Abstract
Background
Traditional screw or plate fixation options can be used to fix the majority of sacral fractures. However, these techniques are unreliable with dysmorphic upper sacral segments, U-fractures, osseous compression of neural elements, and previously failed fixation. Lumbopelvic fixation can potentially address these injuries but is a technically demanding procedure requiring spinal and pelvic fixation and it is unclear whether it reliably corrects the deformity and restores function.
Questions/purposes
We therefore assessed reduction quality and loss of fixation, pain related to prominent hardware, subjective dysfunction measured by the Short Musculoskeletal Function Assessment (SMFA), and complications.
Methods
We retrospectively reviewed 15 patients with unstable sacral fractures treated with lumbopelvic fixation between 2002 and 2010. Patients had radiographic monitoring regarding reduction quality and loss of fixation and clinical followup using the SMFA. The minimum followup was 12 months (mean, 23 months; range, 12–41 months).
Results
Posterior reduction quality was 11 of 15 with less than 5 mm persistent displacement and four of 15 with 5 to 10 mm displacement. Loss of fixation was observed in one patient as a result of a technical error. Prominent hardware resulted in greater pain. Despite daily activity and bother subscores improving over time, we found long-term dysfunction in the SMFA. Eleven of the 15 patients were able to return to previous work or activities.
Conclusion
Complex posterior pelvic ring injuries of the sacrum not amenable to traditional fixation options can be salvaged with adherence to the technical details of lumbopelvic fixation. Hardware prominence and pain are markedly reduced with screw head recession. Long-term impairment is noted in patients with complex pelvic ring injuries requiring lumbopelvic fixation compared with normative data.
Level of Evidence
Level IV, therapeutic study. See Guidelines for Authors for a complete description of levels of evidence.
Introduction
Fixation of sacral fractures continues to be challenging as a result of complex local anatomy, unique biomechanical forces, and poor bone quality [21]. Surgical stabilization of pelvic fractures reportedly decreases mortality [7]. The key requirements of successful posterior pelvic ring repair are proper alignment among the ilium, sacrum, and lumbar spine [13] and sufficient stability to counterbalance translational and rotational forces in vertical and horizontal directions [34]. The combination of a vertical (lumbopelvic) and horizontal (iliosacral) fixation transfers vertical loads from the ilium directly to the lumbar spine and prevents flexion of the pelvis [35]. Most stabilization techniques, including open or percutaneous iliosacral screw fixation, tension band transiliac plate fixation, transiliac bars, and local plate fixation, do not entirely fulfill these requirements and may result in 2% to 17.3% fixation failure [9, 22, 24, 34, 35]. Dysmorphic upper sacral segments (small safe zone), sacral U-fractures (short segment fixation), osseous compression of neural elements requiring decompression (removing osseous interdigitation and increasing instability), and previously failed fixation of sacral fractures (secondary to osteoporosis and noncompliant early weightbearing) further complicate and compromise traditional fixation methods by reducing the available bone stock for direct fixation.
Lumbopelvic fixation in traumatic pelvic ring injuries was introduced in 1994 [17] and has been refined by Schildhauer et al. [34]. Two methods have been advocated: lumbopelvic fixation using a double pedicle screw rod construct with a cross-connector [34, 35] and triangular fixation using a single pedicle screw rod construct with a supplemental iliosacral screw [22, 29]. Lumbopelvic fixation is the only surgical procedure in unstable posterior pelvic ring disruptions that allows immediate weightbearing [34]. In biomechanical testing, the triangular lumbopelvic fixation provides greater immediate postoperative stability than iliosacral screw fixation while unloading the area of injury [9, 35] instead of compression [17]. This ability to perform in situ fixation without the necessity of a load-bearing second fragment makes it also a useful tool for spinopelvic dissociations [15] as a result of horizontal Roy-Camille and Strange-Vognsen type fractures [28, 39, 40]. However, because the fracture deformity often involves rotation of the upper sacrum [28], the use of a single screw may not provide adequate support against the deforming forces [15]. Additionally, the procedure includes immobilization of at least one lumbosacral spinal motion segment as well as rotational immobilization of the sacroiliac joint. Despite the theoretical advantages, it remains unclear whether lumbopelvic fixation reliably allows maintenance of reduction and restoration of function.
We therefore assessed reduction quality and loss of fixation, pain related to prominent hardware, subjective dysfunction, and complications in patients who had lumbopelvic fixation for unstable sacral fractures.
Patients and Methods
Between June 2002 and June 2010 a total of 1635 pelvic fractures were treated at our institution. Of these, we retrospectively identified 896 patients who were operatively treated for pelvic ring injuries and of these, 18 patients with unstable sacral fractures underwent lumbopelvic fixation. Operative indications were (1) unstable pelvic ring injury based on a complete posterior ring injury; (2) displacement of more than 10 mm; (3) instability based on intraoperative fluoroscopic imaging; and/or (4) neural injury with associated intraforaminal debris. Indications for lumbopelvic fixation were based on ongoing instability and displacement after failed operative (four) and nonoperative (two) treatment, upper sacral segment dysmorphism with a small safe zone, and unstable sacral fracture (nine) (Fig. 1) with neural injury (seven) and intraforaminal debris requiring decompression (three). The contraindications were: (1) life-threatening comorbidities and (2) life-threatening associated injuries. For this study we included patients ≥ 16 years. We excluded three patients with a followup less than 1 year and with insufficient medical records or radiographic data. This left 15 patients with a mean age of 39 years (range, 16–68 years). There were seven males and eight females with a mean body mass index (BMI) of 28 kg/m2 (range, 21–39 kg/m2). Three of 15 patients were involved in litigation. Workers compensation was involved with three of 15 of the patients. Most injuries were caused by high-energy mechanisms (Table 1). Four of the 15 patients had an Injury Severity Score > 15 and were classified as polytrauma. The minimum followup was 12 months (mean, 23 months; range, 12–41 months). No patients were recalled specifically for this study; all data were obtained from medical records and radiographs.
Fig. 1.
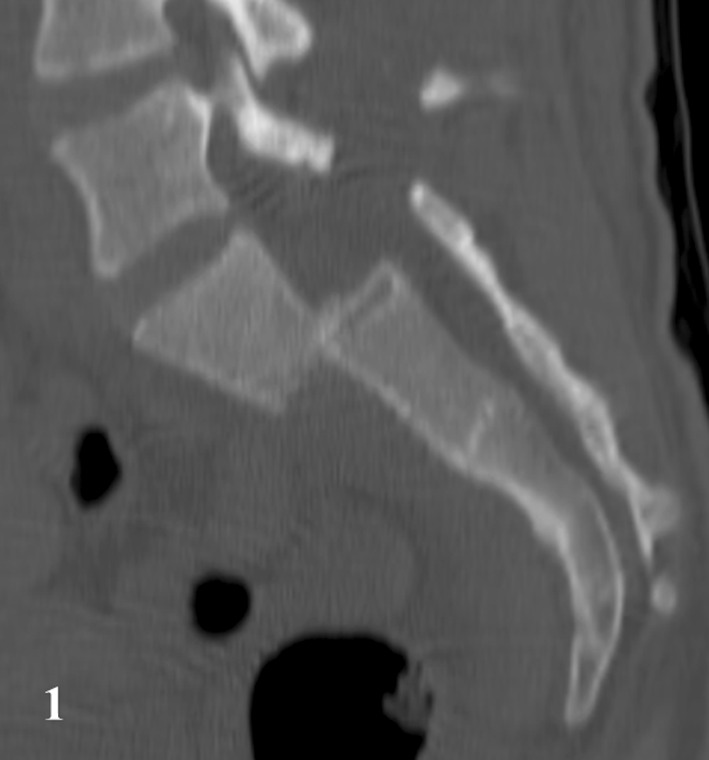
The CT reconstruction demonstrates a Roy-Camille Type 3 fracture.
Table 1.
Injury mechanisms
| Mechanism | Number | Percent (%) |
|---|---|---|
| Low-energy fall | 4 | 26.7 |
| High-energy fall | 1 | 6.7 |
| Pedestrian | 1 | 6.7 |
| Motorcycle accident/bike | 2 | 13.3 |
| Motor vehicle accident | 5 | 33.3 |
| All-terrain vehicle | 1 | 6.7 |
| Crush | 1 | 6.7 |
Each patient had three views of the initially injured pelvis. These were an AP view with the patient supine, a pelvic inlet view, and an outlet view [46]. Inlet and outlet views were performed for assessing rotational, translational, and vertical displacement. Bilateral transforaminal and transverse sacral fractures had lateral (Lat) lumbosacral (LS) imaging to evaluate angulation and translation. Pelvic asymmetry influencing leg length discrepancy was determined as the difference in height of the acetabular dome from a line perpendicular to the long axis of the sacrum on the AP view [20]. Each patient had a CT scan (Fig. 2A) with reconstruction (Fig. 2B) of the injured pelvis that provided information on both extent of the injury and the magnitude of the displacement. Furthermore, the CT defined injury to the lumbar five (L5) transverse process and/or L5-sacral 1 (S1) joint injury extension. Injuries were classified according to Tile [42], OTA/AO [19], Denis et al. [2], Roy-Camille et al. [28]/Strange-Vognsen and Lebech [39], and Isler [16] classification (Table 2).
Fig. 2A–B.
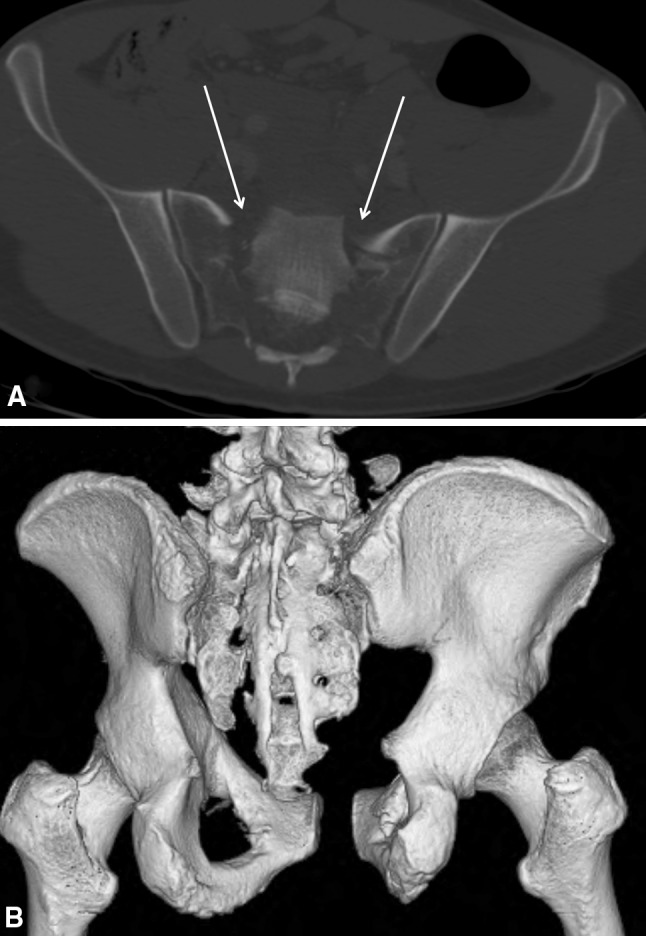
CT scans of all patients have been performed. (A) A bilateral sacral fracture is demonstrated. The three-dimensional reconstruction (B) gives an idea of the displacement and instability.
Table 2.
Patient demographics and fracture patterns
| Patient number | Gender | Age (years) | Body mass index (kg/m2) | Tile | AO/OTA | Roy-Camille/Vognsen | Isler | Denis | Previous treatment | Complication |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Female | 63 | 28.90 | B3 | B3 | 3 | 1 | Bilateral | None | |
| 2 | Male | 55 | 39.25 | B1 | B1 | NC | 1 | Zone 3 | None | Deep vein thrombosis |
| 3 | Female | 32 | 23.36 | B3 | B3 | 1 | 1 | Bilateral | None | Infection, heterotopic ossification, prominent hardware |
| 4 | Female | 32 | 21.09 | B2 | B2 | NC | NC | Zone 2 | Nonoperative | Loss of fixation, nonunion |
| 5 | Female | 68 | 31.84 | B3 | B3 | 4 | NC | Zone 3 | None | Heterotopic ossification |
| 6 | Female | 39 | 25.56 | B2 | B2 | NC | 1 | Zone 2 | Nonoperative | Prominent hardware |
| 7 | Male | 56 | 35.99 | B1 | B1 | NC | 1 | Zone 2 | Iliosacral screw | |
| 8 | Male | 26 | 23.02 | C | C3 | 2 | NC | Bilateral | None | |
| 9 | Male | 41 | 25.93 | B2 | B2 | NC | 3 | Zone 2 | Iliosacral screw | Prominent hardware |
| 10 | Female | 46 | 23.71 | C | C3 | 4 | NC | Zone 3 | None | |
| 11 | Female | 30 | 27.05 | C | C2 | 1 | 3 | Bilateral | None | |
| 12 | Male | 17 | 25.62 | C | C3 | 3 | 1 | Bilateral | None | Infection, prominent hardware |
| 13 | Female | 16 | 29.36 | B2 | B2 | NC | 2 | Bilateral | Iliosacral screw | |
| 14 | Male | 38 | 35.06 | B3 | B3 | 2 | NC | Bilateral | Bilateral iliosacral screws | |
| 15 | Male | 30 | N/A | C | C3 | NC | NC | Bilateral | None |
N/A = not available; NC = not classifiable.
One fellowship-trained orthopedic trauma surgeon (CBJ) performed all operative procedures. Patients were positioned prone on a radiolucent table with appropriate eye protection [37] and sequential compression devices. The operative approaches were tailored to each patient based on the pattern of the injury, location of the fracture, associated injuries, and soft tissue involvement [10]. Attention to detail was maintained to avoid dural or neural injury. Lumbopelvic implants (USS; Synthes, Paoli, PA, USA) were inserted as described by Schildhauer et al. [33]. With the aid of a high-speed burr, a carve out was created at the posteroinferior iliac spine (PIIS) entrance site for the iliac screw. Therefore, recession was created for the screw head, end cap, and connector rod in an attempt to reduce prominence. Screw size was based on length from the recessed entrance of the PIIS along the sciatic buttress and ending at the anteroinferior iliac spine and was 8.0 mm × 110 to 130 mm (USS, titanium; Synthes). Sacroiliac screws (7.0 mm, Magnafx, stainless steel; Zimmer, Warsaw, IN, USA) were inserted if anatomically able and as described by Routt et al. [27]. Patients with ligamentous injury involving the L5-S1 interval or with posterior neural decompression involving the S1 facets and lamina underwent an associated L5-S1 posterolateral arthrodesis. Approaches were closed over drains and in anatomic layers. Anterior fixation was performed when anterior ring motion with examination under anesthesia (EUA) was deemed too great by the surgeon. Anterior instability was assumed when the surgeon manually applied lateral compression forces creating translation greater than approximately 20 mm of the anterior fracture lines confirmed with concomitant inlet-view fluoroscopy. Six patients required additional anterior fixation. One patient had a retrograde ramus screw and five patients underwent additional anterior plating.
Postoperatively, all patients had antibiotic and deep vein thrombosis prophylaxis. Patients were mobilized based on the constellation of injuries and pelvic injury pattern. In general, progressive weightbearing was continued for 3 months on the lower extremity corresponding to the ipsilateral posterior pelvic ring injury. On beginning unrestricted weightbearing, formal therapy was instituted working on core strengthening, dynamic lumbar stabilization, ROM, strengthening, and conditioning. A Morel-Lavellee degloving injury [1, 25] had excisional débridement. Antibiotics for degloving injuries were continued until drains were removed.
Patients were evaluated at regular and consistent intervals of 2 weeks, 6 weeks, 12 weeks, 6 months, 1 year, and 2 years if possible. Complaints of pain with visual analog scale, erectile dysfunction, urinary dysfunction, and sexual problems were recorded. Clinical examination of incisional healing, motor and sensory function, pelvic stability, and ambulation was performed. Radiographs consisting of AP, inlet, and outlet views of the pelvis and Lat LS view were obtained at each interval. One of us (MFH), who was not involved in surgical treatment or patient care, evaluated the initial postoperative radiographs (Fig. 3), the initial postoperative CT (Fig. 4), and final radiographs. Reduction quality was grouped in 0 to 4 mm persistent displacement, 5 to 10 mm displacement, and greater than 10 mm displacement after reduction [20]. Loss of fixation was recorded comparing the initial and final radiographs. Nonunion was defined as radiographic lucency around implants in the setting of a continued fracture line, loss of fixation, and clinical complaints of pain. Deep infection was defined as an infection requiring operative débridement and antibiotic administration. Hardware prominence was defined as a palpable and painful posterior screw head, rod, or connector. Short Musculoskeletal Function Assessment (SMFA) questionnaires were obtained at 6 months, 1 year, and 2 years if possible [41]. The SMFA was chosen as a well-established, validated, and reproducible measure of health status with normative data [14]. The SMFA consists of 46 questions representing indices of dysfunction and bother by functional problems: daily activity, emotional status, arm/hand function, mobility, dysfunction index, and bother index. Questions 22 and 46 were separately analyzed concerning sexual intercourse and pain. Complications noted included infection, hematoma, prominent hardware, and fixation failure with loss of reduction were recorded [3].
Fig. 3.
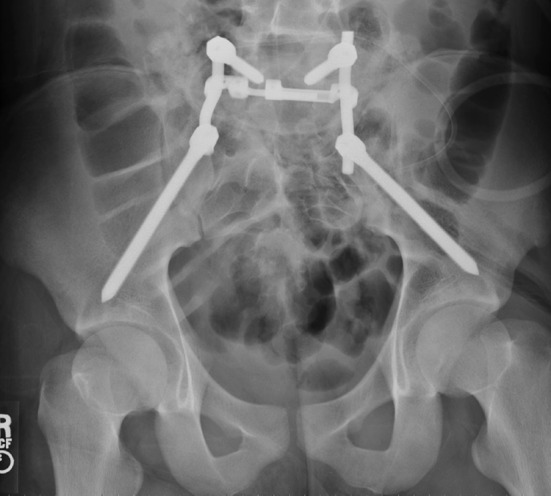
The radiograph demonstrates a bilateral lumbopelvic fixation with a cross-connector and long 130-mm sciatic buttress screws.
Fig. 4.
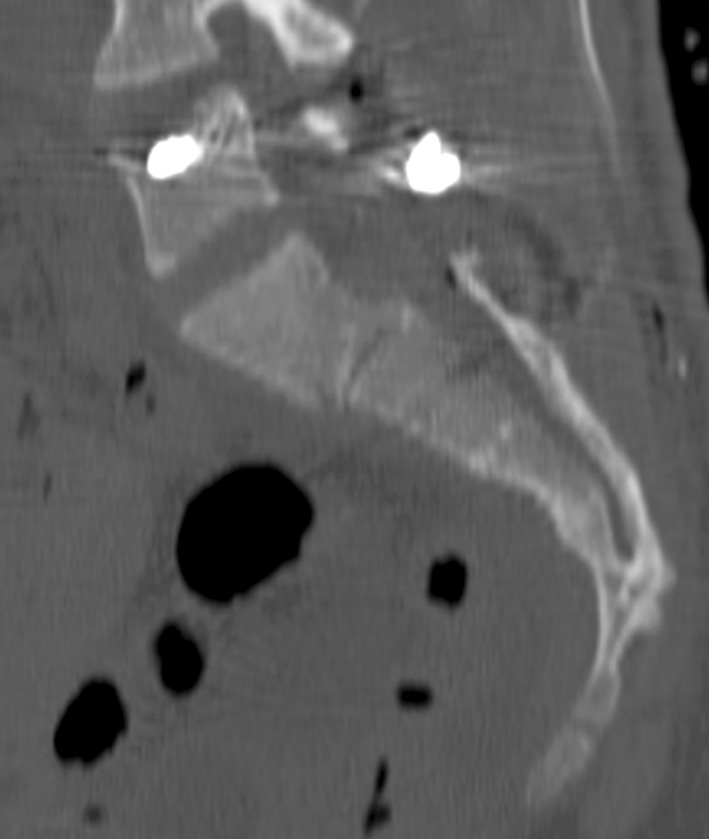
Good reduction quality was achieved after lumbopelvic fixation.
We computed descriptive statistics. However, statistics were otherwise limited as a result of the small patient sample. We used PASW® 18 (IBM, Armonk, NY, USA) for analyses.
Results
The posterior reduction quality was 11 of 15 with < 5 mm displacement and four of 15 with 5 to 10 mm displacement. No displacement greater than 10 mm was measured postoperatively. Of the seven with neurologic injuries, four were anatomically reduced, two had a posterior reduction of 2 to 4 mm, and one had a posterior reduction of 5 to 10 mm. Two of the 15 patients had chronic back pain. Both patients had initial L5/S1 involvement and were classified as Roy-Camille Type 1 and 4. Of the two with chronic low back pain, one was anatomically reduced and the other had postreduction displacement of 5 to 10 mm. Six patients had an acetabular height difference of more than 10 mm but did not have clinical leg length discrepancy. One of the 15 patients had loss of fixation and subsequent nonunion requiring revision surgery and bone grafting. This patient underwent isolated unilateral lumbopelvic fixation without additional sacroiliac screw insertion.
Four of 15 patients had palpable prominent posterior hardware. No differences were noted in SMFA indices between presence of prominent hardware and not (Table 3). However, patients with prominent hardware had greater pain at 1 year compared with patients without prominent hardware. Patients with prominent hardware had a mean pain level of 3.5 (out of 5) compared with patients having a mean pain level of 1.75 (out of 5) without prominent hardware. Four patients had associated lower extremity injuries, which did not affect daily activity, mobility, dysfunction, or bother at any time interval (Table 4). Screw loosening of the anterior ring fixation occurred in two patients but neither was symptomatic and neither patient had surgical revision.
Table 3.
Final followup SMFA, sexual dysfunction, and pain comparison
| SMFA subscore | Daily activity | Emotional | Arm/hand | Mobility | Dysfunction | Bother | Sexual dysfunction | Pain |
|---|---|---|---|---|---|---|---|---|
| Prominent hardware | 34.2 ± 17.6 (18–53) | 44.7 ± 6.4 (39–52) | 2.1 ± 3.6 (0–6) | 35.6 ± 20.3 (19–58) | 29.2 ± 11.4 (18–41) | 35.4 ± 13.7 (21–48) | 2.0 ± 0 (2) | 3.7 ± 1.5 (2–5) |
| No prominent hardware | 38.4 ± 30.5 (0–78) | 34.1 ± 17.4 (7–61) | 9.0 ± 15.1 (0–44) | 34.7 ± 17.4 (7–61) | 29.6 ± 18.4 (2–57) | 32.0 ± 21.0 (8–63) | 2.4 ± 1.8 (1–5) | 3.1 ± 1.0 (2–5) |
SMFA = Short Musculoskeletal Function Assessment.
Table 4.
Patients with additional lower extremity injuries had comparable Short Musculoskeletal Function Assessment scores
| SMFA | 6 months | Significance at 6 months | 12 months | Significance at 12 months | 24 months | Significance at 24 months | Norm | |||
|---|---|---|---|---|---|---|---|---|---|---|
| LE injury | No LE injury | LE injury | No LE injury | LE injury | No LE injury | |||||
| Daily activity | 77.5 | 46.2 | −0.980; 0.430 | 52.5 | 20.1 | −9.500; 0.011 | 15.0 | 34.4 | 0.812; 0.476 | 11.85 ± 19.2 |
| Emotional | 60.7 | 52.4 | −1.322; 0.317 | 42.9 | 44.1 | 0.071; 0.950 | 33.3 | 37.1 | 0.294; 0.788 | 20.54 ± 18.4 |
| Arm/hand | 43.8 | 5.2 | −5.131; 0.036 | 6.3 | 0 | Unable to analyze | 12.5 | 1.0 | −6.708; 0.007 | 6.02 ± 12.26 |
| Mobility | 44.4 | 35.2 | −0.433; 0.707 | 58.3 | 21.9 | −4.073; 0.055 | 30.6 | 30.2 | −0.035; 0.975 | 13.61 ± 18.31 |
| Dysfunction | 57.4 | 34.9 | −1.265; 0.333 | 41.2 | 21.0 | −4.109; 0.054 | 22.3 | 25.9 | 0.414; 0.707 | 12.70 ± 15.59 |
| Bother | 60.4 | 44.4 | −0.781; 0.517 | 47.9 | 18.8 | −2.781; 0.109 | 33.3 | 27.1 | −0.561; 0.614 | 13.77 ± 18.59 |
SMFA = Short Musculoskeletal Function Assessment; LE = lower extremity.
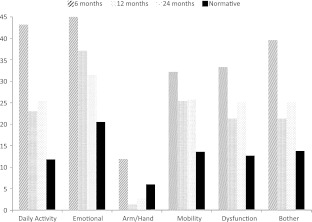
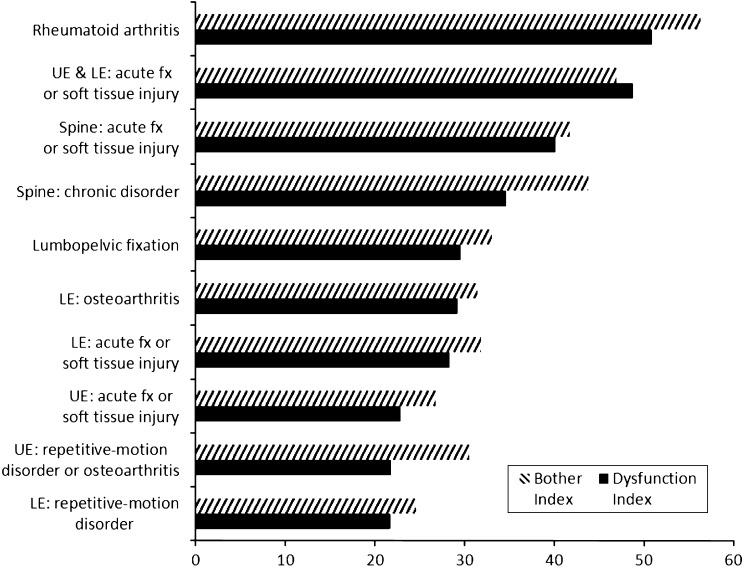
Compared with normative SMFA data, patients with sacral fractures stabilized with lumbopelvic fixation had permanent impairment (Fig. 5). Compared with other orthopaedic conditions, sacral fractures requiring lumbopelvic fixation had permanent dysfunction and bother index subscores similar to conditions with lower extremity osteoarthritis and chronic spinal disorders (Fig. 6). Eleven of the 15 patients returned to their previous work or activities, whereas four of 15 were unable to return to similar work and/or activities.
Fig. 5.
The graph shows changes in all SMFA subscores over time in comparison to a normative population.
Fig. 6.
The graph shows functional outcome of patients with lumbopelvic fixation in comparison with patients with other injuries/diseases. UE = upper extremity; LE = lower extremity; fx = fracture.
Two (including the patient with Morel-Lavellee degloving) of the 15 patients developed a deep infection requiring débridement. Two patients had urologic injuries resulting from their additional anterior pelvic ring injury. One patient developed deep vein thrombosis; none had pulmonary embolism. Seven of the 15 patients had persistent neurologic injuries (Table 5). Six patients had accompanying spinal injuries with five of the six having associated neurological injury. Neurologic injury was affiliated with certain fracture patterns (Table 6) but not related to Isler classification. Ten patients had associated L5-S1 involvement and underwent subsequent posterolateral L5-S1 arthrodesis without signs of nonunion or adjacent-level degeneration.
Table 5.
Persistent neurologic dysfunction may influence outcome
| Persistent neurologic dysfunction | Number | Percent |
|---|---|---|
| None | 8 | 53.3 |
| Sensory injury | 2 | 13.3 |
| Motor injury | 3 | 20 |
| Erectile dysfunction/neurogenic bladder | 1 | 6.7 |
| Paraplegia | 1 | 6.7 |
Table 6.
Classification of fractures with neurologic injuries
| Fracture classification/neurologic injury | Number | Percent |
|---|---|---|
| Tile or OTA/AO Type B | 4 | 57 |
| Tile or OTA/AO Type C | 3 | 43 |
| Denis Zone 2 | 1 | 14 |
| Denis Zone 2 bilateral | 6 | 86 |
| Roy-Camille Type 2 | 2 | 29 |
| Roy-Camille Type 3 | 2 | 29 |
Discussion
Unstable sacral fractures are often not amenable to traditional fixation methods. Different salvage procedures have been reported [5, 8, 17, 44]. Biomechanical studies have confirmed segmental lumbopelvic stabilization provides stable fixation of the posterior pelvic ring while unloading the area of injury [18, 34]. Lumbopelvic fixation provides increased immediate postoperative stability [9, 35] but is a demanding procedure with numerous potential complications. High rates of fixation failure and local pain have been reported and restriction of the technique combined with routine hardware removal has been advocated [31]. High success rates can be achieved when performed systematically and in appropriately selected patients [21]. To enhance the limited literature on lumbopelvic fixation, we assessed reduction quality and loss of fixation, pain related to prominent hardware, subjective dysfunction, and complications.
We acknowledge limitations to our study. First, a small patient cohort is presented and was too small to determine any differences based on potentially confounding variables. Second, the group is heterogeneous. We combined several different injury mechanisms, injury patterns, and associated injuries. All injuries were complex and problematic not being amenable to traditional posterior pelvic ring fixation options. Because these injuries were complex and many associated L5/S1 junctional, spinal, and neural injuries, these fracture patterns are not easily coded by the standard classification systems. We therefore used different classification systems [2, 19, 28, 39]. The small number and large variances could skew outcome measurements and conclusions. Third, some patients had associated posterolateral arthrodesis of the L5/S1 junction. Fourth, because later in the treatment regimen the screw heads were recessed, not all patients had the same problem with screw prominence.
In contrast to the study of Tornetta and Matta [43] who noted worsening function with increased posterior displacement, we did not find worse pelvic pain, low back pain, or function in patients with greater posterior displacement. However, our relatively small sample size and no malreductions greater than 10 mm might have lessened the variance and therefore clustered the results. We suspect a concomitant L5/S1 posterolateral arthrodesis addressed the many L5/S1-associated injuries and could have been associated with less low back pain and improved outcome measurements. Despite the absence of a relationship between displacement and function, the importance of accurate posterior reduction quality should not be underestimated. Avoiding sagittal malalignment of L5/S1, coronal malrotation of the acetabular dome heights, posterior or cephalad translation, or sacral sagittal malrotation should be honored. Furthermore, increasing posterior malreduction decreases the ability to safely insert a sacroiliac screw [26]. One of our patients developed a sacral nonunion. This is consistent with published series [30]. This patient had osteoporotic bone, was a smoker, and required extensive decompression for fracture manipulation and reduction but also only had initial unilateral lumbopelvic fixation. Without insertion of a sacroiliac screw (triangular lumbopelvic fixation) or bilateral lumbopelvic fixation with a locking crossbar, rotational and sagittal plane instability is potentially present. We recommend stable construct fixation in all planes to avoid potential instability and nonunion results.
Prominence of iliac screw heads was a frequent problem in thin patients [32]. In previous studies, up to 95% of the patients had painful and prominent implants [22, 31]. Therefore, routine hardware removal has been required [31]. Following the recommendation of Schildhauer et al. [32] to countersink the iliac screw heads, we demonstrated only four patients with prominent hardware.
In comparison to the only other series evaluating outcomes with SMFA, Sagi et al. [31] treated only unilateral Zone 2 sacral fractures using triangular fixation. Because our patient series had complex sacral fractures requiring sacral neural decompression, upper sacral dysmorphism impeding sacroiliac screw insertion, and sacral U-fractures with bilateral transforaminal fractures, our series is difficult if not impossible to compare. Sagi et al. also demonstrated no improvement with time. In our study, daily activity and bother index measurements improved with time. A trend to lessened problems with pain or difficulty having sexual activity was also noted. As to be expected, patients with prominent hardware had greater pain at the 1-year time point, but no differences were noted in SMFA indices compared with patients without prominent hardware. Compared with normative data, sacral fractures fixed with lumbopelvic fixation had persistent reduction in daily activity, emotional, mobility, dysfunction, and bother outcome measurements. Associated lower extremity, L5/S1 injury, urologic dysfunction, or sexual difficulty did not relate to pain or worse outcome measurements. These findings are different from prior published series of pelvic ring injuries [4]. Our patient population functioned worse than one with lower extremity osteoarthritis and acute lower extremity fractures but better than cohorts with acute spine fractures or chronic spinal disorders. Because these injuries occurred through the sacrum, which is the junction between the spine and the lower extremities, and were fixated with spinal instrumentation, function better than that of patients with isolated lower extremity problems but worse than those with spine problems would be expected. The presence of persistent neurologic injury was related to worsened bother measurements [6]. Further analysis into improvement with time and extent of neurologic injury with larger numbers may allow for determination of which neurologic injuries permanently affect outcome measurements. Comparable to published data, we did demonstrate a return to activity and work in a majority of patients at the 1-year interval [30]. Based on published data on a plethora of subjects, litigation or Workers Compensation claims did have a worse effect on clinical outcomes [11, 12]. Because high-energy pelvic ring injuries occur primarily in patients of employable age and a trend exists for worse pain and functional outcomes, counseling and rehabilitation concerning return to work should be initiated early.
We had no iatrogenic neural injuries. Our study does not support the concern that increased muscle mobilization and potential devitalization increase the risk for hematoma formation and infection [31]. The initial infection rate of two in 15 seems rather high, but these patients have more complex injury patterns than most sacral fractures and one had a degloving injury requiring initial irrigation and débridement [23]. Pelvic fractures are related to higher prevalence of deep vein thrombosis [38, 45]. In our study, only one patient had a diagnosed thrombosis. Early mobilization is one of the crucial steps to prevention for thrombosis [36]. Lumbopelvic fixation provides stable fixation and therefore allows early mobilization [34]. A 1-year clinical followup by Sagi et al. [31] mentioned concern that triangular fixation may lead to sagittal plane deformity and that this deformity may result in overloading the contralateral L5/S1 facet joint and result in future chronic lower back pain. Using a unilateral L5 pedicle screw as the fixation point to achieve sacral translation and length is not recommended. Locking the L5/S1 junction with bilateral L5 and S1 pedicle screws attached to the sciatic buttress screws will provide increased and symmetric fixation for sacral reduction and stabilization methods. We recommend reduction by use of a joystick and clamp methods to avoid stress on the lumbar pedicle screws and facet joints. We did not see the problem of scoliosis or pain with our technique or patient population. This method of reduction and stabilization avoids stress across the L5/S1 junction and could also potentially reduce iatrogenic neural injuries [30].
Complex posterior pelvic ring injuries of the sacrum that are not amenable to traditional fixation options can be salvaged with lumbopelvic fixation. Complications of nonunion are reduced with appropriately detailed stable fixation methods. Hardware prominence and pain are reduced with screw head recession. Long-term functional outcomes in patients with complex pelvic ring injuries requiring lumbopelvic fixation have permanent impairment compared with normative data. Daily activity and bother indices appear to improve with time.
Acknowledgments
We thank Judy Zane CRT for her coordination of imaging studies. We also thank the staff at Orthopaedic Associates of Michigan for their vigilant attention to detail and ability to obtain completed outcome questionnaires.
Footnotes
Each author certifies that he or she, or a member of their immediate family, has no commercial associations (eg, consultancies, stock ownership, equity interest, patent/licensing arrangements, etc) that might pose a conflict of interest in connection with the submitted article.
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research editors and board members are on file with the publication and can be viewed on request.
Clinical Orthopaedics and Related Research neither advocates nor endorses the use of any treatment, drug or device. Readers are encouraged to always seek additional information, including FDA-approval status, of any drug or device prior to clinical use.
Each author certifies that his or her institution approved the human protocol for this investigation, that all investigations were conducted in conformity with ethical principles of research, and that informed consent for participation in the study was obtained.
This work was performed at Orthopaedic Associates of Michigan, Grand Rapids, MI, USA.
References
- 1.Collinge C, Tornetta P., 3rd Soft tissue injuries associated with pelvic fractures. Orthop Clin North Am. 2004;35:451–456. doi: 10.1016/j.ocl.2004.05.003. [DOI] [PubMed] [Google Scholar]
- 2.Denis F, Davis S, Comfort T. Sacral fractures: an important problem. Retrospective analysis of 236 cases. Clin Orthop Relat Res. 1988;227:67–81. [PubMed] [Google Scholar]
- 3.Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205–213. doi: 10.1097/01.sla.0000133083.54934.ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ellison M, Timberlake GA, Kerstein MD. Impotence following pelvic fracture. J Trauma. 1988;28:695–696. doi: 10.1097/00005373-198805000-00026. [DOI] [PubMed] [Google Scholar]
- 5.Gansslen A, Pape HC, Lehmann U, Lange U, Krettek C, Pohlemann T. Open reduction and internal fixation of unstable sacral fractures] [in German. Zentralblatt fur Chirurgie. 2003;128:40–45. doi: 10.1055/s-2003-37318. [DOI] [PubMed] [Google Scholar]
- 6.Gibbons KJ, Soloniuk DS, Razack N. Neurological injury and patterns of sacral fractures. J Neurosurg. 1990;72:889–893. doi: 10.3171/jns.1990.72.6.0889. [DOI] [PubMed] [Google Scholar]
- 7.Goldstein A, Phillips T, Sclafani SJ, Scalea T, Duncan A, Goldstein J, Panetta T, Shaftan G. Early open reduction and internal fixation of the disrupted pelvic ring. J Trauma. 1986;26:325–333. doi: 10.1097/00005373-198604000-00004. [DOI] [PubMed] [Google Scholar]
- 8.Gribnau AJ, Hensbroek PB, Haverlag R, Ponsen KJ, Been HD, Goslings JC. U-shaped sacral fractures: surgical treatment and quality of life. Injury. 2009;40:1040–1048. doi: 10.1016/j.injury.2008.11.027. [DOI] [PubMed] [Google Scholar]
- 9.Griffin DR, Starr AJ, Reinert CM, Jones AL, Whitlock S. Vertically unstable pelvic fractures fixed with percutaneous iliosacral screws: does posterior injury pattern predict fixation failure? J Orthop Trauma. 2003;17:399–405. doi: 10.1097/00005131-200307000-00001. [DOI] [PubMed] [Google Scholar]
- 10.Gruen GS, Leit ME, Gruen RJ, Garrison HG, Auble TE, Peitzman AB. Functional outcome of patients with unstable pelvic ring fractures stabilized with open reduction and internal fixation. J Trauma. 1995;39:838–844. doi: 10.1097/00005373-199511000-00006. [DOI] [PubMed] [Google Scholar]
- 11.Harris I, Dao AT, Young J, Solomon M, Jalaludin BB, Rae H. Factors predicting patient satisfaction following major trauma. Injury. 2007;38:1102–1108. doi: 10.1016/j.injury.2007.05.004. [DOI] [PubMed] [Google Scholar]
- 12.Harris IA, Dao AT, Young JM, Solomon MJ, Jalaludin BB. Predictors of patient and surgeon satisfaction after orthopaedic trauma. Injury. 2009;40:377–384. doi: 10.1016/j.injury.2008.08.039. [DOI] [PubMed] [Google Scholar]
- 13.Henderson RC. The long-term results of nonoperatively treated major pelvic disruptions. J Orthop Trauma. 1989;3:41–47. doi: 10.1097/00005131-198903010-00008. [DOI] [PubMed] [Google Scholar]
- 14.Hunsaker FG, Cioffi DA, Amadio PC, Wright JG, Caughlin B. The American Academy of Orthopaedic Surgeons outcomes instruments: normative values from the general population. J Bone Joint Surg Am. 2002;84:208–215. doi: 10.2106/00004623-200202000-00007. [DOI] [PubMed] [Google Scholar]
- 15.Hunt N, Jennings A, Smith M. Current management of U-shaped sacral fractures or spino-pelvic dissociation. Injury. 2002;33:123–126. doi: 10.1016/S0020-1383(00)00179-0. [DOI] [PubMed] [Google Scholar]
- 16.Isler B. Lumbosacral lesions associated with pelvic ring injuries. J Orthop Trauma. 1990;4:1–6. doi: 10.1097/00005131-199003000-00001. [DOI] [PubMed] [Google Scholar]
- 17.Kaech K, Trenz O. Distraction spondylodesis of the sacrum in ‘vertical shear lesions’ of the pelvis. Der Unfallchirurg. 1994;97:28–38. [PubMed] [Google Scholar]
- 18.Keel MJ, Benneker LM, Siebenrock KA, Bastian JD. Less invasive lumbopelvic stabilization of posterior pelvic ring instability: technique and preliminary results. J Trauma. 2011;71:E62–E70. doi: 10.1097/TA.0b013e3182218c99. [DOI] [PubMed] [Google Scholar]
- 19.Marsh JL, Slongo TF, Agel J, Broderick JS, Creevey W, DeCoster TA, Prokuski L, Sirkin MS, Ziran B, Henley B, Audige L. Fracture and dislocation classification compendium—2007: Orthopaedic Trauma Association classification, database and outcomes committee. J Orthop Trauma. 2007;21:S1–S133. doi: 10.1097/00005131-200711101-00001. [DOI] [PubMed] [Google Scholar]
- 20.Matta JM, Tornetta P., 3rd Internal fixation of unstable pelvic ring injuries. Clin Orthop Relat Res. 1996;329:129–140. doi: 10.1097/00003086-199608000-00016. [DOI] [PubMed] [Google Scholar]
- 21.Moshirfar A, Rand FF, Sponseller PD, Parazin SJ, Khanna AJ, Kebaish KM, Stinson JT, Riley LH., 3rd Pelvic fixation in spine surgery. Historical overview, indications, biomechanical relevance, and current techniques. J Bone Joint Surg Am. 2005;87(Suppl 2):89–106. doi: 10.2106/JBJS.E.00453. [DOI] [PubMed] [Google Scholar]
- 22.Mouhsine E, Wettstein M, Schizas C, Borens O, Blanc CH, Leyvraz PF, Theumann N, Garofalo R. Modified triangular posterior osteosynthesis of unstable sacrum fracture. Eur Spine J. 2006;15:857–863. doi: 10.1007/s00586-004-0858-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mullis BH, Sagi HC. Minimum 1-year follow-up for patients with vertical shear sacroiliac joint dislocations treated with iliosacral screws: does joint ankylosis or anatomic reduction contribute to functional outcome? J Orthop Trauma. 2008;22:293–298. doi: 10.1097/BOT.0b013e31816b6b4e. [DOI] [PubMed] [Google Scholar]
- 24.Papakostidis C, Kanakaris NK, Kontakis G, Giannoudis PV. Pelvic ring disruptions: treatment modalities and analysis of outcomes. Int Orthop. 2009;33:329–338. doi: 10.1007/s00264-008-0555-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Powers ML, Hatem SF, Sundaram M. Morel-Lavallee lesion. Orthopedics. 2007;30(250):322–353. doi: 10.3928/01477447-20070401-10. [DOI] [PubMed] [Google Scholar]
- 26.Reilly MC, Bono CM, Litkouhi B, Sirkin M, Behrens FF. The effect of sacral fracture malreduction on the safe placement of iliosacral screws. J Orthop Trauma. 2003;17:88–94. doi: 10.1097/00005131-200302000-00002. [DOI] [PubMed] [Google Scholar]
- 27.Routt ML, Jr, Kregor PJ, Simonian PT, Mayo KA. Early results of percutaneous iliosacral screws placed with the patient in the supine position. J Orthop Trauma. 1995;9:207–214. doi: 10.1097/00005131-199506000-00005. [DOI] [PubMed] [Google Scholar]
- 28.Roy-Camille R, Saillant G, Gagna G, Mazel C. Transverse fracture of the upper sacrum. Suicidal jumper’s fracture. Spine. 1985;10:838–845. doi: 10.1097/00007632-198511000-00011. [DOI] [PubMed] [Google Scholar]
- 29.Sagi HC. Technical aspects and recommended treatment algorithms in triangular osteosynthesis and spinopelvic fixation for vertical shear transforaminal sacral fractures. J Orthop Trauma. 2009;23:354–360. doi: 10.1097/BOT.0b013e3181a1143a. [DOI] [PubMed] [Google Scholar]
- 30.Sagi HC, Lindvall EM. Inadvertent intraforaminal iliosacral screw placement despite apparent appropriate positioning on intraoperative fluoroscopy. J Orthop Trauma. 2005;19:130–133. doi: 10.1097/00005131-200502000-00010. [DOI] [PubMed] [Google Scholar]
- 31.Sagi HC, Militano U, Caron T, Lindvall E. A comprehensive analysis with minimum 1-year follow-up of vertically unstable transforaminal sacral fractures treated with triangular osteosynthesis. J Orthop Trauma. 2009;23:313–319. doi: 10.1097/BOT.0b013e3181a32b91. [DOI] [PubMed] [Google Scholar]
- 32.Schildhauer TA, Bellabarba C, Nork SE, Barei DP, Routt ML, Jr, Chapman JR. Decompression and lumbopelvic fixation for sacral fracture-dislocations with spino-pelvic dissociation. J Orthop Trauma. 2006;20:447–457. doi: 10.1097/00005131-200608000-00001. [DOI] [PubMed] [Google Scholar]
- 33.Schildhauer TA, Chapman JR, Mayo KA. Multisegmental open sacral fracture due to impalement: a case report. J Orthop Trauma. 2005;19:134–139. doi: 10.1097/00005131-200502000-00011. [DOI] [PubMed] [Google Scholar]
- 34.Schildhauer TA, Josten C, Muhr G. Triangular osteosynthesis of vertically unstable sacrum fractures: a new concept allowing early weight-bearing. J Orthop Trauma. 1998;12:307–314. doi: 10.1097/00005131-199806000-00002. [DOI] [PubMed] [Google Scholar]
- 35.Schildhauer TA, Ledoux WR, Chapman JR, Henley MB, Tencer AF, Routt ML., Jr Triangular osteosynthesis and iliosacral screw fixation for unstable sacral fractures: a cadaveric and biomechanical evaluation under cyclic loads. J Orthop Trauma. 2003;17:22–31. doi: 10.1097/00005131-200301000-00004. [DOI] [PubMed] [Google Scholar]
- 36.Selby R, Geerts W. Prevention of venous thromboembolism: consensus, controversies, and challenges. Hematology Am Soc Hematol Educ Program. 2009:286–292. [DOI] [PubMed]
- 37.Stambough JL, Dolan D, Werner R, Godfrey E. Ophthalmologic complications associated with prone positioning in spine surgery. J Am Acad Orthop Surg. 2007;15:156–165. doi: 10.5435/00124635-200703000-00005. [DOI] [PubMed] [Google Scholar]
- 38.Stannard JP, Singhania AK, Lopez-Ben RR, Anderson ER, Farris RC, Volgas DA, McGwin GR, Jr, Alonso JE. Deep-vein thrombosis in high-energy skeletal trauma despite thromboprophylaxis. J Bone Joint Surg Br. 2005;87:965–968. doi: 10.1302/0301-620X.87B7.15989. [DOI] [PubMed] [Google Scholar]
- 39.Strange-Vognsen HH, Lebech A. An unusual type of fracture in the upper sacrum. J Orthop Trauma. 1991;5:200–203. doi: 10.1097/00005131-199105020-00014. [DOI] [PubMed] [Google Scholar]
- 40.Suzuki K, Mochida J. Operative treatment of a transverse fracture-dislocation at the S1–S2 level. J Orthop Trauma. 2001;15:363–367. doi: 10.1097/00005131-200106000-00011. [DOI] [PubMed] [Google Scholar]
- 41.Swiontkowski MF, Engelberg R, Martin DP, Agel J. Short musculoskeletal function assessment questionnaire: validity, reliability, and responsiveness. J Bone Joint Surg Am. 1999;81:1245–1260. doi: 10.1302/0301-620X.81B6.9794. [DOI] [PubMed] [Google Scholar]
- 42.Tile M. Fractures of the Pelvis and Acetabulum. Baltimore, MD, USA: Lippincott Williams & Wilkins; 1984. [Google Scholar]
- 43.Tornetta P, 3rd, Matta JM. Outcome of operatively treated unstable posterior pelvic ring disruptions. Clin Orthop Relat Res. 1996;329:186–193. doi: 10.1097/00003086-199608000-00022. [DOI] [PubMed] [Google Scholar]
- 44.Vanderschot P, Meuleman C, Lefevre A, Broos P. Trans iliac-sacral-iliac bar stabilisation to treat bilateral lesions of the sacro-iliac joint or sacrum: anatomical considerations and clinical experience. Injury. 2001;32:587–592. doi: 10.1016/S0020-1383(01)00039-0. [DOI] [PubMed] [Google Scholar]
- 45.White RH, Goulet JA, Bray TJ, Daschbach MM, McGahan JP, Hartling RP. Deep-vein thrombosis after fracture of the pelvis: assessment with serial duplex-ultrasound screening. J Bone Joint Surg Am. 1990;72:495–500. [PubMed] [Google Scholar]
- 46.Young JW, Burgess AR, Brumback RJ, Poka A. Pelvic fractures: value of plain radiography in early assessment. Radiology. 1986;160:445–451. doi: 10.1148/radiology.160.2.3726125. [DOI] [PubMed] [Google Scholar]