovirus | (134) | |
| BMP-2 | Adenovirus | (135) | |
| BMP-2 | Adenovirus; lentivirus | (136) | |
| BMP-2 | Adenovirus | Mouse spine fusion | (137) |
| BMP-2 | Adenovirus | Rabbit spine fusion | (135) |
| BMP-2 | Liposomes, Adenovirus | Rat mandibular defect | (138) |
| LMP-3 | Adenovirus | (139) | |
| Hepatocyte growth factor | Adenovirus | Avascular necrosis | (140) |
| BMP-2 | Adenovirus | Goat Tibial defect | (141, 142) |
| BMP-2 | Adenovirus | Horse Metatarsus/metacarpus Rib | (143, 144) |
| BMP-2 | Adenovirus | Goat, avascular necrosis | (145) |
| BMP-2 | Adenovirus | Pig, cranial defect | (146) |
| BMP-6 | Plasmid (nucleofection) | Rat, vertebral defect | (147) |
stably transfected cell line
3.2.2. Expedited Ex Vivo Delivery
One disadvantage of the traditional ex vivo approach is the time, laboriousness and expense of establishing cultures of autologous cells. For clinical use, cells would need to be grown under Good Manufacturing Practice (GMP) conditions, which is very expensive. Moreover, the patient is subject to two invasive procedures, one to harvest cells and the other to reimplant their expanded and genetically modified progeny (61). One way to address these issues is to use allograft cells, and there is presently much interest in this possibility. Interest in allografted MSCs has been enhanced by the recent perception that these cells need not persist in the host to effect tissue repair and regeneration (30). Allografted chondrocytes expressing TGF-β1 have been used to improve the healing of fractures in osteoporotic rats (62). Nevertheless Tsuchida et al. (63), using a rat femoral defect model, were only able to achieve healing with allogeneic MSCs expressing BMP-2 in the presence of immunosuppression.
As an alternative approach, expedited ex vivo techniques are being developed where tissue is harvested, genetically modified and reimplanted within a single operative session. Our group is very interested in the use of autologous muscle, fat and marrow in this regard, because these tissues contain progenitor cells that can be genetically modified in situ (61). Moreover, they have intrinsic scaffolding properties and thus do not require matrices for reimplantation. In preliminary experiments, samples of fat and muscle were genetically modified with adenovirus expressing BMP-2 and implanted into critical size femoral defects in rat femora. The data confirmed rapid healing of the defects in all animals (20). We have also used clotted bone marrow for gene delivery to osteochondral lesions (64).
In a slightly different approach, Viggeswarapu et al. (65) isolated buffy coat cells from the peripheral blood of rabbits, transduced them intra-operatively with adenovirus carrying LMP-1 cDNA, and implanted the cells on a collagen-ceramic sponge in a spine fusion model. All animals that underwent this procedure achieved solid spinal fusion. Following a similar logic, Lieberman’s group have also obtained very good results using buffy coat cells obtained from the bone marrow of rats and transduced intraoperatively with a lentivirus expressing BMP-2 (66). All rats receiving the genetically modified cells healed a critical sized, femoral defect.
4. PROPECTS FOR CLINICAL APPLICATION
Of the candidate indications listed in Table 1, two areas of application offer the greatest potential for clinical application: osteogenesis imperfecta and bone healing. Although osteoporosis is a tempting target several new, non-genetic drugs have recently become available and these seem very effective.
4.1. Osteogenesis imperfecta
After a long delay and many setbacks, the field of gene therapy is finally registering some major successes (67, 68). These are all in the treatment of rare, genetic diseases that are difficult to treat by conventional means. Some of these gene therapies have a persistence that suggests a possible cure. With such encouraging outcomes, it might be worth investing in the most common Mendelian disease of bone, osteogenesis imperfecta, even though the pre-clinical work in this area is still evolving (69, 70).
Most, but not all, cases of osteogenesis imperfecta result from mutations in type I collagen genes. Because they are predominantly dominant negative disorders, in most cases the mutant gene will need to be silenced before the beneficial effects of a wild-type gene can be realized. This is complicated because the primary amino acid sequence of each alpha chain of type I collagen largely consists of repeating triplets with glycine at every third residue. Thus gene silencing with anti-sense RNA affects expression of wild-type, as well as mutant, alpha chains (71). Ribozymes and RNA interference have greater precision and offer better prospects in this regard (72, 73). However, because many different mutations can cause osteogenesis imperfecta, specific RNA molecules will need to be designed for each mutation.
A more generalized approach is suggested by the work of Chamberlain et al (74, 75) using a novel strategy in which AAV is targeted to the collagen alpha chain gene, where it inserts and disrupts expression. Although this does not discriminate between mutant and wild-type genes, it is possible to select for the appropriately modified cells, which can then be expanded and used for transplant. As noted (29), the resistance of iPS cells to replicative senescence is an advantage when generating modified MSCs for repeated use in osteogenesis imperfecta. In this context, Deyle et al (76) have isolated MSCs from patients with osteogenesis imperfecta, inactivated mutation collagen genes with AAV and generated iPS cells which were then re-differentiated into MSCs. These cells formed normal collagen and bone in immunodeficient mice.
Because osteogenesis imperfecta affects the entire skeleton, ex vivo gene therapy using osteoprogenitor cells is an attractive strategy. This has received a boost from reports claiming that allo-transplants of bone marrow or MSCs from normal donors improves certain clinical measures in patients with osteogenesis imperfecta (77). Such studies are, however, controversial because the engraftment rate of the MSCs is very low and the cells are cleared with time, possibly as a result of an allograft response. As discussed earlier in this review, the ability of MSCs to home to bone is an issue that remains to be resolved. Nevertheless, if the patient’s own cells were used and engraftment of MSCs improved, there would be considerable potential for clinical application in the more severe forms of osteogenesis imperfecta, a rare and troubling condition with orphan drug status. Because osteogenesis imperfecta can be diagnosed soon after birth, there is the possibility to administer cell-based gene therapy in very young individuals in whom the ability of MSCs to engraft in bone after injection seems much higher than in adults (78). It is also possible to contemplate in utero delivery, where engraftment rates seem particularly high (79, 80).
4.2. Long Bone Healing
As noted above, there is convincing proof of principle in animal models that gene transfer can be successfully used to heal segmental defects in bone (81). Most experimenters have used BMP-2. The significance of this has recently achieved additional prominence with the revelation that when used clinically to promote spine fusion, recombinant human BMP-2 causes a number of previously unreported adverse side effects, including osteolysis, infections, boney overgrowth, radiculitis, malignancy, and retrograde ejaculation and other urogenital events (82, 83).
Because of the problems associated with achieving the slow release of recombinant proteins at sites of bone healing, Infuse™ deposits milligram quantities of rhBMP-2 directly into the lesion. Most of this diffuses away within hours. Gene transfer, in contrast, achieves the sustained secretion of nanogram quantities of newly synthesized BMP-2 in a local and focal manner. It is highly likely that all the nascent BMP-2 is consumed locally, suggesting that the adverse side-effects seen at distant anatomical locations when using Infuse™ will not occur with gene transfer. Ironically, gene therapy thus may turn out to be safer than protein therapy in this application. The major impediments to clinical application of gene therapy are the lack of large animal studies, which are expensive and take a long time, and the restrictive regulatory environment (13, 84).
5. SAFETY
Clinical application of gene therapy, especially for non-lethal diseases, is hindered by its pervasive reputation for being unsafe. This is more perception than reality. Table 7 lists the known cases of the death of a subject in a gene therapy trial (14). Of these 4 instances, only 2 were attributable to gene transfer. Given that over 1,700 human gene therapy trials have been initiated, this is a remarkably low mortality.
Table 7.
Published deaths of subjects in human gene therapy trials
| Year | Disease target | Vector | Comment | Death related to gene transfer? | Ref |
|---|---|---|---|---|---|
| 1999 | Ornithine transcarbamylase deficiency | Adenovirus | Patient died within 4 days from cytokine storm | Yes | (18) |
| 2002 | X-linked severe combined immunodeficiency disease | Retrovirus | Leukemia developed, linked to insertion of retrovirus adjacent to oncogene | Yes | (16) |
| 2006 | X-linked chronic granulomatous disease | Retrovirus | Loss of transgene expression led to death from underlying disease | No | (148) |
| 2007 | Rheumatoid arthritis | AAV | Histoplasmosis and retroperitoneal aneurysm | No | (15) |
Adapted from Evans et al., 2008(14)
AAV: Adeno-associated virus
The use of viral vectors makes a major psychological contribution to the belief that gene therapy is inherently more risky than non-genetic therapy. This is especially the case for lentiviral vectors derived from HIV. It is often overlooked that non-genetic medicines can have fatal side effects too. For example, at least 16,500 NSAID-related deaths occur each year in the US among arthritis patients alone. It is likely that the first human gene therapy trials in the context of this article will be for bone healing. Adenovirus is a probable vector, which suffers from the epithet of being the vector that killed Jesse Gelsinger. However, in this case an enormous amount of virus was infused directly into the liver. For bone healing, a relatively small dose will be delivered locally and, if an ex vivo protocol is used, no viral particles will enter the body.
6. CONCLUSIONS
Genes can be transferred to the bones of experimental animals with a number of different viral and non-viral vectors using in vivo and ex vivo strategies. The choice of vector and strategy are largely determined by the biology of the indication. For systemic, disseminated diseases ex vivo strategies using modified osteoprogenitor cells that home to bone hold advantages; for local application, in vivo or expedited ex vivo methods may be preferred. Recombinant adenoviral vectors usually provide a high level of transgene expression for up to 3 weeks in vivo and so are appropriate for short term need, such as providing a growth factor to heal a fracture or supplying an agent to kill a tumor. Integrating vectors, such as retrovirus and lentivirus, provide the potential for the long-term transgene expression needed to treat a genetic disease. For clinical translation safety issues also determine the choice of vector, with AAV and non-viral vectors favored in this regard. Given the widespread, general concern about the safety of gene therapy it is worth noting that genetic delivery of BMP-2 has the potential to be much safer than delivery of the large amounts of recombinant protein presently administered in the clinic. However, gene therapy approaches to bone healing are still hindered by incomplete understanding of how much BMP-2 is needed to be produced and at what time(s) during the healing process.
Clinical application of gene transfer to bone is not yet on the near horizon, but certain applications hold potential. Osteogenesis imperfecta is a rare Mendelian condition that could benefit from gene therapy, especially as genetically unmodified MSCs have already been used experimentally to treat patients. Bone healing, another possible application, has an impressive body of supportive pre-clinical data in animal models using simple, readily available technologies. In this case, the route to the clinic is less likely to be retarded by science and technology, than by funding and regulatory issues. These are large, but under-appreciated barriers to progress (13).
Acknowledgments
The author’s work in this area has been funded by the Orthopaedic Trauma Association, NIH (NIAMS grant R01 AR050243) and the AO Foundation. I thank Prof. Hamish Simpson and Dr. Michael Kelly for permission to cite their unpublished data, and Prof. Topher Niyibizi for reading a draft of the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Warnock JN, Daigre C, Al-Rubeai M. Introduction to viral vectors. Methods Mol Biol. 2011;737:1–25. doi: 10.1007/978-1-61779-095-9_1. [DOI] [PubMed] [Google Scholar]
- 2.Heilbronn R, Weger S. Viral vectors for gene transfer: current status of gene therapeutics. Handb Exp Pharmacol. 2011:143–170. doi: 10.1007/978-3-642-00477-3_5. [DOI] [PubMed] [Google Scholar]
- 3.Elsabahy M, Nazarali A, Foldvari M. Non-viral nucleic acid delivery: key challenges and future directions. Curr Drug Deliv. 2011;8:235–244. doi: 10.2174/156720111795256174. [DOI] [PubMed] [Google Scholar]
- 4.Schleef M, Blaesen M, Schmeer M, Baier R, Marie C, Dickson G, Scherman D. Production of non viral DNA vectors. Curr Gene Ther. 2010;10:487–507. doi: 10.2174/156652310793797711. [DOI] [PubMed] [Google Scholar]
- 5.Ghosh SS, Gopinath P, Ramesh A. Adenoviral vectors: a promising tool for gene therapy. Appl Biochem Biotechnol. 2006;133:9–29. doi: 10.1385/abab:133:1:9. [DOI] [PubMed] [Google Scholar]
- 6.Aronovich EL, McIvor RS, Hackett PB. The Sleeping Beauty transposon system: a non-viral vector for gene therapy. Hum Mol Genet. 2011;20:R14–20. doi: 10.1093/hmg/ddr140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Heinz N, Schambach A, Galla M, Maetzig T, Baum C, Loew R, Schiedlmeier B. Retroviral and transposon-based tet-regulated all-in-one vectors with reduced background expression and improved dynamic range. Hum Gene Ther. 2011;22:166–176. doi: 10.1089/hum.2010.099. [DOI] [PubMed] [Google Scholar]
- 8.Walther W, Stein U. Heat-responsive gene expression for gene therapy. Adv Drug Deliv Rev. 2009;61:641–649. doi: 10.1016/j.addr.2009.02.009. [DOI] [PubMed] [Google Scholar]
- 9.Weber W, Bacchus W, Gruber F, Hamberger M, Fussenegger M. A novel vector platform for vitamin H-inducible transgene expression in mammalian cells. J Biotechnol. 2007;131:150–158. doi: 10.1016/j.jbiotec.2007.06.008. [DOI] [PubMed] [Google Scholar]
- 10.Cai G, Nie X, Guo P, Guan Z, Zhang J, Shen Q. A new inducible adenoviral expression system that responds to inflammatory stimuli in vivo. J Gene Med. 2006;8:1369–1378. doi: 10.1002/jgm.983. [DOI] [PubMed] [Google Scholar]
- 11.Miagkov AV, Varley AW, Munford RS, Makarov SS. Endogenous regulation of a therapeutic transgene restores homeostasis in arthritic joints. J Clin Invest. 2002;109:1223–1229. doi: 10.1172/JCI14536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gafni Y, Pelled G, Zilberman Y, Turgeman G, Apparailly F, Yotvat H, Galun E, Gazit Z, Jorgensen C, Gazit D. Gene therapy platform for bone regeneration using an exogenously regulated, AAV-2-based gene expression system. Mol Ther. 2004;9:587–595. doi: 10.1016/j.ymthe.2003.12.009. [DOI] [PubMed] [Google Scholar]
- 13.Evans CH, Ghivizzani SC, Robbins PD. Orthopaedic gene therapy - lost in translation? J Cell Physiol. 2012;227:416–420. doi: 10.1002/jcp.23031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Evans CH, Ghivizzani SC, Robbins PD. Arthritis gene therapy’s first death. Arthritis Res Ther. 2008;10:110. doi: 10.1186/ar2411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Frank KM, Hogarth DK, Miller JL, Mandal S, Mease PJ, Samulski RJ, Weisgerber GA, Hart J. Investigation of the cause of death in a gene-therapy trial. N Engl J Med. 2009;361:161–169. doi: 10.1056/NEJMoa0801066. [DOI] [PubMed] [Google Scholar]
- 16.Hacein-Bey-Abina S, von Kalle C, Schmidt M, Le Deist F, Wulffraat N, McIntyre E, Radford I, Villeval JL, Fraser CC, Cavazzana-Calvo M, et al. A serious adverse event after successful gene therapy for X-linked severe combined immunodeficiency. N Engl J Med. 2003;348:255–256. doi: 10.1056/NEJM200301163480314. [DOI] [PubMed] [Google Scholar]
- 17.Sarkis C, Philippe S, Mallet J, Serguera C. Non-integrating lentiviral vectors. Curr Gene Ther. 2008;8:430–437. doi: 10.2174/156652308786848012. [DOI] [PubMed] [Google Scholar]
- 18.Raper SE, Chirmule N, Lee FS, Wivel NA, Bagg A, Gao GP, Wilson JM, Batshaw ML. Fatal systemic inflammatory response syndrome in a ornithine transcarbamylase deficient patient following adenoviral gene transfer. Mol Genet Metab. 2003;80:148–158. doi: 10.1016/j.ymgme.2003.08.016. [DOI] [PubMed] [Google Scholar]
- 19.Ersching J, Hernandez MI, Cezarotto FS, Ferreira JD, Martins AB, Switzer WM, Xiang Z, Ertl HC, Zanetti CR, Pinto AR. Neutralizing antibodies to human and simian adenoviruses in humans and New-World monkeys. Virology. 2010;407:1–6. doi: 10.1016/j.virol.2010.07.043. [DOI] [PubMed] [Google Scholar]
- 20.Evans CH, Liu FJ, Glatt V, Hoyland JA, Kirker-Head C, Walsh A, Betz O, Wells JW, Betz V, Porter RM, et al. Use of genetically modified muscle and fat grafts to repair defects in bone and cartilage. Eur Cell Mater. 2009;18:96–111. doi: 10.22203/ecm.v018a09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Barouch DH, Pau MG, Custers JH, Koudstaal W, Kostense S, Havenga MJ, Truitt DM, Sumida SM, Kishko MG, Arthur JC, et al. Immunogenicity of recombinant adenovirus serotype 35 vaccine in the presence of pre-existing anti-Ad5 immunity. J Immunol. 2004;172:6290–6297. doi: 10.4049/jimmunol.172.10.6290. [DOI] [PubMed] [Google Scholar]
- 22.Gao J, Dennis JE, Muzic RF, Lundberg M, Caplan AI. The dynamic in vivo distribution of bone marrow-derived mesenchymal stem cells after infusion. Cells Tissues Organs. 2001;169:12–20. doi: 10.1159/000047856. [DOI] [PubMed] [Google Scholar]
- 23.Granero-Molto F, Weis JA, Miga MI, Landis B, Myers TJ, O’Rear L, Longobardi L, Jansen ED, Mortlock DP, Spagnoli A. Regenerative effects of transplanted mesenchymal stem cells in fracture healing. Stem Cells. 2009;27:1887–1898. doi: 10.1002/stem.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lien CY, Chih-Yuan Ho K, Lee OK, Blunn GW, Su Y. Restoration of bone mass and strength in glucocorticoid-treated mice by systemic transplantation of CXCR4 and cbfa-1 co-expressing mesenchymal stem cells. J Bone Miner Res. 2009;24:837–848. doi: 10.1359/jbmr.081257. [DOI] [PubMed] [Google Scholar]
- 25.Wang X, Li F, Niyibizi C. Progenitors systemically transplanted into neonatal mice localize to areas of active bone formation in vivo: implications of cell therapy for skeletal diseases. Stem Cells. 2006;24:1869–1878. doi: 10.1634/stemcells.2005-0430. [DOI] [PubMed] [Google Scholar]
- 26.Shen FH, Visger JM, Balian G, Hurwitz SR, Diduch DR. Systemically administered mesenchymal stromal cells transduced with insulin-like growth factor-I localize to a fracture site and potentiate healing. J Orthop Trauma. 2002;16:651–659. doi: 10.1097/00005131-200210000-00007. [DOI] [PubMed] [Google Scholar]
- 27.Kumar S, Ponnazhagan S. Bone homing of mesenchymal stem cells by ectopic alpha 4 integrin expression. FASEB J. 2007;21:3917–3927. doi: 10.1096/fj.07-8275com. [DOI] [PubMed] [Google Scholar]
- 28.Li F, Wang X, Niyibizi C. Bone marrow stromal cells contribute to bone formation following infusion into femoral cavities of a mouse model of osteogenesis imperfecta. Bone. 2010;47:546–555. doi: 10.1016/j.bone.2010.05.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li F, Bronson S, Niyibizi C. Derivation of murine induced pluripotent stem cells (iPS) and assessment of their differentiation toward osteogenic lineage. J Cell Biochem. 2010;109:643–652. doi: 10.1002/jcb.22440. [DOI] [PubMed] [Google Scholar]
- 30.Caplan AI, Dennis JE. Mesenchymal stem cells as trophic mediators. J Cell Biochem. 2006;98:1076–1084. doi: 10.1002/jcb.20886. [DOI] [PubMed] [Google Scholar]
- 31.Pignolo RJ, Kassem M. Circulating osteogenic cells: implications for injury, repair, and regeneration. J Bone Miner Res. 2011;26:1685–1693. doi: 10.1002/jbmr.370. [DOI] [PubMed] [Google Scholar]
- 32.Fang J, Zhu YY, Smiley E, Bonadio J, Rouleau JP, Goldstein SA, McCauley LK, Davidson BL, Roessler BJ. Stimulation of new bone formation by direct transfer of osteogenic plasmid genes. Proc Natl Acad Sci U S A. 1996;93:5753–5758. doi: 10.1073/pnas.93.12.5753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bonadio J, Smiley E, Patil P, Goldstein S. Localized, direct plasmid gene delivery in vivo: prolonged therapy results in reproducible tissue regeneration. Nat Med. 1999;5:753–759. doi: 10.1038/10473. [DOI] [PubMed] [Google Scholar]
- 34.Chang PC, Cirelli JA, Jin Q, Seol YJ, Sugai JV, D’Silva NJ, Danciu TE, Chandler LA, Sosnowski BA, Giannobile WV. Adenovirus encoding human platelet-derived growth factor-B delivered to alveolar bone defects exhibits safety and biodistribution profiles favorable for clinical use. Hum Gene Ther. 2009;20:486–496. doi: 10.1089/hum.2008.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jin Q, Anusaksathien O, Webb SA, Printz MA, Giannobile WV. Engineering of tooth-supporting structures by delivery of PDGF gene therapy vectors. Mol Ther. 2004;9:519–526. doi: 10.1016/j.ymthe.2004.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Blume P, Driver VR, Tallis AJ, Kirsner RS, Kroeker R, Payne WG, Wali S, Marston W, Dove C, Engler RL, et al. Formulated collagen gel accelerates healing rate immediately after application in patients with diabetic neuropathic foot ulcers. Wound Repair Regen. 2011;19:302–308. doi: 10.1111/j.1524-475X.2011.00669.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mulder G, Tallis AJ, Marshall VT, Mozingo D, Phillips L, Pierce GF, Chandler LA, Sosnowski BK. Treatment of nonhealing diabetic foot ulcers with a platelet-derived growth factor gene-activated matrix (GAM501): results of a phase 1/2 trial. Wound Repair Regen. 2009;17:772–779. doi: 10.1111/j.1524-475X.2009.00541.x. [DOI] [PubMed] [Google Scholar]
- 38.Yazici C, Takahata M, Reynolds DG, Xie C, Samulski RJ, Samulski J, Beecham EJ, Gertzman AA, Spilker M, Zhang X, et al. Self-complementary AAV2.5-BMP2-coated femoral allografts mediated superior bone healing versus live autografts in mice with equivalent biomechanics to unfractured femur. Mol Ther. 2011;19:1416–1425. doi: 10.1038/mt.2010.294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Koefoed M, Ito H, Gromov K, Reynolds DG, Awad HA, Rubery PT, Ulrich-Vinther M, Soballe K, Guldberg RE, Lin AS, et al. Biological effects of rAAV-caAlk2 coating on structural allograft healing. Mol Ther. 2005;12:212–218. doi: 10.1016/j.ymthe.2005.02.026. [DOI] [PubMed] [Google Scholar]
- 40.Ito H, Koefoed M, Tiyapatanaputi P, Gromov K, Goater JJ, Carmouche J, Zhang X, Rubery PT, Rabinowitz J, Samulski RJ, et al. Remodeling of cortical bone allografts mediated by adherent rAAV-RANKL and VEGF gene therapy. Nat Med. 2005;11:291–297. doi: 10.1038/nm1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jang JH, Schaffer DV, Shea LD. Engineering biomaterial systems to enhance viral vector gene delivery. Mol Ther. 2011;19:1407–1415. doi: 10.1038/mt.2011.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Baltzer AW, Lattermann C, Whalen JD, Wooley P, Weiss K, Grimm M, Ghivizzani SC, Robbins PD, Evans CH. Genetic enhancement of fracture repair: healing of an experimental segmental defect by adenoviral transfer of the BMP-2 gene. Gene Ther. 2000;7:734–739. doi: 10.1038/sj.gt.3301166. [DOI] [PubMed] [Google Scholar]
- 43.Betz OB, Betz VM, Nazarian A, Pilapil CG, Vrahas MS, Bouxsein ML, Gerstenfeld LC, Einhorn TA, Evans CH. Direct percutaneous gene delivery to enhance healing of segmental bone defects. J Bone Joint Surg Am. 2006;88:355–365. doi: 10.2106/JBJS.E.00464. [DOI] [PubMed] [Google Scholar]
- 44.Ishihara A, Shields KM, Litsky AS, Mattoon JS, Weisbrode SE, Bartlett JS, Bertone AL. Osteogenic gene regulation and relative acceleration of healing by adenoviral-mediated transfer of human BMP-2 or -6 in equine osteotomy and ostectomy models. J Orthop Res. 2008;26:764–771. doi: 10.1002/jor.20585. [DOI] [PubMed] [Google Scholar]
- 45.Egermann M, Lill CA, Griesbeck K, Evans CH, Robbins PD, Schneider E, Baltzer AW. Effect of BMP-2 gene transfer on bone healing in sheep. Gene Ther. 2006;13:1290–1299. doi: 10.1038/sj.gt.3302785. [DOI] [PubMed] [Google Scholar]
- 46.Egermann M, Baltzer AW, Adamaszek S, Evans C, Robbins P, Schneider E, Lill CA. Direct adenoviral transfer of bone morphogenetic protein-2 cDNA enhances fracture healing in osteoporotic sheep. Hum Gene Ther. 2006;17:507–517. doi: 10.1089/hum.2006.17.507. [DOI] [PubMed] [Google Scholar]
- 47.Baltzer AW, Lattermann C, Whalen JD, Braunstein S, Robbins PD, Evans CH. A gene therapy approach to accelerating bone healing. Evaluation of gene expression in a New Zealand white rabbit model. Knee Surg Sports Traumatol Arthrosc. 1999;7:197–202. doi: 10.1007/s001670050147. [DOI] [PubMed] [Google Scholar]
- 48.Musgrave DS, Bosch P, Ghivizzani S, Robbins PD, Evans CH, Huard J. Adenovirus-mediated direct gene therapy with bone morphogenetic protein-2 produces bone. Bone. 1999;24:541–547. doi: 10.1016/s8756-3282(99)00086-1. [DOI] [PubMed] [Google Scholar]
- 49.Southwood LL, Frisbie DD, Kawcak CE, Ghivizzani SC, Evans CH, McIlwraith CW. Evaluation of Ad-BMP-2 for enhancing fracture healing in an infected defect fracture rabbit model. J Orthop Res. 2004;22:66–72. doi: 10.1016/S0736-0266(03)00129-3. [DOI] [PubMed] [Google Scholar]
- 50.Lattermann C, Baltzer AW, Zelle BA, Whalen JD, Niyibizi C, Robbins PD, Evans CH, Gruen GS. Feasibility of percutaneous gene transfer to an atrophic nonunion in a rabbit. Clin Orthop Relat Res. 2004:237–243. doi: 10.1097/00003086-200408000-00034. [DOI] [PubMed] [Google Scholar]
- 51.Pelled G, Ben-Arav A, Hock C, Reynolds DG, Yazici C, Zilberman Y, Gazit Z, Awad H, Gazit D, Schwarz EM. Direct gene therapy for bone regeneration: gene delivery, animal models, and outcome measures. Tissue Eng Part B Rev. 2010;16:13–20. doi: 10.1089/ten.teb.2009.0156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lieberman JR, Daluiski A, Stevenson S, Wu L, McAllister P, Lee YP, Kabo JM, Finerman GA, Berk AJ, Witte ON. The effect of regional gene therapy with bone morphogenetic protein-2-producing bone-marrow cells on the repair of segmental femoral defects in rats. J Bone Joint Surg Am. 1999;81:905–917. doi: 10.2106/00004623-199907000-00002. [DOI] [PubMed] [Google Scholar]
- 53.Shen HC, Peng H, Usas A, Gearhart B, Fu FH, Huard J. Structural and functional healing of critical-size segmental bone defects by transduced muscle-derived cells expressing BMP4. J Gene Med. 2004;6:984–991. doi: 10.1002/jgm.588. [DOI] [PubMed] [Google Scholar]
- 54.Hsu WK, Sugiyama O, Park SH, Conduah A, Feeley BT, Liu NQ, Krenek L, Virk MS, An DS, Chen IS, et al. Lentiviral-mediated BMP-2 gene transfer enhances healing of segmental femoral defects in rats. Bone. 2007;40:931–938. doi: 10.1016/j.bone.2006.10.030. [DOI] [PubMed] [Google Scholar]
- 55.Li R, Stewart DJ, von Schroeder HP, Mackinnon ES, Schemitsch EH. Effect of cell-based VEGF gene therapy on healing of a segmental bone defect. J Orthop Res. 2009;27:8–14. doi: 10.1002/jor.20658. [DOI] [PubMed] [Google Scholar]
- 56.Alaee F, Sugiyama O, Virk MS, Tang Y, Wang B, Lieberman JR. In vitro evaluation of a double-stranded self-complementary adeno-associated virus type2 vector in bone marrow stromal cells for bone healing. Genet Vaccines Ther. 2011;9:4. doi: 10.1186/1479-0556-9-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kumar S, Mahendra G, Nagy TR, Ponnazhagan S. Osteogenic differentiation of recombinant adeno-associated virus 2-transduced murine mesenchymal stem cells and development of an immunocompetent mouse model for ex vivo osteoporosis gene therapy. Hum Gene Ther. 2004;15:1197–1206. doi: 10.1089/hum.2004.15.1197. [DOI] [PubMed] [Google Scholar]
- 58.Stender S, Murphy M, O’Brien T, Stengaard C, Ulrich-Vinther M, Soballe K, Barry F. Adeno-associated viral vector transduction of human mesenchymal stem cells. Eur Cell Mater. 2007;13:93–99. doi: 10.22203/ecm.v013a10. discussion 99. [DOI] [PubMed] [Google Scholar]
- 59.Peterson B, Zhang J, Iglesias R, Kabo M, Hedrick M, Benhaim P, Lieberman JR. Healing of critically sized femoral defects, using genetically modified mesenchymal stem cells from human adipose tissue. Tissue Eng. 2005;11:120–129. doi: 10.1089/ten.2005.11.120. [DOI] [PubMed] [Google Scholar]
- 60.Zippel N, Schulze M, Tobiasch E. Biomaterials and mesenchymal stem cells for regenerative medicine. Recent Pat Biotechnol. 2010;4:1–22. doi: 10.2174/187220810790069497. [DOI] [PubMed] [Google Scholar]
- 61.Evans CH, Palmer GD, Pascher A, Porter R, Kwong FN, Gouze E, Gouze JN, Liu F, Steinert A, Betz O, et al. Facilitated endogenous repair: making tissue engineering simple, practical, and economical. Tissue Eng. 2007;13:1987–1993. doi: 10.1089/ten.2006.0302. [DOI] [PubMed] [Google Scholar]
- 62.Yi Y, Choi KB, Lim CL, Hyun JP, Lee HY, Lee KB, Yun L, Ayverdi A, Hwang S, Yip V, et al. Irradiated human chondrocytes expressing bone morphogenetic protein 2 promote healing of osteoporotic bone fracture in rats. Tissue Eng Part A. 2009;15:2853–2863. doi: 10.1089/ten.TEA.2008.0578. [DOI] [PubMed] [Google Scholar]
- 63.Tsuchida H, Hashimoto J, Crawford E, Manske P, Lou J. Engineered allogeneic mesenchymal stem cells repair femoral segmental defect in rats. J Orthop Res. 2003;21:44–53. doi: 10.1016/S0736-0266(02)00108-0. [DOI] [PubMed] [Google Scholar]
- 64.Pascher A, Palmer GD, Steinert A, Oligino T, Gouze E, Gouze JN, Betz O, Spector M, Robbins PD, Evans CH, et al. Gene delivery to cartilage defects using coagulated bone marrow aspirate. Gene Ther. 2004;11:133–141. doi: 10.1038/sj.gt.3302155. [DOI] [PubMed] [Google Scholar]
- 65.Viggeswarapu M, Boden SD, Liu Y, Hair GA, Louis-Ugbo J, Murakami H, Kim HS, Mayr MT, Hutton WC, Titus L. Adenoviral delivery of LIM mineralization protein-1 induces new-bone formation in vitro and in vivo. J Bone Joint Surg Am. 2001;83-A:364–376. doi: 10.2106/00004623-200103000-00008. [DOI] [PubMed] [Google Scholar]
- 66.Virk MS, Sugiyama O, Park SH, Gambhir SS, Adams DJ, Drissi H, Lieberman JR. “Same day” ex-vivo regional gene therapy: a novel strategy to enhance bone repair. Mol Ther. 2011;19:960–968. doi: 10.1038/mt.2011.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Herzog RW, Cao O, Srivastava A. Two decades of clinical gene therapy--success is finally mounting. Discov Med. 2010;9:105–111. [PMC free article] [PubMed] [Google Scholar]
- 68.Sheridan C. Gene therapy finds its niche. Nat Biotechnol. 2011;29:121–128. doi: 10.1038/nbt.1769. [DOI] [PubMed] [Google Scholar]
- 69.Niyibizi C, Li F. Potential implications of cell therapy for osteogenesis imperfecta. Int J Clin Rheumtol. 2009;4:57–66. doi: 10.2217/17584272.4.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Niyibizi C, Wang S, Mi Z, Robbins PD. Gene therapy approaches for osteogenesis imperfecta. Gene Ther. 2004;11:408–416. doi: 10.1038/sj.gt.3302199. [DOI] [PubMed] [Google Scholar]
- 71.Wang Q, Marini JC. Antisense oligodeoxynucleotides selectively suppress expression of the mutant alpha 2(I) collagen allele in type IV osteogenesis imperfecta fibroblasts. A molecular approach to therapeutics of dominant negative disorders. J Clin Invest. 1996;97:448–454. doi: 10.1172/JCI118434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lindahl K, Rubin CJ, Kindmark A, Ljunggren O. Allele dependent silencing of COL1A2 using small interfering RNAs. Int J Med Sci. 2008;5:361–365. doi: 10.7150/ijms.5.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Millington-Ward S, McMahon HP, Allen D, Tuohy G, Kiang AS, Palfi A, Kenna PF, Humphries P, Farrar GJ. RNAi of COL1A1 in mesenchymal progenitor cells. Eur J Hum Genet. 2004;12:864–866. doi: 10.1038/sj.ejhg.5201230. [DOI] [PubMed] [Google Scholar]
- 74.Chamberlain JR, Deyle DR, Schwarze U, Wang P, Hirata RK, Li Y, Byers PH, Russell DW. Gene targeting of mutant COL1A2 alleles in mesenchymal stem cells from individuals with osteogenesis imperfecta. Mol Ther. 2008;16:187–193. doi: 10.1038/sj.mt.6300339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Chamberlain JR, Schwarze U, Wang PR, Hirata RK, Hankenson KD, Pace JM, Underwood RA, Song KM, Sussman M, Byers PH, et al. Gene targeting in stem cells from individuals with osteogenesis imperfecta. Science. 2004;303:1198–1201. doi: 10.1126/science.1088757. [DOI] [PubMed] [Google Scholar]
- 76.Deyle DR, Khan IF, Ren G, Wang PR, Kho J, Schwarze U, Russell DW. Normal collagen and bone production by gene-targeted human osteogenesis imperfecta iPSCs. Mol Ther. 2012;20:204–213. doi: 10.1038/mt.2011.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Horwitz EM, Gordon PL, Koo WK, Marx JC, Neel MD, McNall RY, Muul L, Hofmann T. Isolated allogeneic bone marrow-derived mesenchymal cells engraft and stimulate growth in children with osteogenesis imperfecta: Implications for cell therapy of bone. Proc Natl Acad Sci U S A. 2002;99:8932–8937. doi: 10.1073/pnas.132252399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Li F, Wang X, Niyibizi C. Distribution of single-cell expanded marrow derived progenitors in a developing mouse model of osteogenesis imperfecta following systemic transplantation. Stem Cells. 2007;25:3183–3193. doi: 10.1634/stemcells.2007-0466. [DOI] [PubMed] [Google Scholar]
- 79.Guillot PV, Abass O, Bassett JH, Shefelbine SJ, Bou-Gharios G, Chan J, Kurata H, Williams GR, Polak J, Fisk NM. Intrauterine transplantation of human fetal mesenchymal stem cells from first-trimester blood repairs bone and reduces fractures in osteogenesis imperfecta mice. Blood. 2008;111:1717–1725. doi: 10.1182/blood-2007-08-105809. [DOI] [PubMed] [Google Scholar]
- 80.Panaroni C, Gioia R, Lupi A, Besio R, Goldstein SA, Kreider J, Leikin S, Vera JC, Mertz EL, Perilli E, et al. In utero transplantation of adult bone marrow decreases perinatal lethality and rescues the bone phenotype in the knockin murine model for classical, dominant osteogenesis imperfecta. Blood. 2009;114:459–468. doi: 10.1182/blood-2008-12-195859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Evans CH. Gene therapy for bone healing. Expert Rev Mol Med. 2010;12:e18. doi: 10.1017/S1462399410001493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Carragee EJ, Ghanayem AJ, Weiner BK, Rothman DJ, Bono CM. A challenge to integrity in spine publications: years of living dangerously with the promotion of bone growth factors. Spine J. 2011;11:463–468. doi: 10.1016/j.spinee.2011.06.001. [DOI] [PubMed] [Google Scholar]
- 83.Carragee EJ, Hurwitz EL, Weiner BK. A critical review of recombinant human bone morphogenetic protein-2 trials in spinal surgery: emerging safety concerns and lessons learned. Spine J. 2011;11:471–491. doi: 10.1016/j.spinee.2011.04.023. [DOI] [PubMed] [Google Scholar]
- 84.Evans CH. Barriers to the Clinical Translation of Orthopedic Tissue Engineering. Tissue Eng Part B Rev. 2011;17:437–441. doi: 10.1089/ten.teb.2011.0228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Baltzer AW, Whalen JD, Wooley P, Latterman C, Truchan LM, Robbins PD, Evans CH. Gene therapy for osteoporosis: evaluation in a murine ovariectomy model. Gene Ther. 2001;8:1770–1776. doi: 10.1038/sj.gt.3301594. [DOI] [PubMed] [Google Scholar]
- 86.Bolon B, Carter C, Daris M, Morony S, Capparelli C, Hsieh A, Mao M, Kostenuik P, Dunstan CR, Lacey DL, et al. Adenoviral delivery of osteoprotegerin ameliorates bone resorption in a mouse ovariectomy model of osteoporosis. Mol Ther. 2001;3:197–205. doi: 10.1006/mthe.2001.0245. [DOI] [PubMed] [Google Scholar]
- 87.Kostenuik PJ, Bolon B, Morony S, Daris M, Geng Z, Carter C, Sheng J. Gene therapy with human recombinant osteoprotegerin reverses established osteopenia in ovariectomized mice. Bone. 2004;34:656–664. doi: 10.1016/j.bone.2003.12.006. [DOI] [PubMed] [Google Scholar]
- 88.Nauth A, Miclau T, 3rd, Li R, Schemitsch EH. Gene therapy for fracture healing. J Orthop Trauma. 2010;24(Suppl 1):S17–24. doi: 10.1097/BOT.0b013e3181cec6fb. [DOI] [PubMed] [Google Scholar]
- 89.Carofino BC, Lieberman JR. Gene therapy applications for fracture-healing. J Bone Joint Surg Am. 2008;90(Suppl 1):99–110. doi: 10.2106/JBJS.G.01546. [DOI] [PubMed] [Google Scholar]
- 90.Tan ML, Choong PF, Dass CR. Osteosarcoma: Conventional treatment vs. gene therapy. Cancer Biol Ther. 2009;8:106–117. doi: 10.4161/cbt.8.2.7385. [DOI] [PubMed] [Google Scholar]
- 91.Witlox MA, Lamfers ML, Wuisman PI, Curiel DT, Siegal GP. Evolving gene therapy approaches for osteosarcoma using viral vectors: review. Bone. 2007;40:797–812. doi: 10.1016/j.bone.2006.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Elhamess H, Bertrand JR, Maccario J, Maksimenko A, Malvy C. Antitumor vectorized oligonucleotides in a model of ewing sarcoma: unexpected role of nanoparticles. Oligonucleotides. 2009;19:255–264. doi: 10.1089/oli.2009.0197. [DOI] [PubMed] [Google Scholar]
- 93.Doran PM, Turner RT, Chen D, Facteau SM, Ludvigson JM, Khosla S, Riggs BL, Russell SJ. Native osteoprotegerin gene transfer inhibits the development of murine osteolytic bone disease induced by tumor xenografts. Exp Hematol. 2004;32:351–359. doi: 10.1016/j.exphem.2004.01.006. [DOI] [PubMed] [Google Scholar]
- 94.Yang SY, Nasser S, Markel DC, Robbins PD, Wooley PH. Human periprosthetic tissues implanted in severe combined immunodeficient mice respond to gene transfer of a cytokine inhibitor. J Bone Joint Surg Am. 2005;87:1088–1097. doi: 10.2106/JBJS.D.02052. [DOI] [PubMed] [Google Scholar]
- 95.Ulrich-Vinther M, Carmody EE, Goater JJ, K Sb, O’Keefe RJ, Schwarz EM. Recombinant adeno-associated virus-mediated osteoprotegerin gene therapy inhibits wear debris-induced osteolysis. J Bone Joint Surg Am. 2002;84-A:1405–1412. doi: 10.2106/00004623-200208000-00016. [DOI] [PubMed] [Google Scholar]
- 96.de Poorter JJ, Hoeben RC, Hogendoorn S, Mautner V, Ellis J, Obermann WR, Huizinga TW, Nelissen RG. Gene therapy and cement injection for restabilization of loosened hip prostheses. Hum Gene Ther. 2008;19:83–95. doi: 10.1089/hum.2007.111. [DOI] [PubMed] [Google Scholar]
- 97.Evans CH, Ghivizzani SC, Robbins PD. Getting arthritis gene therapy into the clinic. Nat Rev Rheumatol. 2011;7:244–249. doi: 10.1038/nrrheum.2010.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Lu SS, Zhang X, Soo C, Hsu T, Napoli A, Aghaloo T, Wu BM, Tsou P, Ting K, Wang JC. The osteoinductive properties of Nell-1 in a rat spinal fusion model. Spine J. 2007;7:50–60. doi: 10.1016/j.spinee.2006.04.020. [DOI] [PubMed] [Google Scholar]
- 99.Wang X, Oyane A, Tsurushima H, Sogo Y, Li X, Ito A. BMP-2 and ALP gene expression induced by a BMP-2 gene-fibronectin-apatite composite layer. Biomed Mater. 2011;6:045004. doi: 10.1088/1748-6041/6/4/045004. [DOI] [PubMed] [Google Scholar]
- 100.Zhang Y, Fan W, Nothdurft L, Wu C, Zhou Y, Crawford R, Xiao Y. In vitro and in vivo evaluation of adenovirus combined silk fibroin scaffolds for bone morphogenetic protein-7 gene delivery. Tissue Eng Part C Methods. 2011;17:789–797. doi: 10.1089/ten.tec.2010.0453. [DOI] [PubMed] [Google Scholar]
- 101.Luo T, Zhang W, Shi B, Cheng X, Zhang Y. Enhanced bone regeneration around dental implant with bone morphogenetic protein 2 gene and vascular endothelial growth factor protein delivery. Clin Oral Implants Res. 2012;23:467–473. doi: 10.1111/j.1600-0501.2011.02164.x. [DOI] [PubMed] [Google Scholar]
- 102.Chew SA, Kretlow JD, Spicer PP, Edwards AW, Baggett LS, Tabata Y, Kasper FK, Mikos AG. Delivery of plasmid DNA encoding bone morphogenetic protein-2 with a biodegradable branched polycationic polymer in a critical-size rat cranial defect model. Tissue Eng Part A. 2011;17:751–763. doi: 10.1089/ten.tea.2010.0496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Keeney M, van den Beucken JJ, van der Kraan PM, Jansen JA, Pandit A. The ability of a collagen/calcium phosphate scaffold to act as its own vector for gene delivery and to promote bone formation via transfection with VEGF(165) Biomaterials. 2010;31:2893–2902. doi: 10.1016/j.biomaterials.2009.12.041. [DOI] [PubMed] [Google Scholar]
- 104.Sakai S, Tamura M, Mishima H, Kojima H, Uemura T. Bone regeneration induced by adenoviral vectors carrying til-1/Cbfa1 genes implanted with biodegradable porous materials in animal models of osteonecrosis of the femoral head. J Tissue Eng Regen Med. 2008;2:164–167. doi: 10.1002/term.72. [DOI] [PubMed] [Google Scholar]
- 105.Zhang Y, Song J, Shi B, Wang Y, Chen X, Huang C, Yang X, Xu D, Cheng X. Combination of scaffold and adenovirus vectors expressing bone morphogenetic protein-7 for alveolar bone regeneration at dental implant defects. Biomaterials. 2007;28:4635–4642. doi: 10.1016/j.biomaterials.2007.07.009. [DOI] [PubMed] [Google Scholar]
- 106.Itaka K, Ohba S, Miyata K, Kawaguchi H, Nakamura K, Takato T, Chung UI, Kataoka K. Bone regeneration by regulated in vivo gene transfer using biocompatible polyplex nanomicelles. Mol Ther. 2007;15:1655–1662. doi: 10.1038/sj.mt.6300218. [DOI] [PubMed] [Google Scholar]
- 107.Huang YC, Simmons C, Kaigler D, Rice KG, Mooney DJ. Bone regeneration in a rat cranial defect with delivery of PEI-condensed plasmid DNA encoding for bone morphogenetic protein-4 (BMP-4) Gene Ther. 2005;12:418–426. doi: 10.1038/sj.gt.3302439. [DOI] [PubMed] [Google Scholar]
- 108.Endo M, Kuroda S, Kondo H, Maruoka Y, Ohya K, Kasugai S. Bone regeneration by modified gene-activated matrix: effectiveness in segmental tibial defects in rats. Tissue Eng. 2006;12:489–497. doi: 10.1089/ten.2006.12.489. [DOI] [PubMed] [Google Scholar]
- 109.Geiger F, Bertram H, Berger I, Lorenz H, Wall O, Eckhardt C, Simank HG, Richter W. Vascular endothelial growth factor gene-activated matrix (VEGF165-GAM) enhances osteogenesis and angiogenesis in large segmental bone defects. J Bone Miner Res. 2005;20:2028–2035. doi: 10.1359/JBMR.050701. [DOI] [PubMed] [Google Scholar]
- 110.Backstrom KC, Bertone AL, Wisner ER, Weisbrode SE. Response of induced bone defects in horses to collagen matrix containing the human parathyroid hormone gene. Am J Vet Res. 2004;65:1223–1232. doi: 10.2460/ajvr.2004.65.1223. [DOI] [PubMed] [Google Scholar]
- 111.Betz OB, Betz VM, Nazarian A, Egermann M, Gerstenfeld LC, Einhorn TA, Vrahas MS, Bouxsein ML, Evans CH. Delayed administration of adenoviral BMP-2 vector improves the formation of bone in osseous defects. Gene Ther. 2007;14:1039–1044. doi: 10.1038/sj.gt.3302956. [DOI] [PubMed] [Google Scholar]
- 112.Betz VM, Betz OB, Glatt V, Gerstenfeld LC, Einhorn TA, Bouxsein ML, Vrahas MS, Evans CH. Healing of segmental bone defects by direct percutaneous gene delivery: effect of vector dose. Hum Gene Ther. 2007;18:907–915. doi: 10.1089/hum.2007.077. [DOI] [PubMed] [Google Scholar]
- 113.Ashinoff RL, Cetrulo CL, Jr, Galiano RD, Dobryansky M, Bhatt KA, Ceradini DJ, Michaels Jt, McCarthy JG, Gurtner GC. Bone morphogenic protein-2 gene therapy for mandibular distraction osteogenesis. Ann Plast Surg. 2004;52:585–590. doi: 10.1097/01.sap.0000123023.28874.1e. discussion 591. [DOI] [PubMed] [Google Scholar]
- 114.Bertone AL, Pittman DD, Bouxsein ML, Li J, Clancy B, Seeherman HJ. Adenoviral-mediated transfer of human BMP-6 gene accelerates healing in a rabbit ulnar osteotomy model. J Orthop Res. 2004;22:1261–1270. doi: 10.1016/j.orthres.2004.03.014. [DOI] [PubMed] [Google Scholar]
- 115.Alden TD, Beres EJ, Laurent JS, Engh JA, Das S, London SD, Jane JA, Jr, Hudson SB, Helm GA. The use of bone morphogenetic protein gene therapy in craniofacial bone repair. J Craniofac Surg. 2000;11:24–30. doi: 10.1097/00001665-200011010-00005. [DOI] [PubMed] [Google Scholar]
- 116.Rundle CH, Miyakoshi N, Kasukawa Y, Chen ST, Sheng MH, Wergedal JE, Lau KH, Baylink DJ. In vivo bone formation in fracture repair induced by direct retroviral-based gene therapy with bone morphogenetic protein-4. Bone. 2003;32:591–601. doi: 10.1016/s8756-3282(03)00096-6. [DOI] [PubMed] [Google Scholar]
- 117.Rundle CH, Strong DD, Chen ST, Linkhart TA, Sheng MH, Wergedal JE, Lau KH, Baylink DJ. Retroviral-based gene therapy with cyclooxygenase-2 promotes the union of bony callus tissues and accelerates fracture healing in the rat. J Gene Med. 2008;10:229–241. doi: 10.1002/jgm.1148. [DOI] [PubMed] [Google Scholar]
- 118.Strohbach CA, Rundle CH, Wergedal JE, Chen ST, Linkhart TA, Lau KH, Strong DD. LMP-1 retroviral gene therapy influences osteoblast differentiation and fracture repair: a preliminary study. Calcif Tissue Int. 2008;83:202–211. doi: 10.1007/s00223-008-9163-0. [DOI] [PubMed] [Google Scholar]
- 119.Tarkka T, Sipola A, Jamsa T, Soini Y, Yla-Herttuala S, Tuukkanen J, Hautala T. Adenoviral VEGF-A gene transfer induces angiogenesis and promotes bone formation in healing osseous tissues. J Gene Med. 2003;5:560–566. doi: 10.1002/jgm.392. [DOI] [PubMed] [Google Scholar]
- 120.Liu YG, Zhou Y, Hu X, Fu JJ, Pan Y, Chu TW. Effect of vascular endothelial growth factor 121 adenovirus transduction in rabbit model of femur head necrosis. J Trauma. 2011;70:1519–1523. doi: 10.1097/TA.0b013e3181f31595. [DOI] [PubMed] [Google Scholar]
- 121.Helm GA, Alden TD, Beres EJ, Hudson SB, Das S, Engh JA, Pittman DD, Kerns KM, Kallmes DF. Use of bone morphogenetic protein-9 gene therapy to induce spinal arthrodesis in the rodent. J Neurosurg. 2000;92:191–196. doi: 10.3171/spi.2000.92.2.0191. [DOI] [PubMed] [Google Scholar]
- 122.Alden TD, Pittman DD, Beres EJ, Hankins GR, Kallmes DF, Wisotsky BM, Kerns KM, Helm GA. Percutaneous spinal fusion using bone morphogenetic protein-2 gene therapy. J Neurosurg. 1999;90:109–114. doi: 10.3171/spi.1999.90.1.0109. [DOI] [PubMed] [Google Scholar]
- 123.Laurent JJ, Webb KM, Beres EJ, McGee K, Li J, van Rietbergen B, Helm GA. The use of bone morphogenetic protein-6 gene therapy for percutaneous spinal fusion in rabbits. J Neurosurg Spine. 2004;1:90–94. doi: 10.3171/spi.2004.1.1.0090. [DOI] [PubMed] [Google Scholar]
- 124.Bright C, Park YS, Sieber AN, Kostuik JP, Leong KW. In vivo evaluation of plasmid DNA encoding OP-1 protein for spine fusion. Spine (Phila Pa 1976) 2006;31:2163–2172. doi: 10.1097/01.brs.0000232721.59901.45. [DOI] [PubMed] [Google Scholar]
- 125.Kimelman-Bleich N, Pelled G, Zilberman Y, Kallai I, Mizrahi O, Tawackoli W, Gazit Z, Gazit D. Targeted gene-and-host progenitor cell therapy for nonunion bone fracture repair. Mol Ther. 2011;19:53–59. doi: 10.1038/mt.2010.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Lieberman JR, Le LQ, Wu L, Finerman GA, Berk A, Witte ON, Stevenson S. Regional gene therapy with a BMP-2-producing murine stromal cell line induces heterotopic and orthotopic bone formation in rodents. J Orthop Res. 1998;16:330–339. doi: 10.1002/jor.1100160309. [DOI] [PubMed] [Google Scholar]
- 127.Guo X, Zheng Q, Kulbatski I, Yuan Q, Yang S, Shao Z, Wang H, Xiao B, Pan Z, Tang S. Bone regeneration with active angiogenesis by basic fibroblast growth factor gene transfected mesenchymal stem cells seeded on porous beta-TCP ceramic scaffolds. Biomed Mater. 2006;1:93–99. doi: 10.1088/1748-6041/1/3/001. [DOI] [PubMed] [Google Scholar]
- 128.Lee JY, Musgrave D, Pelinkovic D, Fukushima K, Cummins J, Usas A, Robbins P, Fu FH, Huard J. Effect of bone morphogenetic protein-2-expressing muscle-derived cells on healing of critical-sized bone defects in mice. J Bone Joint Surg Am. 2001;83-A:1032–1039. doi: 10.2106/00004623-200107000-00008. [DOI] [PubMed] [Google Scholar]
- 129.Breitbart AS, Grande DA, Mason JM, Barcia M, James T, Grant RT. Gene-enhanced tissue engineering: applications for bone healing using cultured periosteal cells transduced retrovirally with the BMP-7 gene. Ann Plast Surg. 1999;42:488–495. [PubMed] [Google Scholar]
- 130.Peng H, Wright V, Usas A, Gearhart B, Shen HC, Cummins J, Huard J. Synergistic enhancement of bone formation and healing by stem cell-expressed VEGF and bone morphogenetic protein-4. J Clin Invest. 2002;110:751–759. doi: 10.1172/JCI15153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Zhao Z, Wang Z, Ge C, Krebsbach P, Franceschi RT. Healing cranial defects with AdRunx2-transduced marrow stromal cells. J Dent Res. 2007;86:1207–1211. doi: 10.1177/154405910708601213. [DOI] [PubMed] [Google Scholar]
- 132.Tu Q, Valverde P, Li S, Zhang J, Yang P, Chen J. Osterix overexpression in mesenchymal stem cells stimulates healing of critical-sized defects in murine calvarial bone. Tissue Eng. 2007;13:2431–2440. doi: 10.1089/ten.2006.0406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Boden SD, Titus L, Hair G, Liu Y, Viggeswarapu M, Nanes MS, Baranowski C. Lumbar spine fusion by local gene therapy with a cDNA encoding a novel osteoinductive protein (LMP-1) Spine (Phila Pa 1976) 1998;23:2486–2492. doi: 10.1097/00007632-199812010-00003. [DOI] [PubMed] [Google Scholar]
- 134.Hidaka C, Goshi K, Rawlins B, Boachie-Adjei O, Crystal RG. Enhancement of spine fusion using combined gene therapy and tissue engineering BMP-7-expressing bone marrow cells and allograft bone. Spine (Phila Pa 1976) 2003;28:2049–2057. doi: 10.1097/01.BRS.0000091661.11228.C3. [DOI] [PubMed] [Google Scholar]
- 135.Hsu WK, Wang JC, Liu NQ, Krenek L, Zuk PA, Hedrick MH, Benhaim P, Lieberman JR. Stem cells from human fat as cellular delivery vehicles in an athymic rat posterolateral spine fusion model. J Bone Joint Surg Am. 2008;90:1043–1052. doi: 10.2106/JBJS.G.00292. [DOI] [PubMed] [Google Scholar]
- 136.Miyazaki M, Sugiyama O, Zou J, Yoon SH, Wei F, Morishita Y, Sintuu C, Virk MS, Lieberman JR, Wang JC. Comparison of lentiviral and adenoviral gene therapy for spinal fusion in rats. Spine (Phila Pa 1976) 2008;33:1410–1417. doi: 10.1097/BRS.0b013e3181761003. [DOI] [PubMed] [Google Scholar]
- 137.Hasharoni A, Zilberman Y, Turgeman G, Helm GA, Liebergall M, Gazit D. Murine spinal fusion induced by engineered mesenchymal stem cells that conditionally express bone morphogenetic protein-2. J Neurosurg Spine. 2005;3:47–52. doi: 10.3171/spi.2005.3.1.0047. [DOI] [PubMed] [Google Scholar]
- 138.Park J, Ries J, Gelse K, Kloss F, von der Mark K, Wiltfang J, Neukam FW, Schneider H. Bone regeneration in critical size defects by cell-mediated BMP-2 gene transfer: a comparison of adenoviral vectors and liposomes. Gene Ther. 2003;10:1089–1098. doi: 10.1038/sj.gt.3301960. [DOI] [PubMed] [Google Scholar]
- 139.Lattanzi W, Parrilla C, Fetoni A, Logroscino G, Straface G, Pecorini G, Stigliano E, Tampieri A, Bedini R, Pecci R, et al. Ex vivo-transduced autologous skin fibroblasts expressing human Lim mineralization protein-3 efficiently form new bone in animal models. Gene Ther. 2008;15:1330–1343. doi: 10.1038/gt.2008.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Wen Q, Ma L, Chen YP, Yang L, Luo W, Wang XN. Treatment of avascular necrosis of the femoral head by hepatocyte growth factor-transgenic bone marrow stromal stem cells. Gene Ther. 2008;15:1523–1535. doi: 10.1038/gt.2008.110. [DOI] [PubMed] [Google Scholar]
- 141.Dai KR, Xu XL, Tang TT, Zhu ZA, Yu CF, Lou JR, Zhang XL. Repairing of goat tibial bone defects with BMP-2 gene-modified tissue-engineered bone. Calcif Tissue Int. 2005;77:55–61. doi: 10.1007/s00223-004-0095-z. [DOI] [PubMed] [Google Scholar]
- 142.Xu XL, Tang T, Dai K, Zhu Z, Guo XE, Yu C, Lou J. Immune response and effect of adenovirus-mediated human BMP-2 gene transfer on the repair of segmental tibial bone defects in goats. Acta Orthop. 2005;76:637–646. doi: 10.1080/17453670510041709. [DOI] [PubMed] [Google Scholar]
- 143.Ishihara A, Zekas LJ, Weisbrode SE, Bertone AL. Comparative efficacy of dermal fibroblast-mediated and direct adenoviral bone morphogenetic protein-2 gene therapy for bone regeneration in an equine rib model. Gene Ther. 2010;17:733–744. doi: 10.1038/gt.2010.13. [DOI] [PubMed] [Google Scholar]
- 144.Ishihara A, Zekas LJ, Litsky AS, Weisbrode SE, Bertone AL. Dermal fibroblast-mediated BMP2 therapy to accelerate bone healing in an equine osteotomy model. J Orthop Res. 2010;28:403–411. doi: 10.1002/jor.20978. [DOI] [PubMed] [Google Scholar]
- 145.Tang TT, Lu B, Yue B, Xie XH, Xie YZ, Dai KR, Lu JX, Lou JR. Treatment of osteonecrosis of the femoral head with hBMP-2-gene-modified tissue-engineered bone in goats. J Bone Joint Surg Br. 2007;89:127–129. doi: 10.1302/0301-620X.89B1.18350. [DOI] [PubMed] [Google Scholar]
- 146.Chang SC, Lin TM, Chung HY, Chen PK, Lin FH, Lou J, Jeng LB. Large-scale bicortical skull bone regeneration using ex vivo replication-defective adenoviral-mediated bone morphogenetic protein-2 gene-transferred bone marrow stromal cells and composite biomaterials. Neurosurgery. 2009;65:75–81. doi: 10.1227/01.NEU.0000345947.33730.91. discussion 81–73. [DOI] [PubMed] [Google Scholar]
- 147.Sheyn D, Kallai I, Tawackoli W, Cohn Yakubovich D, Oh A, Su S, Da X, Lavi A, Kimelman-Bleich N, Zilberman Y, et al. Gene-modified adult stem cells regenerate vertebral bone defect in a rat model. Mol Pharm. 2011;8:1592–1601. doi: 10.1021/mp200226c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Anonymous. One of three successfully treated CGD patients in a Swiss-German gene therapy trial died due to his underlying disease: A position statement from the European Society of Gene Therapy (ESGT) J Gene Med. 2006;8:1435. doi: 10.1002/jgm.991. [DOI] [PubMed] [Google Scholar]